Abstract
In patients undergoing percutaneous coronary intervention (PCI) for acute coronary syndrome (ACS), both periprocedural acute myocardial infarction and bleeding complications have been shown to be associated with early and late mortality. Current standard antithrombotic therapy after coronary stent implantation consists of lifelong aspirin and clopidogrel for a variable period depending in part on the stent type. Despite its well-established efficacy in reducing cardiac-related death, myocardial infarction, and stroke, dual antiplatelet therapy with aspirin and clopidogrel is not without shortcomings. While clopidogrel may be of little beneficial effect if administered immediately prior to PCI and may even increase major bleeding risk if coronary artery bypass grafting is anticipated, early discontinuation of the drug may result in insufficient antiplatelet coverage with thrombotic complications. Optimal and rapid inhibition of platelet activity to suppress ischemic and thrombotic events while minimizing bleeding complications is an important therapeutic goal in the management of patients undergoing percutaneous coronary intervention. In this article we present an overview of the literature on clinical trials evaluating the different aspects of antithrombotic therapy in patients undergoing PCI and discuss the emerging role of these agents in the contemporary era of early invasive coronary intervention. Clinical trial acronyms and their full names are provided in Table 1.
Keywords: acute coronary syndrome, percutaneous coronary intervention, aspirin, clopidogrel, glycoprotein IIb/IIIa inhibitors, bivalirudin
Antiplatelet agents
Aspirin
Aspirin irreversibly acetylates cyclooxygenase subtype 1 found in platelets and effectively blocks the production of the potent aggregatory agent thromboxane A2. Aspirin is an essential drug in every PCI setting.
In an observational analysis of the PCI-CURE study consisting of 2,658 patients with ACS undergoing PCI stratified into three aspirin dose groups > 200 mg (high, n = 1,064), 101–199 mg (moderate, n = 538), and <100 mg (low, n = 1,056), Jolly et al1 demonstrated similar rates of cardiovascular death, myocardial infarction (MI), or stroke among the three aspirin groups, whereas the rate of major bleeding was increased in those receiving high dose aspirin (Hazard ratio of high- vs low-dose 2.05, P = 0.009). Notably, however, the risk of major bleeding was increased in high-compared to moderate- and low-dose groups [HR: high- vs low-dose 2.05 (1.20–3.50), and moderate- vs low-dose 0.78 (0.34–1.77)]. Similarly, the net adverse clinical events (death, MI, stroke, major bleeding) favored low- over high-dose aspirin (8.4% vs 11.0%, HR 1.31, P = 0.056). Nonetheless, it should be noted that several limitations intrinsic to any observational study exist.
The CURRENT-OASIS-7 was the first large scale, multicenter, multinational, randomized factorial trial designed to simultaneously evaluate the efficacy and safety of a higher loading and maintenance dose of clopidogrel compared with the standard-dose regimen and high-dose ASA compared with low-dose ASA in patients with ACS, UA/NSTEMI, and STEMI, undergoing angiography with intended PCI.2
More than 25,000 patients were randomized in a 2 × 2 factorial design to receive high-dose or standard-dose clopidogrel (600 mg clopidogrel loading dose followed by 150 mg daily for 7 days, then 75 mg daily for high-dose regimen (n = 12,508); 300 mg clopidogrel loading dose followed by 75 mg daily for standard-dose regimen (n = 12,579), respectively). Within each group (ie, high- versus low-dose clopidogrel), patients were further randomized to receive high-dose or low-dose ASA (300–325 mg for high-dose; 75–100 mg for low-dose). The primary outcome was first occurrence of any component of cardiovascular death, MI, or stroke through 30 days. The safety outcome was the specific CURRENT definition of major bleeding through 30 days.
The aspirin analysis showed no difference in the primary outcome between the low- and high-dose aspirin groups among the overall patient cohort, the PCI subgroup, and the no PCI subgroup. There was also no difference in stent thrombosis or increase in bleeding using the CURRENT major or severe bleeding and TIMI major bleeding criteria. In the clopidogrel analysis, there was no significant difference in the primary composite outcome for the overall population between the high- and standard-dose clopidogrel (4.2% vs 4.4% respectively; hazard ratio [HR] 0.95; P = 0.37), and no statistically significant benefit in each individual component of the primary outcome. Conversely, the PCI subgroup had a significant reduction in the primary composite outcome in the high- vs standard-dose clopidogrel (4.5% vs 3.9%; HR 0.85; P = 0.036) and reduction in definite stent thrombosis in those who received a stent (0.7% vs 1.2%; P = 0.001). Both the overall population and PCI subgroup with high-dose clopidogrel had statistically significant increased CURRENT major and severe bleeding but not TIMI major bleeding, fatal bleeding, intracranial bleeding, or CABG-related bleeding. Within the high aspirin cohort, the primary efficacy event rate was lower with the high-dose clopidogrel vs standarddose clopidogrel group (4.6% vs 3.8%, HR 0.83, P = 0.036). There was no difference seen between the high- vs standarddose clopidogrel group within the low aspirin cohort (4.2% vs 4.5%, HR 1.07; P = 0.42). With respect to major bleeding, the interaction between aspirin and clopidogrel did not reach statistical significance (P = 0.099).
The trial showed no clinical benefit of high-dose aspirin or clopidogrel for the entire study group with the exception of the high-dose clopidogrel PCI subgroup who had significantly reduced ischemic events and stent thrombosis at the cost of increased bleeding.
