Abstract
Glioblastoma multiforme (GBM) is a lethal cancer that responds poorly to radiotherapy and chemotherapy. Glioma cancer-initiating cells have been shown to recapitulate the characteristic features of GBM and mediate chemotherapy and radiation resistance. However, it is unknown whether the cancer-initiating cells contribute to the profound immune suppression in GBM patients. Recent studies have found that the activated form of signal transducer and activator of transcription 3 (STAT3) is a key mediator in GBM immunosuppression. We isolated and generated CD133+ cancer-initiating single colonies from GBM patients and investigated their immune suppressive properties. We found that the cancer-initiating cells inhibited T cell proliferation and activation, induced regulatory T cells (Tregs) and triggered T cell apoptosis. The STAT3 pathway is constitutively active in these clones and the immunosuppressive properties were markedly diminished when the STAT3 pathway was blocked in the cancer-initiating cells. These findings indicate that cancer-initiating cells contribute to GBMs’ immune evasion and that blockade of the STAT3 pathway has therapeutic potential.
Keywords: Cancer-initiating cells, glioblastoma multiforme, immune suppression, signal transducer and activator of transcription 3, regulatory T cells
INTRODUCTION
Malignant gliomas express tumor-associated and tumor-specific antigens that should make these tumors detectable to the immune system (1). However, there is a distinct lack of immune-mediated tumor eradication in glioma patients, and most attempts at immunotherapy have met with little clinical success (2). Many factors work in concert to inhibit anti-glioma immunity, including immunosuppressive cytokines such as IL-10, TGF-β, and prostaglandin E2 (PGE2), the induction of Tregs, and down modulation of co-stimulation molecules by antigen presenting cells (APCs) resulting in loss of T cell effector function - all of which that have been shown to be operational in GBM patients (Reviewed in 3). Although central nervous system (CNS) tumors are recognized by the immune system, this is insufficient for their suppression or eradication. Primed CD8+ cytotoxic T cells gain CNS access (4); however, the lack of tumor eradication indicates that the T cells mediating adaptive immune responses are deficient in malignant glioma patients and are functionally impaired within the local tumor microenvironment (5–9).
Cancer-initiating cells are a heterogeneous population of undifferentiated cells with the capacity for self-renewal and a high proliferative potential. GBMs contain cancer-initiating cells that are multi-potent, and can recapitulate the characteristics of GBM including high motility, diversity of progeny, tendency to migrate along white matter tracts, and expression of immature antigenic phenotypes such as epidermal growth factor receptor and nestin (10). Cancer-initiating cells may express CD133 (11), although cancer-initiating cells have been identified that do not express CD133 (12–14) indicating this is not a definitive marker for the identification of stem cells. Furthermore, the expression of CD133 is heterogeneous within neurospheres and can alter with subsequent passage and cloning (15). Characteristics of stem cells also include high levels of the anti-apoptotic genes, the ability to form neurospheres, nonadherence, possession of marker characteristics for astrocytic, neuronal, and oligodendroglial lineages (16) and tumorigencity in vivo. The cancer-initiating cells are believed to confer the resistance to chemotherapy and radiation observed in GBM (17, 18). Since significant T cell immune suppression has been shown to be induced by the GBM and since glioblastoma associated cancer-initiating cells are a therapeutically resistant population, the question arises as to the participation of cancer-initiating cells in inhibiting T cell responses.
The STAT3 pathway has been shown to be a potent regulator of anti-inflammatory responses through its suppression of macrophage activation (19, 20), reduction of the cellular cytotoxicity of natural killer cells and neutrophils, and reduction of the expression of major histocompatibility complex (MHC) II, CD80, CD86, and IL-12 in dendritic cells rendering them unable to stimulate T cells and generate antitumor immunity (21). The ablation of STAT3 activity in only the immune cells results in marked antitumor effects in vivo, indicating that STAT3 expression within the immune cells is what restrains antitumor eradication (21). Recent evidence shows that GBM-associated immunosuppression is linked to the up-regulation of STAT3 in tumor cells (22). Many growth factors and cytokines, including IL-6 and epidermal growth factor, activate Janus kinase 2 (Jak2), which subsequently activates STAT3 by phosphorylation of the tyrosine residue in the transactivation domain. Phosphorylated STAT3 (p-STAT3), which is over-expressed in most cancers including gliomas (23, 24) then translocates into the nucleus and induces a variety of transcriptional factors that propagate tumorigenesis (25) and up-regulate tumor-mediated immunosuppressive factors (26). These factors include IL-10 (27, 28) that adversely influences Th1-mediated cytotoxic immune responses at multiple levels and is essential for Treg function (29, 30), VEGF (31) that inhibits dendritic cell maturation and activation by inhibiting co-stimulatory molecule expression (32), PGE2 (33) that induces the immune suppressive Th17 cell (34), and TGF-β (35) that induces Tregs, inhibits T cell proliferation and down-modulates the IL-2 receptor (reviewed in 36), to name just a few. These STAT3-regulated tumor secreted factors then activate STAT3 in diverse immune cells including both innate immune cells and T cells (21). Because STAT3 target genes encode many factors that activate STAT3 in the immune cells, a feed-forward mechanism for constitutive activation of STAT3 in both the tumor cells and the immune cells within the tumor microenvironment is initiated. Cumulatively, these data indicate that the STAT3 pathway is a molecular hub of tumor mediate immune suppression.
The inhibition of STAT3 activity in only the hematopoietic cells of tumor-bearing mice can induce potent multi-component anti-tumor immune responses (21). We have previously found that inhibition of STAT3 with WP1066, which blocks the Jak2/STAT3 interaction and subsequent phosphorylation of STAT3 at tyrosine705 (p-STAT3) (37), results in the up-regulation of co-stimulatory molecules (CD80 and CD86) on human microglia, secretion of pro-inflammatory cytokines essential for T-effector responses, and activation and proliferation of T cells (22). Thus, STAT3 blockade is a potent approach for modulating multi-factorial immunosuppression and eliciting anti-tumor immune responses. Cancer-initiating cells have been shown to have activated p-STAT3 that can be blocked with p-STAT3 inhibitors, resulting in diminished stem cell proliferation, neurosphere formation, and depletion of CD133 positive (CD133+) cells (38). Specifically, the STAT3 inhibitor WP1066 can induce apoptosis of human glioma cells both in vitro and in vivo (39), reverse glioma mediated immune suppression (22), is orally bioavailable, achieves excellent CNS penetration, exerts efficacy against established intracerebral tumors with minimal toxicity (40) and inhibits Tregs (41). Based on the aforementioned properties (42) and the pending investigational new drug application of WP1066, we selected WP1066 and siRNA for these studies to test the hypothesis that if cancer-initiating cells mediate T cell immune suppression that the p-STAT3 pathway would likely be a key mediator of this immune suppression which could be reversed by blockade of the STAT3 pathway.
MATERIALS AND METHODS
Human glioma cell lines
Human normal astrocytes and glioma cell lines U-251 and U87 were purchased from the American Type Culture Collection (Manassas, VA) and cultured in Royal Park Memorial Institute (RPMI) 1640 medium (astrocytes), modified Eagle’s medium (MEM) (U-251) or MEM plus 0.1 mM nonessential amino acids (U-87). To all media, 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin were added.
Human tumors
Tumor tissues from newly diagnosed GBM patients (n=9) were obtained from surgery specimens and were graded pathologically according to the World Health Organization’s classification system by a neuropathologist. Each patient provided written informed consent for tumor tissues and this study was conducted under protocol #LAB03-0687, which was approved by the institutional review board of The University of Texas M. D. Anderson Cancer Center.
