Abstract
Background
Initial clinical trials from Europe have demonstrated that the administration of bone marrow-derived mononuclear cells (BMCs) may improve left-ventricular (LV) function in patients following ST-elevation myocardial infarction (STEMI). However, results from trials performed in the United States have not yet been presented.
Methods
We developed a Phase 1, randomized, placebo-controlled, double-blind trial to investigate the effects of BMC administration in patients following STEMI on recovery of LV function using cardiac MRI (cMRI). Forty patients with moderate to large anterior STEMIs were randomized to 100 million intracoronary BMCs vs placebo 3 to 10 days following successful primary angioplasty and stenting (PCI) of the left-anterior descending coronary artery.
Results
BMC administration was safely performed in a high-risk cohort with minimal MACE rates and all patients remain alive to date. LV ejection fraction (LVEF) increased from 49.0±9.5% at baseline to 55.2±9.8% at 6 months by cMRI in the BMC group (p < 0.05) that was not different from the increase in the placebo group (48.6 ± 8.5% to 57.0 ± 13.4%, p<0.05). LV end-diastolic volume (LVEDV) decreased by 4 ml/m2 in the BMC group at 6 months but increased significantly in the placebo group (17 ml/m2, p < 0.01).
Conclusions
This Phase 1 study from the United States confirms the ongoing safety profile of BMC administration in patients following STEMI. The improvement in LVEF at 6 months by cMRI in the cell therapy group was not different than the placebo group. However, BMC administration had a favorable effect on LV remodeling at 6 months.
INTRODUCTION
Recently, considerable attention has been focused on the use of stem cells to repair the heart following STEMI. In 2002, Strauer et al. (1) introduced the technique of intracoronary delivery of bone marrow mononuclear cells (BMCs) to STEMI patients based on initial observations in animals that stem cells derived from bone marrow could significantly improve left-ventricular (LV) function following coronary ligation and infarction (2). These findings led to the initiation of multiple clinical trials, predominantly in Europe, that administered intracoronary BMCs within several days following STEMI (3–6). Meta-analyses of these trials demonstrate that BMC delivery post-STEMI results in a small improvement in ejection fraction and attenuation of adverse LV remodeling compared to placebo (7–9). However, individual trial results have not been uniform, as several studies have failed to demonstrate an improvement in LV function following cell therapy compared to placebo (5,6). This has been attributed to a variety of factors including methodological differences of cell isolation (10) or insufficient dosing of cells. Furthermore, it is presently unclear why some patients appear to demonstrate significant recovery of LV function in response to cell therapy while other patients do not.
We initiated one of the first randomized, placebo-controlled cell therapy trials in the United States using BMCs following STEMI. We made several modifications in our protocol compared to the European studies. Whereas no previous study had delivered the same number of cells to their patients; we delivered a uniform dose of 100 million BMCs. Secondly, we elected to deliver cells by an intracoronary infusion as opposed to the stop-flow technique that had been used in all the preceding trials. This eliminated the potential confounding effects of repeated episodes of ischemia and reperfusion induced by this method of cell delivery (11). Here, we report the results of this Phase 1, randomized, double-blind, placebo-controlled trial in a high-risk cohort of patients with moderate to large anterior STEMIs.
METHODS
The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents.
Patient Enrollment
Forty patients (31 M, 9 F) were enrolled in this single center Phase 1 trial beginning in December 2005 following FDA approval (BB-IND-12480). All patients gave written, informed consent. The study was approved by the Institutional Review Boards of Abbott Northwestern Hospital and the University of Minnesota. Although 60 patients were approved for the trial, enrollment was stopped when our institution became a member of the NHLBI Cardiovascular Cell Therapy Research Network (CCTRN) and began enrollment in the TIME Trial (12). Eligible patients included those who presented with their first anterior STEMI who underwent successful angioplasty and stenting (PCI) of the LAD coronary artery. Enrolled patients were required to have at least two hours of chest pain and EKG changes consistent with anterior STEMI or new LBBB and an initial ejection fraction < 50% by echocardiography following PCI. Key exclusions included history of CABG or significant co-morbidities. Because the trial focused on enrolling patients at high risk for developing CHF or LV dysfunction, we included patients with cardiac arrest or cardiogenic shock. All patients were screened for the presence of pre-infarction angina prior to their STEMI presentation.
