Abstract
Macaques provide excellent models for preclinical testing and safety assessment of female reproductive toxicants. Currently, cynomolgus monkeys are the predominant species for (reproductive) toxicity testing. Marmosets and rhesus monkeys are being used occasionally. The authors provide a brief review on physiology and endocrinology of the cynomolgus monkey ovarian cycle, practical guidance on assessment and monitoring of ovarian cyclicity, and new data on effects of social housing on ovarian cyclicity in toxicological studies. In macaques, cycle monitoring is achieved using daily vaginal smears for menstruation combined with cycle-timed frequent sampling for steroid and peptide hormone analysis. Owing to requirements of frequent and timed blood sampling, it is not recommended to incorporate these special evaluations into a general toxicity study design. Marmosets lack external signs of ovarian cyclicity, and cycle monitoring is done by regular determinations of progesterone. Cynomolgus and marmoset monkeys do not exhibit seasonal variations in ovarian activity, whereas such annual rhythm is pronounced in rhesus monkeys. Studies on pair- and group-housed cynomolgus monkeys revealed transient alterations in the duration and endocrinology of the ovarian cycle followed by return to normal cyclicity after approximately six months. This effect is avoided if the animals had contact with each other prior to mingling. These experiments also demonstrated that synchronization of ovarian cycles did not occur.
Keywords: cynomolgus monkey, rhesus monkey, marmoset, reproduction, female
RATIONALE AND INTRODUCTION
From the viewpoint of reproductive toxicology, female macaques provide excellent models for preclinical testing and safety assessment of female fertility. Currently, the predominant species in toxicity testing is the cynomolgus monkey (Macaca fascicularis, synonymous with Macaca cynomolgus, Macaca irus, long-tailed macaque, or crab-eating macaque). Occasionally, the rhesus monkey (Macaca mulatto) and the (common) marmoset (Callithrix jacchus) are also used for reproductive toxicity evaluation. At Covance Laboratories GmbH, Muenster, Germany, over the past years, approximately 80% of toxicity studies have been conducted in the cynomolgus monkey model, approximately 15% in the marmoset model, and the remainder in the rhesus monkey model. The purpose of this article is (1) to briefly review the physiology and endocrinology of the primate ovarian cycle, (2) to provide practical guidance on how to assess and monitor ovarian cyclicity in toxicity studies, and (3) to describe new data on the effects of psychosocial and environmental enrichment on ovarian cyclicity in the context of toxicological studies. The latter is considered particularly relevant in light of the current changes in housing recommendations for nonhuman primates (Council of Europe 2007). Special emphasis is given to the cynomolgus monkey and some comparative information is provided for other nonhuman primate species currently used in toxicology (i.e., rhesus monkey and marmoset). Animal experiments conducted at Covance Laboratories were performed in accordance with the German Animal Welfare Act.
In terms of ovarian cycle characteristics and regulation, primates exhibit substantial differences from other mammalian species, in particular rodents or ruminants. Primates have a comparatively long life span of the corpus luteum—about two weeks or longer—irrespective of whether conception occurs. If a pregnancy is established, the corpus luteum has an extended duration of function and delayed luteal regression to permit implantation and the luteal-placental shift. Unlike in rodents, prolactin is not considered to play a decisive role during the luteal phase, and luteolysis does not involve a uterine signal.
A wealth of studies is available to demonstrate that the ovarian cycle in various macaque species shows close similarities to that in women. The precise endocrine sequelae and control of the primate ovarian cycle were unravelled initially in rhesus monkeys (Zeleznik and Pohl 2006), and insights from nonhuman primate research have yielded significant insights into women’s health (Archer 2004; Cline et al. 2001; Kaplan 2004). Macaque models have been specifically useful for research on ovarian cycle control, endometriosis, anovulatory infertility, osteoporosis, stress-associated infertility, menopausal changes in physiology, and related metabolic disorders (diabetes, atherosclerosis). Overall, excellent reviews on the endocrinology and physiology of the primate ovarian cycle are available (Gougeon 1996; Jabbour et al. 2006; Stouffer 2003; Zeleznik and Pohl 2006). A wealth of information and new insights into endocrine requirements and local control of the ovarian cycle were also gained from experimental studies in macaques using controlled ovarian (hyper)stimulation protocols and/or selective steroid/steroid receptor modulation (Cline 2007; Macklon et al. 2006).
ENDOCRINE CIRCUITS
Menstrual cycles, cycle-related changes in hormone production, and the provision of a mature and fertilization-competent oocyte are governed by a complex but delicate interplay among hypothalamus, pituitary, and ovary. This brain-gonadal axis represents the core unit for the maintenance of the endocrine balance and fertility. Ovarian functions (i.e., production of steroid hormones and of ova) are entirely subject to regulation by endocrine factors derived from the brain. GnRH is secreted from the hypothalamus and stimulates the synthesis and release of the gonadotropic hormones, luteinizing hormone (LH), and follicle-stimulating hormone (FSH) from the pituitary gland. LH acts on ovarian theca cells and governs the production and secretion of androgens by these cells. Within the follicle, androgens act on the somatic granulosa cells and are converted by them into estradiol. FSH acts directly on the granulosa cells to stimulate follicular growth, and receptors for FSH have been identified only on this cell type. Estrogens exert a positive feedback effect on gonadotropin release prior to ovulation and provoke the ovulatory LH peak, whereas during the postovulatory luteal phase and in the early follicular phase, estrogens inhibit gonadotropin levels. During the luteal phase, LH stimulates progesterone production leading to further negative feedback effects on gonadotropin secretion and, along with estrogen, is believed to result in the luteal inhibition of early follicular development.
Hypothalamo-Hypophyseal Loop
Among primates, the decapeptide GnRH is predominantly synthesized in neurons of the mediobasal hypothalamus and the arcuate nucleus. Two forms of GnRH (GnRH-I and GnRH-II) have been described for primates that have receptors in the gonadotropic cells but are encoded by different genes (Cheng and Leung 2005). GnRH is produced by successive cleavage stages from a longer precursor, called preproGnRH, transported along the axons to the median eminence and there released into portal blood. GnRH-I is involved in gonadotropin regulation. A second GnRH receptor gene was identified in marmosets (GnRH type II receptor), which is structurally and functionally distinct from the classical, type I receptor. The GnRH type II receptor, however, is not functional in the human and many other species, and its role is as yet unknown (Millar 2005). Both GnRH-I and GnRH-II were shown to signal through the GnRH type I receptor. While GnRH-I regulates gonadotropins, GnRH-II appears to be a neuromodulator and stimulates sexual behavior.
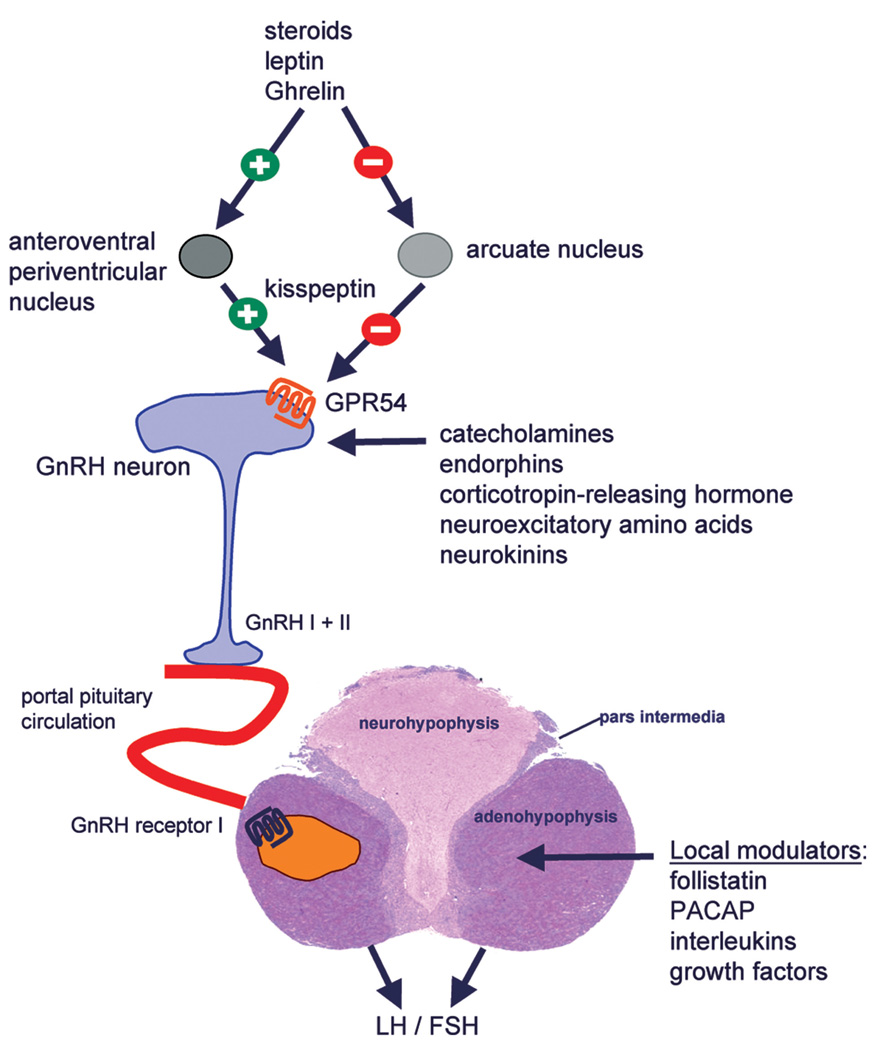
GnRH is released from the hypothalamus in a pulsatile manner and this pulsatility is indispensable for intact pituitary gonadotropin secretion and, subsequently, ovarian function. Experimental manipulation provoking increased (including continuous exposure) or decreased frequency of GnRH pulses reduces or abolishes gonadotropin secretion and thus disturbs the ovarian cycle. It has become clear over the past years that the kisspeptin/GPR54 system is pivotal for the regulation of GnRH secretion (Figure 1). Kisspeptin-expressing neurons are located in the anteroventral periventricular nucleus, in the periventricular nucleus, in the anterodorsal preoptic nucleus, and in the arcuate nucleus (AN). Outside the nervous system, the KISS1 gene is expressed in placenta, testis, pancreas, liver, and intestine (Popa, Clifton, and Steiner 2008). Kisspeptin is sensitive to steroid levels within the circulation and mediates the negative feedback regulation of gonadotropin secretion. In fact, although androgens, estrogens, and progesterone suppress gonadotropin secretion through androgen receptor–, estrogen receptor-α–, and progesterone receptor–dependent mechanisms, respectively, none of these sex steroids affects GnRH secretion by direct action on GnRH neurons. On the contrary, kisspeptin neurons in the arcuate nucleus are direct targets of sex steroids in all species and should be viewed as the site of the negative feedback control of GnRH production. In addition, kisspeptin produced by the anteroventral periventricular nucleus, a sexually dimorphic nucleus rich in steroid-sensitive neurons in the female, may mediate the positive feedback effects of estrogen on GnRH secretion. Expression of KISS1 is also influenced by adiposity and satiety factors including leptins and Ghrelin (Tena-Sempere 2008).
FIGURE 1.
Regulation of hypothalamic GnRH system and pituitary gonadotropin synthesis and release. Kisspeptin, the ligand of the GPR54 receptor located on GnRH neurons, is a key regulator of GnRH production. Steroids, leptin, and Ghrelin are believed to modulate GnRH secretion via the kisspeptin/GPR54 system. GnRH I and II molecules are present in primates, and both molecules act via GnRH I receptors in gonadotropes. Synthesis and secretion of LH and FSH are locally modulated by a variety of paracrine factors.
The hypothalamic release of GnRH is modulated by a variety of factors including estradiol, progesterone, catecholamines, dopamine, and endorphins (Figure 1). Norepinephrine, glutamate, and neuropeptide Y stimulate, whereas endogenous opioids, GABA, and corticotrophin-releasing hormone inhibit GnRH secretion (Zeleznik and Pohl 2006). Ovarian steroid hormones can exert positive (e.g., during ovulation trigger) and negative feedback effects (e.g., during follicular and luteal phases) and can act at the hypothalamic and/or pituitary level. In the ovariectomized monkey, estradiol reduced hypothalamic pulsatile multiunit activity compatible with a direct hypothalamic effect (O’Byrne and Knobil 1993). The inhibitory effects of progesterone might be mediated via an inhibitory factor (Zeleznik and Pohl 2006).
