Abstract
BACKGROUND
Abnormal fetal testis development can result in disorders of sex development (DSDs) and predispose to later testicular dysgenesis syndrome (TDS) disorders such as testicular germ cell tumours. Studies of human fetal testis development are hampered by the lack of appropriate model, and intervention systems. We hypothesized that human fetal testis xenografts can recapitulate normal development.
METHODS
Human fetal testes (at 9 weeks, n = 4 and 14–18 weeks gestation, n = 6) were xenografted into male nude mice for 6 weeks, with or without hCG treatment of the host, and evaluated for normal cellular development and function using immunohistochemistry, triple immunofluorescence and testosterone assay. The differentiation and proliferation status of germ cells within xenografts was quantified and compared with age-matched controls.
RESULTS
Xenografts showed >75% survival with normal morphology. In the first-trimester xenografts seminiferous cord formation was initiated and in first- and second-trimester grafts normal functional development of Sertoli, Leydig and peritubular myoid cells was demonstrated using cell-specific protein markers. Grafts produced testosterone when hosts were treated with hCG (P = 0.004 versus control). Proliferation of germ cells and differentiation from gonocytes (OCT4+) into pre-spermatogonia (VASA+) occurred in grafts and quantification showed this progressed comparably with age-matched ungrafted controls.
CONCLUSIONS
Human fetal testis tissue xenografts demonstrate normal structure, function and development after xenografting, including normal germ cell differentiation. This provides an in vivo system to study normal human fetal testis development and its susceptibility to disruption by exogenous factors (e.g. environmental chemicals). This should provide mechanistic insight into the fetal origins of DSDs and TDS disorders.
Keywords: fetal testis, disorders of sexual differentiation, testicular dysgenesis syndrome, xenografts, fetal germ cell differentiation
Introduction
Formation of a testis from the indifferent gonad is orchestrated by the SRY gene, resulting in differentiation of Sertoli cells (Wilhelm et al., 2007). These then enclose the fetal germ cells (gonocytes) to form seminiferous cords, starting at 7–9 weeks' gestation (Wartenberg, 1981; Tilmann and Capel, 1999; Hanley et al., 2000; Gaskell et al., 2004; Ostrer et al., 2007). Fetal Leydig cells then differentiate and via hormone secretions, primarily testosterone, bring about bodywide masculinization (Scott et al., 2009).
Disorders of sex development (DSDs) may result from failure of normal gonadal development or subnormal androgen production/action (Hughes, 2008). Such disorders can predispose to development of testicular germ cell tumours (TGCTs) in young adulthood from pre-malignant carcinoma in situ (CIS) cells (Skakkebaek, 1972; Rajpert-De Meyts, 2006). CIS cells are thought to originate because of failure of normal differentiation of fetal germ cells into prespermatogonia (Rajpert-De Meyts, 2006), a process that encompasses fetal and early post-natal life in humans and involves the loss of expression of pluripotency factors (OCT4 and NANOG) and expression of proteins indicative of differentiation (VASA and MAGE-A4) (Gaskell et al., 2004; Anderson et al., 2007; Mitchell et al., 2008). TGCT may be the most serious manifestation of a testicular dysgenesis syndrome (TDS), which also includes some cases of undescended testis, hypospadias and low sperm counts; these TDS disorders are all thought to result from maldevelopment of the fetal testis (Skakkebaek et al., 2001).
Much of our understanding of normal and disrupted testicular development derives from rodent models (Wilhelm et al., 2007; Gassei et al., 2008), because only retrospective studies are usually possible in patients with DSDs or TDS disorders, preventing identification of the mechanisms linking fetal testis maldevelopment to altered cell development/function. However, there are major fundamental differences in the pace and duration of fetal testis development between primates and rodents (Ehmcke et al., 2006), especially regarding fetal germ cell differentiation (Mitchell et al., 2008). Its long duration, in particular, limits the use of in vitro testis explants as an investigative approach. Such differences may explain why rodents do not develop TGCT equivalent to that in humans (Oosterhuis and Looijenga, 2005; Mitchell et al., 2008; Anderson et al., 2009).
Testicular xenografting has become increasingly utilized as a technique in germ/stem cell biology, fertility preservation and the production of transgenic sperm (Wistuba and Schlatt, 2002; Dobrinski, 2008; Ehmcke and Schlatt, 2008; Schlatt et al. 2010). Xenografting of immature testis tissue from several species, including the rhesus macaque, into a nude mouse host results in full spermatogenesis within the grafted tissue (Honaramooz et al., 2004; Rodriguez-Sosa and Dobrinski, 2009). Previous reports of human testis xenografts show limited survival when post-natal tissue is grafted (Geens et al., 2006; Schlatt et al., 2006) and studies of fetal testis xenografts have been limited to descriptive reports of small numbers of second-trimester grafts (Povlsen et al., 1974; Skakkebaek et al., 1974; Yu et al., 2006). Therefore, the aim of the present study was to investigate the suitability of human fetal testis xenografting as a technique to recapitulate normal fetal testis development, including germ cell differentiation. Such a system would be invaluable for mechanistic investigation of the origins of DSD and TDS disorders such as CIS (TGCT).
Materials and Methods
Tissue collection
First-trimester (9 weeks, n = 4) and second-trimester (14–18 weeks, n = 6) human fetal testes were obtained following termination of pregnancy. Women gave consent in accordance with national guidelines (Polkinghorne, 1989), and ethical approval was obtained from the Lothian Research Ethics Committee. No terminations were related to fetal abnormalities. A small portion of each testis was fixed as a pre-graft control, while the remainder was placed immediately into ice-cold media containing Liebowitz L-15 with glutamine, 10% fetal bovine serum, 1% penicillin/streptomycin and 1% non-essential amino acids (all Sigma, Poole, UK) for xenografting. Fetal testis specimens were also obtained at gestational ages equivalent to the end of the grafting period and fixed as age-matched controls.