Study Protocol
Forty patients consented to participate in the study and were randomized in a 3:1 ratio to 100 million autologous BMCs versus placebo. A 3:1 ratio was utilized to increase our understanding of the safety profile of this therapy and to encourage enrollment based on the limited number of patients approved for the trial. Randomization was based on an algorithm developed by a biostatistician and was performed at the cell therapy facility following preparation of the BMCs. Cell therapy administration occurred when the patients were clinically stable, 3 to 10 days following their PCI and patients were routinely discharged the following day. On the morning of the procedure the patients were lightly sedated and bone marrow aspiration (50–70 ml) was performed at the posterior iliac crest by a hematopathologist. The aspirate was heparinized and transported within one hour to the University of Minnesota Cell Therapy Laboratory. BMCs were isolated by Ficoll density centrifugation (Gallard-Schlessinger; Plainview, NY) at 450g using a COBE 2991 Cell Processor (Gambro BCT; Lakewood Co.). The cell suspension was counted with an automated cell counter and the volume adjusted to obtain a final product of 100 million BMCs with 5% human serum albumin (HSA) in 20 ml. The placebo product was a solution of 0.9% normal saline and 5% HSA in an identical volume. The final product was delivered within 8 hours of the initial aspiration.
Lot release criteria included > 90% viability by trypan blue exclusion, negative gram stain and endotoxin < 5.0 EU/kg. Routine infectious disease testing included anaerobic, aerobic and fungal cultures, and virology assays for detection of HIV, HCV and Hepatitis B. Flow cytometry for measurement of CD34, CD45 and CD133 was performed in all samples.
Patients were returned to the cardiac catheterization laboratory in the afternoon following bone marrow aspiration. Patients were heparinized and a 6 Fr guiding catheter was placed in the left main coronary ostium. LAD stent patency and TIMI 3 flow was confirmed by angiography in all patients. A 3 Fr infusion catheter was positioned in the stented segment (TurboTracker, Boston Scientific Corp.) and the cellular product or placebo was infused at a rate of 1 ml/min over 20-min. Following completion of the infusion, angiography was again performed to confirm normal arterial flow. No patient experienced chest pain or ECG changes during the infusion.
Endpoints
The primary endpoints of this Phase 1 study was safety as assessed by major adverse clinical events (death, repeat target vessel revascularization, recurrent MI, hospitalization for CHF and ICD placement). Although the study was not sufficiently powered, we examined the absolute change in LVEF between baseline and 6 months as assessed by cardiac MRI (cMRI). Secondary end-points included changes in LV end-diastolic (LVEDV) and end-systolic volumes (LVESV).
cMRI measurements were performed at baseline, 3 and 6 months in all patients using a 1.5 T scanning unit (Avanto, Siemens Medical Solutions). All cMRI readers were blinded to treatment randomization. Commercial software was used for calculations of LVEF and end-systolic and -diastolic volumes and myocardial mass. Infarct size was quantified by delayed (20 min), contrast-enhancement with gadolinium (0.2 mmol/kg) using diastolic 2D flash imaging with the TI adjusted to “null” normal myocardium. The entire ventricle was covered with multiple breath-holds using a slice thickness of 5 mm.
Baseline clinical data is expressed as the median and inter-quartile range while cMRI data of LVEF and LV volumes are presented as mean ± SD. Categorical variables were compared using Fischer’s Exact testing while continuous variables were compared using a k-sample median test or Wilcoxin rank-sum test. The change in LVEF over time was analyzed by ANCOVA. The statistical software used for these calculations was STATA/IC 11.0.
Funding Sources
This study was supported by the John Dehaan Foundation. Cell processing was supported by the Production Assistance for Cellular Therapies (PACT), N01-HB-37164. Infusion catheters were generously supplied by the Boston Scientific Corporation (Natick, MA).
