Abstract
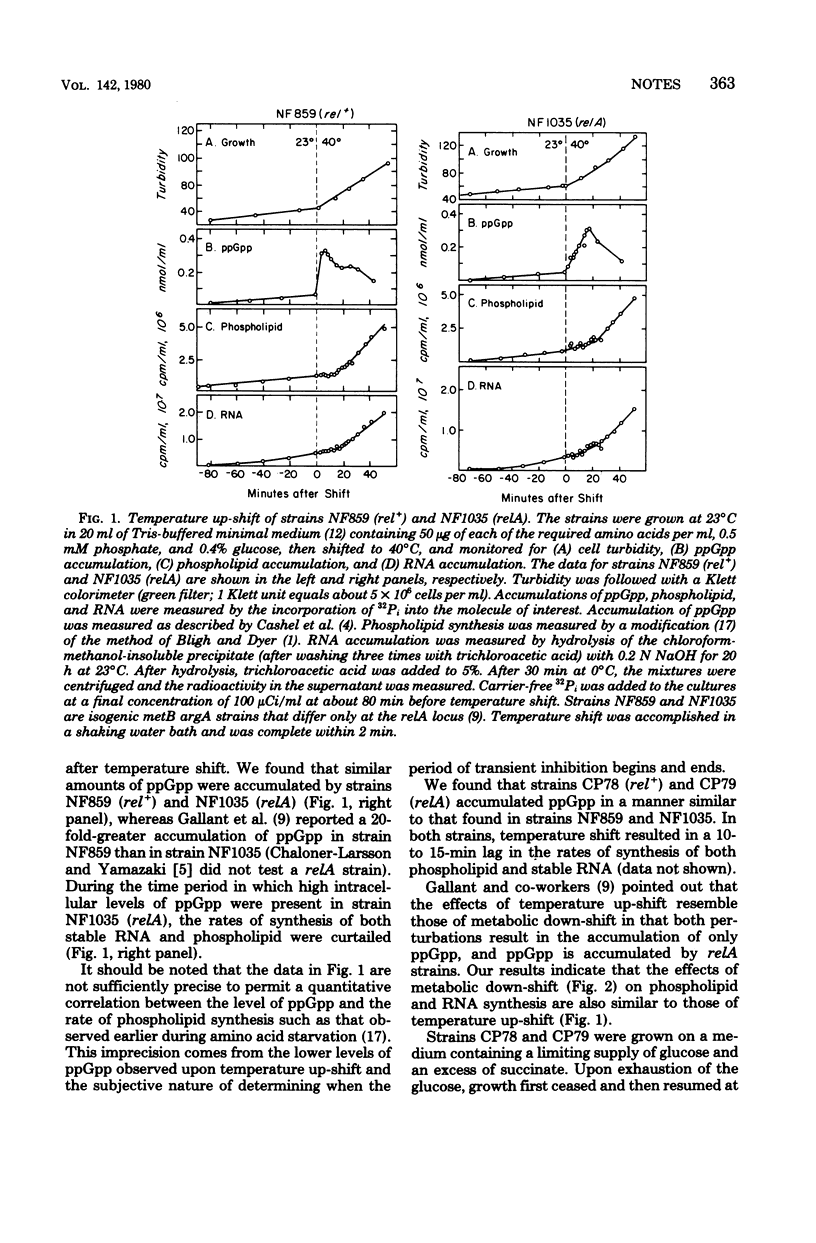
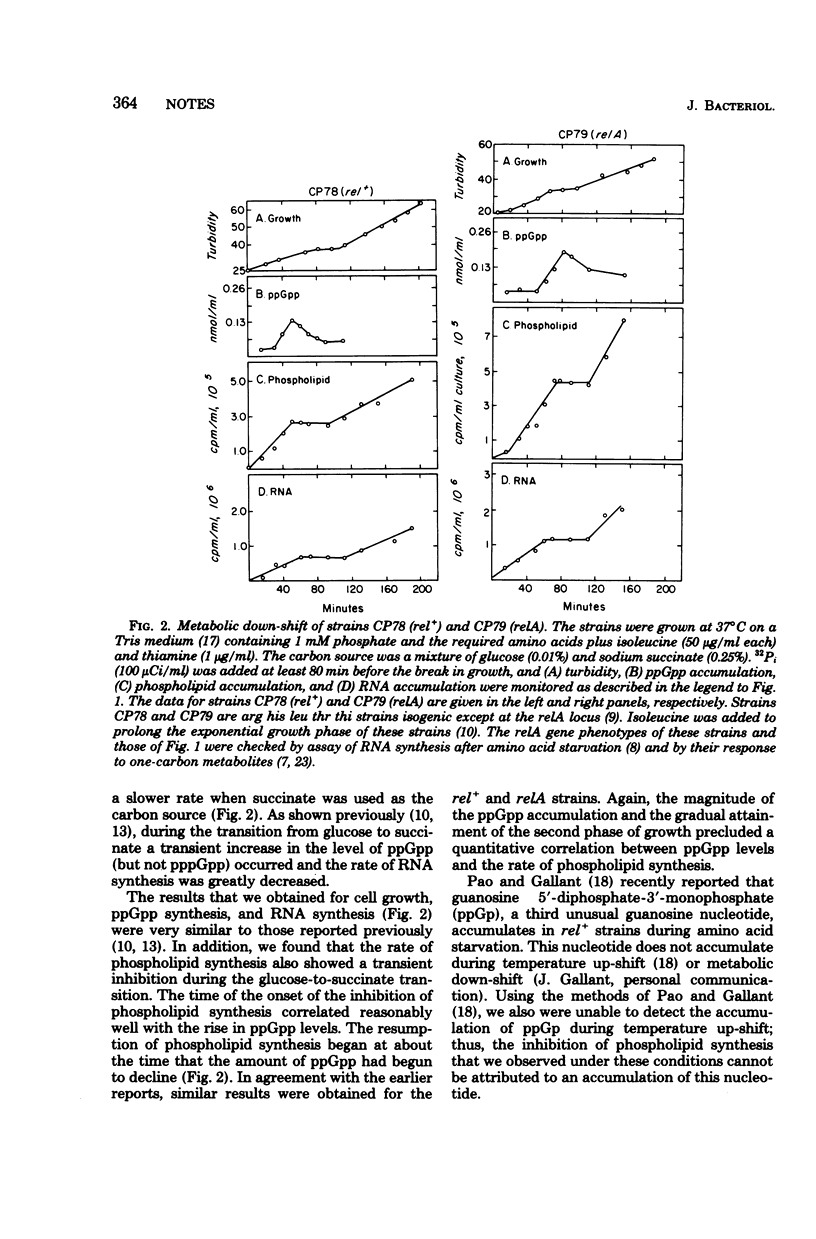
The synthesis of membrane phospholipids and that of stable ribonucleic acid were inhibited during temperature up-shift of both rel+ and relA strains of Escherichia coli. The kinetics of the inhibition of the synthesis of both molecules were correlated with the kinetics of guanosine 5'-diphosphate-3'-diphosphate synthesis. Metabolic down-shift experiments gave similar results.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Cashel M., Gallant J. Two compounds implicated in the function of the RC gene of Escherichia coli. Nature. 1969 Mar 1;221(5183):838–841. doi: 10.1038/221838a0. [DOI] [PubMed] [Google Scholar]
- Cashel M., Lazzarini R. A., Kalbacher B. An improved method for thin-layer chromatography of nucleotide mixtures containing 32P-labelled orthophosphate. J Chromatogr. 1969 Mar 11;40(1):103–109. doi: 10.1016/s0021-9673(01)96624-5. [DOI] [PubMed] [Google Scholar]
- Cashel M. Regulation of bacterial ppGpp and pppGpp. Annu Rev Microbiol. 1975;29:301–318. doi: 10.1146/annurev.mi.29.100175.001505. [DOI] [PubMed] [Google Scholar]
- Chaloner-Larsson G., Yamazaki H. Adjustment of RNA content during temperature upshift in Escherichia coli. Biochem Biophys Res Commun. 1977 Jul 25;77(2):503–508. doi: 10.1016/s0006-291x(77)80008-9. [DOI] [PubMed] [Google Scholar]
- Cronan J. E., Jr Molecular biology of bacterial membrane lipids. Annu Rev Biochem. 1978;47:163–189. doi: 10.1146/annurev.bi.47.070178.001115. [DOI] [PubMed] [Google Scholar]
- Danchin A. A new technique for selection of sensitive and auxotrophic mutants of E. coli: isolation of a strain sensitive to an excess of one-carbon metabolites. Mol Gen Genet. 1977 Feb 15;150(3):293–299. doi: 10.1007/BF00268128. [DOI] [PubMed] [Google Scholar]