Xenografting procedure
Xenografting was performed following ethical approval from Lothian Research Ethics Committee and animals were maintained in accordance with UK Home Office guidelines. Male CD1 nude mice (n = 18, Charles River UK, Margate, England) were anaesthetized by inhalation of isofluorane, castrated and small pieces (1 mm3 approx.) of donor human testis tissue were inserted subcutaneously under the dorsal skin using a 13G cancer implant needle (Popper and Sons, NY, USA). Between three and six testis grafts from a single fetus were inserted on either side of the midline. Mice were housed individually and received analgesia (Rimadyl LA, Pfizer, NY, USA) and antibiotics (Baytril, Bayer, Germany) in the drinking water for 5 days post-surgery. After 7 days, mice with second-trimester xenografts were randomly assigned to receive s.c. injections of either hCG (n = 6, 20 IU, three times a week, Pregnyl, Organon Laboratories, Cambridge, UK) in 0.9% saline containing 1% fetal bovine serum, or the vehicle. Grafts were retrieved after 6 weeks. Host mice were killed by cervical dislocation and blood obtained by cardiac puncture for measurement of testosterone. Seminal vesicles were removed and weighed, and xenografts were retrieved and weighed.
Tissue fixation and processing
Pre-graft, xenograft and equivalent age-matched control tissues were fixed for 2 h in Bouins, transferred to 70% ethanol and then processed into paraffin blocks using standard procedures.
Immunohistochemistry
Antibodies, dilutions and requirement for antigen retrieval are shown in Table I. Immunohistochemistry was performed with Tris-buffered saline (TBS, 0.05 M Tris and 0.85% NaCl, pH 7.6) washes between each step (Mitchell et al., 2008). Sections (5 µm) were subjected to heat-induced antigen retrieval in 0.1 M citrate buffer (pH 6) and endogenous peroxidase blocked with 3% (v/v) H2O2 in methanol for 30 min. Endogenous biotin was blocked using an avidin/biotin blocking kit (Vector Laboratories, Inc., Peterborough, UK), according to manufacturers' instructions. Sections were incubated in appropriate normal serum diluted 1:5 with TBS containing 5% (w/v) bovine serum albumin (BSA) for 30 min. Sections were incubated overnight with primary antibody diluted in serum at 4°C in a humidified chamber and then incubated for 30 min with the appropriate biotinylated secondary antibody, diluted in normal serum, followed by 30 min incubation with Streptavidin-horse-radish peroxidase (DAKO, Ely, Cambridgeshire, UK) at 1:1000, diluted in TBS. Visualization was performed using 3,3′-diaminobenzidine tetrahydrochloride (DAB, DAKO) and sections were counterstained with haematoxylin before mounting in Pertex mounting medium (CellPath plc, Hemel Hempstead, UK). Negative controls were sections in which the primary antibody was replaced with the appropriate normal serum. Images were captured as previously described (Mitchell et al., 2008).
Table I.
Antibodies and conditions used for immunohistochemistry in xenograft study using human fetal testis tissue in nude mice.
| Antigen | Source | Species | Dilution | Retrieval |
|---|---|---|---|---|
| AMH | Santa Cruza | Goat | 1:500 | N |
| AR | Santa Cruza | Rabbit | 1:200 | Y |
| MAGE-A4 | Giftc | Mouse | 1:20 | N |
| OCT 4 | Santa Cruza | Goat | 1:50 | Y |
| SOX9 | Chemicone | Rabbit | 1:80 | Y |
| SMA | Sigmaf | Mouse | 1:5000 | Y |
| 3β-HSD | Giftd | Rabbit | 1:1000 | N |
| VASA | Abcamb | Rabbit | 1:500 | Y |
All antibodies were raised against human peptide sequences. AMH, anti-Mullerian hormone; AR, androgen receptor; SMA, smooth muscle actin; 3β-HSD, 3β-hydroxysteroid dehydrogenase.
aSanta Cruz Biotechnology, CA, USA.
bAbcam, Cambridge, UK.
cDr Guilio Spagnoli, University Hospital, Basel, Switzerland.
dProf. Ian Mason, The Queen's Medical Research Institute, Edinburgh, UK.
eChemicon/Upstate/Linco.
fSigma, Poole, UK.
For double immunohistochemistry, the first primary antibody was detected using Streptavidin alkaline phosphatase (DAKO) at 1:400 for 30 min and visualized with 1 mg/ml Fast Blue (Sigma) in fast blue buffer, until desired staining was achieved (typically 10–15 min). The second primary antibody was then applied and subsequent steps were as described above for single immunostaining.
Triple immunofluorescence
Details of antibodies and detection reagents are shown in Table II. Antigen retrieval, blocking and incubation with the first primary antibody were performed as described for immunohistochemistry, with phosphate-buffered saline (PBS) replacing TBS. Sections were incubated with a peroxidise-conjugated secondary antibody diluted in normal serum/PBS/BSA for 30 min and kept in the dark thereafter. Slides were then incubated with labelled Tyramide diluted in buffer at 1:50 for 10 min before placing in citrate buffer and microwaving on full power for 2.5 min and left to cool for a further 30 min. The incubation of primary antibody, peroxidise-conjugated secondary and Tyramide (with different fluorescent labels), was repeated for the second and third primary antibodies, with a second microwave citrate retrieval prior to the third primary antibody. Sections were counterstained with 4′,6-diamidino-2-phenylindole (DAPI, Sigma) diluted 1:1000 in PBS for 10 min. Slides were mounted using Permafluor (Immunotech, Marseille, France). Images were captured using an LSM 510 Meta Confocal microscope (Carl Zeiss, Hertfordshire, UK).
Table II.