RESULTS
All 40 patients that consented to the study underwent successful bone marrow aspiration and infusion of BMCs (n=30) or placebo (n=10) without complication. There were no procedural complications associated with cell processing. No patient experienced a rise in troponin or procedure-related complication following the intracoronary infusion. Thirty-three patients presented with an occluded artery and TIMI 0 flow at the time of PCI and 5 patients underwent aspiration thrombectomy. A total of 12 patients experienced pre-infarction angina in the 24 hours prior to their STEMI (BMC = 10, placebo = 2) and 6 patients underwent postconditioning at the time of their PCI (BMC=3, placebo = 3). The majority of patients (38/40) received drug-eluting stents. Seven patients required placement of an intra-aortic balloon pump for cardiogenic shock and two patients underwent hypothermia treatment in the setting of cardiac arrest.
Major Adverse Cardiovascular Events (MACE)
Two patients in the placebo group experienced a MACE including one patient who received an ICD for palpitations and syncope, and a second patient who underwent repeat stenting in the LAD for in-stent restenosis at 15 months followed by CABG one month later after admission for a NSTEMI due to stent thrombosis. No patients in the BMC group experienced a MACE although two patients underwent non-target revascularization including CABG 8 months following cell therapy for an anomalous right coronary artery that was found to course between the aorta and pulmonary artery. A second patient underwent scheduled circumflex PCI two months following cell therapy infusion due to a pre-existing stenosis. No patients were hospitalized with CHF and all patients remained alive to date (Table 1).
Table I.
Major Adverse Events in Trial
| BMCs (n=30) |
Placebo (n=10) |
|
|---|---|---|
| Death | 0 | 0 |
| Myocardial Infarction | 0 | 1 |
| Revascularization- Target / Non Target | 0 / 2 | 1 / 0 |
| Hospitalization for CHF | 0 | 0 |
| ICD Implantation | 0 | 1 |
CHF = congestive heart failure, ICD = internal cardiac defibrillator
Clinical Presentation
This trial sought to enroll patients with moderate to large anterior infarctions who were at risk of developing significant LV dysfunction and CHF. In this high-risk cohort, the average baseline ejection fraction measured immediately following PCI by left-ventriculography was 35.7 ± 8.0% and 38.7± 10.5% by echocardiography 1–2 days following PCI. The average ischemic time for the entire group was 6.5 ± 4.3 hrs and the peak CK and CKMB was 3074 ± 2212 and 282 ± 230 IU/ml respectively. Patients received cell therapy on day 5.2 ± 2.3 days following PCI when they were clinically stable. There was no significant difference in baseline characteristics such as age, medications, risk factors, ischemic time or day of cell delivery post-AMI between the cell therapy and placebo groups (Table 2).