Hypothalamo-Hypophyseal-Ovarian Loop
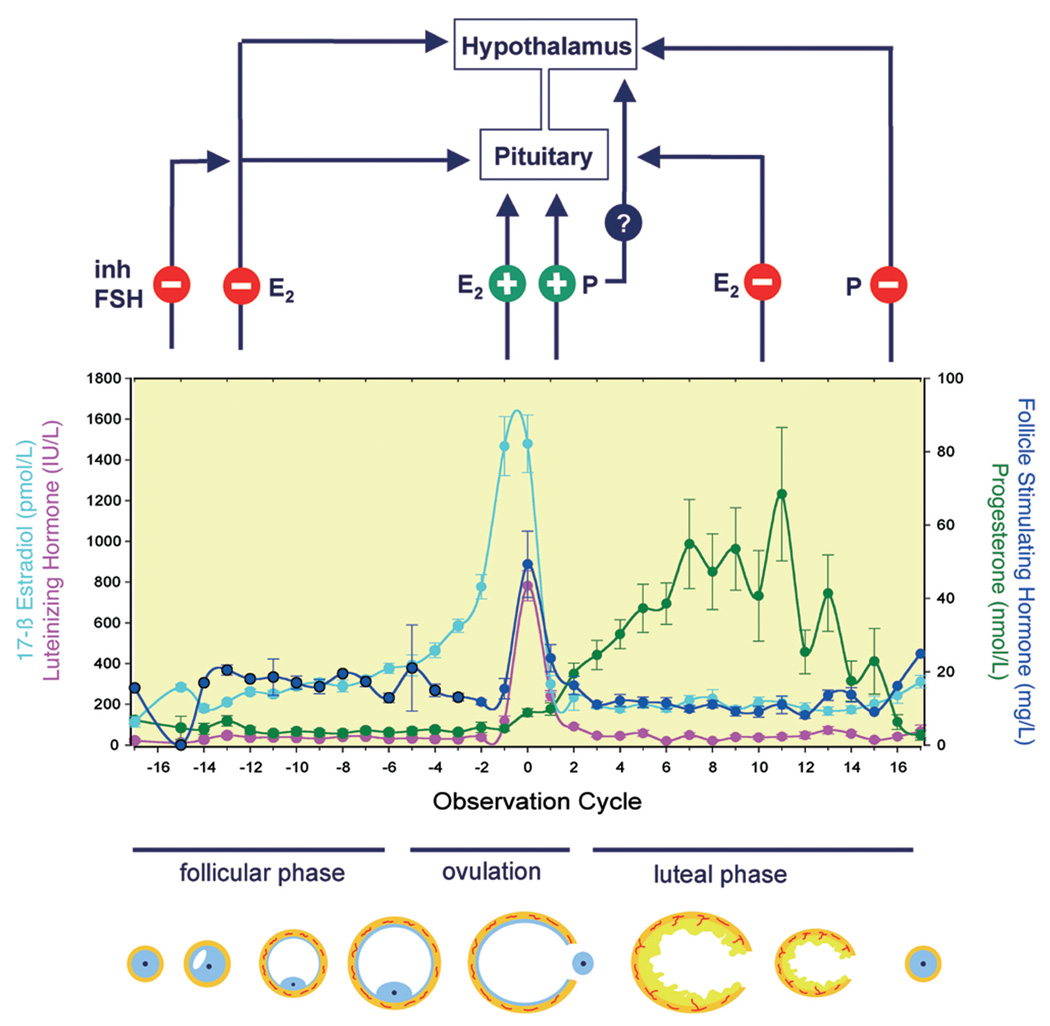
The GnRH-driven synthesis and secretion of gonadotropic hormones is modulated by ovarian steroid and protein factors (Figure 2). During the follicular phase, increased estradiol production by the maturing follicles exerts a negative feedback action and decreases FSH secretion (and LH secretion) by lowering pulse amplitudes. Once serum estradiol concentration surpasses a certain threshold over a defined period, a positive feedback action of estradiol on FSH and LH release occurs, thus provoking the midcycle peak of gonadotropin secretion and inducing ovulation. This effect is assumed to involve actions at the hypothalamic level (increase of GnRH release) and at the pituitary level. It is assumed that the midcycle progesterone increase is also involved in this positive feedback activity since progesterone antagonists delay or even block ovulation, an effect that is reversible on provision of progesterone. On the other hand, work in rhesus monkeys suggests that progesterone has a direct beneficial effect on oocyte maturation/follicle survival but is unable to elicit ovulation in the absence of LH (Borman et al. 2004). Substance P, a neurokinin, may be involved in the control of the preovulatory LH release since administration of a specific substance P antagonist acting via the NK1 receptor significantly lowered LH concentrations (Kerdelhue et al. 2000). Following ovulation, estradiol and progesterone exert negative feedback actions on both gonadotropic hormones, ultimately resulting in restoration of preovulatory levels via reduction of pulse frequency rather than pulse amplitude. It seems that pulse amplitude and frequency are inversely related, yielding comparable LH output during the follicular and the luteal phases (Veldhuis and Johnson 1990).
FIGURE 2.
Endocrine profiles of estradiol (E2), LH, FSH, and progesterone (P) during the ovarian cycle in the cynomolgus monkey and presumed feedback actions of ovarian hormones. The day of ovulation is denoted as zero, and days for follicular and luteal phases are counted relative to ovulation time point. Endocrine data represent M ± SD of forty-four animals except for FSH with data from fourteen animals. During ovulation, ovarian steroids are assumed to exert primarily a direct and positive feedback effect on pituitary gonadotropin secretion, whereas negative feedback actions occur during the follicular and luteal phases. Studies in ovariectomized monkeys also suggested a hypothalamic site of E2 action. Whether progesterone also has a direct positive effect on hypothalamus is unclear.
In addition, a peptide-mediated feedback loop has been described for FSH that is mediated by the effects of inhibins/activins and follistatin (Bilezikjian et al. 2006; Messinis 2006). Inhibin secretion from granulosa cells is stimulated by LH and FSH and in turn inhibits FSH secretion. Activin and follistatin are also involved in FSH feedback regulation but act more as local regulators than as endocrine factors. Within the pituitary, local and differential regulation of FSH versus LH synthesis might also involve pituitary-adenylase cyclase-activating peptide produced in folliculostellate cells (Winters and Moore 2007). Peripheral feedback effects of inhibins and activins occur at the level of the pituitary. Activins selectively stimulate FSH secretion, and follistatin binds to activin and, presumably, determines and regulates activin-associated effects through this mechanism.
OVARIAN CYCLE
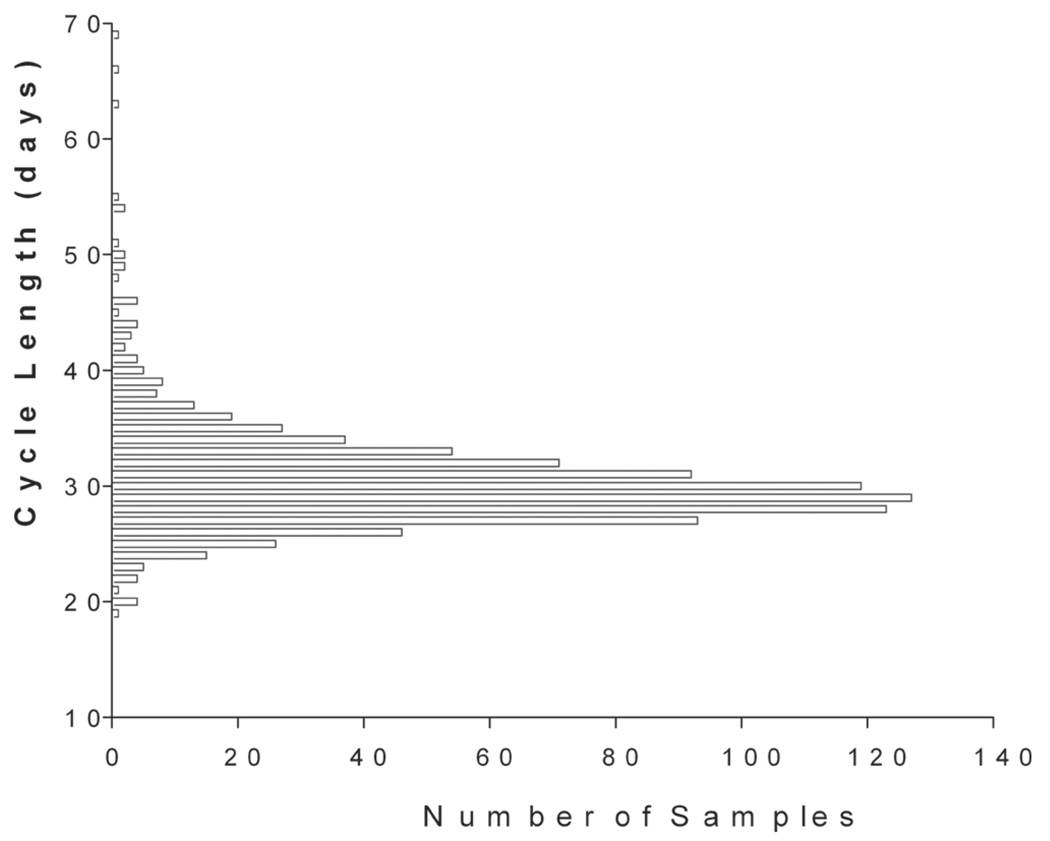
The term ovarian triad—that is, follicle, oocyte, and corpus luteum for the primate menstrual cycle—was coined many years ago (Goodman and Hodgen 1983). The ovarian cycle comprises follicular maturation during the follicular phase and ovulation and establishment of the corpus luteum during the luteal phase, followed by corpus luteum regression and menstruation. The first day of menstrual bleeding is designated as the first day of the ovarian cycle. The entire duration of the ovarian cycle is 28 to 32 days in the cynomolgus monkey and the rhesus monkey. The follicular phase lasts 12 to 14 days, the periovulatory interval is approximately 3 days, and the luteal phase lasts 14 to 16 days. For practical purposes and experimental constraints imposed by frequent and repeated blood sampling, ovarian cycle duration is determined from daily vaginal smears in toxicology studies. Analysis of menstrual bleeding patterns from vaginal smears over a total of 926 spontaneous ovarian cycles in untreated and individually housed cynomolgus monkeys at the Covance laboratory yielded an average cycle duration of 30.4 ± 4.7 (SD) days (median value: 30.0 days) with a range of 19 to 69 days (Figure 3). Another set of data derived from more than 200 cycles of pair-housed animals revealed a vaginal smear-based cycle duration of 30.3 ± 0.7 days (Table 1). Shaikh, Naqvi, and Shaikh (1978) observed a cycle duration of 29.2 days (range 22–37 days) in a total of 647 cycles. Butterstein et al. (1997) reported an average cycle duration of 30.2 ± 1.4 (range 29–35) days. Another study on twenty individually housed cynomolgus monkeys reported a vaginal smear-based average cycle duration of 35.9 ± 1.6 days (range 26–45 days; Attia 1998). Duration of menstruation varied from 1 to 8 days with 85% of cycles exhibiting bleeding duration of 3 to 5 days (Shaikh, Naqvi, and Shaikh 1978).
FIGURE 3.
Duration of the ovarian cycle in the cynomolgus monkey based on daily vaginal smears. Data represent 926 spontaneous cycles. Average cycle (± SD) duration is 30.4 ± 4.7 days with a range of 19 to 69 days. Median cycle duration is 30 days, indicating a normal distribution of cycle duration.
TABLE 1.
Duration of the ovarian cycle based on daily vaginal smears in the cynomolgus monkey.
| Duration (days) | Range (days) | No. of cycles | Housing | Reference |
|---|---|---|---|---|
| 30.4 ± 4.7 | 16–69 | 926 | Single, indoor | Present work |
| 30.3 ±3.9 | 22–71 | 200 | Pair, outdoor/indoor | Present work |
| 29.2 | 22–37 | 647 | Single, indoor | Shaikh, Naqvi, and Shaikh (1978) |
| 30.2 ± 1.4 | 29–35 | 6 | NA | Butterstein et al. (1997) |
| 35.9 ± 1.6 | 26–45 | 20 | Single, indoor | Attia (1998) |
| 30.3 | NA | 395 | 5–6 female and 1 male, outdoor | Adams, Kaplan, and Koritnik (1985) |
Data represent M ± SD. NA = not available.
The subsequent description and discussion focuses on rhesus monkeys and cynomolgus monkeys unless other macaque species are specifically mentioned. Ovarian cyclicity and endocrine control are essentially identical among macaques (Hotchkiss and Knobil 1994). The description and discussion of the New World marmoset monkey is provided as a separate section because of the essential physiological and endocrine differences of the ovarian cycle control and monitoring in this neotropical primate compared with Old World species.
Endocrine Profiles
During the follicular phase, estradiol stemming from the growing and the Graafian follicle is the predominant ovarian steroid. As estradiol levels produced by the growing follicle continue to increase, LH and FSH levels remain low or even decrease. Progesterone levels remain very low throughout the follicular phase. The beginning rise of estradiol levels toward the end of the follicular phase is accompanied by a corresponding decrease of levels of FSH. The midcycle peak initiates with a sharp rise of estradiol levels, followed by LH, FSH, and some increase of progesterone levels (Figure 2). Estradiol concentrations peak on day 12 of the cycle, followed by LH (day 12.5) and FSH (day 13), whereas progesterone levels peak during day 22 of the cycle (Table 2). During the luteal phase, gonadotropin levels return to levels comparable to those seen during the follicular phase. Progesterone levels produced by the rapidly developing corpus luteum are clearly elevated during the midluteal phase. Estradiol levels decline following ovulation. Levels of testosterone and prolactin concentrations vary widely but are unrelated to the phases of the ovarian cycle; in the Covance laboratory, testosterone levels, on average, range between 1 and 4 nmol/L and prolactin levels, on average, between 50 and 250 mU/L. Inhibin B concentrations are high in the early follicular phase, whereas levels of inhibin A are elevated during the luteal phase (Fraser, Groome, and McNeilly 1999).
TABLE 2.