Antibodies and conditions for triple immunofluorescence in xenograft study using human fetal testis tissue in nude mice.
| Antigen | Dilution | Secondary antibody | Visualization |
|---|---|---|---|
| OCT4 | 1:150 | aCAG-p | cTyr-Cy3 (10 min) |
| MAGE-A4 | 1:100 | bCAM-p | dTyr-Cy5 (10 min) |
| Ki67 | 1:200 | bCAM-p | eTyr Fluor (10 min) |
aCAG-p, Chicken anti-goat peroxidase (Sigma, Poole, UK).
bCAM-p, Chicken anti-mouse peroxidase (Sigma, Poole, UK).
cTyr Cy3, Tyramide Cy3 (Perkin Elmer, MA, USA).
dTyr Cy5, Tyramide Cy5 (Perkin Elmer).
eTyr Fluor, Tyramide Fluorescien (Perkin Elmer).
Quantification of germ cell differentiation and proliferation
Quantification of germ cell subpopulations and proliferation indices were performed for the triple-stained sections. Images were obtained using an Axiovert 200 M microscope with attached Axiocam HRc camera and Axiovision 4.6 software (all Carl Zeiss). All germ cells within each section were counted and quantified according to their protein expression profile and proliferation status using Adobe Photoshop 7.0 (Adobe, San Jose, CA, USA).
Testosterone assay
Host serum testosterone levels were measured by competitive radioimmunoassay using radiolabelled testosterone (I125, MP Biomedicals, UK) and a rabbit primary antibody (1:600 000, AMS Biotech, Abingdon, UK). Residual I125 was measured with a gamma counter (WIZARD 1470, Perkin Elmer, Turku, Finland) and testosterone levels were expressed as ng/ml.
Statistics
Statistical analysis was performed using Graphpad Prism 5 software (La Jolla, CA, USA). Multiple groups were analysed using one-way analysis of variance, while comparisons between pre-graft control and xenografted material taken from the same fetus were compared using a paired t-test. Statistical significance was set at P < 0.05.
Results
Retrieval rates, growth and gross morphology of human fetal testis xenografts
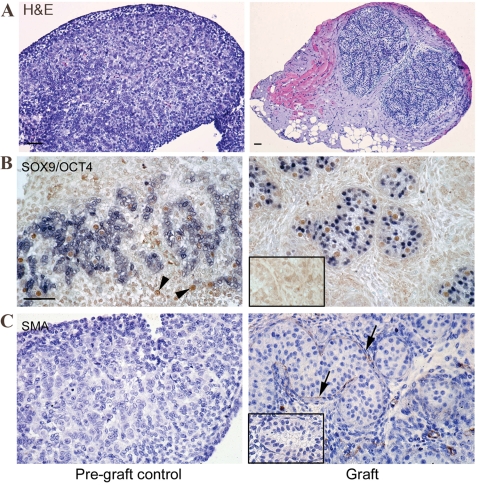
Surviving grafts were easily identifiable as a distinct mass on the under surface of the dorsal skin. Retrieval rates were similar with 9/12 (75%) first-trimester and 50/65 (77%) second-trimester grafts recovered. Most xenografts had grown from a diameter of ∼1–3 mm3 and their weights ranged from 0.1 to 5.2 mg (mean 2.1 mg). Grafts were enclosed by a capsule, separating the testis material from the surrounding s.c. tissue of the host (Fig. 1A). The recovered grafts exhibited normal gross appearance, with recognizable seminiferous cords (containing Sertoli cells and germ cells) surrounded by an interstitium containing Leydig cells in 9/9 (100%) of first-trimester and 47/50 (94%) of second-trimester grafts (Fig. 1A).
Figure 1.
(A) Haematoxylin and eosin. Seminiferous cord formation in first-trimester human fetal testis xenografts. Pre-graft control testes (left) lacked completed seminiferous cord formation, while grafts had developed seminiferous cords with normal appearance (right). (B) Identification of cell types within newly formed seminiferous cords in first-trimester human fetal testis xenografts (right) in comparison with pre-graft controls (left) based on immunoexpression of SOX9/OCT4. Isolated OCT4+ (brown) germ cells can be identified (arrowheads) in areas devoid of SOX9 (blue) expressing Sertoli cells in pre-graft controls, but after grafting all germ cells are enclosed within clearly defined seminiferous cords. (C) The cords are separated from the interstitial compartment by a basement membrane outlined by the expression of SMA, (arrows), while the pre-graft control tissue is negative for SMA. Scale bar = 50 µm. Negative controls are also shown (inset).
Formation of seminiferous cords in first-trimester human fetal testis xenografts
At the time of grafting, seminiferous cords had not fully formed in first trimester testes, but cords formed during the grafting period and exhibited a normal appearance (Fig. 1A). At grafting, Sertoli cells (identified by expression of SOX9) had aggregated but not formed obvious cords and many of the germ cells (identified by expression of OCT4) were still located in areas devoid of Sertoli cells (Fig. 1B). After grafting, Sertoli cells had enclosed the germ cells in seminiferous cords surrounded by a layer of cells expressing smooth muscle actin (SMA; presumptive peritubular myoid cells), which was not evident in pre-graft controls (Fig. 1C).
Functional development of Sertoli and Leydig cells in first-trimester human fetal testis xenografts
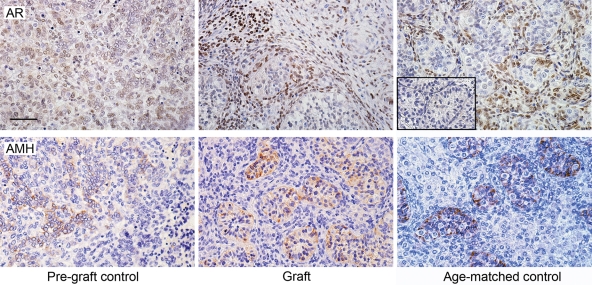
Androgen receptor (AR) immunoexpression was not evident in first trimester pre-graft control testis tissue, but after grafting for 6 weeks AR expression was observed in peritubular myoid cells and in some interstitial cells, a pattern comparable with equivalent age-matched controls (Fig. 2). Anti-Müllerian hormone (AMH) was expressed in presumptive Sertoli cells in the first trimester pre-graft control and expression was maintained in the cytoplasm of presumptive Sertoli cells after grafting, and was similar in localization to equivalent age-matched controls (Fig. 2).