TABLE II.
Baseline Characteristics - BMC vs Placebo
| BMC (n=30) |
Placebo (n=10) |
P Value | |
|---|---|---|---|
| Age (years) | 52.5 (43, 64) | 57.5 (54, 59) | .193 |
| Male / Female | 25 / 5 | 6 / 4 | .665 |
| Diabetes | 6 | 2 | 1.00 |
| IABP | 5 | 2 | 1.00 |
| TIMI Flow = 0 | 26 / 30 | 8 / 10 | 1.00 |
| Ischemic Time (hrs) | 4.6 (2, 12) | 2.9 (2.8, 10.6) | .508 |
| Pre-Infarction Angina |
10 | 2 | .330 |
| Postconditioning | 3 | 3 | .230 |
| Peak Troponin (ng/ml) | 7.7 (3.9, 10.8) | 8.8 (5.6, 10.2) | .832 |
| Peak CK (IU/ml) | 2752 (1687, 4074) | 2049 (852, 3987) | .446 |
| Peak CKMB (IU/ml) | 202 (149, 320) | 291 (87, 368) | .804 |
| Time from PCI to BMC infusion (Days) |
4.5 (4,7) | 5.5 (3, 7) | 1.00 |
| Number of BMCs injected |
100 × 106 | _____ | |
| CD34+ (%) | 1.60 ± 0.73 | ||
| CD34+/CD133+ (%) | 0.77 ± 0.44 | ||
| CD133+ (%) | 0.16 ± 0.05 |
Data are expressed as the median with interquartile range
IABP – intra-aortic balloon pump
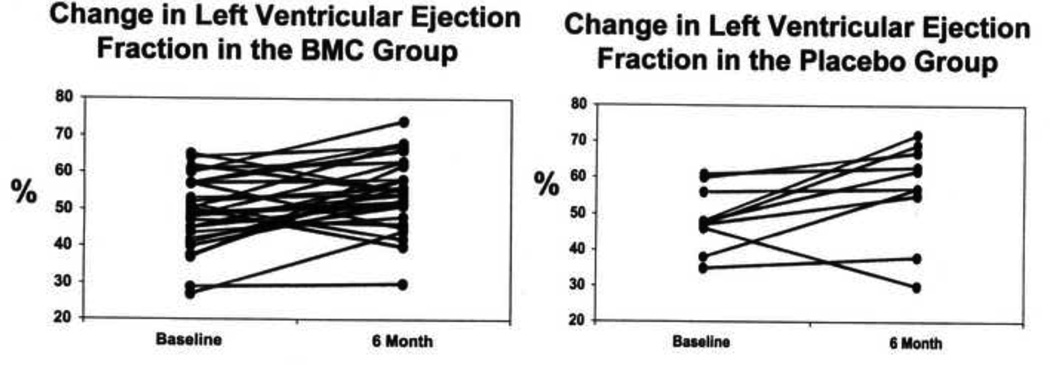
Patients underwent baseline cMRI at 3.3 ± 2.3 days following PCI. The average baseline LVEF was 49.0±9.5 % in the cell therapy group and 48.6 ± 8.5 % in the placebo group (Table 3). In our MRI laboratory, the reported LVEF is 5–10% higher than that measured by echocardiography. At 6 months there was a significant increase in LVEF in the cell therapy group to 55.1 ± 9.6 %, (p < 0.05) (Figure 1). However, this improvement was not significantly different than the improvement observed in the placebo group. (56.7 ± 13.9 %, p<0.05).
TABLE III.
LV Function and Volumes - BMC vs Placebo
| BMC (n=30) |
Placebo (n=10) |
P-Value | |
|---|---|---|---|
| LVEF – LV-gram (%) | 35.2 ± 8.4 | 36.5 ± 7.1 | .494 |
| Baseline LVEF (%)- ECHO | 38.9 ± 10.9 | 37.4 ± 9.8 | .331 |
| Baseline LVEF (%)-cMRI | 49.0 ± 9.5 | 48.6 ± 8.5 | .731 |
| Baseline LVEDV (ml/m2) | 88 ±31 | 77 ± 12 | .703 |
| Baseline LVESV (ml/m2) | 46 ± 26 | 40 ± 11 | .894 |
| Change in LVEF (%) from Baseline to 6 months |
6.2 ± 9.8 | 9.4 ± 10.0 | .425 |
| Change in LVEDV (ml/m2) from Baseline to 6 months |
−4 ± 22 | 17 ± 11 | 0.001 |
| Change in LVESV (ml/m2) from Baseline to 6 months |
−7 ± 3.3 | −2 ± 8.4 | .330 |
Data are expressed as mean ± SD.
Figure 1.
Change in left-ventricular ejection fraction (LVEF) by cardiac MRI between baseline and 6 months in the 30 individual patients following bone marrow mononuclear cell (BMC) administration and in the 10 individual placebo patients (both, p < 0.05).