Day of peak hormone levels during the cynomolgus monkey ovarian cycle.
| Hormone | Peak M ± SD (days) | Peak median (days) |
|---|---|---|
| Estradiol | 12.5 ± 2.45 | 12.0 |
| BioLH | 12.9 ± 2.5 | 12.5 |
| FSH | 12.5 ± 1.2 | 13.0 |
| Progesterone | 21.8 ± 2.8 | 22.0 |
Data are from forty-four animals, except for FSH, which comprises data from fourteen animals.
Follicular Phase and Follicle Selection
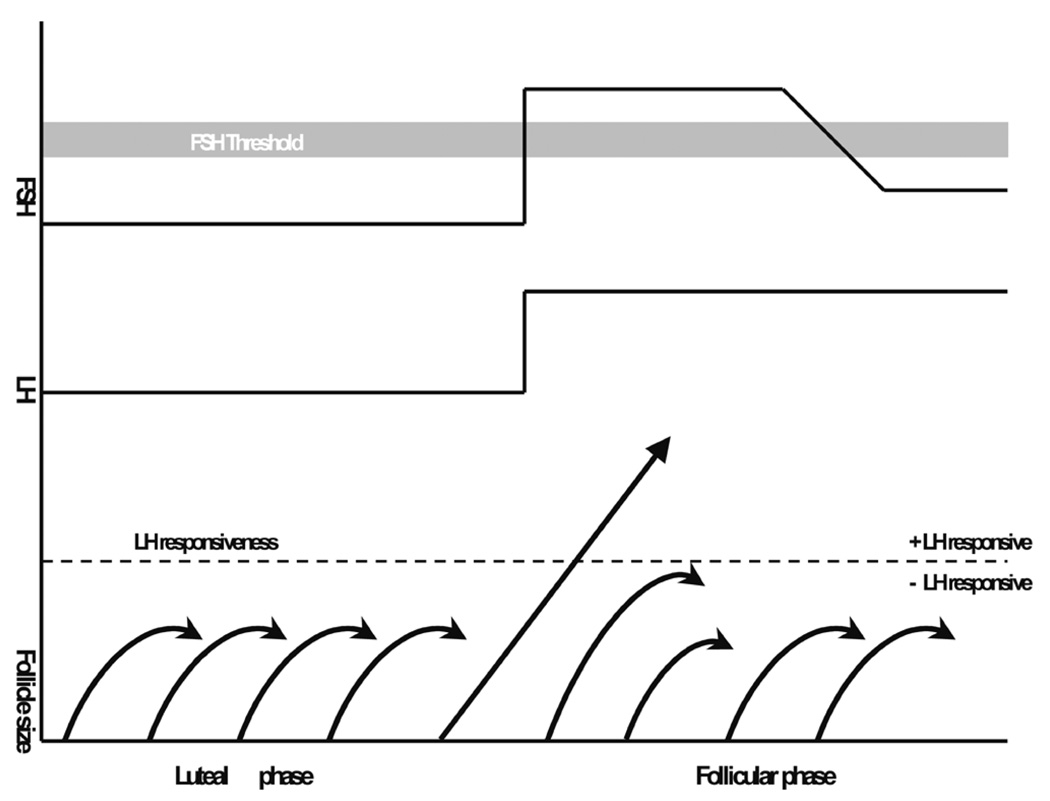
Preovulatory follicular growth is initiated during the perimenstrual rise of FSH secretion. This insight was originally developed by Brown in 1978 (quoted in Zeleznik 2001) during gonadotropin therapy investigations. The “two-cell/ two gonadotropin” control of follicular estradiol production and maturation (Hillier, Whitelaw, and Smyth 1994) is as follows: LH stimulates androstenedione and testosterone production in theca cells. These steroid hormones enter the granulosa cells and are aromatized into estrone and estradiol under the influence of FSH. Estrone and estradiol are released into the circulation but also into the follicular antrum. FSH and estrogens are the key local factors during follicular growth by acting on granulosa cell multiplication. Beyond that, FSH also stimulates FSH receptor formation. As a net result, follicles will grow at different rates depending on the intrafollicular estradiol levels and FSH receptor numbers. At some point during the follicular phase, the increasing estradiol concentrations can also be detected in peripheral blood and provoke a negative feedback on FSH release resulting in reduced aromatization and less follicular growth. As a net result, the largest grown follicle is assumed to have acquired sufficient internal aromatase activity to sustain estradiol production and, hence, will become the dominant (leading) follicle. Possibly, the dominant follicle is less dependent on FSH stimulation through the expression of LH receptors on granulosa cells, thereby increasing follicular estradiol production further. Because of the reduced FSH activity, less developed follicles will fall short of the required FSH support and undergo atresia rather than continue development. Hence, the dominant follicle gains advantage by becoming less dependent on FSH and inhibits maturation of other follicles by depriving them of FSH stimulation (Figure 4; Zeleznik 2001). Peripheral inhibin B levels can also serve as a major indicator of follicular maturation (Fraser, Groome, and McNeilly 1999). The selected follicle acquires dominance four to eight days after the demise of the corpus luteum and—given the onset of menses two to four days after luteolysis—follicle selection is completed between the second and sixth day of the ovarian cycle (Goodman and Hodgen 1983). In the rhesus monkey, selection of the ovary with the dominant follicle is at random. There is also evidence that LH might be a critical factor in the very final stage of follicular maturation (Young et al. 2003). Progesterone also supports oocyte maturation (Borman et al. 2004) and survival of the dominant follicle.
FIGURE 4.
Schematic representation of the concept of follicle selection in macaques (modified from Zeleznik 2001). For explanation, the reader is referred to section “Follicular Phase and Follicle Selection.”
Ovulation
Ovulation is induced by the midcycle gonadotropin surge, which triggers and initiates oocyte maturation, follicle rupture, and luteinization of follicles. Once serum estradiol concentrations exceed a threshold concentration of approximately 150 to 200 ng/L for at least 36 hours in the rhesus monkey and 160 to 210 ng/L in the cynomolgus monkey (Figure 2), a positive feedback action of estradiol is observed: FSH and LH are released and induce ovulation, with LH being the most likely primary stimulator. Following this surge, granulosa cells progressively become mitotically inactive. During the periovulatory interval of approximately 36 hours, follicular steroidogenesis shifts from the predominant production of estrogen and androgens to production of progesterone. The corresponding shift of enzyme expression and activity is primarily regulated by gonadotropins. Local progesterone plays a critical role during the periovulatory period by preventing atresia of the stimulated follicle, promoting follicle remodelling and permitting oocyte maturation (Stouffer 2003).
The follicular contraction and release of the oocyte during ovulation is potentially influenced by a variety of local factors such as prostaglandins, plasminogen activator, leucotrienes, angiotensins, catecholamines, and vasoactive growth factors. The precise interrelationships are not yet delineated. Indomethacin—in the rhesus monkey—prevented oocyte release in four out of eight animals without inhibiting follicular rupture and luteal function (Duffy and Stouffer 2002). In this study, addition of prostaglandin E2 to the indomethacin regimen provoked oocyte release in all three animals, indicating an important role for this prostaglandin during primate ovulation.
Luteal Phase
Luteinization of granulosa cells occurs within a few hours after ovulation. During luteinization, the follicle further develops into a progesterone-producing structure. Hormone production of the corpus luteum is primarily under LH control. FSH also induces LH receptor synthesis on granulosa cells and LH is indispensable for development and function of the corpus luteum. FSH alone in a regimen of controlled ovarian stimulation failed to maintain normal luteal function (Zelinski-Wooten et al. 1998). Following binding of LH to its receptor, granulosa cells luteinize and start production of progesterone (and estradiol) under the influence of LH. Production of inhibin during the luteal phase is also dependent on LH. In this phase, development of other follicles does not progress beyond the early antral stage, and it is assumed that this inhibition of follicular development is indirect and mediated via modulation of FSH activity (for a review, see Zeleznik 2001). Inhibin A might also be involved in the suppression of FSH release (Molskness et al. 1996). The precise role of local androgens during the luteal phase is unclear as well as whether estrogens take part directly in promoting luteal regression.
In the case of pregnancy, progesterone supports the early implantation phase until chorionic gonadotropin produced by the placenta replaces LH activity and sustains progesterone production by the corpus luteum. In case of a nonpregnant cycle, luteolysis and menses will occur around fourteen to sixteen days following ovulation. Luteal regression is not always directly linked to LH, and it is unclear what controls the life span of the primate corpus luteum in a normal (i.e., nonpregnant) cycle. It has been suggested that the corpus luteum’s sensitivity to LH diminishes over time, and this insensitivity could be a determining factor for the trigger of luteolysis since mimicking pregnancy-related rescue of the corpus luteum in nonpregnant monkeys requires more intense LH/CG bioactivity (Zeleznik 1998).
OVARIAN CYCLE MONITORING IN CYNOMOLGUS MONKEYS
Daily assessment of vaginal smears provides a convenient, reliable, and minimally invasive approach for monitoring the ovarian cycle and determining its duration (Figure 5). Females are easily trained to present the vagina, and smears are collected using a cotton swab. Smears are collected on a daily basis. Collection of vaginal smears also provides a simple but reliable tool for assessing menarche and sexual maturity in female cynomolgus monkeys.
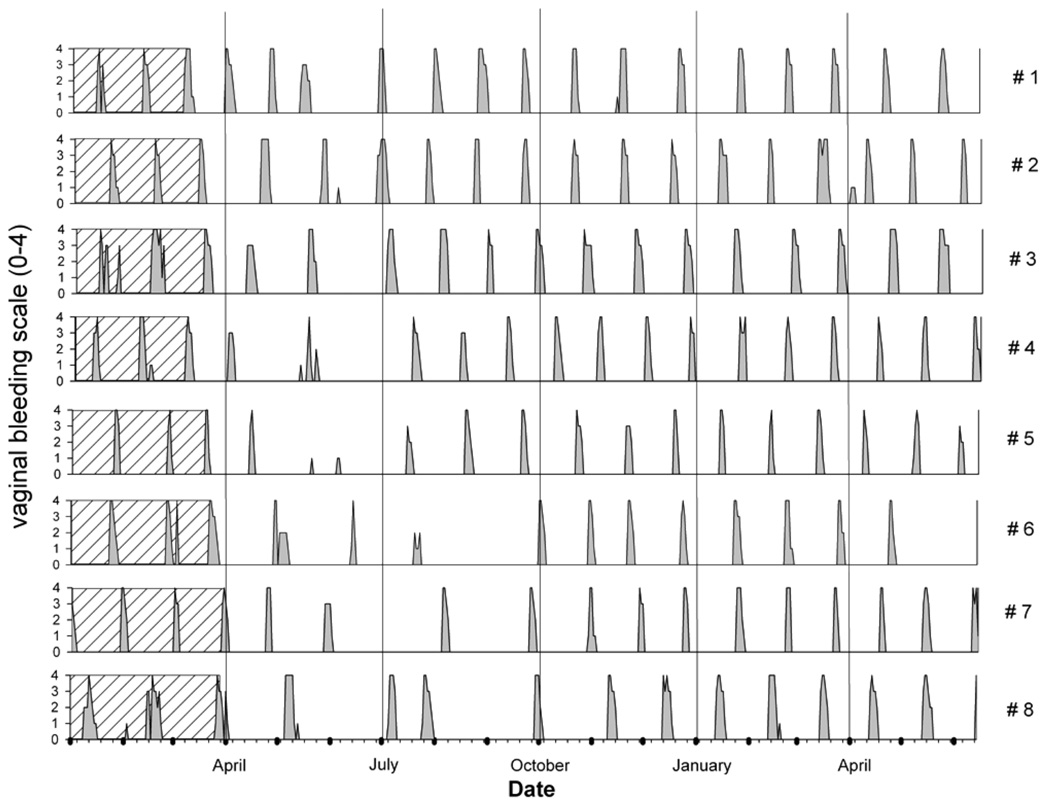
FIGURE 5.
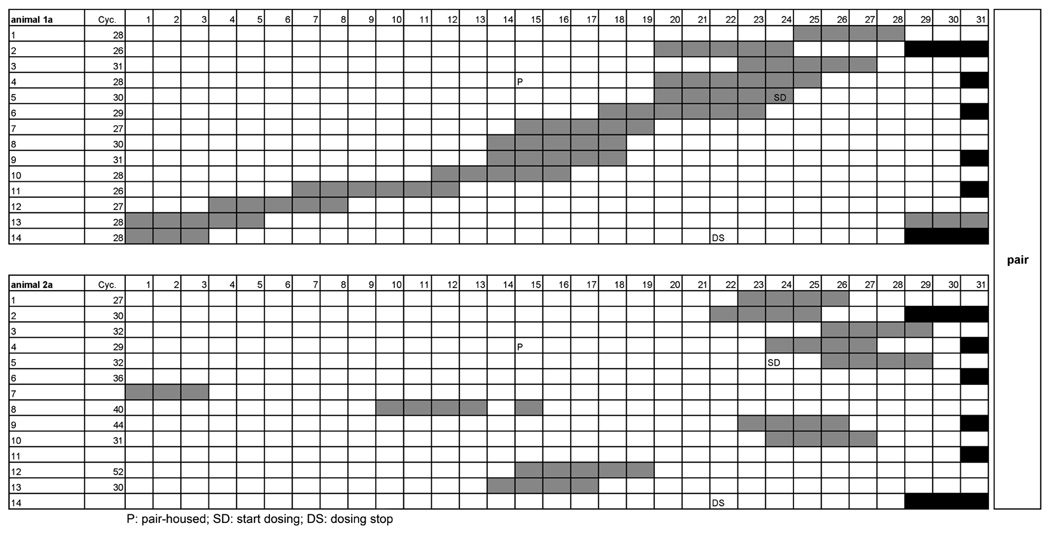
Graphic representation of daily bleeding records from vaginal smears that are used to determine ovarian cycle duration in the cynomolgus monkey. The data correspond to those shown in Figure 15 (animals la and 2a). X-axis denotes days, and y-axis denotes the number of cycles. Grey boxes indicate bleeding. Black boxes aid in cycle determination by denoting the number of days for every month. Cycle duration is determined by the interval between first day of bleeding and last day prior to onset of next bleeding. Note that animal la maintained a rather constant cycle duration (26–31 days), whereas its companion, animal 2a, showed cycle irregularity (increased cycle length up to 52 days or lack of cyclicity) during pair housing. Cyc. = cycle duration in days; P = shift from single to pair housing; SD = start of dosing; DS = dosing stopped.