Figure 2.
Expression of AR and AMH in first-trimester human fetal testis xenografts. Androgen receptor (AR, upper panels) is not expressed in the pre-graft control, but is expressed in the grafts and equivalent age-matched controls. A negative control is also shown (inset). AMH (lower panels) is expressed in first trimester pre-graft controls and localized to Sertoli cells within the newly formed seminiferous cords after grafting and in an equivalent age-matched control. Scale bar = 50 µm.
Steroidogenesis and testosterone production by human fetal testis xenografts
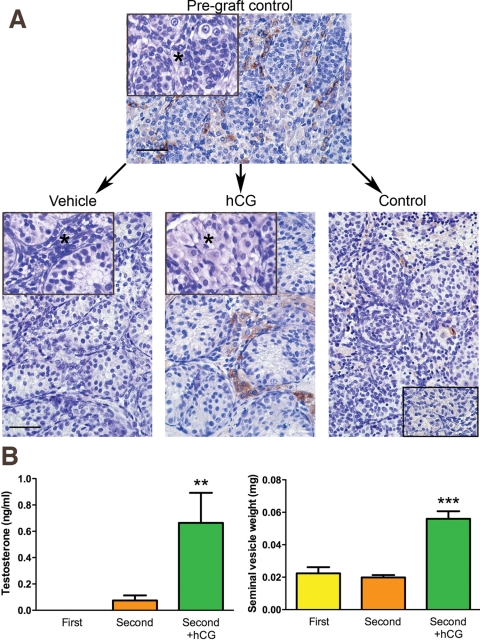
Differentiation and functional activation of Leydig cells was assessed in the xenografts. In the absence of hCG treatment, Leydig cell cytoplasm was scanty compared with the conspicuous cytoplasm in Leydig cells from second-trimester grafts of hCG-treated animals (Fig. 3A). In vehicle-treated controls, the Leydig cells within grafts were mostly immunonegative for 3β-hydroxysteroid dehydrogenase (3β-HSD), but this enzyme was strongly expressed in Leydig cells from hCG-exposed grafts, indicating induction of steroidogenesis (Fig. 3A). Testosterone production was below the level of detection in (untreated) hosts bearing first-trimester xenografts, while low levels were detected (mean 0.07 ± 0.04 ng/ml) in vehicle-treated hosts bearing second-trimester grafts. Treatment of hosts bearing second-trimester xenografts with hCG resulted in nearly a 10-fold increase (mean 0.68 ± 0.23 ng/ml, P = 0.004) in serum testosterone levels (Fig. 3B). Seminal vesicle weight was also significantly (3-fold) increased in hCG-treated animals compared with vehicle-treated controls (Fig. 3B).
Figure 3.
(A) Comparison of Leydig cell 3β-HSD expression (brown) in a second trimester pre-graft control (upper panel) and tissue from the same testis after grafting into a vehicle-treated (lower panel; left) or hCG-treated mouse host (lower panel, middle). An age-matched control testis has also been shown (lower panel, right). Note the presence of 3β-HSD expression in the hCG-exposed xenograft, similar to the age-matched control. Leydig cell hypertrophy (*) was demonstrated in the hCG-treated grafts (lower middle panel, inset), compared with the vehicle-treated control graft (lower left panel, inset) and the pre-graft control (upper panel, inset). Scale bar = 50 µm. A negative control is shown (lower right panel, inset). (B) Testosterone production and seminal vesicle weights in untreated/vehicle-treated mice bearing human fetal testis xenografts from first and second trimester human fetal testes in comparison with hCG-treated mice bearing second-trimester grafts (second+hCG, n = 3). Note that hCG treatment results in a significant increase in both testosterone (left panel) and seminal vesicle weight (right panel). Mean ± SEM. **P < 0.01, ***P < 0.001, in comparison with vehicle-treated mice bearing second-trimester grafts.
Germ cell differentiation in human fetal testis xenografts
The gonocyte pluripotency marker OCT4 was expressed in most germ cells of first trimester testes prior to grafting, while after grafting some of the germ cells had ceased to express this protein, coincident with emerging expression of proteins associated with differentiation into pre-spermatogonia, such as MAGE-A4 and VASA (Fig. 4A). The latter were only rarely expressed in germ cells in first trimester testes prior to grafting. Similar results were obtained using second-trimester grafts. These results suggested that germ cells differentiate during the grafting period similar to that which occurs normally in the intact testis. To establish if this was the case, quantification of the change in germ cell differentiation marker expression was performed on sections co-stained for OCT4 and VASA (Fig. 4B). The results confirmed that the % of cells expressing OCT4 decreased during the grafting period while the % of cells expressing VASA increased. The % of cells expressing OCT4 after grafting was significantly reduced when compared with the pre-graft control tissue for both first and second trimester testes, while the % of cells expressing VASA significantly increased in the first-trimester grafts, compared with the pre-graft control. Further evidence for normal germ cell differentiation after grafting is that the % of cells expressing OCT4 or VASA in xenografts was not significantly different from that found in equivalent age-matched controls (Fig. 4B). Comparable results were also obtained when germ cells were co-stained for OCT4 and MAGE-A4 (not shown).
Figure 4.