Human glioma cancer-initiating cell derivation
GBM specimens were processed within 4 hours after resection. They were washed and disassociated mechanically. After a single-cell suspension was prepared, erythrocytes were lysed using 1x RBC lysis buffer (eBioscience, San Diego, CA). Trypan blue staining confirmed >80% cell viability. Glioma-associated cancer-initiating cells were cultured in Dulbecco’s Modified Eagle Medium F-12 containing 20 ng/ml of EGF, basic fibroblast growth factor (bFGF) (Sigma, St Louis, MO), and B27 (1:50; Invitrogen, Calsbad, CA) as a neural stem cell-permissive medium (neurosphere medium) and passaged every 5–7 days. CD133 expression on glioma-associated cancer-initiating cells was confirmed prior to use.
Intracranial xenografts
Single cell suspensions of glioma-associated cancer-initiating cells in serum-free medium at 2 × 102 cells per 5 μL were injected into the right frontal lobes of 58-week-old nude mice using a stereotactic frame system (Kopf Instruments, Tujunga, CA) as previously described (43). Mice were maintained in the M. D. Anderson Isolation Facility in accordance with Laboratory Animal Resources Commission standards and conducted according to an approved protocol, 08-06-11831.
Cloning of glioma-associated cancer-initiating cells
Accutase (Sigma, St Louis, MO) dissociated glioma-associated cancer-initiating cells were sorted using the CD133 cell isolation kit (Miltenyi Biotech, Auburn, CA), and > 90% purity CD133+ sorted cells were seeded into 96-well plates at a theoretical density of 1 cell per well. After overnight culture, microscopic observation was utilized to identify wells that contained a single cell. These wells were monitored and the medium changed every 5–7 days for 45 days before immune functional analysis.
Human peripheral blood mononuclear cells (PBMCs)
PBMCs were prepared from healthy donor blood (Gulf Coast Blood Center, Houston, TX) and GBM patients’ blood (the same patients whose glioma-associated cancer-initiating cells were isolated) by centrifugation on a Ficoll-Hypaque density gradient (Sigma-Aldrich, St. Louis, MO). Aliquots of the isolated PBMCs were frozen and stored at −180°C until use. Prior to co-culture experiments, frozen PBMCs were thawed at 37°C for 5 min and then washed with warm 10% FBS in RPMI 1640 medium. CD3+ T cells were purified from PBMCs by negative selection using a Pan T Cell Isolation Kit II (Miltenyi Biotech, Auburn, CA), according to the manufacturer’s instructions.
Antibodies and reagents
Tissue culture grade monoclonal antibodies to CD3 (OKT3) and CD28 (28.6) were obtained from eBioscience. Anti-human IL-6 (1936) and anti-human TGF-β1 (27235) antibodies were obtained from R&D Systems (Minneapolis, MN). WP1066 was synthesized and supplied by Waldemar Priebe, and was stored as a 10 mM stock in dimethyl sulfoxide and diluted with phosphate buffered saline (PBS) when used. WP1066 was used at doses that could be achieved in vivo (1–5 μM) (22). The cell surface was stained with phycoerythrin (PE), fluorescein isothiocyanate (FITC), or allophycocyanin (APC)-conjugated antibodies against the following proteins: CD3, CD4, CD8, MHC I, MHC II, CD40, CD80, CD86, and B7-H1 (BD Pharmingen, San Diego, CA) and CD133 (Miltenyi Biotech, Auburn, CA). To detect intracellular cytokines, PE-conjugated antibodies against IL-2 and interferon (IFN)-γ (R&D Systems) were used. Intracellular STAT3 was assessed using PE-conjugated p-STAT3 (pY705) (BD Pharmingen). Appropriate isotype controls were used for each antibody.
Flow cytometry
FITC-conjugated anti-CD4 (RPA-T4) and APC-conjugated anti-CD8 (RPA-T8) antibodies were used for cell surface staining. Sub-analysis of the T cell populations was based on the gated surface expression of CD4 and CD8. To detect forkhead box P3 (FoxP3) protein expression, the surface stained cells were further subjected to intracellular staining with PE-conjugated monoclonal antibodies to human FoxP3 (clone PCH101, eBioscience) using staining buffers and conditions specified by the manufacturer. For intracellular cytokine staining, cells were stimulated for 6 hours in the presence of 50 ng/ml phorbol myristate acetate, 500 ng/ml ionomycin (Sigma-Aldrich), and 2 μM monensin (GolgiStop, BD Pharmingen). Then the cells were incubated with FITC-conjugated anti-CD4 and APC-conjugated anti-CD8 (RPA-T8) antibodies for surface staining followed by intracellular staining using PE-conjugated anti-mouse IFN-γ (4S.B3) or PE-conjugated anti-mouse IL-2 (MQ1-17H12) antibodies and FIX/PERM buffers (BD Pharmingen) according to the manufacturer’s instructions. Intracellular p-STAT3 intracellular staining was performed as previously described (41). Flow cytometry acquisition was done with a FACSCaliber (Becton Dickinson, San Diego, CA) and data analysis was with FlowJo software (TreeStar, Ashland, OR).
Glioma-associated cancer-initiating cell differentiation
Accutase-dissociated glioma-associated cancer-initiating cells were cultured in differentiation medium consisting of 10% FBS, 10 ng/ml retinoic acid, and 20 ng/ml PDGF-AA (both from Sigma-Aldrich) (44). Confluent monolayer cells were detached every 5–7 days by trypsinization, and retinoic acid and PDGF-AA were replenished during the culture.
Immunohistochemistry
Differentiated glioma-associated cancer-initiating cells were cultured on eight-chamber slides (Nunc, Rochester, NY) at 5,000/well. After 3 days, cells were fixed with 4% paraformaldehyde, permeabilized with 3% Triton X-100 in PBS, and then blocked with 5% horse serum. Primary antibodies were rabbit anti-GFAP (1:40; Dako, Golstrup, Denmark), mouse anti-galactosylceramidase (GalC) (1:100; Chemicon, Ramona, CA), and mouse anti-microtubule associate protein 2 (MAP2) (1:50; Chemicon). After incubation for 90 min, the slides were washed with 5% horse serum. Secondary antibodies, goat anti-rabbit Alexa 546 (1:300; Invitrogen) and donkey anti-mouse Alexa 488 (1:300; Invitrogen), were added for 30 min. Slides were mounted using Vectashield Hard Set mounting medium with DAPI (Vector Laboratories, Burlingame, CA).
Enzyme-linked immunosorbent assay (ELISA)
Supernatants from the human glioma cell lines U-87 and U-251, and the glioma-associated cancer-initiating cells were measured for cytokine concentrations using ELISA kits per manufacturer’s instructions (R&D Systems). These supernatants were collected after 5 days in culture and stored at −20°C. The supernatants were added in duplicate to appropriate pre-coated plates. The optical density was measured at 450 nm with a microplate reader (Spectra Max 190; Molecular Devices, Sunnyvale, CA), and chemokine concentrations were quantitated with SoftMax Pro software (Molecular Devices). The detection limits for chemokine C-C motif-2 (CCL-2) were 5 pg/ml; TGF-β1, 16 pg/ml; IL-10, 5 pg/ml; IL-6, 1 pg/ml; PGE2, 10 pg/ml; soluble Fas, 10 pg/ml, and Galectin-3, 10 pg/ml.
Cell proliferation assay and Treg induction assay
Glioma-associated cancer-initiating cells were plated into 48-well plates (3 × 104 cells/ml) containing 3 × 105 PBMCs/ml in the presence of 1 μg/ml pre-bound anti-CD3/anti-CD28 antibodies or 2.5 μg/ml phytohemagglutinin (PHA, Sigma-Aldrich). Alternatively, conditioned media from the glioma-associated cancer-initiating cells were also added to the stimulated PBMCs. After 72 hours, 100 μl of cells from each well was transferred to new 96-well plates with 10 μl of Cell Counting Kit-8 (Dojindo Laboratories, Rockville, MD). After incubation for 4 hours at 37°C, absorbance was measured at 450 nm with a microplate reader (Spectra Max 190). To detect FoxP3+ Tregs, CD4 surface staining and then FoxP3 intracellular staining were performed on immune cells cultured for 96 hours.