We use a four-grade subjective scale for recording menstrual bleeding patterns:
No menstrual bleeding.
Slight menstrual bleeding.
Heavy menstrual bleeding.
Very heavy (i.e., visible) menstrual bleeding.
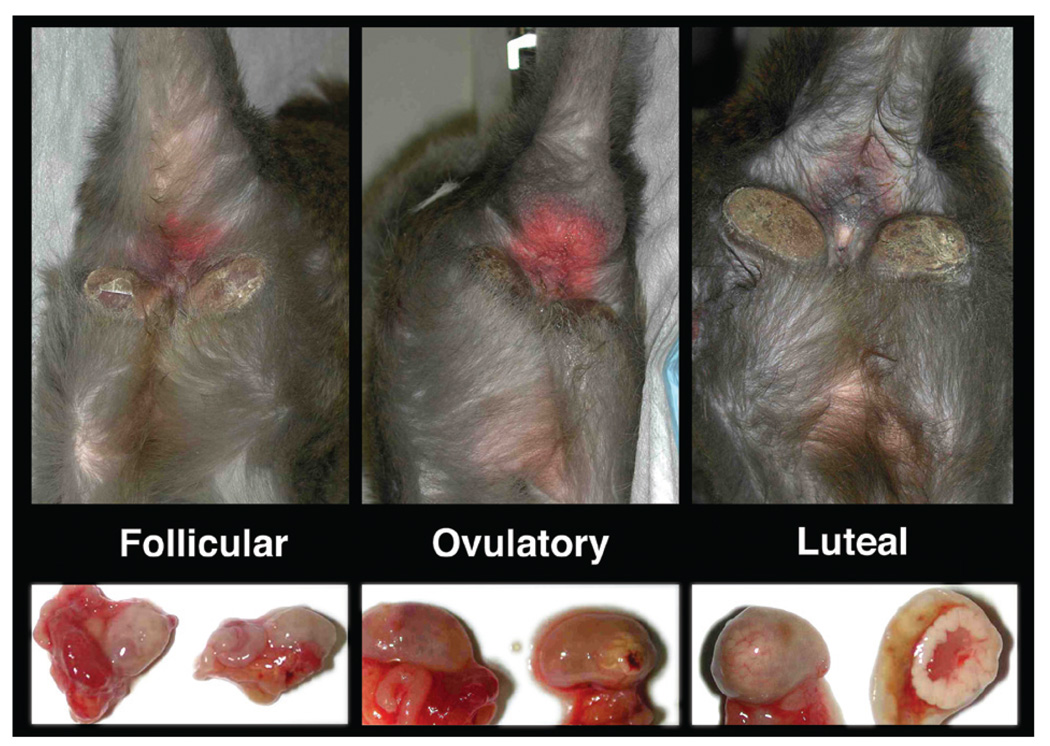
Perineal sex-skin swelling and erythema is present in macaques and shows correlation with ovarian cycles for species with seasonal reproduction. Although some female cynomolgus monkeys display sex-skin swelling and reddening, this holds true only for some animals, and in such cases, the sex-skin changes correlate with the ovarian cycle phase (Figure 6). However, these observations cannot be generalized, and evaluation of sex-skin alterations is not recommended for external monitoring of the ovarian cycle.
FIGURE 6.
Perineal sex-skin color changes and swelling in adult cynomolgus monkeys during the follicular, ovulatory, and luteal phases of the ovarian cycle. Paired photographs of the sex skin and ovaries from individual animals were taken at the time of experimental ovariectomy. Note that these changes occur only in some animals and cannot be used to generally monitor ovarian cyclicity in this species.
Another but more demanding approach is to monitor the endocrinology of the ovarian cycle. This approach requires frequent blood collections and access to validated assays for gonadotropic hormones and gonadal steroid hormones. Typically, the cyclicity pattern is initially determined from vaginal smears, and subsequently, blood sampling for the new cycle starts on the day of first bleeding. Blood samples (about 2 mL) are taken on days 1, 4, 7, 10, 11, 12, 13, 14, 15, 16, 18, 20, 22, 24, and 27 of menstrual cycle. A typical female reproductive toxicity study comprises six ovarian cycles—that is, two predose cycles, two cycles with exposure to test item, and two recovery cycles following cessation of test-item administration. Owing to restraints imposed by the available blood volume, the blood-withdrawal exercise is done only during predose observation cycle 2, test-item treatment cycles 1 and 2 (corresponding to cycles 3 and 4), and recovery cycle 2 (corresponding to cycle 6 of the study). If menstrual cyclicity is impaired, blood samples are obtained according to the above scheme, assuming a nominal cycle length of thirty days. Serum is prepared and analyzed for concentrations of progesterone, 17-β estradiol, LH, and FSH. Optionally, inhibins, testosterone, prolactin, and adrenal steroids can be determined.
RECOMMENDATIONS FOR ASSESSING FEMALE REPRODUCTIVE TOXICITY IN CYNOMOLGUS MONKEYS
Vaginal smears for menstruation are sufficient for identifying normally cycling female animals. For a special female fertility study, it is important to use only animals with a regular menstrual cycle—that is, cycle monitoring should be available for three to five consecutive cycles prior to recruiting the animals into a toxicity study. Only 30% to 50% of cycling females may present with a regular ovarian cycle. If it is decided to include endocrine measurements, frequent blood-sampling schedules (e.g., as described above) should be included both during predose, during test-item exposure, and during the recovery period. Typically, one observation and two treatment cycles are used for closely monitoring hormone levels, whereas inclusion of further sampling cycles might impose limitations on blood-volume availability. The use of weekly or monthly determinations of female reproductive hormones is not recommended, as these intervals might prove to be too long for making judgments about endocrine effects. At a minimum, estradiol and progesterone levels should be determined since these two steroids are the main regulators of the primate ovarian cycle. Preferably, LH and FSH should be included in the analysis. In our experience, it is not advisable to combine vaginal smears with weekly or monthly hormone determinations. Should vaginal smear analysis indicate ovarian irregularities, it is usually not possible to reconcile or interpret these alterations in conjunction with infrequent hormone data.
OVARIAN CYCLES IN THE MARMOSET
The basic aspects of the reproductive physiology of the common marmoset were described in the seventies (Hearn and Lunn 1975). It is evident that the features of the marmoset ovarian cycle differ substantially from those of macaques and humans (Abbott et al. 2003). Among these differences are lack of external signs of menstruation or changes in the vaginal epithelium that would permit cycle-stage assessment, multiovulation, postpartum conception, and differently timed follicular and luteal phases. Marmosets and tamarins are the only anthropoid primate species that exhibit multiple ovulation (Tardif et al. 2003). Reported cycle length ranges from 15 to 28 days with a rather short follicular phase of around 8 days and a luteal phase of around 20 days (Fraser and Lunn 1999). We observed a cycle duration of 28.7 ± 6.3 (SD) days in 110 ovarian cycles from eighteen animals (Fuchs and Weinbauer 2006). Between two and four eggs are ovulated, and recent observations suggest that multiple dominant follicles are present (Gilchrist et al. 2001). For the marmoset, it was suggested that the follicle could be the determinant for spontaneous luteinization (Wehrenberg and Rune 2000). Angiogenesis is essential for luteal development, and this process is initiated by LH activity (e.g., chorionic gonadotropin) in this species (Dickson and Fraser 2000).
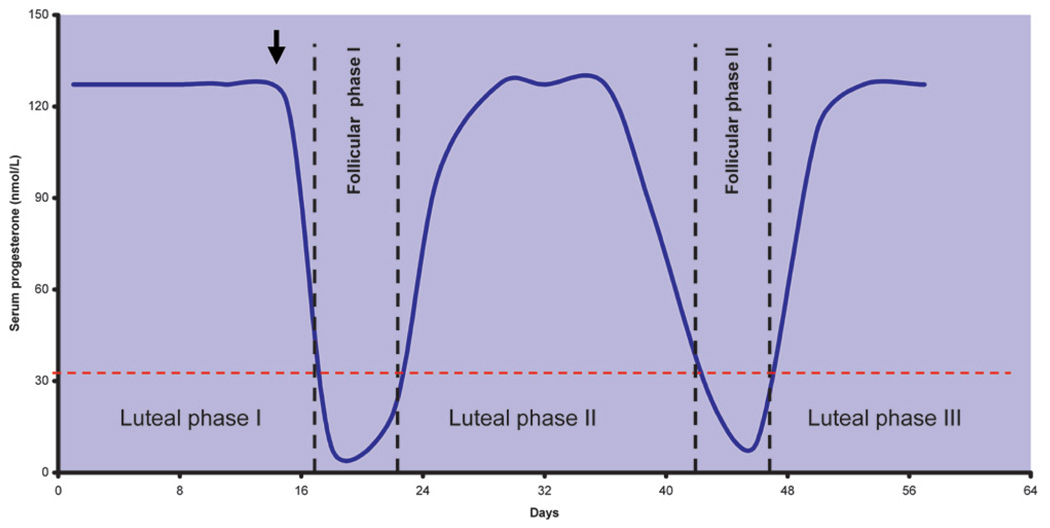
To be able to monitor the ovarian cycle in the absence of external signs, it is necessary to experimentally trigger luteolysis, which is commonly achieved by administration of prostaglandin F2-alpha (Harlow, Hearn, and Hodges 1984). The day after prostaglandin administration is denoted as day 1 of the follicular phase of the next cycle, and blood progesterone levels serve to monitor the ovarian cycle. A progesterone peak above 10 ng/L is used as hallmark of ovulation on the prior day. Since individual variability and fluctuations of progesterone can be enormous, it is advisable to monitor several ovarian cycles to determine individual ovarian cyclicity (Figure 7).
FIGURE 7.
Progesterone-based ovarian cycle monitoring in the marmoset. Note that luteal-phase duration is about twice as long as follicular phase. To initiate cycle monitoring, prostaglandin F2-alpha is used for induction of luteolysis in the first cycle (arrow).
In the intact marmoset, the LH receptor lacks exon 10, which clinically would mean an inactive LH receptor that cannot respond to LH in terms of steroid production (Gromoll et al. 2000; Zhang, Kero, and Huhtaniemi 1998). A surprising difference between marmosets and Old World primates including humans resides in the fact that marmosets lack LH (Gromoll et al. 2003). Recent work even suggests that this could be a common feature of New World monkeys (Muller et al. 2004), and according to recent concepts, the pituitary produces and releases chorionic gonadotropin instead of LH (Henke et al. 2007). Studies on the relationship between GnRH release and induced chorionic gonadotropin release revealed a discordant pattern when compared with the GnRH and LH congruence described for rats and other primates (Tannenbaum et al. 2007). These data imply some differences in the interplay between hypothalamus and pituitary in marmoset versus macaques and human. Among group-housed female marmosets, typically only the socially most dominant female reproduces, whereas subordinate females exhibit endocrine suppression associated with anovulation (for further details, Abbott et al. 1997). These inhibitory effects on reproduction seem to be mediated via visual, behavioral, and olfactory signals. It also appears that infanticide is used for inbreeding avoidance since females—if they are pregnant—may kill their daughters’ infants (Saltzman et al. 2008).
OVARIAN MENARCHE AND MENOPAUSE
At approximately twenty weeks following birth, LH and FSH levels in circulation peak sharply followed by a decline toward prepubertal levels in macaques (Mann et al. 2000; Plant 2006). This neonatal gonadotropin peak is reduced in females ovariectomized at 1 week of age compared with males orchidectomized at the same age. The onset of puberty is triggered by initiation of pulsatile GnRH release, with a corresponding increase in the tonic level of LH secretion, particularly at night (Terasawa and Fernandez 2001; Wilson, Fisher, and Chikazawa 2004). Neither adrenalectomy nor pinealectomy alter the timing of the prepubertal/pubertal onset of gonadotropin release (Plant, Ramaswamy, and Dipietro 2006). Activation of the G protein–coupled GPR54 receptor on GnRH neurons by metastin/ kisspeptin triggers GnRH release on onset of puberty (Plant, Ramaswamy, and Dipietro 2006; Seminara et al. 2006). In the juvenile and prepubertal ovary, follicle development continues to the preantral and early antral follicle stage but does not lead to formation of preovulatory follicles. Instead, many primordial follicles are lost through atresia during these periods. Follicles in the prepubertal ovary are steroidogenically active as suggested by several-fold higher estradiol concentrations in the ovarian vein compared with circulating levels and by the fact that ovariectomy in prepubertal animals decreases circulating estradiol levels (Winter et al. 1978). Menarche in female cynomolgus monkeys and rhesus monkeys occurs at an age of two to three years (Honjo, Cho, and Terao 1984; Watanabe et al. 2006; Wilen and Naftolin 1976). The earliest onset of vaginal bleeding in our experience among cynomolgus monkeys was encountered in an approximately eighteen-month-old female.