(A) Immunoexpression (brown) of OCT4 (top), VASA (middle) and MAGE-A4 (bottom) in germ cells in a first trimester human testis prior to grafting (Pre-graft control) and 6 weeks after grafting into a mouse host (graft). Note that after grafting, the proportion of germ cells expressing OCT4 decreases as demonstrated by the presence of unstained germ cells (arrowhead), compared with the pre-graft control, whereas expression of MAGE-A4 and VASA (arrows, inset) increase (neither are expressed in the pre-graft control). Scale bar = 50 µm. (B) Quantification of germ cell differentiation in human fetal testis xenografts. The proportion of germ cells expressing OCT4 (red bars) or VASA (blue bars) in first (upper panels) and second (lower panels) trimester human fetal testes for pre-graft control, grafts and equivalent non-grafted controls. Values are Mean ± SEM for n = 3 fetuses. *P < 0.05, **P < 0.01, ns, not significant.
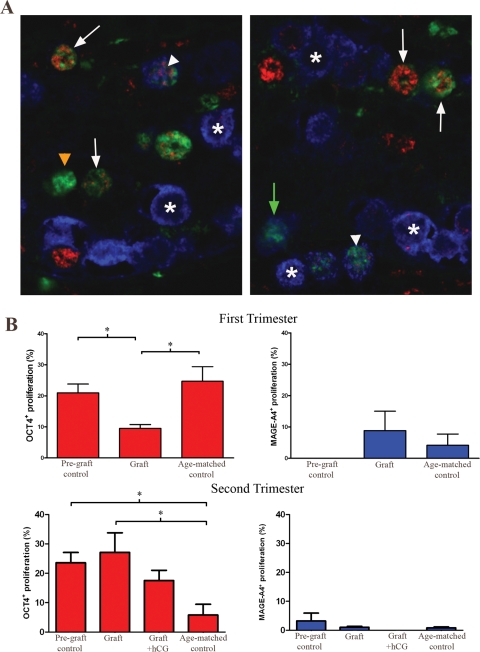
Proliferation of cells within human fetal testis xenografts
Development stage-specific germ cell proliferation was evaluated in the human fetal testis xenografts by triple staining for OCT4, MAGE-A4 and the proliferation marker Ki67. This demonstrated that a proportion of all three subpopulations of germ cells (OCT4+/MAGE-A4−, OCT4+/MAGE-A4+ and OCT4−/MAGE-A4+) were proliferating (Fig. 5A). Quantification of the proliferation index (PI) for the different germ cell subpopulations showed that OCT4+/MAGE-A4− germ cells (i.e. undifferentiated) had the highest PI, while the subpopulations that expressed MAGE-A4 (i.e. differentiated) had a lower PI. This was the case for both first and second trimester testis grafts and was similarly evident in pre-graft controls and equivalent age-matched controls (Fig. 5B). However, there was a significantly decreased PI in OCT4+ cells from first-trimester grafts compared with the pre-graft control and equivalent age-matched control, while in second trimester samples proliferation of OCT4+ germ cells was reduced in the equivalent age-matched control compared with the pre-graft and grafted tissues. There were no significant differences in the PI for MAGE-A4+ cells in grafts when compared with pre-graft and equivalent age-matched controls.
Figure 5.
(A) Proliferation (Ki67, green) of germ cell subpopulations in a second trimester human fetal testis xenograft based on co-immunoexpression with either OCT4 (red), or MAGE-A4 (blue). Examples of proliferating germ cells expressing OCT4+/MAGE-A4− (white arrows), OCT4+/MAGE-A4+ (white arrowheads) and OCT4−/MAGE-A4+ (green arrows) can be seen. Many of the OCT4−/MAGE-A4+ population are not proliferating (asterisk). The cells stained for Ki67 that are negative for OCT4 and MAGE-A4 represent proliferating Sertoli cells (orange arrowhead). (B) Quantification of the proportion of OCT4+ (red bars) or MAGE-A4+ (blue bars) germ cells that are proliferating in first (upper panels) and second (lower panels) trimester human fetal testes for pre-graft control, grafts (vehicle or hCG treated) and equivalent non-grafted controls. Note the higher proliferation rate of OCT4+ germ cells compared with MAGE-A4+ cells. Values are Mean ± SEM for n = 3 fetuses. *P < 0.05.
Discussion
The present study has demonstrated that human fetal testis development can be recapitulated using the xenografting approach and that overall testis (graft) growth and cellular proliferation/differentiation appear to progress normally. Graft survival rates were excellent (>75%) and the vast majority of retrieved grafts had normal testis structure with seminiferous cords of normal appearance.
Our study provides the first demonstration of ex situ seminiferous cord formation in the human testis as we have shown that first-trimester human fetal testis xenografts can form normal seminiferous cords, the timing of which matches that described previously for the normal intact human fetal testis (Wartenberg, 1981; Hanley et al., 2000; Ostrer et al., 2007). Xenografting of first trimester fetal testes thus has potential for investigating the mechanisms of normal human fetal testis development, and may also be useful for studies that aim to assess the consequences of disrupting seminiferous cord formation (dysgenesis). However, in order to establish validity of the xenografting model for use in such studies, it was considered vital to first determine whether xenografts continue to develop normally from a structural, functional and hormonal perspective. The present studies have shown that testis structure is maintained throughout the first and second trimester and that expression of functional markers of Sertoli (AMH), Leydig (3β-HSD) and peritubular myoid cells (SMA) are comparable with those of equivalent age-matched controls. AR and 3β-HSD were not expressed in first trimester testes at 9 weeks of gestation, but were expressed in the same testes 6 weeks after xenografting, indicating that steroidogenesis is beginning to occur and that the testes are becoming androgen responsive: SMA expression in peritubular myoid cells is considered to be androgen-dependent (Schlatt et al., 1993). In addition to these changes, weak expression of AMH in first trimester testes became stronger within the Sertoli cells after xenografting. The change in expression patterns of AR, 3β-HSD and AMH in testis xenografts is comparable with that described previously for the human fetal testis between 7–9 and 14 weeks of gestation (Gaskell et al., 2004).