- Fiil N., Friesen J. D. Isolation of "relaxed" mutants of Escherichia coli. J Bacteriol. 1968 Feb;95(2):729–731. doi: 10.1128/jb.95.2.729-731.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallant J., Palmer L., Pao C. C. Anomalous synthesis of ppGpp in growing cells. Cell. 1977 May;11(1):181–185. doi: 10.1016/0092-8674(77)90329-4. [DOI] [PubMed] [Google Scholar]
- Harshman R. B., Yamazaki H. Formation of ppGpp in a relaxed and stringent strain of Escherichia coli during diauxie lag. Biochemistry. 1971 Oct 12;10(21):3980–3982. doi: 10.1021/bi00797a027. [DOI] [PubMed] [Google Scholar]
- Heinemeyer E. A., Richter D. Characterization of the guanosine 5'-triphosphate 3'-diphosphate and guanosine 5'-diphosphate 3'-diphosphate degradation reaction catalyzed by a specific pyrophosphorylase from Escherichia coli. Biochemistry. 1978 Dec 12;17(25):5368–5372. doi: 10.1021/bi00618a007. [DOI] [PubMed] [Google Scholar]
- Kaempfer R. O., Magasanik B. Effect of infection with T-even phage on the inducible synthesis of beta-glactosidase in Escherichia coli. J Mol Biol. 1967 Aug 14;27(3):453–468. doi: 10.1016/0022-2836(67)90051-4. [DOI] [PubMed] [Google Scholar]
- Lazzarini R. A., Cashel M., Gallant J. On the regulation of guanosine tetraphosphate levels in stringent and relaxed strains of Escherichia coli. J Biol Chem. 1971 Jul 25;246(14):4381–4385. [PubMed] [Google Scholar]
- Merlie J. P., Pizer L. I. Regulation of phospholipid synthesis in Escherichia coli by guanosine tetraphosphate. J Bacteriol. 1973 Oct;116(1):355–366. doi: 10.1128/jb.116.1.355-366.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunn W. D., Cronan J. E., Jr Evidence for a direct effect on fatty acid synthesis in rela gene control of membrane phospholipid synthesis. J Mol Biol. 1976 Mar 25;102(1):167–172. doi: 10.1016/0022-2836(76)90080-2. [DOI] [PubMed] [Google Scholar]