Female reproductive aging has been described for a variety of nonhuman primate species (Nozaki, Mitsunaga, and Shimizu 1995). In that context, the rhesus monkey has been studied in particular detail. Overall, the processes and consequences of female reproductive aging display similarities between Old World monkeys and women (for a review, see Bellino and Wise 2003). This, for example, holds true for endocrine profiles, menstrual-cycle irregularities, fertility decline, dyslipidemia, body weight gain, and altered body composition. Hot flush–like phenomena were observed in rhesus monkeys ovariectomized for simulation of menopause (Dierschke 1985). What appears different is the timing and duration of menopause and a more abrupt onset of cycle irregularities in monkeys (Shideler et al. 2001) when compared with women. In rhesus monkeys, menopausal changes occur very late in life (e.g., third decade of life; Walker 1995; Shideler et al. 2001), and consequently, duration of menopause is shorter relative to life span in the rhesus monkey than in women. For the seasonal Japanese monkey (Macaca fuscata), menopause has been observed at approximately twenty-seven years of age (Nozaki, Mitsunaga, and Shimizu 1995).
Endocrinologically, menopausal monkeys have elevated gonadotropin levels, reduced estradiol levels, and unaltered or slightly reduced progesterone levels. Recent work using pushpull perfusion from stalk-median eminence in rhesus monkeys indicates that during menopause, GnRH secretion is increased (Gore, Windsor-Engnell, and Terasawa 2004). Whether this is the consequence of reduced estradiol or progesterone feedback and/or inherent aging in GnRH-synthesizing hypothalamic neurons remains to be clarified. Histologically, ovarian senescence has recently been characterized in rhesus monkeys throughout a twenty-five-year period (Nichols et al. 2005) and in aged cynomolgus monkeys (Buse, Zöller, and van Esch, 2008 [this issue]). The proportion of primary follicles decreased from approximately 80% in one-year-old ovaries to approximately 55% in more than twenty-year-old ovaries in the rhesus monkey. Accordingly, total number of primary and antral follicles per ovarian section also decreased several-fold until the age of more than twenty years. In terms of reproduction, female monkeys lost fertility a couple of years prior to ovarian endocrine senescence, a feature qualitatively similar to women (Nozaki, Mitsunaga, and Shimizu 1995). Unlike for the rhesus monkey, information on female reproductive aging in cynomolgus monkeys is limited. However, a more recent report identified naturally occurring menopause in cynomolgus monkeys (Kavanagh, Williams, and Wagner 2005). In this study of sixteen animals, age estimates, based on dentition, were twenty-two to thirty-one years (M = 29) for seven postmenopausal females and nine to twenty-one years (M = 16) for nine premenopausal females. Postmenopausal animals had elevated FSH and reduced estradiol levels.
PSYCHOSOCIAL AND ENVIRONMENTAL EFFECTS
Recent developments in regulations governing the use of primates demand social housing whenever possible (Council of Europe 2007). Although it is evident that nonhuman primates generally benefit from social housing (Capitanio, Kyes, and Fairbanks 2006), systematic studies on the effects of social housing of cynomolgus monkeys in the context of reproductive toxicity evaluation are scarce. Earlier work in this species indicated that subordinate females have fewer ovulatory menstrual cycles and more cycles with deficient luteal-phase progesterone production (Adams, Kaplan, and Koritnik 1985). In these studies, however, group housing involved a male along with several females, whereas during the conduct of toxicity studies, the females were housed in isosexual pairs or groups. This experimental settings raise the question about effects of social housing on the endocrinology and duration of the menstrual cycle. Also, based on an article on “menstrual synchrony and suppression” by McClintock (1971), a series of studies were performed to evaluate the original hypothesis that close physical contact as well as pheromonal mechanisms may trigger the synchronization of human ovarian cycles. Comparative analysis of these studies yielded ambiguous results (Wilson, Kiefhaber, and Gravel 1991) leading to the proposition that avoiding synchrony might rather be a strategy to prevent female-female competition. We performed a series of studies to evaluate the effects of social housing on female reproductive endocrinology and to clarify whether ovarian cycle synchronization occurs in the cynomolgus monkey model during pair housing and group housing.
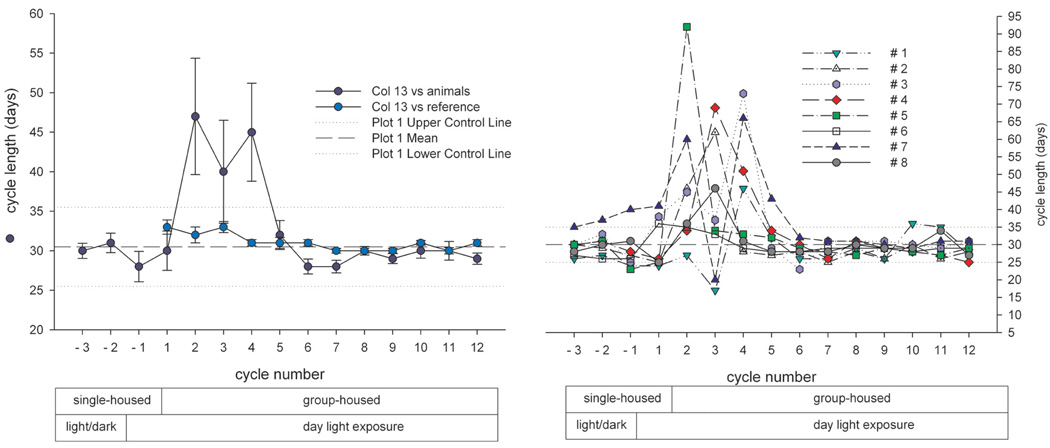
In one experiment, a newly formed group of eight previously single-housed animals was studied over fifteen ovarian cycles—that is, up to three cycles under single housing and twelve cycles following group formation. Cyclicity was assessed by daily vaginal smears and cycles were numbered consecutively. Data were compared to cycle data from seventy-eight animals housed individually during a comparable period. Blood samples were taken during cycle 1 (single housing) and during cycles 3, 5, 7, 9, and 11 (all group housing) on cycle days 1, 4, 7, 10, 11, 12, 13, 14, 15, 16, 18, 20, 22, 24, and 27 for estradiol and progesterone determination. Social rank was determined by food challenge test in regular intervals (Rilling, Winslow, and Kilts 2004). Clear effects on variation in cycle length were encountered when animals were transferred from individual housing to group housing. During group housing, average cycle duration became prolonged from thirty-one to forty-six days for about six months but subsequently returned to baseline duration from cycle 6 onward (Figure 8). The prolongation of menstrual-cycle duration corresponded to a statistically significant absent or decreased preovulatory estradiol peak and loss of the normal luteal progesterone rise in cycle 3 (Figure 9), whereas from cycle 6 onward, hormone patterns appeared normal. These observations have a significant bearing on the design of female reproductive toxicity studies, suggesting a need for either individual housing or use of pairs and groups with an established history of social contact for at least six months.