FoxP3+ Treg suppression assay
Healthy donor PBMCs were labeled with 2 μM carboxyfluorescein diacetate succinimidyl ester (CFSE) for 5 min at room temperature in PBS with 0.1% bovine serum albumin, and then the reaction was quenched with RPMI 1640 medium with 10% FBS for 10 min at 37°C. 1 × 106/ml CFSE-labeled PBMCs, and 1 × 105/ml autologous T cells which were cultured with conditioned media from glioma-associated cancer-initiating cells for 4 days, were plated into 96-well plates in the presence of 2 × 106/ml allogeneic irradiated PBMCs in RPMI 1640 medium with10% FBS in a total volume of 0.2 ml. After 72 hours, the cells were harvested, and analysis of cell division was performed by flow cytometry.
Apoptosis assay
The T cell apoptosis assay was performed with the Annexin V/7AAD staining kit (BD Pharmingen). Healthy donor PBMCs were cultured with medium or glioma-associated cancer-initiating cell supernatants and then harvested by centrifugation at day 3 and day 5. The cells were stained with APC-conjugated anti-CD3 antibodies and then washed twice with cold PBS and resuspended in 1x binding buffer (BD Pharmingen) at a concentration of 1 × 106 cells/ml. Next, PE-conjugated annexin V and 7-AAD were added, the cells were incubated for 20 min at 25°C in the dark, and CD3+ T cell apoptosis was analyzed by flow cytometry within one hour.
STAT3 siRNA transfection
To knock down STAT3 gene expression in glioma-associated cancer-initiating cells, STAT3 siRNA was transfected into two of our established glioma-associated cancer-initiating cell lines, as described (Santa Cruz Biotech, Santa Cruz, CA). Briefly, 2 × 105 glioma-associated cancer-initiating cells per well were seeded in 2 ml antibiotic-free medium in six-well plates and incubated for 8 h. The siRNA duplex solution (1 ug STAT3 siRNA or control siRNA) in 100 ul siRNA transfection medium were prepared and gently mixed with diluted siRNA transfection reagent and incubated for 45 min at room temperature. The mixture was then over layed onto the cells washed by transfection medium. The cells were incubated for 5 h at 37°C, then 1 ml of neurosphere medium was added and the cells cultured for an additional 72 h prior to conducting the immune functional assays.
Statistical analysis
All values were calculated as means and 95% confidence intervals (CIs) from at least three independent experiments The Student t test was used to test for differences in the means between two groups. P values less than 0.05 were considered to be statistically significant. All statistical analyses were performed using the Statistical Package for the Social Sciences v.12.0.0 (SPSS, Chicago, IL). Error bars represent SD.
RESULTS
Isolation of cancer-initiating cells from GBM patients
From newly diagnosed GBM patients (n=9) at the time of surgery, we isolated cancer-initiating cells and the patients’ autologous T cells. The cancer-initiating cells from the patients expressed CD133, formed neurospheres (Fig. 1A) in serum-free medium containing EGF and bFGF after 5–10 days of culture, and these CD133+ cells express CD34 but not CD45 indicating that they are of endothelial origin (Fig. 1B) (45). The established cancer-initiating cells were capable of differentiating into glial fibrillary acidic protein (GFAP+) astrocyte-like cells, neuron-like cells that were immunoreactive for MAP2, and GalC -immunoreactive oligodendrocyte-like cells (Fig. 1C). When the cancer-initiating cells were injected in the right frontal lobes of 5–8-week-old nude mice, the mice developed tumors that were highly infiltrative along white matter tracts--a characteristic of human GBM (Fig. 1D). To further characterize the cancer-initiating cells, CD133+ cancer-initiating cells were sorted from the neurospheres and diluted for single colony formation. Over 80% of seeded single cells grew out and ten clones from each neurosphere were selected at random and expanded for further immunological characterization.
Figure 1. Characterization of human glioma-associated cancer-initiating cells.
A. A representative image of neurospheres from one glioma-associated cancer-initiating cell is shown. B. The glioma-associated cancer-initiating cells were surface stained with antibodies to CD133, CD34 and CD45. Representative FACS histogram plots for CD133+ cells are shown for target staining (shaded line) with associated isotype controls (gray line). Percentages of the positive populations are shown. C. After 7 days of culture in differentiating medium, the glioma-associated cancer-initiating cells differentiated into GFAP+ astroglial lineage cells, MAP2+ neuronal lineage cells, and GalC+ oligodendroglial lineage cells (magnification X 40) indicating the glioma-associated cancer-initiating cells have multi-potent differentiation potential. D. A representative image of a glioma-associated cancer-initiating cell xenografted into the frontal lobe of a nude mouse. The tumor that developed from the glioma-associated cancer-initiating cell caused enlargement of the brain and were diffusely infiltrative (arrow on right), including into white matter tracts such as the corpus callosum (arrow on left).
Immunological characterization of cancer-initiating cells
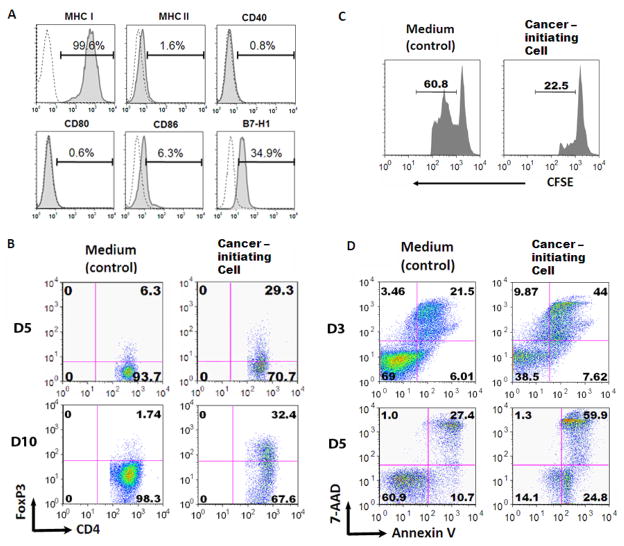
To characterize their immunological phenotype, the cancer-initiating cells (n=5) were assessed for their expression of MHC I, MHC II, CD40, CD80, CD86, and B7-H1, by flow cytometry. The cancer-initiating cells expressed high levels of MHC I (mean 99.3%, range 98.5–99.8%) and low levels of CD86 (mean 6.7%, range 5.9–7.9%) and CD40 (mean 5.8%, range 0.7–15.8%), but not MHC II (mean 2.4%, range 1.6–3.2%) or CD80 (mean 0.6%, range 0.2–0.6%) (a representative example is shown in Fig 2A), indicating that cancer-initiating cells lack the capacity for antigen presentation necessary to stimulate T cell activation or proliferation. Furthermore, the inhibitory co-stimulatory molecule B7-H1 (mean 31.2%, range 28.5–34.9%) was expressed, indicating that direct contact between T cells and cancer-initiating cells would be inhibitory on immune cells.
Figure 2. Glioma-associated cancer-initiating cells are immunosuppressive of human T cells.
A. Immune surface phenotype of a representative glioma-associated cancer-initiating cell. The glioma-associated cancer-initiating cells were surface stained with antibodies to MHC I, MHC II, CD40, CD80, CD86, and B7-H1. Representative FACS histogram plots for one glioma-associated cancer-initiating cell are shown for target staining (solid line) with associated isotype controls (dotted line). Percentages of the positive populations are shown. B. The supernatants from the glioma-associated cancer-initiating cells induce an increase in the number of CD4+FoxP3+ Tregs on both day 5 and 10. Representative FACS plots are shown. C. The glioma-associated cancer-initiating cell induced FoxP3+ Tregs suppress T cell proliferation. T cells that were treated with glioma-associated cancer-initiating cell supernatants were harvested, co-cultured for 3 days with autologous PBMCs (labeled with CFSE, responder cells) at a 1:1 ratio in the presence of soluble anti-CD3 and subsequently analyzed via FACScan. The number above the line in each histogram represents proliferating responder cells. D. The glioma-associated cancer-initiating cell supernatants induce T cell apoptosis after 3 days of exposure to the supernatants. Similar data was obtained after 5 days of exposure. After culturing with the glioma-associated cancer-initiating cell supernatants, T cells were stimulated with anti-CD3/CD28 and stained with 7-AAD and Annexin V. Compared to medium alone (control), the glioma-associated cancer-initiating cells enhanced T cell apoptosis.