Production of androgens is crucial for other aspects of testis development such as Sertoli cell proliferation (Scott et al., 2007, 2008), as well as for masculinization of the reproductive organs (Auchus, 2004; Scott et al., 2009). Our studies used measurement of serum testosterone and seminal vesicle weight in the host animal to determine the steroidogenic function of the xenografts and showed that second-trimester xenografts are capable of producing testosterone and that this could be significantly increased by hCG treatment. However, first-trimester xenografts did not produce detectable levels of testosterone in host serum, which may be related to the relatively small size and number of grafts in each host, or may be a result of age-related differences in the responsiveness of human grafts to host mouse LH, as has been described in Rhesus monkey testis xenografts (Rathi et al., 2008). We anticipate that hCG treatment of host mice carrying first-trimester human fetal testis xenografts would result in the production of detectable levels of testosterone.
Previous studies have demonstrated that germ cells are present in second-trimester human fetal testis xenografts, but the identity and differentiation of these cells was not demonstrated (Povlsen et al., 1974; Skakkebaek et al., 1974; Yu et al., 2006). The present study has shown that germ cell differentiation from gonocyte (OCT4+/MAGE-A4−/VASA−) to pre-spermatogonium (OCT4−/MAGE-A4+/VASA+), a process that normally takes place during fetal and early post-natal life in the human, occurred normally in xenografts and was comparable with that of equivalent age-matched controls (Gaskell et al., 2004; Anderson et al., 2007; Mitchell et al., 2008). One minor exception was that OCT4+ germ cells in the first trimester xenografts had a significantly lower PI than did the pre-graft or equivalent age-matched controls, and this could artifactually affect the proportion of germ cells expressing OCT4 in the xenografts. However, this could not account for the emergence of differentiated (VASA+ or MAGE-A4+) germ cells in first-trimester grafts compared with pre-graft controls (most of which had no VASA or MAGE-A4 expressing cells prior to grafting) or for the increase in proportion of differentiated germ cells in the second-trimester grafts. Taken together these results indicate that germ cell differentiation occurs in the xenografts and that this is broadly comparable with the normal situation in vivo.
Having established xenografting as a suitable model for human fetal testis seminiferous cord formation and germ cell differentiation, we propose that it may be especially useful for studies investigating the origins of DSDs and TDS in humans. DSDs often result from genetic abnormalities involving the sex chromosomes or genes involved in gonad development (Hughes et al., 2006). Genetic manipulation of testis tissue or isolated cells prior to grafting has been shown to be possible in studies that introduced the β-galactosidase gene into bovine testis tissue that was subsequently xenografted (Oatley et al., 2004). The xenografting technique may be modified to introduce genes that either promote or disrupt normal cord formation and testis development in human fetal testis xenografts and thus provide an in vivo model of these conditions. DSDs may also result from impaired androgen production or action. Several environmental chemicals, such as certain phthalate esters, have been shown to inhibit testosterone production by the fetal rat testis and to result in a TDS-like phenotype in male offspring, but it remains unclear whether these chemicals can exert similar effects in the human (Scott et al., 2009). For example, in vitro studies with first and second-trimester human fetal testis explants have shown no inhibition of testosterone production by either of two different phthalates (Hallmark et al., 2007; Lambrot et al., 2009), whereas in vivo studies have provided (indirect) evidence that such phthalates might impair androgen production (Swan et al., 2005). The present results show that testis xenografting would be a relevant approach via which to investigate the effects of these chemicals on the development of the seminiferous cords, germ cell development and steroidogenesis by the human fetal testis under conditions in which normal cell development and function appears to be occurring. Investigating the effects of phthalate esters in this system may help to resolve the uncertainty about whether these chemicals affect steroidogenesis (or other functions) of the fetal human testis (Scott et al., 2009).
In conclusion, we have demonstrated for the first time that human fetal testis xenografts are a comparable in vivo ex situ model of normal seminiferous cord formation, germ cell development and testosterone production. Germ cells within xenografts differentiate from gonocytes into pre-spermatogonia and proliferate in a manner similar to that in normal age-matched control testes. In addition, grafts are capable of producing testosterone, and an increase from basal levels can be induced by hCG treatment of the host animal. We propose that this system can be used to dissect the cellular mechanisms that underpin normal human fetal testis development, the disruption of which can lead to common male reproductive disorders: these mechanisms are otherwise inaccessible for study in situ and may be compromised when studied in vitro. Genetic disruption of the initial stages of testis development or manipulation of testosterone production by proposed endocrine disruptors, using the xenograft approach, may be useful for studies relating to DSD or the development of TDS and TGCT.
Funding
This study was funded by the UK Medical Research Council (WBS U.1276.00.003.00003.01 and WBS U.1276.00.002.00001). The 3β-HSD antibody was a kind gift from Prof. Ian Mason (University of Edinburgh, Edinburgh, UK). The MAGE-A4 antibody was a kind gift from Dr Giulio Spagnoli (University Hospital, Basel, Switzerland).
Acknowledgements
We gratefully acknowledge the work of Anne Saunderson and the staff of the Bruntsfield Suite of the Royal Infirmary of Edinburgh in provision of tissue for these studies. We would also like to thank Marion Walker (HRSU, Edinburgh, UK) for her technical assistance. Special thanks to Prof. Stefan Schlatt (University of Münster, Germany) for his assistance in providing training in the xenografting technique.
Conflict of interest: none declared.