FIGURE 8.
Group mean cycle length (left panel) and individual menstrual-cycle length (right) from eight animals under individual and group-housing conditions. Data are M ± SD and are displayed in Figure 13 for every animal. The reference line (blue circles) in the left panel depicts the mean cycle duration in seventy-eight single-housed animals (M ± SD). Dotted lines indicate the normal variation (30.4 ± 4.7 days) of menstrual cycle length. Note the absence of seasonal variation in cycle duration. The right panel depicts the individual data for the eight individual animals. Note the increased variability and cycle duration after transition from single to group housing and the fact that these cycle irregularities were transient. Following approximately six cycles, ovarian cycle duration had returned to normal. The x-axis denotes the numbers of successive ovarian cycles. Negative cycles numbers are those prior to having all eight animals transferred into one group.
FIGURE 9.
Group mean (± SD) progesterone (left panel) and estradiol (right panel) levels during ovarian cycles from eight animals under individual and group-housing conditions (cycle duration is shown in Figure 8). Data for cycle 1 were collected during single housing, whereas subsequent cycle data are derived under group-housing conditions (cycles 3, 5, 7, 9, and 11). The asterisks indicate statistical significances. Note that during cycle 3—within the period of cycle irregularity and prolongation—the ovulatory estradiol peak and luteal progesterone peak were suppressed, suggesting a transient lack of ovulation.
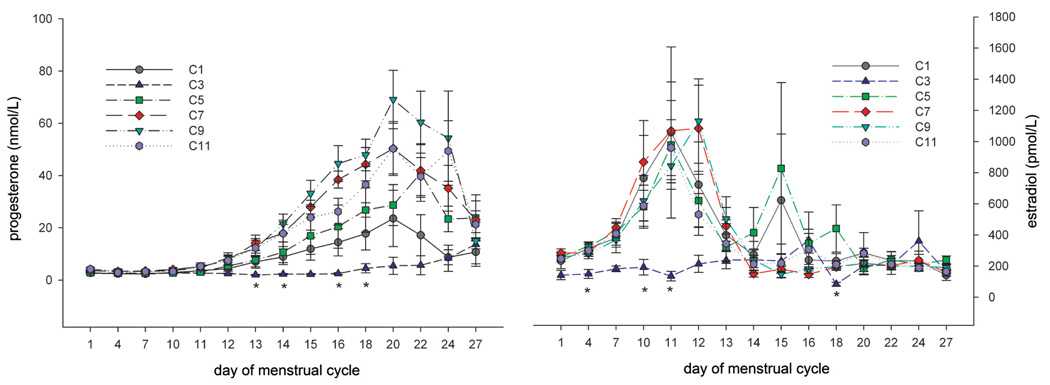
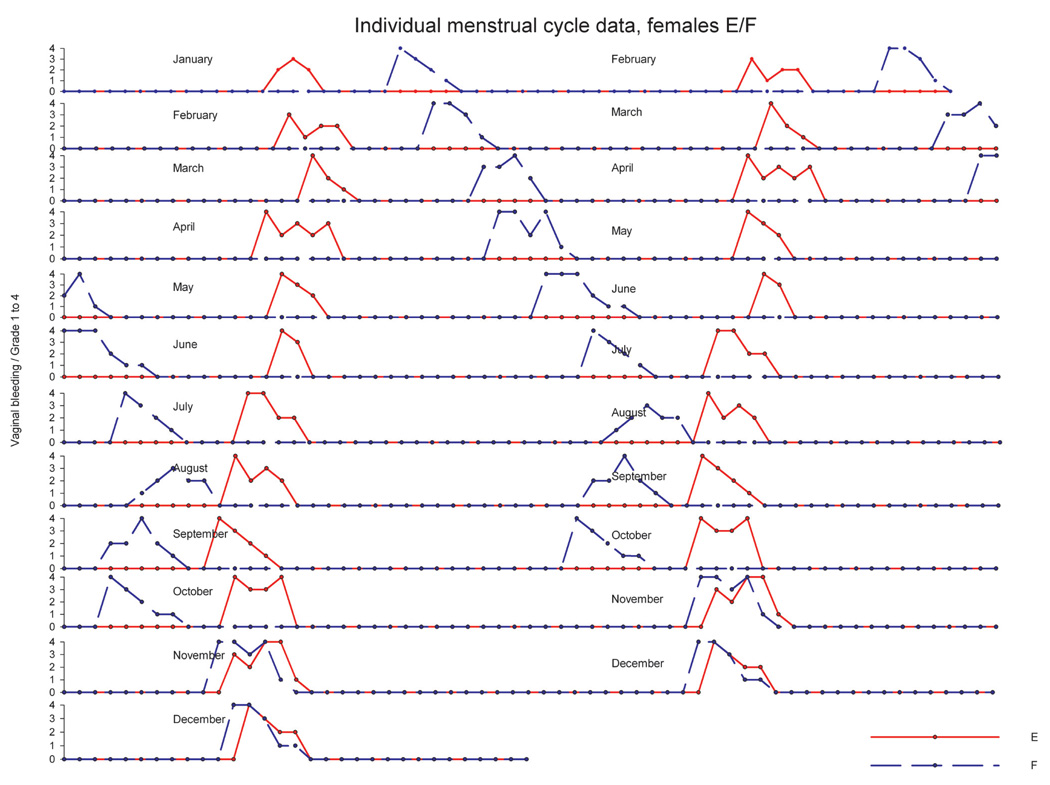
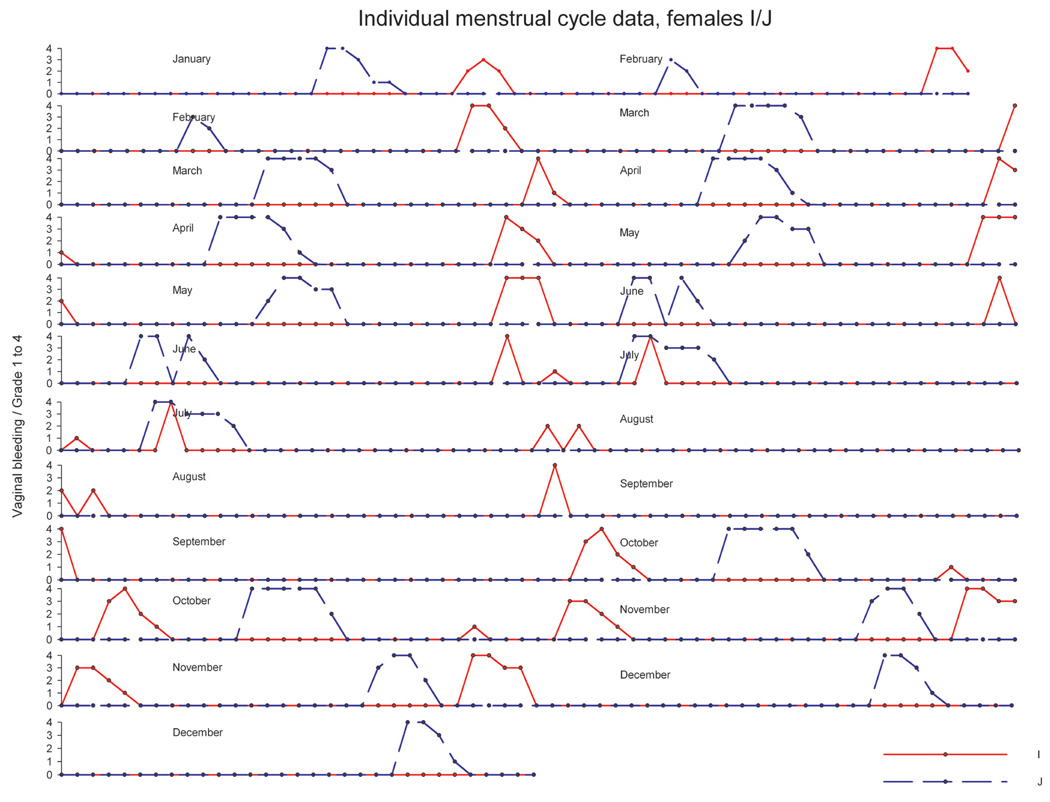
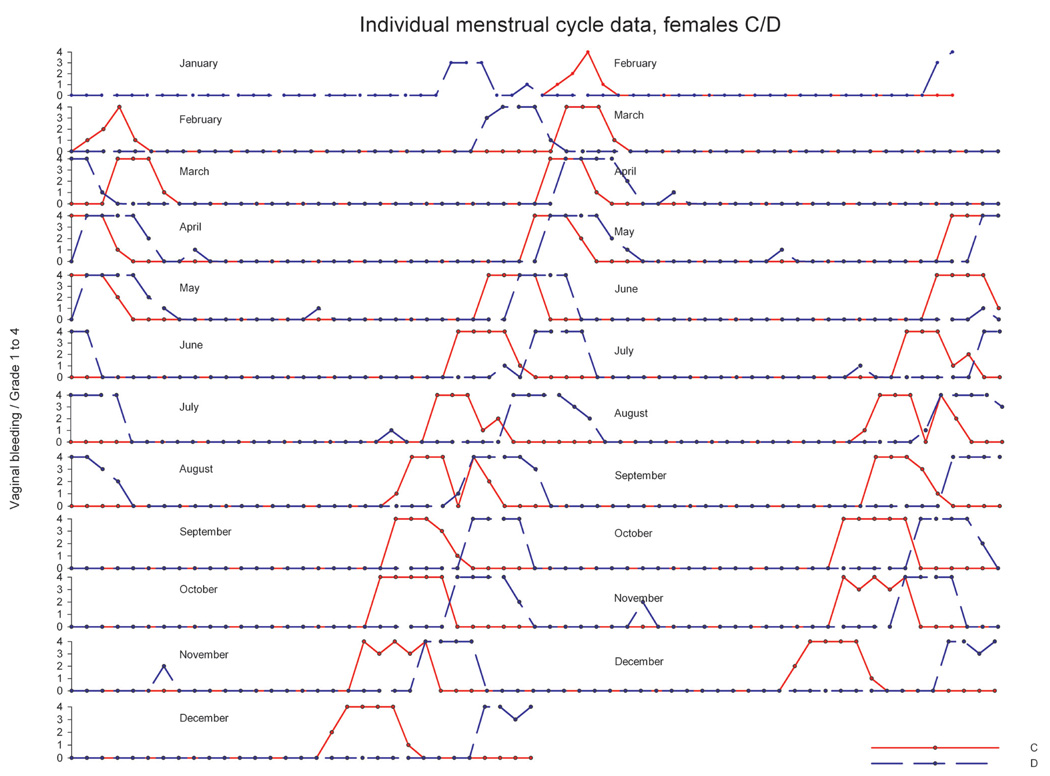
In another experiment, twenty mature animals—already accustomed to each other—were pair housed (pairs were denoted as A/B, C/D, E/F, G/H, I/J, K/L, M/N, O/P, R/S, T/U) in a climate-controlled room over a period of approximately twelve months. Artificial lighting was controlled automatically to provide a cycle of twelve hours of light and twelve hours of dark exposure. During the observation period, the menstrual cycle was monitored using daily vaginal smears for menses detection. Average cycle duration across the year ranged between 29.3 and 31.3 days, and no evidence for seasonal variation of ovarian cycles was obtained (data not shown). Analysis of individual menstrual cycle patterns revealed no evidence for a menstrual-cycle synchrony in pair-housed (examples in Figure 10–Figure 12) animals. For pair E/F (Figure 10), cycle timing was different but very robust and not influenced by the cage mate. Pair C/D (Figure 11) maintained a parallel cycle, whereas pair I/J maintained an irregular cyclicity (Figure 12) irrespective of the cage mate. Generally, all pairs presented robust and regular cycle durations. It has been reported earlier that female proximity or social contact might stabilize ovarian cycles (Wallis, King, and Roth-Meyer 1986). Our data set was not sufficiently large to ascertain such effect. During the group-housing experiment comprising eight animals described above and shown in Figure 8 and Figure 9, ovarian cycles were studied throughout fifteen cycles. These data were replotted to evaluate whether seasonal or synchronization effects were present (Figure 13). Neither seasonality nor synchronization of ovarian cycles could be detected in these experiments. Hence, there is no evidence for the synchronization of ovarian cycles in female cynomolgus monkeys housed in pairs or groups.
FIGURE 10.
Individual ovarian cycle pattern (double plot) of pair-housed cynomolgus monkeys with separate cycle patterns and not influenced by the cage mate. The y-axis denotes the grading of vaginal smear evaluation.
FIGURE 12.
Individual ovarian cycle pattern (double plot) of pair-housed cynomolgus monkeys with irregular cycle patterns and not influenced by the cage mate. The y-axis denotes the grading of vaginal smear evaluation.
FIGURE 11.
Individual ovarian cycle pattern (double plot) of pair-housed cynomolgus monkeys with parallel cycle patterns and not influenced by the cage mate. The y-axis denotes the grading of vaginal smear evaluation.
FIGURE 13.
Individual cyclicity pattern of eight cynomolgus monkeys throughout a period of approximately seventeen months (data were replotted from Figure 8). Animals were initially single housed (shaded area) and then co-housed as a group. Every plot represents one animal. The y-axis denotes the grading of vaginal smear evaluation. Note the absence of synchronization and seasonality and the transient irregularity/absence of cycling in some animals during group housing.
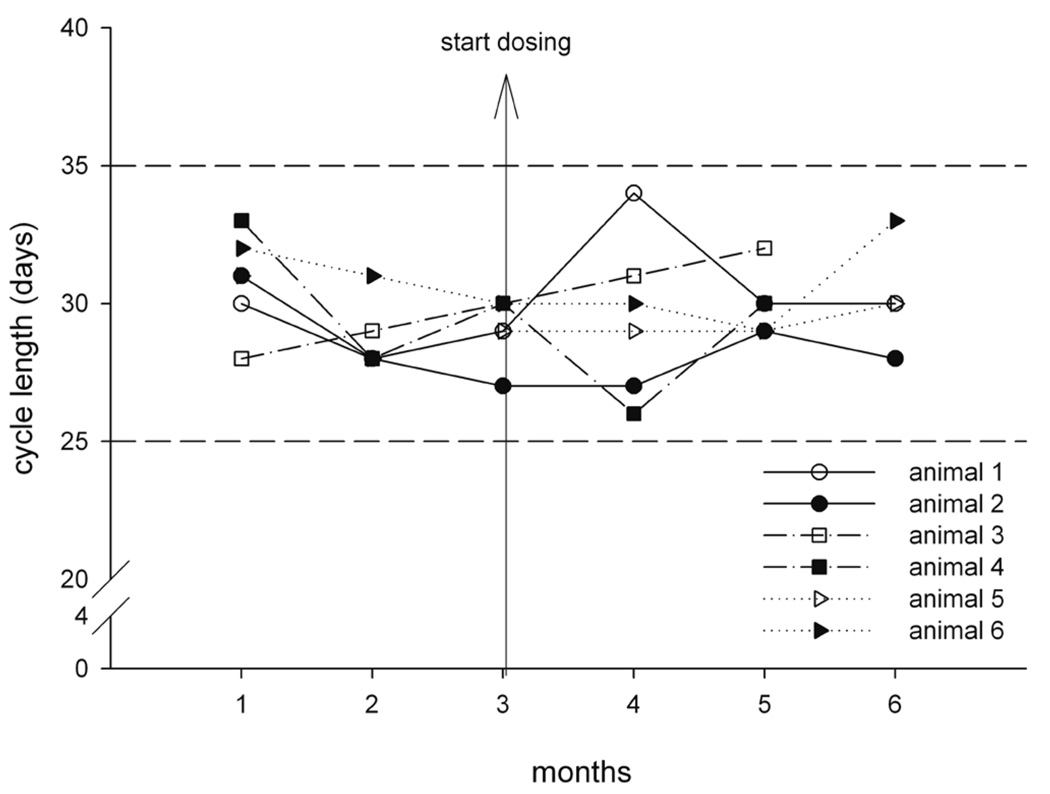
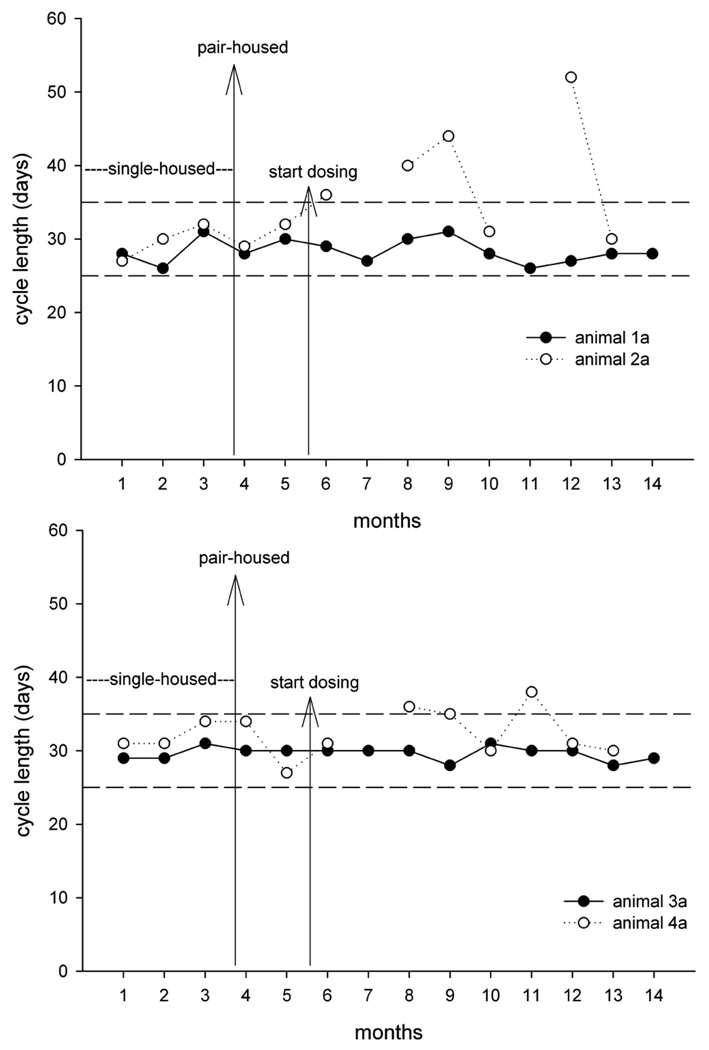
In further experiments, cycle duration was studied throughout a six-month period in three pairs that had been in contact for more than twelve months and in two pairs that had not had contact previously. Artificial lighting was controlled automatically to provide a cycle of twelve hours of light and twelve hours of dark exposure. These animals were also subjected to daily vehicle administration during toxicity evaluation. Ovarian cycle duration remained constant in the three accustomed pairs (Figure 14). In contrast, in the two pairs without previous contact, one animal each of the newly formed pairs presented with prolonged and irregular cycle (Figure 15). These observations along with the findings of the first two experiments clearly demonstrate that for the study of ovarian cycle length and reproductive endocrinology in socially housed female cynomolgus monkeys, it is essential to consider the housing history and familiarity between the animals prior to pair or group formation.
FIGURE 14.