To evaluate what immunosuppressive cytokines the cancer-initiating cells were elaborating, the cancer-initiating cells (n=4) and established glioma cell lines were assayed for immunosuppressive cytokines such as TGF-β1, IL-6, IL-10, PGE2, soluble Fas, and VEGF by ELISA. The cancer-initiating cells did not produce any appreciable IL-6, IL-10, or soluble Fas, but did produce TGF-β1 (38.5 -118 pg/ml) and the Treg chemokine attractant CCL-2 (12.8 –1134 pg/ml) (Table 1).
Table 1.
Immunosuppressive cytokines and chemokines are elaborated by glioma cell lines and glioma-associated cancer-initiating cells
| Source | Cytokine (pg/ml) |
|||||||
|---|---|---|---|---|---|---|---|---|
| TGF-β1 | VEGF | IL-6 | PGE2 | CCL-2 | Fas | IL-10 | Galectin-3 | |
| U-87 | 2473 | 3920 | 43,568 | 231 | 4401 | 0 | 0 | 467 |
| U-251 | 1597 | 1708 | 70 | 80 | 10,586 | 0 | 0 | 175 |
| Cancer-initiating cells (n=4) | 76 (38.5–118) | 49 (22–98) | 0 | 75 (54–95) | 457 (12.8–1134) | 0 | 0 | 2440 (1645–3183) |
For U-87 and U-251, the values are actual values from one experiment while the cancer-initiating cell values are means with ranges.
Cancer-initiating cells inhibit T cell activation and proliferation
To determine if the cancer-initiating cells could inhibit the proliferation and elaboration of pro-inflammatory responses of immune cells, PBMCs from healthy donors were activated with anti-CD3/CD28 or PHA in the presence of conditioned medium obtained from 3-day cultures of cancer-initiating cells and T cell proliferation was assessed by flow cytometry. The media from all of the CD133+ clones (n=20, generated from two GBM specimens, and 10 clones from each) potently inhibited T cell proliferation by 91 ± 12% (p=0.0005). No inhibition of T cell proliferation was detected when the conditioned medium was obtained from either normal human astrocytes or the U-87 cell line. To ascertain if the supernatants could also inhibit the elaboration of the pro-inflammatory cytokines IL-2 and IFN-γ from the CD4+ T cell helper and the CD8+ T cell effector subset, the cancer-initiating cell supernatants were co-incubated with healthy donors’ PBMCs in the presence of anti-CD3/CD28 stimulation, and the percentages of CD4+ and CD8+ T cells producing IL-2 and IFN-γ were determined by intracellular staining via flow cytometry. The production of both IFN-γ and IL-2 by the CD4+ and CD8+ T cell subsets was suppressed by the cancer-initiating cell supernatants (Table 2). These data demonstrate that cancer-initiating cells suppress T cell proliferative and pro-inflammatory responses.
Table 2.
Effects of cancer-initiating supernatants on cytokine production by CD4 or CD8 T cell subsets
| Experimental condition | Cancer- initiating cell supernatant | T cell subset | IL-2 producing cells (%) | Change compared to medium (%) | γ-IFN producing cells (%) | Change compared to medium (%) |
|---|---|---|---|---|---|---|
| Medium | None | CD4 | 60.5 | --- | 28.3 | --- |
| Medium | None | CD8 | 64.2 | --- | 53.9 | --- |
| Supernatant | 9–29 | CD4 | 21.4 | ↓ 65 | 17.5 | ↓ 38 |
| Supernatant | 9–29 | CD8 | 6.1 | ↓ 90 | 49.4 | ↓ 8 |
| Supernatant | 6–15 | CD4 | 36.5 | ↓ 40 | 19.9 | ↓ 30 |
| Supernatant | 6–15 | CD8 | 14.5 | ↓ 76 | 43.5 | ↓ 19 |
| Supernatant | 11–28 | CD4 | 31.8 | ↓ 47 | 22.3 | ↓ 21 |
| Supernatant | 11–28 | CD8 | 9.2 | ↓ 85 | 50.3 | ↓ 7 |
Cancer-initiating cells induce Tregs and T cell apoptosis
Since the cancer-initiating cells were producing TGF-β, we next determined if these cells could induce Tregs. Incubation with supernatants from the cancer-initiating cells (18 out of 20) markedly expanded the number of CD4+FoxP3+ Tregs in healthy donor PBMCs by 128 ± 51 % (p=0.0007)(representative example in Fig. 2B). These FoxP3+ Tregs were functionally suppressive in autologous T cell proliferation assays (Fig. 2C). All of the cancer-initiating supernatants (n=20) were able to increase immune cell apoptosis by 144 ± 29% (p=0.0001) in healthy donor PBMCs. Furthermore, when GBM patients’ PBMCs were co-incubated with the respective patients’ cancer-initiating cells, as predicted from the phenotypic expression of B7-H1, both pre-apoptosis and apoptosis were induced in the immune cells (representative example in Fig. 2D). Conditioned media from normal human astrocytes and the U-87 glioma cell line did not induce T cell apoptosis. Activated immune cells also underwent apoptosis when co-cultured with conditioned medium from cancer-initiating cells, indicating that activation did not protect immune cells from the apoptosis induced by conditioned medium from cancer-initiating cells. This indicates that cancer-initiating cells can mediate immunosuppression by apoptotic elimination of immune cells, regardless of their activation state, likely by both secretion of product(s) and direct cell-to-cell contact.
Cancer-initiating cells induce the expression of p-STAT3 in immune cells
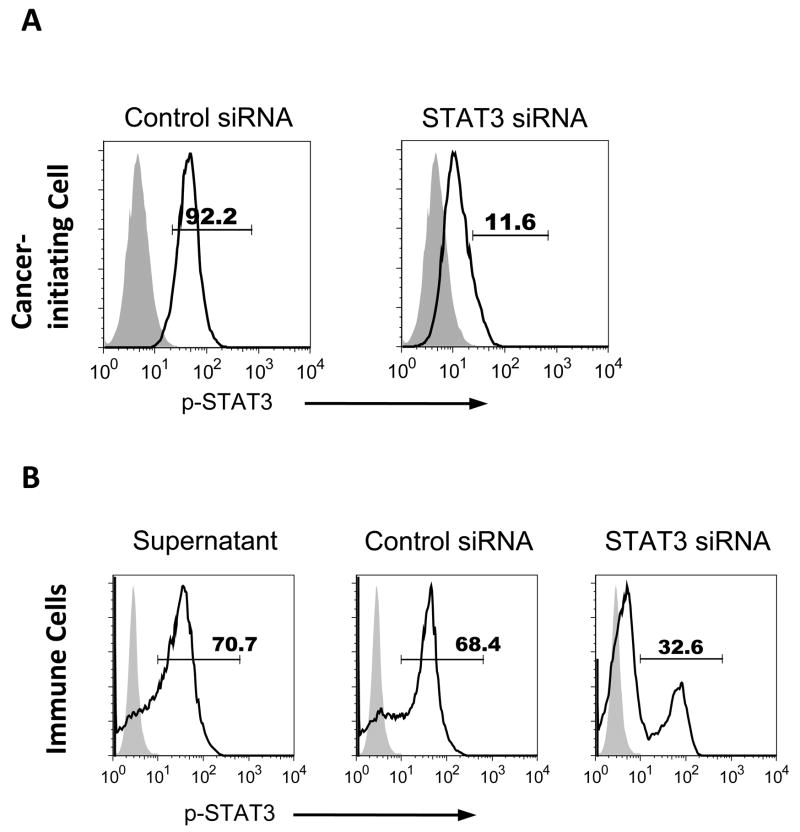
The expression of p-STAT3 by tumor cells triggers a cascade of transcriptional factors that have been shown to activate p-STAT3 in immune cells (26) and cancer-initiating cells have been shown to express p-STAT3 (38). The cancer-initiating cells p-STAT3 expression can be inhibited with the STAT3 siRNA (Fig. 3A), and this treatment did not affect cell viability based on trypan blue exclusion. The supernatants from cancer-initiating cells (n=3) were capable of increasing p-STAT3 expression in immune cells by 64.7 ± 12.5% (p=0.006) (representative in Fig. 3B). When the cancer-initiating cells were treated with the STAT3 siRNA and then healthy donor PBMCs (n=3) were subsequently exposed to conditioned media from these glioma-associated cancer-initiating cells, the induction of p-STAT3 was significantly diminished in the immune cells (Fig. 3B). Similar data was obtained with the small molecular inhibitor of the p-STAT3 pathway WP1066.