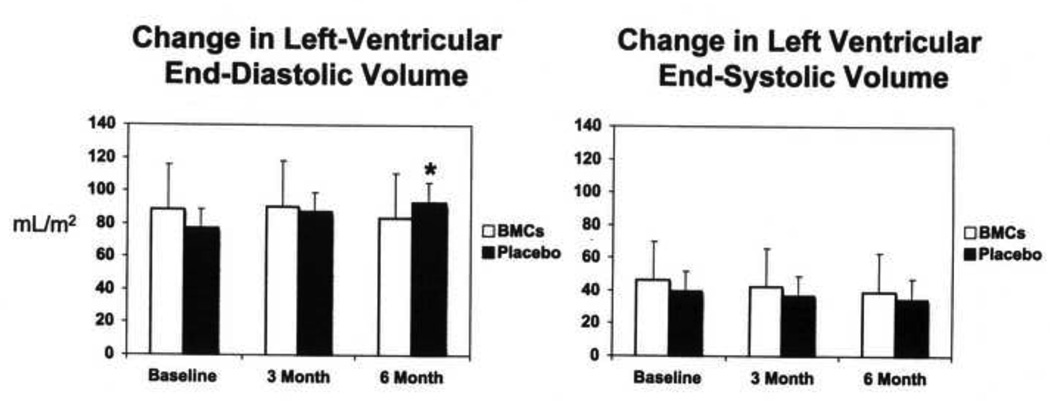
There was no significant difference in LV end-diastolic (LVEDV) or end-systolic volumes (LVESV) at baseline. (Table III, Figure 2). At 6 months there was a small decrease in LVEDV in the BMC group (−4 ml/m2), but a significant increase in the placebo group of 17 ml/m2 (p <0.01) suggesting a potential benefit of cell therapy on LV remodeling. No significant differences were observed between the two groups with respect to changes in LVESV at 6 months.
Figure 2.
Change in left-ventricular end-diastolic volume (LVEDV) and end-systolic volume (LVESV) by cardiac MRI between baseline, 3 and 6 months following bone marrow mononuclear cell (BMC) administration or placebo administration. BMC administration was associated with a significant improvement in LVEDV compared to the placebo group (p < 0.01)
DISCUSSION
In this Phase 1 trial we observed that cell therapy with intracoronary BMCs following moderate to large anterior STEMI can be safely performed with a low MACE rate in a high-risk patient cohort. This finding of safety is consistent with the European experience. Cell therapy was associated with a significant improvement in LVEF at 6 months following moderate to large anterior STEMI. However, we failed to demonstrate that cell therapy was superior to placebo due to the similar improvement in the small placebo group. We did observe that cell therapy had a beneficial effect on LV remodeling as LVEDV was significantly improved in the BMC group. Another important finding was that despite a high-risk subgroup of patients with large STEMI, MACE rates were low, including none in the BMC group.
Comparison with Previous Trials
The results from major European randomized trials administering BMCs one to seven days following STEMI have yielded mixed results in regard to their ability to improve LV function. The REPAIR-AMI trial (3) administered 236±174×106 BMCs vs placebo 4.3 days post-AMI in 204 patients and found a small but significant (2.5%) improvement in LVEF as measured by left-ventriculography compared to placebo. Importantly, they found that the benefit of cell therapy was sustained and resulted in a reduction in major adverse events at one year compared to placebo (13). The BOOST trial (4) administered 24.6 ± 9.4×108 total nucleated cells 4.8 days post-AMI and found a significant improvement in LVEF at 6 months compared to placebo as measured by cMRI. However, the statistical benefit was lost at 18 months due to further improvement in the placebo group (14). The ASTAMI trial (5) administered 54 to 130 ×106 BMCs (interquartile range) at a median of 6 days post-AMI and observed no improvement in LVEF at 6 months as measured by echocardiography, SPECT or cMRI. Janssens et al. (6) administered 172±72×106 BMCs one day following successful PCI for STEMI. Although they observed that cell therapy did not improve LVEF compared to placebo; they found that BMCs significantly improved regional systolic function in the infarct region by strain rate imaging with echocardiography at 4 months (15).
Although the mean total dose delivered has varied significantly in these studies, it is worth noting that none of these studies has delivered a uniform dose to its cohort. As a result, some patients may have received three times the amount of cells compared to other patients within the same trial. Although a clear dose-response relationship to recovery of LV function has not been observed, the failure to deliver a consistent dose within these trials remains a potential limitation in interpretation of the results. To overcome this limitation, all of the patients in our trial received the same number of BMCs at a dose sufficient to affect LV recovery as determined by a recent meta-analysis (9).