References
- Anderson RA, Fulton N, Cowan G, Coutts S, Saunders PTK. Conserved and divergent patterns of expression of DAZL, VASA and OCT4 in the germ cells of the human fetal ovary and testis. BMC Dev Biol. 2007;7:136. doi: 10.1186/1471-213X-7-136. doi:10.1186/1471-213X-7-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson PD, Lam MY, Poirier C, Bishop CE, Nadeau JH. The role of the mouse y chromosome in susceptibility to testicular germ cell tumors. Cancer Res. 2009;69:3614–3618. doi: 10.1158/0008-5472.CAN-08-4881. doi:10.1158/0008-5472.CAN-08-4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auchus RJ. The backdoor pathway to dihydrotestosterone. Trends Endocrinol Metab. 2004;15:432–438. doi: 10.1016/j.tem.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Dobrinski I. Male germ cell transplantation. Reprod Dom Anim. 2008;43(Suppl. 2):288–294. doi: 10.1111/j.1439-0531.2008.01176.x. [DOI] [PubMed] [Google Scholar]
- Ehmcke J, Schlatt S. Animal models for fertility preservation in the male. Reproduction. 2008;136:717–723. doi: 10.1530/REP-08-0093. doi:10.1530/REP-08-0093. [DOI] [PubMed] [Google Scholar]
- Ehmcke J, Wistuba S, Schlatt S. Spermatogonial stem cells: questions, models and perspectives. Hum Reprod Update. 2006;12:275–282. doi: 10.1093/humupd/dmk001. doi:10.1093/humupd/dmk001. [DOI] [PubMed] [Google Scholar]
- Gaskell TL, Esnal A, Robinson LL, Anderson RA, Saunders PTK. Immunohistochemical profiling of germ cells within the human fetal testis: identification of three subpopulations. Biol Reprod. 2004;71:2012–2021. doi: 10.1095/biolreprod.104.028381. doi:10.1095/biolreprod.104.028381. [DOI] [PubMed] [Google Scholar]
- Gassei K, Ehmcke J, Schlatt S. Initiation of testicular tubulogenesis is controlled by neurotrophic tyrosine receptor kinases in a three-dimensional Sertoli cell aggregation assay. Reproduction. 2008;136:459–469. doi: 10.1530/REP-08-0241. doi:10.1530/REP-08-0241. [DOI] [PubMed] [Google Scholar]
- Geens M, De Block G, Goossens E, Frederickx V, Van Steirteghem A, Tournaye H. Spermatogonial survival after grafting human testicular tissue to immunodeficient mice. Hum Reprod. 2006;21:390–396. doi: 10.1093/humrep/dei412. doi:10.1093/humrep/dei412. [DOI] [PubMed] [Google Scholar]
- Hallmark N, Walker M, McKinnell C, Mahood IK, Scott H, Bayne R, Coutts S, Anderson RA, Greig I, Morris K, et al. Effects of monobutyl and di(n-butyl) phthalate in vitro on steroidogenesis and Leydig cell aggregation in fetal testis explants from the rat: comparison with effects in vivo in the fetal rat and neonatal marmoset and in vitro in the human. Environ Health Perspect. 2007;115:390–396. doi: 10.1289/ehp.9490. doi:10.1289/ehp.9490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley NA, Hagan DM, Clement-Jones M, Ball SG, Strachan T, Salas-Cortes L, McElreavey K, Lindsay S, Robson S, Bullen P, et al. SRY SOX9 DAX1 expression patterns during human sex determination gonadal development. Mech Dev. 2000;91:403–407. doi: 10.1016/s0925-4773(99)00307-x. doi:10.1016/S0925-4773(99)00307-X. [DOI] [PubMed] [Google Scholar]
- Honaramooz A, Li MW, Penedo MC, Meyers S, Dobrinski I. Accelerated maturation of primate testis by xenografting into mice. Biol Reprod. 2004;70:1500–1503. doi: 10.1095/biolreprod.103.025536. doi:10.1095/biolreprod.103.025536. [DOI] [PubMed] [Google Scholar]
- Hughes IA. Disorders of sex development: a new definition and classification. Best Pract Res Clin Endocrinol Metab. 2008;22:119–134. doi: 10.1016/j.beem.2007.11.001. doi:10.1016/j.beem.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Hughes IA, Houk C, Ahmed SF, Lee PA. Consensus statement on management of intersex disorders. Arch Dis Child. 2006;91:554–563. doi: 10.1136/adc.2006.098319. doi:10.1136/adc.2006.098319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrot R, Muczynski V, Lecureuil C, Angenard G, Coffigny H, Pairault C, Moison D, Frydman R, Habert R, Rouiller-Fabre V. Phthalates impair germ cell development in the human fetal testis in vitro without change in testosterone production. Environ Health Perspect. 2009;117:32–37. doi: 10.1289/ehp.11146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell RT, Cowan G, Morris KD, Anderson RA, Fraser HM, McKenzie KJ, Wallace WH, Kelnar CJ, Saunders PT, Sharpe RM. Germ cell differentiation in the marmoset (Callithrix jacchus) during fetal and neonatal life closely parallels that in the human. Hum Reprod. 2008;23:2755–2765. doi: 10.1093/humrep/den295. doi:10.1093/humrep/den295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oatley JM, de Avila DM, Reeves JJ, McLean DJ. Spermatogenesis and germ cell transgene expression in xenografted bovine testicular tissue. Biol Reprod. 2004;71:494–501. doi: 10.1095/biolreprod.104.027953. doi:10.1095/biolreprod.104.027953. [DOI] [PubMed] [Google Scholar]
- Oosterhuis JW, Looijenga LH. Testicular germ-cell tumours in a broader perspective. Nature Rev. 2005;5:210–222. doi: 10.1038/nrc1568. [DOI] [PubMed] [Google Scholar]
- Ostrer H, Huang HY, Masch RJ, Shapiro E. A cellular study of human testis development. Sex Dev. 2007;1:286–292. doi: 10.1159/000108930. doi:10.1159/000108930. [DOI] [PubMed] [Google Scholar]