Figure 3. Glioma-associated cancer-initiating cells express p-STAT3 and induce p-STAT3 expression in human immune cells.
A. STAT3 siRNA treated glioma-associated cancer-initiating cells express lower p-STAT3 compared to control siRNA. B. Glioma-associated cancer-initiating cell supernatants enhance the expression of p-STAT3 in PBMCs, and the supernatants from STAT3 siRNA treated cancer-initiating cells reduce p-STAT3 level in PBMCs. p-STAT3 expression in normal donor PBMCs was measured by intracellular p-STAT3 (pY705) staining via flow cytometry after 3 days of co-culture with cancer-initiating cell supernatants in the presence of anti-CD3/CD28 stimulation. Representative histograms are shown from three independent experiments. Black line: p-STAT3; Gray shade: isotype control.
Blockade of p-STAT3 can restore T cell function
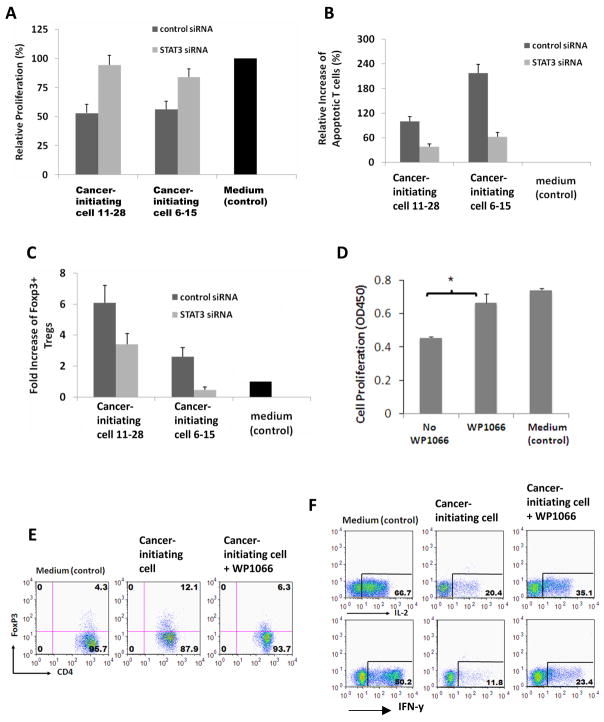
To determine if p-STAT3 blockade can change the immunosuppressive functions of the cancer-initiating cells, the cancer-initiating cells were treated with the STAT3 siRNA and phenotypically and functionally characterized. Treatment did not alter the expression of MHC II, CD40, CD80, CD86, or B7-H1 (data not shown). In contrast to the marked inhibition of T cell proliferation with the supernatant from the cancer-initiating cells, the supernatants from siRNA-treated cancer-initiating cells did not inhibit T cell proliferation (Fig. 4A), there was restoration of the number of T cells secreting IL-2 and IFN-γ (Fig. 4B), T cell apoptosis was inhibited (Fig. 4C), and there was a reduction in glioma-associated cancer-initiating cell induced FoxP3+ Tregs (Fig. 4D). Identical data was obtained with the small molecular inhibitor of the p-STAT3 pathway WP1066. The treatment of cancer-initiating cells (n=3) with the STAT3 siRNA decreased the production of Galectin-3 from 1967 ± 1151 to 920 ± 423 pg/ml (p<0.05).
Figure 4. Immunosuppression mediated by glioma-associated cancer-initiating cells is reversed with p-STAT3 inhibition.
A. Two glioma-associated cancer-initiating cell lines were transfected with STAT3 siRNA or control siRNA. After 3 days, the glioma-associated cancer-initiating cells were harvested for p-STAT3 staining, and conditioned media were collected for conducting T cell immune function assays. Inhibition of T cell proliferation mediated by glioma-associated cancer-initiating cells is reversed with STAT3 siRNA inhibition. Proliferation of T cells from normal donor was measured with cell division of CFSE-labeled T cells via flow cytometry after 5 days of culture. B. siRNA treated glioma-associated cancer-initiating cells reduces T cell apoptosis. Cultured T cells on day 5 from (A) were analyzed for apoptosis. C. siRNA treated glioma-associated cancer-initiating cells reduces the generation of FoxP3+ Tregs. Cultured T cells on day 5 from (A) were stained for CD4 and FoxP3, and FACS data were converted into bar graphs showing fold change in the percentage of FoxP3+ Tregs versus medium alone (control; set at baseline of 1). In A–C, media only served as negative controls represented by black bars. The results are averages from three independent experiments, with error bars demonstrating standard deviation. P < 0.05 for all control and STAT3 siRNA comparisons. D. Similar to the STAT-3 siRNA, the inhibition of T cell proliferation mediated by glioma-associated cancer-initiating cells is reversed by WP1066. Proliferation of the T cells was measured by CCK-8 staining after culturing the T cells with the supernatants from the cancer-initiating cells or the cancer-initiating cells treated with WP1066 for 4 days. The results are averages from three independent experiments. *P <0.05. E. Treatment of glioma-associated cancer-initiating cells with WP1066 reduces the generation of FoxP3+ Tregs. F. Inhibition of the pro-inflammatory cytokine IL-2 and IFN-y by the T cells in the presence of supernatant from glioma-associated cancer-initiating cells is partially reversed by WP1066. Intracellular cytokine staining of CD3+ T cells from (D) for IL-2 and IFN-y was performed on day 4. In E and F, FACS plots from one representative glioma-associated cancer-initiating cell experiment were shown but similar results were obtained with glioma-associated cancer-initiating cells from three other patients.
DISCUSSION
The immunological properties of human cancer-initiating cells have not been defined previously, and to our knowledge this is the first study to show that these cells mediate many of the key features of immunosuppression and explains a possible mechanism for resistance to immunotherapy. To investigate the immune properties of glioma-associated cancer-initiating cells, we used two different experimental approaches. In the first approach, the supernatants from glioma-associated cancer-initiating cells were used in immunological assays with T cells from healthy donors to determine the effects of glioma-associated cancer-initiating cells in the absence of pre-existing T cell immunosuppression while avoiding allogeneic responses that could confound the interpretation of the data. In the second approach, using GBM patients’ T cells and the respective patients’ glioma-associated cancer-initiating cells, allogeneic interactions would not confound the data, allowing for analysis of direct cell-to-cell contact; however, pre-existing immune suppression in the patient T cells might dampen the extent of immunosuppression exerted by the glioma-associated cancer-initiating cells. Regardless of the experimental approaches, the data consistently demonstrated that the glioma-associated cancer-initiating cells inhibit T cell mediated responses.