The “stop-flow” method of cell delivery previously utilized in all of these trials relies on the infusion of cells through an inflated PTCA catheter placed in the infarct artery delivered over multiple cycles of several minutes duration under the unproven assumption that this may improve cell retention. Several preclinical studies comparing cell retention between the stop-flow method versus continuous infusion used in our study have been performed yielding mixed conclusions. A recent pre-clinical study that labeled cultured mononuclear cells with 18F-FDG observed that a single bolus of cells produced superior myocardial retention at one hour compared to delivery of the cells using 3 cycles of stop-flow (16). In contrast, Meluzin et al. (17) administered allogeneic BMCs to pigs 3 days following infarction and observed that cell retention was improved with the stop-flow technique, but only in the infarct border zone. Tussios et al. (18) administered 11Indium-labeled BMCs to pigs following myocardial infarction and found no difference in retention between the two techniques and concluded that the stop-flow method was unnecessary. This technique, which produces brief periods of ischemia and reperfusion, has been demonstrated in models of ischemic preconditioning to result in the recruitment of progenitor cells to the myocardium (11,19) that may independently contribute to recovery of LV function. Because we utilized an intracoronary infusion, our study was free of this potentially confounding effect.
Limitations of STEMI Cell Therapy Trials
The small sample size and unbalanced distribution between the treatment and placebo groups may have contributed to the larger than expected increase in the LVEF observed in our placebo group. Additionally, half of the patients in the placebo group experienced either pre-infarction angina or underwent postconditioning at the time of their PCI that may have also increased their LVEF. It is important to note that the mean improvement in the placebo group of the 4 major randomized European trials (3–6) was only 2.6 ± 1.6 %, significantly less than the 8.4% improvement in our placebo group. Conversely, the mean absolute improvement in LVEF in our treatment group of 6.2% was significant, and similar to the improvement observed in the treatment groups of the positive REPAIR-AMI (3) and the initial BOOST Trial. (4).
In retrospect, it is not surprising that cell therapy administration with BMCs following STEMI has yielded such disparate results in regards to its ability to enhance the recovery of LV function (3–6). Although claims of suboptimal cell processing or insufficient dose have been suggested as a cause of negative trials, there are other important factors that have yet to be considered. The resolution of myocardial stunning represents an underappreciated issue in these trials, since the measurement of baseline LV function is performed during a period of significant LV recovery during the first week following PCI. In our study, the LVEF measured by echocardiography in the BMC group, increased from 38.9 ± 10.9 % to 44.6 ± 10.4 % in the first week following PCI with no further improvement in LVEF at one month. Serial cMRI studies following STEMI have demonstrated up to a 3% increase in LVEF over the first 7 days following STEMI and up to 5% by 6 weeks (20) We performed our baseline MRI 3.3 ± 2.3 days following PCI so it is possible that some of the recovery of LV function we observed at 6 months may be attributable to the resolution of myocardial stunning in the early post-MI period. Given these observations, it is not surprising that the subgroup of patients with the most depressed LVEF at baseline (3) have demonstrated the greatest improvement following cell therapy since they may possess a larger amount of recoverable LV function through this mechanism.
Patients with pre-infarction angina, in particular, may represent a subgroup of patients that will experience significant recovery of LV function that could be attributed to cell therapy (21,22). In our study, we observed that patients with pre-infarction angina had a 4-fold increase in LVEF at 6 months compared to those without pre-infarction angina. Importantly, this factor has never been accounted for in a cell therapy trial. New interventions performed in the catheterization laboratory to reduce ischemia / reperfusion injury or microvascular obstruction at the time of PCI, such as therapeutic hypothermia (23), postconditioning (24,25) and aspiration thrombectomy (26) may also reduce infarct size and improve LV function independently of cell therapy and impact trial results.
Conclusions
Our Phase 1 cell therapy trial using BMCs in patients with anterior STEMI confirms the ongoing safety profile of this therapy. We observed that the intracoronary infusion of 100 million BMCs was associated with a significant improvement in LV function at 6 months as measured by cMRI, but this improvement was not different than that observed in a small placebo group. However, we did demonstrate that cell therapy was associated with improved LV remodeling (LVEDV) which may have long-term implications in the development of CHF that will need to be assessed in larger trials.