- Polkinghorne J. Review of the Guidance on the Research Use of Fetuses and Fetal Material. London, UK: Her Majesty's Stationary Office; 1989. [Google Scholar]
- Povlsen CO, Skakkebaek NE, Rygaard J, Jensen G. Heterotransplantation of human foetal organs to the mouse mutant nude. Nature. 1974;248:247–249. doi: 10.1038/248247a0. doi:10.1038/248247a0. [DOI] [PubMed] [Google Scholar]
- Rajpert-De Meyts E. Developmental model for the pathogenesis of testicular carcinoma in situ: genetic and environmental aspects. Hum Reprod Update. 2006;12:303–323. doi: 10.1093/humupd/dmk006. doi:10.1093/humupd/dmk006. [DOI] [PubMed] [Google Scholar]
- Rathi R, Zeng W, Megee S, Conley A, Meyers S, Dobrinski I. Maturation of testicular tissue from infant monkeys after xenografting into mice. Endocrinology. 2008;149:5288–5296. doi: 10.1210/en.2008-0311. doi:10.1210/en.2008-0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Sosa JR, Dobrinski I. Recent developments in testis tissue xenografting. Reproduction. 2009;138:187–194. doi: 10.1530/REP-09-0012. doi:10.1530/REP-09-0012. [DOI] [PubMed] [Google Scholar]
- Schlatt S, Weinbauer GF, Arslan M, Nieschlag E. Appearance of alpha-smooth muscle actin in peritubular cells of monkey testes is induced by androgens, modulated by follicle-stimulating hormone, and maintained after hormonal withdrawal. J Androl. 1993;14:340–350. [PubMed] [Google Scholar]
- Schlatt S, Honaramooz A, Ehmcke J, Goebell PJ, Rubben H, Dhir R, Dobrinski I, Patrizio P. Limited survival of adult human testicular tissue as ectopic xenograft. Hum Reprod. 2006;21:384–389. doi: 10.1093/humrep/dei352. doi:10.1093/humrep/dei352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlatt S, Westernstroer B, Gassei K, Ehmcke J. Donor–host involvement in immature rat testis xenografting into nude mouse hosts. Biol Reprod. 2010;82:888–895. doi: 10.1095/biolreprod.109.082073. doi:10.1095/biolreprod.109.082073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott HM, Hutchison GR, Mahood IK, Hallmark N, Welsh M, De Gendt K, Verhoeven G, O'Shaughnessy P, Sharpe RM. Role of androgens in fetal testis development and dysgenesis. Endocrinology. 2007;148:2027–2036. doi: 10.1210/en.2006-1622. doi:10.1210/en.2006-1622. [DOI] [PubMed] [Google Scholar]
- Scott HM, Hutchison GR, Jobling MS, McKinnell C, Drake AJ, Sharpe RM. Relationship between androgen action in the ‘male programming window', fetal Sertoli cell number, and adult testis size in the rat. Endocrinology. 2008;149:5280–5287. doi: 10.1210/en.2008-0413. doi:10.1210/en.2008-0413. [DOI] [PubMed] [Google Scholar]
- Scott HM, Mason JI, Sharpe RM. Steroidogenesis in the fetal testis and its susceptibility to disruption by exogenous compounds. Endocr Rev. 2009;30:883–925. doi: 10.1210/er.2009-0016. doi:10.1210/er.2009-0016. [DOI] [PubMed] [Google Scholar]
- Skakkebaek NE. Abnormal morphology of germ cells in two infertile men. Acta Pathol Microbiol Scand. 1972;80:374–378. doi: 10.1111/j.1699-0463.1972.tb00293.x. [DOI] [PubMed] [Google Scholar]
- Skakkebaek NE, Jensen G, Povlsen CO, Rygaard J. Heterotransplantation of human foetal testicular and ovarian tissue to the mouse mutant nude. A preliminary study. Acta Obstet Gynecol Scand. 1974;29:73–75. doi: 10.3109/00016347409157196. [DOI] [PubMed] [Google Scholar]
- Skakkebaek NE, Rajpert-De Meyts E, Main KM. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum Reprod. 2001;16:972–978. doi: 10.1093/humrep/16.5.972. [DOI] [PubMed] [Google Scholar]
- Swan SH, Main KM, Liu F, Stewart SL, Kruse RL, Calafat AM, Mao CS, Redmon JB, Ternand CL, Sullivan S, et al. Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environ Health Perspect. 2005;113:1056–1061. doi: 10.1289/ehp.8100. doi:10.1289/ehp.8100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilmann C, Capel B. Mesonephric cell migration induces testis cord formation and Sertoli cell differentiation in the mammalian gonad. Development. 1999;126:2883–2890. doi: 10.1242/dev.126.13.2883. [DOI] [PubMed] [Google Scholar]
- Wartenberg H. Differentiation and development of the testes. In: Burger H, De Kretser DM, editors. The Testis. 2nd edn. New York: Raven Press; 1981. pp. 39–81. [Google Scholar]
- Wilhelm D, Palmer S, Koopman P. Sex determination and gonadal development in mammals. Physiol Rev. 2007;87:1–28. doi: 10.1152/physrev.00009.2006. doi:10.1152/physrev.00009.2006. [DOI] [PubMed] [Google Scholar]
- Wistuba J, Schlatt S. Transgenic mouse models and germ cell transplantation: two excellent tools for the analysis of genes regulating male fertility. Mol Genet Metab. 2002;77:61–67. doi: 10.1016/s1096-7192(02)00142-7. doi:10.1016/S1096-7192(02)00142-7. [DOI] [PubMed] [Google Scholar]
- Yu J, Cai ZM, Wan HJ, Zhang FT, Ye J, Fang JZ, Gui YT, Ye JX. Development of neonatal mouse and fetal human testicular tissue as ectopic grafts in immunodeficient mice. Asian J Androl. 2006;8:393–403. doi: 10.1111/j.1745-7262.2006.00189.x. doi:10.1111/j.1745-7262.2006.00189.x. [DOI] [PubMed] [Google Scholar]