More specifically, although the glioma-associated cancer-initiating cells expressed MHC I, they lacked MHC II, CD40, and CD80, which would be anticipated to induce T cell anergy (46) and this was confirmed in our functional assays of T cell proliferation. The glioma-associated cancer-initiating cells expressed the co-stimulatory inhibitory molecule B7-H1, which has previously been shown to be a key factor mediating immune resistance in gliomas (47) and can induce T cell apoptosis (48). Thus, it was not unexpected to find in the direct cell-to-cell contact experiments that the glioma-associated cancer-initiating cells induced T cell apoptosis in the scenario of B7-H1 expression. Additionally, it was not entirely surprising to see that cancer-initiating cells could induce Tregs that were functionally active and have been shown to contribute to the immune suppression in malignant glioma patients (49–51) since the cancer-initiating cells elaborated TGF-β (10–100pg/ml) at levels sufficient to induce Tregs (52). Interestingly, the cancer-initiating cells also produced PGE2, which has been shown to regulate Th17 cell function (34). The Th17 immune cell population has recently been shown to promote tumor growth through the IL-6-STAT3 pathway (53). Likely, additional STAT3 mediated cancer-initiating mechanisms of immune suppression will be identified possibly with cytokine microarrays. Although the glioma cell lines U-87 and U-251 also produce TGF-β1 they failed to demonstrate significant inhibition of T cell proliferation likely secondary to elaborated IL-6 being produced which is a potent driver for T cell proliferation (54) indicating a complex interplay among the various secreted components. Cumulatively, these data indicate that in addition to the previously identified key role of glioma-associated cancer-initiating cells in mediating radiation resistance (17) and chemotherapy resistance (11, 55, 56), glioma-associated cancer-initiating cells also contribute to T cell immune suppression by multiple mechanisms.
In this report we also demonstrate that the supernatants from the glioma-associated cancer-initiating cells could increase the number of immune cells expressing p-STAT3, likely by one or more of the previously characterized STAT3 transcriptional controlled tumor secreted factors. The treatment of the STAT3 glioma-associated cancer-initiating cells with STAT3 siRNA or WP1066 diminished the percentage of immune cells expressing p-STAT3 that were exposed to the supernatant from the glioma-associated cancer-initiating cells. Although treatment of glioma-associated cancer-initiating cells with STAT3 siRNA or WP1066 did not alter the immunological phenotype of the glioma-associated cancer-initiating cells, as reflected by the levels of expression of MHC or co-stimulatory molecules, the treatment of the glioma-associated cancer-initiating cells with either STAT3 siRNA or WP1066 did partially restore T cell proliferation and effector function secondary to decreased T cell apoptosis and Tregs. In the case of the PBMCs exposed to the supernatants from the cancer-initiating cells treated with the STAT3 inhibitors, the decrease in Tregs could possibly be secondary to residual STAT3 siRNA or WP1066 in the media that blocks FoxP3 expression in human CD4+CD25+ Tregs (57). Or alternatively, the blockade of the STAT3 pathway in the cancer-initiating cells resulted in the down-modulation of secreted factors that induce Tregs. In the case of the PBMCs exposed to the supernatants from the cancer-initiating cells treated with the STAT3 inhibitors, the decrease in T cell apoptosis is likely secondary to a decrease in Galectin-3. Soluble Galectin-3 has been shown to induce T cell apoptosis (58), is expressed in glioma cell lines but not normal astrocytes or oligodendrocytes (59), and has been shown to enhance glioma proliferation and migration (60). Upon treatment with the STAT3 inhibitors, we found a decrease in the amount of Galectin-3 production that was responsible for the T cell apoptosis. However, since we observed only partial blockade of T cell apoptosis with the STAT-3 inhibitors, thus accounting for the incomplete restoration of T cell proliferation and effector function, a STAT-3 independent pathway such as B7-H1 expression is likely also operational in the cancer-initiating cell mediated T cell apoptosis.
Although we have shown that blockade of the STAT3 pathway with siRNA and the small molecule inhibitor WP1066 could reverse cancer-initiating cell mediated immune suppression, other molecules such as JSI-124 (61), and NSC 74859 (62), among many others, targeting the STAT3 pathway could also potentially exert the same effects. A variety of molecular inhibitors of STAT3 have been devised that interfere with the signaling of STAT3 by blocking the ligand-receptor interaction and activation sites of STAT3, dimerization, nuclear translocation, DNA binding, and gene transcription. Conceivably, the inhibitors of the STAT3 pathway may be able to overcome cancer-initiating cell-mediated chemo- and radiation therapeutic resistance in addition to immune suppression but this awaits more detailed study. Further investigations will be necessary to ascertain how to optimize the STAT3 inhibitors in combination with other immune therapeutics. Finally, since our data indicates that the STAT3 pathway is a significant molecular pathway mediating cancer-initiating cell mediated immune suppression, newer murine model systems are under development that are immune competent that have constitutive over activation of the STAT3 pathway under the GFAP promoter that may ultimately be more applicable to glioma tumorigenesis and more accurately recapitulate heterogeneous human gliomas as opposed to clonotypic xenografts. These murine models may also provide an enriched population of murine glioma cancer-initiating cells in which agents that block the STAT3 pathway could be tested.
Acknowledgments
Grant support: The Anthony Bullock III Foundation (ABH), the Dr Marnie Rose Foundation (ABH), the University of Texas M.D. Anderson Cancer Center (ABH), and the National Institutes of Health (CA120813-01) (ABH).
We thank Lamonne Crutcher for assistance in obtaining tissue specimens and Melissa Burkett and Adelina “Keats” Fuentes for editorial assistance.
Abbreviations
- APC
allophycocyanin
- bFGF
basic fibroblast growth factor
- CFSE
carboxyfluorescein diacetate succinimidyl ester
- CCL-2
chemokine C-C motif-2
- ELISA
Enzyme-linked immunosorbent assay
- FBS
fetal bovine serum
- FITC
fluorescein isothiocyanate
- FoxP3
forkhead box P3
- GalC
galactosylceramidase
- GBM
glioblastoma multiforme
- GFAP
glial fibrillary acidic protein
- IFN
interferon
- IL
interleukin
- Jak2
Janus kinase 2
- MAP2
microtubule associate protein 2
- MEM
modified Eagle’s medium
- MHC
major histocompatibility complex
- p
phosphorylated
- PBMCs
peripheral blood mononuclear cells
- PE
Phycoerythrin
- PGE2
prostaglandin E2
- PHA
phytohemagglutinin
- RPMI
Royal Park Memorial Institute
- STAT3
signal transducer and activator of transcription 3
- TGF
transforming growth factor
- Tregs
regulatory T cells
- VEGF
vascular endothelial growth factor
Footnotes
Parts of these data were presented at the American Society of Clinical Oncology Annual Meeting, May 2008, Chicago, IL.