Our study highlights some of the many factors that may modify infarct size (pre-infarction angina) and reperfusion injury (postconditioning) that could improve LV function independent of cell therapy. The ongoing advances in the treatment of ischemia / reperfusion injury in the STEMI population recruited for stem cell trials may create additional challenges in determining if cell therapy administration provides a therapeutic benefit.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None
REFERENCES
- 1.Strauer BE, Brehm M, Zeus T, et al. Repair of infracted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation. 2002;106:1913–1918. doi: 10.1161/01.cir.0000034046.87607.1c. [DOI] [PubMed] [Google Scholar]
- 2.Orlic D, Kajstura J, Chimenti S, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 3.Schächinger V, Erbs S, Elsässer A, et al. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. NEJM. 2006;355:1210–1221. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- 4.Wollert KC, Meyer GP, Lotz J, et al. Intracoronary autologous bone-marrow cell ransfer after myocardial infarction: the BOOST randomized controlled clinical trial. Lancet. 2004;364:141–148. doi: 10.1016/S0140-6736(04)16626-9. [DOI] [PubMed] [Google Scholar]
- 5.Lunde K, Solheim S, Aakhus S, et al. Intracoronary injection of mononuclear bone marrow cells in acute wall infarction. NEJM. 2006;355:1199–1209. doi: 10.1056/NEJMoa055706. [DOI] [PubMed] [Google Scholar]
- 6.Janssens S, Dubois C, Bogaert J, et al. Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: doubleblind, randomised controlled trial. Lancet. 2006;367:113–121. doi: 10.1016/S0140-6736(05)67861-0. [DOI] [PubMed] [Google Scholar]
- 7.Abdel-Latif A, Bolli R, Tleyjeh IM, et al. Adult bone marrow-derived cells for cardiac repair: a systemic review and meta-analysis. Arch Int Med. 2007;167:989–997. doi: 10.1001/archinte.167.10.989. [DOI] [PubMed] [Google Scholar]
- 8.Lipinski MJ, Biondi-Zoccai GGL, Abbate A, et al. Impact of intracoronary cell therapy on left ventricular function in the setting of acute myocardial infarction. JACC. 2007;50:1761–1767. doi: 10.1016/j.jacc.2007.07.041. [DOI] [PubMed] [Google Scholar]
- 9.Martin-Rendon E, Brunskill SJ, Hyde CJ, et al. Autologous bone marrow stem cells to treat acute myocardial infarction: a systematic review. Eur Heart J. 2008;29:1807–1818. doi: 10.1093/eurheartj/ehn220. [DOI] [PubMed] [Google Scholar]
- 10.Seeger FH, Tonn T, Krzossok N, et al. Cell isolation procedures matter: a comparison of different isolation protocols of bone marrow mononuclear cells used for cell therapy in patients with acute myocardial infarction. Eur Heart J. 2007;28:766–772. doi: 10.1093/eurheartj/ehl509. [DOI] [PubMed] [Google Scholar]
- 11.Ii M, Nishimura H, Iwakura A, et al. Endothelial progenitor cells are rapidly recruited to myocardium and mediate protective effect of ischemic preconditioning via “imported” nitric oxide synthase activity. Circ. 2005;111:1114. doi: 10.1161/01.CIR.0000157144.24888.7E. [DOI] [PubMed] [Google Scholar]
- 12.Traverse JH, Henry TD, Vaughan DE, et al. Rationale and design for TIME: a phase II, randomized, double-blind, placebo-controlled pilot trial evaluating the safety and effect of timing of administration of bone marrow mononuclear cells after acute myocardial infarction. Am Heart J. 2009;158:356–363. doi: 10.1016/j.ahj.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schachinger V, Erbs S, Elsasser A, et al. Improved clinical outcome after intracoronary administration of bone marrow-derived progenitor cells in acute myocardial infarction: final 1-year results of the REPAIR-AMI trial. Eur Heart J. 2006;27:2775–2783. doi: 10.1093/eurheartj/ehl388. [DOI] [PubMed] [Google Scholar]