Cycle duration before and during treatment in six cynomolgus monkeys housed in pairs (animal 1/animal 2, animal 3/animal 4, animal 5/animal 6). Prior to formation of pairs, the animals knew each other. Vertical arrow represents the onset of oral daily dosing of vehicle in a toxicity study. Dashed horizontal lines represent the upper and lower limits of spontaneous ovarian cycle duration. Note that cycle duration remained within normal limits for all animals.
FIGURE 15.
Cycle duration before and during treatment in four cynomolgus monkeys housed in pairs (upper panel: animal la/animal 2a; lower panel: animal 3a/animal 4a). Prior to formation of pairs (long vertical arrow), the animals were single housed and did not know each other. Short vertical arrow represents the onset of oral daily dosing of vehicle in a toxicity study. Dashed horizontal lines represent the upper and lower limits of spontaneous ovarian cycle duration. Note that cycle duration remained within normal limits in one animal of each pair, whereas in the other animal, cycle duration transiently was prolonged or menstrual bleeding was absent.
SEASONALITY OF OVARIAN FUNCTION
It is important to recognize that some nonhuman primate species exhibit distinct seasonal variation in reproductive functions depending on the latitude of their habitat. Species residing in equatorial habitats and not exposed to widely varying seasons lack such clear-cut annual variation of reproductive functions. Marmosets reside in the canopy of tropical forests and do not show reproductive seasonality of fertility or breeding. Cynomolgus monkeys are sexually active and fertile throughout the entire year and are not considered to express distinct and significant reproductive seasonality. Under feral conditions, pregnant females were discovered throughout the entire year (Kavanagh and Laursen 1984) with the incidence of birth being somewhat related to the rainy season. Menstrual cyclicity was unrelated to seasonal environment (Dang 1977). Our own observations in seventy-eight cynomolgus monkeys housed individually (Figure 8 and Figure 13; data for reference group) and twenty animals housed in ten pairs (Figure 10–Figure 12) for at least twelve months are concordant with the absence of ovarian seasonality in the cynomolgus monkey under laboratory conditions and under natural-light conditions.
In contrast, rhesus monkeys display pronounced seasonality of reproductive activity for both sexes (Ghosh and Sengupta 1992; Herndon et al. 1996). In this primate, reproductive functions are entirely shut off or severely diminished for approximately half of the year. In our latitudes, sexual activity and gonadal activity are present roughly throughout October until March, but this may also vary substantially for individual animals. It is crucial to consider that the annual rhythmicity of reproductive cycles can persist over years in captivity and under indoor artificial-light conditions (Wickings and Nieschlag 1980). Hence, in these circumstances, seasonality of reproduction may be uncoupled from actual season, and eventually, an animal may display periodic reproductive activation that is uncoupled to outside season. During the out-of-season periods, reproductive hormone secretion and gonadal activity are at a complete halt. Therefore, the seasonal status of rhesus monkeys should be assessed carefully in general toxicity studies and obviously in special toxicity studies when reproductive parameters are a suspected target of the test article.
ACKNOWLEDGMENTS
We are grateful to the technical and operational staff at Covance Laboratories GmbH, Muenster, Germany, for skillful and dedicated assistance during experimental conduct and data capture and analysis.
Abbreviations
- GnRH
gonadotropin-releasing hormone
- LH
luteinizing hormone
- FSH
follicle-stimulating hormone
- AN
arcuate nucleus
Footnotes
REFERENCES
- Abbott DH, Barnett DK, Colman RJ, Yamamoto ME, Schultz-Darken NJ. Aspects of common marmoset basic biology and life history important for biomedical research. Comp Med. 2003;53:339–350. [PubMed] [Google Scholar]
- Abbott DH, Saltzman W, Schultz-Darken NJ, Smith TE. Specific neuroendocrine mechanisms not involving generalized stress mediate social regulation of female reproduction in cooperatively breeding marmoset monkeys. Ann NY Acad Sci. 1997;807:219–238. doi: 10.1111/j.1749-6632.1997.tb51923.x. [DOI] [PubMed] [Google Scholar]
- Adams MR, Kaplan JR, Koritnik DR. Psychosocial influences on ovarian endocrine and ovulatory function in Macaca fascicularis. Physiol Behav. 1985;35:935–940. doi: 10.1016/0031-9384(85)90262-8. [DOI] [PubMed] [Google Scholar]
- Archer DF. Role of the nonhuman primate for research related to women’s health. Ilar J. 2004;45:212–219. doi: 10.1093/ilar.45.2.212. [DOI] [PubMed] [Google Scholar]
- Attia MA. Cyclic changes in genital organs and vaginal cytology in cynomolgus monkeys (Macaca fascicularis) Dtsch Tierarztl Wochenschr. 1998;105:399–404. [PubMed] [Google Scholar]
- Bellino FL, Wise PM. Nonhuman primate models of menopause workshop. Biol Reprod. 2003;68:10–18. doi: 10.1095/biolreprod.102.005215. [DOI] [PubMed] [Google Scholar]
- Bilezikjian LM, Blount AL, Donaldson CJ, Vale WW. Pituitary actions of ligands of the TGF-beta family: Activins and inhibins. Reproduction. 2006;132:207–215. doi: 10.1530/rep.1.01073. [DOI] [PubMed] [Google Scholar]
- Borman SM, Chaffin CL, Schwinof KM, Stouffer RL, Zelinski-Wooten MB. Progesterone promotes oocyte maturation, but not ovulation, in nonhuman primate follicles without a gonadotropin surge. Biol Reprod. 2004;71:366–373. doi: 10.1095/biolreprod.103.023390. [DOI] [PubMed] [Google Scholar]
- Buse E, Zöller M, van Esch E. The macaque ovary, with special reference to the cynomolgus macaque (Macaca fascicularis) Toxicol Pathol. 2008;36:24S–66S. [Google Scholar]
- Butterstein GM, Mann DR, Gould K, Castracane VD. Prolonged inhibition of normal ovarian cycles in the rat and cynomolgus monkeys following a single sc. injection of danazol. Hum Reprod. 1997;12:1409–1415. doi: 10.1093/humrep/12.7.1409. [DOI] [PubMed] [Google Scholar]
- Capitanio JP, Kyes RC, Fairbanks LA. Considerations in the selection and conditioning of Old World monkeys for laboratory research: Animals from domestic sources. ILAR J. 2006;47:294–306. doi: 10.1093/ilar.47.4.294. [DOI] [PubMed] [Google Scholar]
- Cheng CK, Leung PC. Molecular biology of gonadotropin-releasing hormone (GnRH)-I, GnRH-II, and their receptors in humans. Endocr Rev. 2005;26:283–306. doi: 10.1210/er.2003-0039. [DOI] [PubMed] [Google Scholar]
- Cline JM. Assessing the mammary gland of nonhuman primates: Effects of endogenous hormones and exogenous hormonal agents and growth factors. Birth Defects Res B Dev Reprod Toxicol. 2007;80:126–146. doi: 10.1002/bdrb.20112. [DOI] [PubMed] [Google Scholar]
- Cline JM, Soderqvist G, Register TC, Williams JK, Adams MR, Von Schoultz B. Assessment of hormonally active agents in the reproductive tract of female nonhuman primates. Toxicol Pathol. 2001;29:84–90. doi: 10.1080/019262301301418883. [DOI] [PubMed] [Google Scholar]
- Council of Europe. Appendix A of the European convention for the protection of vertebrate animals used for experimental and other scientific purposes (ETS no. 123)—Guidelines for the accommodation and care of animals (article 5 of the convention) Cons. 2006;123 effective July 15, 2007. [Google Scholar]
- Dang DC. Absence of seasonal variation in the length of the menstrual cycle and the fertility of the crab-eating macaque (Macaca fascicularis) raised under natural daylight ratio. Ann Biol Biochim Biophys. 1977;17:1–7. [Google Scholar]
- Dickson SE, Fraser HM. Inhibition of early luteal angiogenesis by gonadotropin-releasing hormone antagonist treatment in the primate. J Clin Endocrinol Metab. 2000;85:2339–2344. doi: 10.1210/jcem.85.6.6621. [DOI] [PubMed] [Google Scholar]
- Dierschke DJ. Temperature changes suggestive of hot flushes in rhesus monkeys: Preliminary observations. J Med Primatol. 1985;14:271–280. [PubMed] [Google Scholar]
- Duffy DM, Stouffer RL. Follicular administration of a cyclooxygenase inhibitor can prevent oocyte release without alteration of normal luteal function in rhesus monkeys. Hum Reprod. 2002;17:2825–2831. doi: 10.1093/humrep/17.11.2825. [DOI] [PubMed] [Google Scholar]
- Fraser HM, Groome NP, McNeilly AS. Follicle-stimulating hormone-inhibin B interactions during the follicular phase of the primate menstrual cycle revealed by gonadotropin-releasing hormone antagonist and antiestrogen treatment. J Clin Endocrinol Metab. 1999;84:1365–1369. doi: 10.1210/jcem.84.4.5586. [DOI] [PubMed] [Google Scholar]
- Fraser HM, Lunn SF. Nonhuman primates and female reproductive medicine. In: Weinbauer GF, F Vogel, editors. Novel approaches towards primate toxicology. Germany: Waxmann, Münster; 1999. pp. 27–59. [Google Scholar]
- Fuchs A, Weinbauer GF. Feasibility of Embryofetal Development (EFD) studies in marmoset monkeys (Callithrix jacchus) Birth Defects Res B Dev Reprod Toxicol. 2006;76:387. [Google Scholar]
- Ghosh D, Sengupta J. Patterns of ovulation, conception and preimplantation embryo development during the breeding season in rhesus monkeys kept under semi-natural conditions. Acta Endocrinol (Copenh) 1992;127:168–173. doi: 10.1530/acta.0.1270168. [DOI] [PubMed] [Google Scholar]
- Gilchrist RB, Wicherek M, Heistermann M, Nayudu PL, Hodges JK. Changes in follicle-stimulating hormone and follicle populations during the ovarian cycle of the common marmoset. Biol Reprod. 2001;64:27–35. doi: 10.1095/biolreprod64.1.127. [DOI] [PubMed] [Google Scholar]
- Goodman AL, Hodgen GD. The ovarian triad of the primate menstrual cycle. Rec Prog Horm Res. 1983;39:1–73. doi: 10.1016/b978-0-12-571139-5.50005-7. [DOI] [PubMed] [Google Scholar]
- Gore AC, Windsor-Engnell BM, Terasawa E. Menopausal increases in pulsatile gonadotropin-releasing hormone release in a non-human primate (Macaca mulatta) Endocrinology. 2004;145:4653–4659. doi: 10.1210/en.2004-0379. [DOI] [PubMed] [Google Scholar]
- Gougeon A. Regulation of ovarian follicular development in primates: Facts and hypotheses. Endocr Rev. 1996;17:121–155. doi: 10.1210/edrv-17-2-121. [DOI] [PubMed] [Google Scholar]
- Gromoll J, Eiholzer U, Nieschlag E, Simoni M. Male hypogonadism caused by homozygous deletion of exon 10 of the luteinizing hormone (LH) receptor: Differential action of human chorionic gonadotropin and LH. J Clin Endocrinol Metab. 2000;85:2281–2286. doi: 10.1210/jcem.85.6.6636. [DOI] [PubMed] [Google Scholar]
- Gromoll J, Wistuba J, Terwort N, Godmann M, Muller T, Simoni M. A new subclass of the luteinizing hormone/chorionic gonadotropin receptor lacking exon 10 messenger RNA in the New World monkey (Platyrrhini) lineage. Biol Reprod. 2003;69:75–80. doi: 10.1095/biolreprod.102.014902. [DOI] [PubMed] [Google Scholar]
- Harlow CR, Hearn JP, Hodges JK. Ovulation in the marmoset monkey: Endocrinology, prediction and detection. J Endocrinol. 1984;103:17–24. doi: 10.1677/joe.0.1030017. [DOI] [PubMed] [Google Scholar]
- Hearn JP, Lunn SF. The endocrinology of the ovarian cycle and of pregnancy in the marmoset monkey, Callithrix jaccus. J Endocrinol. 1975;65:1P. [PubMed] [Google Scholar]
- Henke A, Luetjens CM, Simoni M, Gromoll J. Chorionic gonadotropin beta-subunit gene expression in the marmoset pituitary is controlled by steroidogenic factor 1, early growth response protein 1, and pituitary homeobox factor 1. Endocrinology. 2007;148:6062–6072. doi: 10.1210/en.2007-0825. [DOI] [PubMed] [Google Scholar]
- Herndon JG, Bein ML, Nordmeyer DL, Turner JJ. Seasonal testicular function in male rhesus monkeys. Horm Behav. 1996;30:266–271. doi: 10.1006/hbeh.1996.0032. [DOI] [PubMed] [Google Scholar]