References
- 1.Kurpad SN, Zhao XG, Wikstrand CJ, Batra SK, McLendon RE, Bigner DD. Tumor antigens in astrocytic gliomas [Review] Glia. 1995 Nov;15(3):244–56. doi: 10.1002/glia.440150306. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004 Sep;10(9):909–15. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dey M, Hussain SF, Heimberger AB. The role of glioma microenvironment in immune modulation: potential targets for intervention. Lett Drug Des Discov. 2006;3(7):443–51. [Google Scholar]
- 4.Hickey WF, Hsu BL, Kimura H. T-lymphocyte entry into the central nervous system. J Neurosci Res. 1991 Feb;28(2):254–60. doi: 10.1002/jnr.490280213. [DOI] [PubMed] [Google Scholar]
- 5.Morford LA, Elliott LH, Carlson SL, Brooks WH, Roszman TL. T cell receptor-mediated signaling is defective in T cells obtained from patients with primary intracranial tumors. J Immunol. 1997 Nov 1;159(9):4415–25. [PubMed] [Google Scholar]
- 6.Fontana A, Hengartner H, de Tribolet N, Weber E. Glioblastoma cells release interleukin 1 and factors inhibiting interleukin 2-mediated effects. J Immunol. 1984 Apr;132(4):1837–44. [PubMed] [Google Scholar]
- 7.Elliott LH, Brooks WH, Roszman TL. Cytokinetic basis for the impaired activation of lymphocytes from patients with primary intracranial tumors. J Immunol. 1984 Mar;132(3):1208–15. [PubMed] [Google Scholar]
- 8.Miescher S, Whiteside TL, de Tribolet N, von Fliedner V. In situ characterization, clonogenic potential, and antitumor cytolytic activity of T lymphocytes infiltrating human brain cancers. J Neurosurg. 1988 Mar;68(3):438–48. doi: 10.3171/jns.1988.68.3.0438. [DOI] [PubMed] [Google Scholar]
- 9.Ashkenazi E, Deutsch M, Tirosh R, Weinreb A, Tsukerman A, Brodie C. A selective impairment of the IL-2 system in lymphocytes of patients with glioblastomas: increased level of soluble IL-2R and reduced protein tyrosine phosphorylation. Neuroimmunomodulation. 1997 Jan–Feb;4(1):49–56. doi: 10.1159/000097315. [DOI] [PubMed] [Google Scholar]
- 10.Sanai N, Alvarez-Buylla A, Berger MS. Neural stem cells and the origin of gliomas. N Engl J Med. 2005 Aug 25;353(8):811–22. doi: 10.1056/NEJMra043666. [DOI] [PubMed] [Google Scholar]
- 11.Liu G, Yuan X, Zeng Z, et al. Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol Cancer. 2006 December 2;5(5):67–79. doi: 10.1186/1476-4598-5-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun Y, Kong W, Falk A, et al. CD133 (Prominin) negative human neural stem cells are clonogenic and tripotent. PLoS One. 2009;4(5):e5498. doi: 10.1371/journal.pone.0005498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shmelkov SV, Butler JM, Hooper AT, et al. CD133 expression is not restricted to stem cells, and both CD133+ and CD133- metastatic colon cancer cells initiate tumors. J Clin Invest. 2008 Jun;118(6):2111–20. doi: 10.1172/JCI34401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bleau AM, Hambardzumyan D, Ozawa T, et al. PTEN/PI3K/Akt pathway regulates the side population phenotype and ABCG2 activity in glioma tumor stem-like cells. Cell Stem Cell. 2009 Mar 6;4(3):226–35. doi: 10.1016/j.stem.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gunther HS, Schmidt NO, Phillips HS, et al. Glioblastoma-derived stem cell-enriched cultures form distinct subgroups according to molecular and phenotypic criteria. Oncogene. 2008 May 1;27(20):2897–909. doi: 10.1038/sj.onc.1210949. [DOI] [PubMed] [Google Scholar]
- 16.Galli R, Binda E, Orfanelli U, et al. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004 Oct 1;64(19):7011–21. doi: 10.1158/0008-5472.CAN-04-1364. [DOI] [PubMed] [Google Scholar]
- 17.Bao S, Wu Q, McLendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006 Dec 7;444(7120):756–60. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 18.Murat A, Migliavacca E, Gorlia T, et al. Stem cell-related “self-renewal” signature and high epidermal growth factor receptor expression associated with resistance to concomitant chemoradiotherapy in glioblastoma. J Clin Oncol. 2008;26(18):3015–24. doi: 10.1200/JCO.2007.15.7164. [DOI] [PubMed] [Google Scholar]
- 19.Lang R, Patel D, Morris JJ, Rutschman RL, Murray PJ. Shaping gene expression in activated and resting primary macrophages by IL-10. J Immunol. 2002 Sep 1;169(5):2253–63. doi: 10.4049/jimmunol.169.5.2253. [DOI] [PubMed] [Google Scholar]
- 20.O’Farrell AM, Liu Y, Moore KW, Mui AL. IL-10 inhibits macrophage activation and proliferation by distinct signaling mechanisms: evidence for Stat3-dependent and -independent pathways. EMBO J. 1998 Feb 16;17(4):1006–18. doi: 10.1093/emboj/17.4.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kortylewski M, Kujawski M, Wang T, et al. Inhibiting Stat3 signaling in the hematopoietic system elicits multicomponent antitumor immunity. Nat Med. 2005 Dec;11(12):1314–21. doi: 10.1038/nm1325. [DOI] [PubMed] [Google Scholar]
- 22.Hussain SF, Kong L-Y, Jordan J, et al. A novel small molecule inhibitor of signal transducers and activators of transcription 3 reverses immune tolerance in malignant glioma patients. Cancer Res. 2007 Oct 15;67(20):9630–6. doi: 10.1158/0008-5472.CAN-07-1243. [DOI] [PubMed] [Google Scholar]
- 23.Brantley EC, Nabors LB, YGG, et al. Loss of protein inhibitors of activated STAT-3 expression in glioblastoma multiforme tumors: implications for STAT-3 activation and gene expression. Clin Cancer Res. 2008 Aug 1;14(15):4694–704. doi: 10.1158/1078-0432.CCR-08-0618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abou-Ghazal M, Yang DS, Qiao W, et al. The incidence, correlation with tumor-infiltrating inflammation, and prognosis of phosphorylated STAT3 expression in human gliomas. Clin Cancer Res. 2008 Dec 15;14(24):8228–35. doi: 10.1158/1078-0432.CCR-08-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu H, Jove R. The STATs of cancer--new molecular targets come of age. Nat Rev Cancer. 2004 Feb;4(2):97–105. doi: 10.1038/nrc1275. [DOI] [PubMed] [Google Scholar]
- 26.Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol. 2007 Jan;7(1):41–51. doi: 10.1038/nri1995. [DOI] [PubMed] [Google Scholar]
- 27.Steinbrink K, Wolfl M, Jonuleit H, Knop J, Enk AH. Induction of tolerance by IL-10-treated dendritic cells. J Immunol. 1997 Nov 15;159(10):4772–80. [PubMed] [Google Scholar]
- 28.Williams L, Bradley L, Smith A, Foxwell B. Signal transducer and activator of transcription 3 is the dominant mediator of the anti-inflammatory effects of IL-10 in human macrophages. J Immunol. 2004 Jan 1;172(1):567–76. doi: 10.4049/jimmunol.172.1.567. [DOI] [PubMed] [Google Scholar]
- 29.DiMeo D, Tian J, Zhang J, Narushima S, Berg DJ. Increased interleukin-10 production and Th2 skewing in the absence of 5-lipoxygenase. Immunology. 2008 Feb;123(2):250–62. doi: 10.1111/j.1365-2567.2007.02694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med. 1999 Oct 4;190(7):995–1004. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Niu G, Bowman T, Huang M, et al. Roles of activated Src and Stat3 signaling in melanoma tumor cell growth. Oncogene. 2002 Oct 10;21(46):7001–10. doi: 10.1038/sj.onc.1205859. [DOI] [PubMed] [Google Scholar]
- 32.Gabrilovich DI, Chen HL, Girgis KR, et al. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells.[erratum appears in Nat Med 1996 Nov;2(11):1267] Nat Med. 1996 Oct;2(10):1096–103. doi: 10.1038/nm1096-1096. [DOI] [PubMed] [Google Scholar]