- 14.Meyer GP, Wollert KC, Lotz J, et al. Intracoronary bone marrow cell transfer after myocardial infarction: 18 months’ follow-up data from randomized, controlled BOOST (BOne marrOw transfer to enhance ST-elevation infarct regeneration) trial. Circulation. 2006;113:1287–1294. doi: 10.1161/CIRCULATIONAHA.105.575118. [DOI] [PubMed] [Google Scholar]
- 15.Herbots L, D’hooge J, Eroglu E, et al. Improved regional function after autologous bone marrow-derived stem cell transfer in patients with acute myocardial infarction: a randomized, double-blind strain rate imaging study. Eur Heart J. 2009;30:662–670. doi: 10.1093/eurheartj/ehn532. [DOI] [PubMed] [Google Scholar]
- 16.Doyle B, Kemp BJ, Chareonthaitawee P, et al. Dynamic tracking during intracoronary injection of 18F-FDG-labeled progenitor cell therapy for acute myocardial infarction. J Nucl Med. 2007;48:1708–1714. doi: 10.2967/jnumed.107.042838. [DOI] [PubMed] [Google Scholar]
- 17.Meluzin J, Vlasin M, Groch L, et al. Intracoronary delivery of bone marrow cells to the acutely infracted myocardium. Optimization of the delivery technique. Cardiology. 2009;112:98–106. doi: 10.1159/000141462. [DOI] [PubMed] [Google Scholar]
- 18.Tossios P, Krausgrill B, Schmidt M, et al. Role of balloon occlusion for mononuclear bone marrow cell deposition after intracoronary injection in pigs with reperfused myocardial infarction. Eur Heart J. 2008;29:1911–1921. doi: 10.1093/eurheartj/ehn218. [DOI] [PubMed] [Google Scholar]
- 19.Kamota T, Li T-S, Morikage N, et al. Ischemic pre-conditioning enhances mobilization and recruitment of bone marrow stem cells to protect against ischemia/reperfusion injury in the late phase. JACC. 2009;19:1814–1822. doi: 10.1016/j.jacc.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 20.Engblom H, Hedstrom E, Heiberg E, et al. Rapid initial reduction of hyperenhanced myocardium after reperfused first myocardial infarction suggests recovery of the peri-infarction zone. One-year follow-up by MRI. Circ Cardiovasc Imaging. 2009;2:47–55. doi: 10.1161/CIRCIMAGING.108.802199. [DOI] [PubMed] [Google Scholar]
- 21.Ottani F, Galli M, Zerboni S, et al. Prodromal angina limits infarct size in the setting of acute myocardial infarction treated with primary percutaneous intervention. JACC. 2005;45:1545–1547. doi: 10.1016/j.jacc.2005.02.033. [DOI] [PubMed] [Google Scholar]
- 22.Kloner RA, Shook T, Przyklenk K, et al. Previous angina alters in-hospital outcome in TIMI 4: a clinical correlate to preconditioning? Circulation. 1995;91:37–45. doi: 10.1161/01.cir.91.1.37. [DOI] [PubMed] [Google Scholar]
- 23.Tissier R, Couvreur N, Ghaleh B, et al. Rapid cooling preserves the ischaemic myocardium against mitochondrial damage and left-ventricular dysfunction. Cardiovasc Res. 2009;83:345–353. doi: 10.1093/cvr/cvp046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lonborg J, Kelbaek H, Vejlstrup N, et al. Cardioprotective effects of ischemic postconditioning in patients treated with primary percutaneous intervention, evaluated by magnetic resonance. Circ Cardiovasc Interv. 2010;3:34–41. doi: 10.1161/CIRCINTERVENTIONS.109.905521. [DOI] [PubMed] [Google Scholar]
- 25.Traverse JH, Wang YL, Chavez IJ, et al. Post-conditioning reduces infarct size and improves LV function during acute myocardial infarction. Circulation. 2006;114:II–344. [Google Scholar]
- 26.Sardella G, Mancone M, Bucciarelli-Ducci C, et al. Thrombus aspiration during primary percutaneous coronary intervention improves myocardial reperfusion and reduces infarct size: The EXPIRA (thrombectomy with export catheter in infarct-related artery during primary percutaneous coronary intervention) prospective, randomized trial. J Am Coll Cardiol. 2009;53:309–315. doi: 10.1016/j.jacc.2008.10.017. [DOI] [PubMed] [Google Scholar]