- Hillier SG, Whitelaw PF, Smyth CD. Follicular oestrogen synthesis: The “two-cell, two-gonadotrophin” model revisited. Mol Cell Endocrinol. 1994;100:51–54. doi: 10.1016/0303-7207(94)90278-x. [DOI] [PubMed] [Google Scholar]
- Honjo S, Cho F, Terao K. Establishing the cynomolgus monkey as a laboratory animal. Adv Vet Sci Comp Med. 1984;28:51–80. doi: 10.1016/b978-0-12-039228-5.50008-5. [DOI] [PubMed] [Google Scholar]
- Hotchkiss J, Knobil E. The menstrual cycle and its neuroendocrine control. In: Knobil E, Neill JD, editors. The physiology of reproduction. New York: Raven Press; 1994. pp. 711–733. [Google Scholar]
- Jabbour HN, Kelly RW, Fraser HM, Critchley HO. Endocrine regulation of menstruation. Endocr Rev. 2006;27:17–46. doi: 10.1210/er.2004-0021. [DOI] [PubMed] [Google Scholar]
- Kaplan JR. Modeling women’s health with nonhuman primates and other animals. ILAR J. 2004;45:83–88. doi: 10.1093/ilar.45.2.83. [DOI] [PubMed] [Google Scholar]
- Kavanagh K, Williams KJ, Wagner JD. Naturally occurring menopause in cynomolgus monkeys: Changes in hormone, lipid, and carbohydrate measures with hormonal status. J Med Primatol. 2005;34:171–177. doi: 10.1111/j.1600-0684.2005.00114.x. [DOI] [PubMed] [Google Scholar]
- Kavanagh M, Laursen E. Breeding seasonality among long-tailed macaques, Macaca fascicularis, in Peninsular Malaysia. Int J Primatol. 1984;5:17–29. [Google Scholar]
- Kerdelhue B, Williams RF, Lenoir V, Fardin V, Kolm P, Hodgen GD, Jones GS, Scholler R, Jones HW., Jr Variations in plasma levels of substance P and effects of a specific substance P antagonist of the NK(1) receptor on preovulatory LH and FSH surges and progesterone secretion in the cycling cynomolgus monkey. Neuroendocrinology. 2000;71:228–236. doi: 10.1159/000054540. [DOI] [PubMed] [Google Scholar]
- Macklon NS, Stouffer RL, Giudice LC, Fauser BC. The science behind 25 years of ovarian stimulation for in vitro fertilization. Endocr Rev. 2006;27:170–207. doi: 10.1210/er.2005-0015. [DOI] [PubMed] [Google Scholar]
- Mann DR, Akinbami MA, Lunn SF, Fraser HM, Gould KG, Ansari AA. Endocrine-immune interaction: Alteractions in immune function resulting from neonatal treatment with a GnRH antagonist and seasonality in male primates. Am J Reprod Immunol. 2000;44:30–40. doi: 10.1111/j.8755-8920.2000.440105.x. [DOI] [PubMed] [Google Scholar]
- McClintock MK. Menstrual synchrony and suppression. Nature. 1971;229:244–245. doi: 10.1038/229244a0. [DOI] [PubMed] [Google Scholar]
- Messinis IE. Ovarian feedback, mechanism of action and possible clinical implications. Hum Reprod Update. 2006;12:557–571. doi: 10.1093/humupd/dml020. [DOI] [PubMed] [Google Scholar]
- Millar RP. GnRHs and GnRH receptors. Anim Reprod Sci. 2005;88:5–28. doi: 10.1016/j.anireprosci.2005.05.032. [DOI] [PubMed] [Google Scholar]
- Molskness TA, Woodruff TK, Hess DL, Dahl KD, Stouffer RL. Recombinant human inhibin-A administered early in the menstrual cycle alters concurrent pituitary and follicular, plus subsequent luteal, function in rhesus monkeys. J Clin Endocrinol Metab. 1996;81:4002–4006. doi: 10.1210/jcem.81.11.8923851. [DOI] [PubMed] [Google Scholar]
- Muller T, Simoni M, Pekel E, Luetjens CM, Chandolia R, Amato F, Norman RJ, Gromoll J. Chorionic gonadotrophin beta subunit mRNA but not luteinising hormone beta subunit mRNA is expressed in the pituitary of the common marmoset (Callithrix jacchus) J Mol Endocrinol. 2004;32:115–128. doi: 10.1677/jme.0.0320115. [DOI] [PubMed] [Google Scholar]
- Nichols SM, Bavister BD, Brenner CA, Didier PJ, Harrison RM, Kubisch HM. Ovarian senescence in the rhesus monkey (Macaca mulatta) Hum Reprod. 2005;20:79–83. doi: 10.1093/humrep/deh576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozaki M, Mtsunaga F, Shimizu K. Reproductive senescence in female Japanese monkeys (Macaca fuscata): Age- and season-related changes in hypothalamic-pituitary-ovarian functions and fecundity rates. Biol Reprod. 1995;52:1250–1257. doi: 10.1095/biolreprod52.6.1250. [DOI] [PubMed] [Google Scholar]
- O’Byrne KT, Knobil E. Electrophysiological approaches to gonadotrophin releasing hormone pulse generator activity in the rhesus monkey. Hum Reprod. 1993;8(Suppl. 2):37–40. doi: 10.1093/humrep/8.suppl_2.37. [DOI] [PubMed] [Google Scholar]
- Plant TM. The male monkey as a model for the study of the neurobiology of puberty onset in man. Mol Cell Endocrinol. 2006;254–55:97–102. doi: 10.1016/j.mce.2006.04.022. [DOI] [PubMed] [Google Scholar]
- Plant TM, Ramaswamy S, Dipietro MJ. Repetitive activation of hypothalamic G protein-coupled receptor 54 with intravenous pulses of kisspeptin in the juvenile monkey (Macaca mulatta) elicits a sustained train of gonadotropin-releasing hormone discharges. Endocrinology. 2006;147:1007–1013. doi: 10.1210/en.2005-1261. [DOI] [PubMed] [Google Scholar]
- Popa SM, Clifton DK, Steiner RA. The role of kisspeptins and GPR54 in the neuroendocrine regulation of reproduction. Ann Rev Physiol. 2008;70:213–238. doi: 10.1146/annurev.physiol.70.113006.100540. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Winslow JT, Kilts CD. The neural correlates of mate competition in dominant male rhesus macaques. Biol Psych. 2004;56:364–375. doi: 10.1016/j.biopsych.2004.06.027. [DOI] [PubMed] [Google Scholar]
- Saltzman W, Liedl KJ, Salper OJ, Pick RR, Abbott DH. Post-conception reproductive competition in cooperatively breeding common marmosets. Horm Behav. 2008;53:274–286. doi: 10.1016/j.yhbeh.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Seminara SB, Dipietro MJ, Ramaswamy S, Crowley WF, Jr, Plant TM. Continuous human metastin 45–54 infusion desensitizes G protein-coupled receptor 54-induced gonadotropin-releasing hormone release monitored indirectly in the juvenile male Rhesus monkey (Macaca mulatta): A finding with therapeutic implications. Endocrinology. 2006;147:2122–2126. doi: 10.1210/en.2005-1550. [DOI] [PubMed] [Google Scholar]
- Shaikh AA, Naqvi RH, Shaikh SA. Concentrations of oestradiol-17beta and progesterone in the peripheral plasma of the cynomolgus monkey (Macaca fascicularis) in relation to the length of the menstrual cycle and its component phases. J Endocrinol. 1978;79:1–7. doi: 10.1677/joe.0.0790001. [DOI] [PubMed] [Google Scholar]
- Shideler SE, Gee NA, Chen J, Lasley BL. Estrogen and progesterone metabolites and follicle-stimulating hormone in the aged macaque female. Biol Reprod. 2001;65:1718–1725. doi: 10.1095/biolreprod65.6.1718. [DOI] [PubMed] [Google Scholar]
- Stouffer RL. Progesterone as a mediator of gonadotrophin action in the corpus luteum: Beyond steroidogenesis. Hum Reprod Update. 2003;9:99–117. doi: 10.1093/humupd/dmg016. [DOI] [PubMed] [Google Scholar]
- Tannenbaum PL, Schultz-Darken NJ, Saltzman W, Terasawa E, Woller MJ, Abbott DH. Gonadotrophin-releasing hormone (GnRH) release in marmosets I: In vivo measurement in ovary-intact and ovariectomised females. J Neuroendocrinol. 2007;19:342–353. doi: 10.1111/j.1365-2826.2007.01534.x. [DOI] [PubMed] [Google Scholar]
- Tardif SD, Smucny DA, Abbott DH, Mansfield K, Schultz-Darken N, Yamamoto ME. Reproduction in captive common marmosets (Callithrix jacchus) Comp Med. 2003;53:364–368. [PubMed] [Google Scholar]
- Tena-Sempere M. Ghrelin and reproduction: Ghrelin as novel regulator of the gonadotropic axis. Vitam Horm. 2008;77:285–300. doi: 10.1016/S0083-6729(06)77012-1. [DOI] [PubMed] [Google Scholar]
- Terasawa E, Fernandez DL. Neurobiological mechanisms of the onset of puberty in primates. Endocr Rev. 2001;22:111–151. doi: 10.1210/edrv.22.1.0418. [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Johnson ML. A review and appraisal of deconvolution methods to evaluate in vivo neuroendocrine secretory events. J Neuroendocrinol. 1990;2:755–771. doi: 10.1111/j.1365-2826.1990.tb00638.x. [DOI] [PubMed] [Google Scholar]
- Walker ML. Menopause in female rhesus monkeys. Am J Primatol. 1995;35:59–71. doi: 10.1002/ajp.1350350106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis J, King BJ, Roth-Meyer C. The effect of female proximity and social interaction on the menstrual cycle of crab-eating monkeys (Macaca fascicularis) Primates. 1986;27:83–94. [Google Scholar]
- Watanabe D, Hoshiya T, Sato J, Yamaguchi Y, Horiguchi K, Nagashima Y, Okaniwa A, Yoshikawa H. Changes in the reproductive organs depending on phases of reproductive cycle and aging in female cynomolgus monkeys. J Toxicol Pathol. 2006;19:169–177. [Google Scholar]
- Wehrenberg U, Rune GM. Spontaneous luteinization of antral marmoset follicles in vitro. Mol Hum Reprod. 2000;6:504–509. doi: 10.1093/molehr/6.6.504. [DOI] [PubMed] [Google Scholar]
- Wickings EJ, Nieschlag E. Seasonality in endocrine and exocrine testicular function of the adult rhesus monkey (Macaca mulatta) maintained in a controlled laboratory environment. Int J Androl. 1980;3:87–104. doi: 10.1111/j.1365-2605.1980.tb00099.x. [DOI] [PubMed] [Google Scholar]
- Wilen R, Naftolin F. Age, weight and weight gain in the individual pubertal female rhesus monkey (Macaca mulatta) Biol Reprod. 1976;15:356–360. doi: 10.1095/biolreprod15.3.356. [DOI] [PubMed] [Google Scholar]
- Wilson ME, Fisher J, Chikazawa K. Estradiol negative feedback regulates nocturnal luteinizing hormone and follicle-stimulating hormone secretion in prepubertal female rhesus monkeys. J Clin Endocrinol Metab. 2004;89:3973–3978. doi: 10.1210/jc.2004-0148. [DOI] [PubMed] [Google Scholar]
- Wilson HC, Kiefhaber SH, Gravel V. Two studies of menstrual synchrony: Negative results. Psychoneuroendocrinology. 1991;16:353–359. doi: 10.1016/0306-4530(91)90021-k. [DOI] [PubMed] [Google Scholar]
- Winter JS, Faiman C, Reyes FI, Hobson WC. Gonadotrophins and steroid hormones in the blood and urine of prepubertal girls and other primates. J Clin Endocrinol Metab. 1978;7:513–530. doi: 10.1016/s0300-595x(78)80007-3. [DOI] [PubMed] [Google Scholar]
- Winters SJ, Moore JP. Paracrine control of gonadotrophs. Semin Reprod Med. 2007;25:379–387. doi: 10.1055/s-2007-984744. [DOI] [PubMed] [Google Scholar]
- Young KA, Chaffin CL, Molskness TA, Stouffer RL. Controlled ovulation of the dominant follicle: A critical role for LH in the late follicular phase of the menstrual cycle. Hum Reprod. 2003;18:2257–2263. doi: 10.1093/humrep/deg467. [DOI] [PubMed] [Google Scholar]
- Zeleznik AJ. In vivo responses of the primate corpus luteum to luteinizing hormone and chorionic gonadotropin. Proc Natl Acad Sci USA. 1998;95:11002–11007. doi: 10.1073/pnas.95.18.11002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeleznik AJ. Follicle selection in primates: Many are called but few are chosen. Biol Reprod. 2001;65:655–659. doi: 10.1095/biolreprod65.3.655. [DOI] [PubMed] [Google Scholar]
- Zeleznik AJ, Pohl CR. Control of follicular development, corpus luteum function, the maternal recognition of pregnancy, and the neuroendocrine regulation of the menstrual cycle in higher primates. In: Neill JD, editor. Knobil and Neill’s Physiology of Reproduction. Louis, MO: Elsevier, St; 2006. pp. 2449–2510. [Google Scholar]
- Zelinski-Wooten MB, Hutchison JS, Hess DL, Wolf DP, Stouffer RL. A bolus of recombinant human follicle stimulating hormone at midcycle induces periovulatory events following multiple follicular development in macaques. Hum Reprod. 1998;13:554–560. doi: 10.1093/humrep/13.3.554. [DOI] [PubMed] [Google Scholar]
- Zhang FP, Kero J, Huhtaniemi I. The unique exon 10 of the human luteinizing hormone receptor is necessary for expression of the receptor protein at the plasma membrane in the human luteinizing hormone receptor, but deleterious when inserted into the human follicle-stimulating hormone receptor. Mol Cell Endocrinol. 1998;142:165–174. doi: 10.1016/s0303-7207(98)00108-7. [DOI] [PubMed] [Google Scholar]