- 33.Rummel C, Sachot C, Poole S, Luheshi GN. Circulating interleukin-6 induces fever through a STAT3-linked activation of COX-2 in the brain. Am J Physiol Regul Integr Comp Physiol. 2006 Nov;291(5):R1316–26. doi: 10.1152/ajpregu.00301.2006. [DOI] [PubMed] [Google Scholar]
- 34.Boniface K, Bak-Jensen KS, Li Y, et al. Prostaglandin E2 regulates Th17 cell differentiation and function through cyclic AMP and EP2/EP4 receptor signaling. J Exp Med. 2009 Mar 16;206(3):535–48. doi: 10.1084/jem.20082293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kinjyo I, Inoue H, Hamano S, et al. Loss of SOCS3 in T helper cells resulted in reduced immune responses and hyperproduction of interleukin 10 and transforming growth factor-β1. J Exp Med. 2006;203(4):1021–31. doi: 10.1084/jem.20052333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heimberger AB, Bigner DD, Sampson JJ. Biological principles of brain tumor immunotherapy. In: Liau LM, Becker DP, Cloughesy TF, Bigner DD, editors. Brain Tumor Immunotherapy. Totowa, NJ: Humana Press Inc; 2000. pp. 101–30. [Google Scholar]
- 37.Ferrajoli A, Faderl S, Van Q, et al. WP1066 disrupts Janus kinase-2 and induces caspase-dependent apoptosis in acute myelogenous leukemia cells. Cancer Res. 2007;67(23):11291–9. doi: 10.1158/0008-5472.CAN-07-0593. [DOI] [PubMed] [Google Scholar]
- 38.Colman H, Sai K, Wang S, et al. Effect of a small molecule inhibitor of the JAK2/STAT3 pathway on self-renewal of glioblastoma stem cells. J Clin Oncol. 2008 May 20;26(15S, Part I of II suppl):89s. [Google Scholar]
- 39.Iwamaru A, Szymanski S, Iwado E, et al. A novel inhibitor of the STAT3 pathway induces apoptosis in malignant glioma cells both in vitro and in vivo. Oncogene. 2007 April 12;26(17):2435–44. doi: 10.1038/sj.onc.1210031. [DOI] [PubMed] [Google Scholar]
- 40.Kong LY, Abou-Ghazal MK, Wei J, et al. A novel inhibitor of signal transducers and activators of transcription 3 activation is efficacious against established central nervous system melanoma and inhibits regulatory T cells. Clin Cancer Res. 2008 Sep 15;14(18):5759–68. doi: 10.1158/1078-0432.CCR-08-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kong L-K, Wei J, Sharma AK, et al. A novel phosphorylated STAT3 inhibitor enhances T cell cytotoxicity against melanoma through inhibition of regulatory T cells. Cancer Immunol Immunother. 2008;58(7):1023–32. doi: 10.1007/s00262-008-0618-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heimberger AB, Priebe W. Small molecular inhibitors of p-STAT3: novel Agents for treatment of primary and metastatic CNS cancers. Recent Patents CNS Drug Discov. 2008;3(3):179–88. doi: 10.2174/157488908786242489. [DOI] [PubMed] [Google Scholar]
- 43.Heimberger AB, Crotty LE, Archer GE, et al. Epidermal growth factor receptor VIII peptide vaccination is efficacious against established intracerebral tumors. Clin Cancer Res. 2003 Sep 15;9(11):4247–54. [PubMed] [Google Scholar]
- 44.Lee A, Kessler JD, Read TA, et al. Isolation of neural stem cells from the postnatal cerebellum. Nat Neurosci. 2005 Jun;8(6):723–9. doi: 10.1038/nn1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parant O, Dubernard G, Challier JC, et al. CD34+ cells in maternal placental blood are mainly fetal in origin and express endothelial markers. Lab Invest. 2009 Aug;89(8):915–23. doi: 10.1038/labinvest.2009.55. [DOI] [PubMed] [Google Scholar]
- 46.Gomez GG, Kruse CA. Mechanisms of malignant glioma immune resistance and sources of immunosuppression. Gene Ther Mol Biol. 2006;10a:133–46. [PMC free article] [PubMed] [Google Scholar]
- 47.Parsa AT, Waldron JS, Panner A, et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med. 2007 Jan;13(1):84–8. doi: 10.1038/nm1517. [DOI] [PubMed] [Google Scholar]
- 48.Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002 Aug;8(8):793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 49.Fecci PE, Mitchell DA, Whitesides JF, et al. Increased regulatory T-cell fraction amidst a diminished CD4 compartment explains cellular immune defects in patients with malignant glioma. Cancer Res. 2006 Mar 15;66(6):3294–302. doi: 10.1158/0008-5472.CAN-05-3773. [DOI] [PubMed] [Google Scholar]
- 50.Heimberger AB, Reina-Ortiz C, Yang DS, et al. Incidence and prognostic impact of FoxP3+ regulatory T cells in human gliomas. Clin Cancer Res. 2008 Aug 15;14(16):5166–72. doi: 10.1158/1078-0432.CCR-08-0320. [DOI] [PubMed] [Google Scholar]
- 51.Jacobs JF, Idema AJ, Bol KF, et al. Regulatory T cells and the PD-L1/PD-1 pathway mediate immune suppression in malignant human brain tumors. Neuro Oncol. 2009 Aug;11(4):394–402. doi: 10.1215/15228517-2008-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wei J, Duramad O, Perng OA, Reiner SL, Liu YJ, Qin FX. Antagonistic nature of T helper 1/2 developmental programs in opposing peripheral induction of Foxp3+ regulatory T cells. Proc Natl Acad Sci U S A. 2007;104(46):18169–74. doi: 10.1073/pnas.0703642104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang L, Yi T, Kortylewski M, Pardoll DM, Zeng D, Yu H. IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. J Exp Med. 2009;206(7):1457–64. doi: 10.1084/jem.20090207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sawa S, Kamimura D, Jin GH, et al. Autoimmune arthritis associated with mutated interleukin (IL)-6 receptor gp130 is driven by STAT3/IL-7-dependent homeostatic proliferation of CD4+ T cells. J Exp Med. 2006 Jun 12;203(6):1459–70. doi: 10.1084/jem.20052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ghods AJ, Irvin D, Liu G, et al. Spheres isolated from 9L gliosarcoma rat cell line possess chemoresistant and aggressive cancer stem-like cells. Stem Cells. 2007;25(7):1645–53. doi: 10.1634/stemcells.2006-0624. [DOI] [PubMed] [Google Scholar]
- 56.Kang SK, Park JB, Cha SH. Multipotent, dedifferentiated cancer stem-like cells from brain gliomas. Stem Cells Dev. 2006 Jun;15(3):423–35. doi: 10.1089/scd.2006.15.423. [DOI] [PubMed] [Google Scholar]
- 57.Zorn E, Nelson EA, Mohseni M, et al. IL-2 regulates FOXP3 expression in human CD4+CD25+ regulatory T cells through a STAT-dependent mechanism and induces the expansion of these cells in vivo. Blood. 2006;108(5):1571–9. doi: 10.1182/blood-2006-02-004747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peng W, Wang HY, Miyahara Y, Peng G, Wang RF. Tumor-associated galectin-3 modulates the function of tumor-reactive T cells. Cancer Res. 2008 Sep 1;68(17):7228–36. doi: 10.1158/0008-5472.CAN-08-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kuklinski S, Pesheva P, Heimann C, et al. Expression pattern of galectin-3 in neural tumor cell lines. J Neurosci Res. 2000 Apr 1;60(1):45–57. doi: 10.1002/(SICI)1097-4547(20000401)60:1<45::AID-JNR5>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 60.Strik HM, Schmidt K, Lingor P, et al. Galectin-1 expression in human glioma cells: modulation by ionizing radiation and effects on tumor cell proliferation and migration. Oncol Rep. 2007 Aug;18(2):483–8. [PubMed] [Google Scholar]
- 61.Turkson J, Jove R. STAT proteins: novel molecular targets for cancer drug discovery. Oncogene. 2000;19(56):6613–26. doi: 10.1038/sj.onc.1204086. [DOI] [PubMed] [Google Scholar]
- 62.Lin L, Amin R, Gallicano GI, et al. The STAT3 inhibitor NSC 74859 is effective in hepatocellular cancers with disrupted TGF-beta signaling. Oncogene. 2009 Feb 19;28(7):961–72. doi: 10.1038/onc.2008.448. [DOI] [PMC free article] [PubMed] [Google Scholar]