- Nunn W. D., Cronan J. E., Jr Regulation of membrane phospholipid synthesis by the relA gene: dependence on ppGpp levels. Biochemistry. 1976 Jun 15;15(12):2546–2550. doi: 10.1021/bi00657a009. [DOI] [PubMed] [Google Scholar]
- Nunn W. D., Cronan J. E., Jr rel Gene control of lipid synthesis in Escherichia coli. Evidence for eliminating fatty acid synthesis as the sole regulatory site. J Biol Chem. 1974 Jun 25;249(12):3994–3996. [PubMed] [Google Scholar]
- Pao C. C., Gallant J. A new nucleotide involved in the stringent response in Escherichia coli. Guanosine 5'-diphosphate-3'-monophosphate. J Biol Chem. 1979 Feb 10;254(3):688–692. [PubMed] [Google Scholar]
- Polakis S. E., Guchhait R. B., Lane M. D. Stringent control of fatty acid synthesis in Escherichia coli. Possible regulation of acetyl coenzyme A carboxylase by ppGpp. J Biol Chem. 1973 Nov 25;248(22):7957–7966. [PubMed] [Google Scholar]
- Raetz C. R. Enzymology, genetics, and regulation of membrane phospholipid synthesis in Escherichia coli. Microbiol Rev. 1978 Sep;42(3):614–659. doi: 10.1128/mr.42.3.614-659.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokawa Y., Nakao E., Kaziro Y. On the nature of the control by RC gene in e. coli: amino acid-dependent control of lipid synthesis. Biochem Biophys Res Commun. 1968 Oct 10;33(1):108–112. doi: 10.1016/0006-291x(68)90263-5. [DOI] [PubMed] [Google Scholar]
- Spencer A., Muller E., Cronan J. E., Jr, Gross T. A. relA gene control of the synthesis of lipid A fatty acyl moieties. J Bacteriol. 1977 Apr;130(1):114–117. doi: 10.1128/jb.130.1.114-117.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzan M., Danchin A. A rapid test for the rel A mutation in E. coli. Biochem Biophys Res Commun. 1976 Apr 5;69(3):751–758. doi: 10.1016/0006-291x(76)90939-6. [DOI] [PubMed] [Google Scholar]