Aspirin current status
The American College of Cardiology and American Heart Association (ACC/AHA) guidelines advocate chewing aspirin (162–325 mg) by patients who have not taken aspirin before presenting with an ST elevation myocardial infarction (STEMI). Post-PCI STEMI patients should continue aspirin 162–325 mg daily for at least 1 month after bare metal stent (BMS) implantation, 3 months after sirolimus-eluting stent (SES) implantation, and 6 months after paclitaxel-eluting stent (PES) implantation. Thereafter, aspirin is continued indefinitely at a dose of 75 mg to 162 mg daily (Class I).
Patients with unstable angina (UA) or non-ST elevation myocardial infarction (NSTEMI) should receive aspirin as soon as possible after hospital presentation and be maintained on aspirin indefinitely (Class I).3
Thienopyridines
The thienopyridines are platelet P2Y12 receptor antagonists that irreversibly inhibit adenosine diphosphate (ADP)-induced platelet aggregation. Aspirin and thienopyridine combination therapy has been shown to have synergistic antiplatelet effect and has become standard treatment for the prevention of ischemic events in patients with ACS and in those undergoing PCI.
Ticlopidine
Ticlopidine is a first generation thienopyridine that has largely been replaced by clopidogrel. Studies suggest that clopidogrel has a more favorable side effect profile and is a safe and effective alternative to ticlopidine. Although uncommon, serious ticlopidine-associated adverse effects include neutropenia, thrombocytopenia, thrombotic thrombocytopenic purpura, rash, hepatic cholestasis, and in rare cases, aplastic anemia, pancytopenia, and agranulocytosis.
Clopidogrel
The beneficial effects of aspirin and clopidogrel combination therapy in the prevention of major ischemic events after PCI have been well described. Nonetheless, studies in which the optimal dosing and timing for clopidogrel treatment before PCI have been evaluated have yielded variable and conflicting results owing in part to the broad range in the duration of pretreatment (hours to days), wide interindividual variability in the inhibitory response to clopidogrel, and differences in study designs. The following section contains a discussion of selected clinical trials evaluating the effectiveness of clopidogrel pretreatment in patients undergoing PCI.
The PCI-CURE trial was among the first study in which the beneficial effect of clopidogrel pretreatment in patients undergoing PCI was demonstrated.4 Of more than 12,000 patients with non-ST elevation ACS in the CURE study who were randomly assigned to receive either clopidogrel or placebo, 1,313 in the clopidogrel- and 1,345 in the placebo-treated groups underwent PCI. A loading dose of clopidogrel 300 mg or matching placebo was given a median of 10 days before PCI. After PCI, more than 80% of patients in both groups received open-labeled thienopyridine for 4 weeks, after which the study drug was restarted and continued for a mean of 8 months. Compared to placebo, clopidogrel reduced the risk of composite primary endpoint (cardiovascular death, MI, or urgent revascularization by 30 days after PCI) by nearly one third (RR 0.70, P = 0.03). The beneficial effect of clopidogrel was seen before PCI, in the 4 weeks after PCI, and in the months thereafter when clopidogrel was continued long-term. There was no significant difference in major bleeding between the two treatment groups at last follow-up (3–12 months after randomization).
In contrast to the PCI-CURE study, results gained from the CREDO trial demonstrated that clopidogrel pretreatment did not significantly reduce adverse cardiac events at 28 days.5 However, subgroup analysis suggested that at least 6 hours of pretreatment is necessary to demonstrate a beneficial effect of clopidogrel. In this study, aspirin-treated patients who underwent elective PCI were randomized to receive 300 mg clopidogrel loading dose (n = 1,053) or matching placebo (n = 1,063) 3–24 hours before PCI followed by clopidogrel 75 mg/day through day 28 in each arm. Thereafter, patients in the loading dose group received clopidogrel 75 mg/day through month 12 whereas those in the control group received a placebo. In the overall cohort, administration of a clopidogrel loading dose did not significantly reduce the combined risks of death, MI, or urgent target-vessel revascularization at 28 days. However, subgroup analysis demonstrated that among patients in whom the study drug was initiated at least 6 hours prior to PCI, those randomized to clopidogrel experienced a 38.6% relative reduction in the combined end points that was of borderline statistical significance (P = 0.051). However, post hoc analysis of the CREDO trial demonstrated that the difference in outcomes between placebo and clopidogrel pretreated patients was not significant until at least 15 hours pre-treatment, with the optimal duration approaching 24 hours.6 Following PCI, long-term clopidogrel treatment (1 year) was associated with a 26.9% relative reduction in the combined risks of death, MI, or stroke (P = 0.02).
The beneficial effect of increasing clopidogrel loading dose to 600 mg in achieving a more rapid and intense platelet suppression and ameliorating outcomes has not been consistently demonstrated. Results of the ARMYDA-2 study demonstrated that pretreatment with a 600 mg loading dose of clopidogrel 4–8 hours before PCI (n = 126) significantly reduced periprocedural adverse cardiac events compared with the conventional 300 mg dose (n = 129). The primary end point of death, MI, or target vessel revascularization at 30 days occurred in 4% and 12% of patients in the high- vs conventional-loading dose groups, respectively (P = 0.041). Multivariate analysis demonstrated that the 600 mg loading dose was associated with a 50% reduction in the risk of periprocedural MI (OR 0.48, P = 0.044).7 Safety data were comparable between the two treatment groups. However, the methodological aspects of the ARMYDA-2 trial have been challenged by experts in the field because only a per-protocol analysis (only patients who underwent PCI were included) rather than an intention-to-treat analysis was performed.8 Furthermore, the study sample consisted of a small number of patients (n = 255) and only moderate-risk patients were enrolled in the study.
In contrast to the ARMYDA-2 study, a retrospective study of 445 patients with stable angina who underwent PCI failed to demonstrate a beneficial effect of a high- vs conventionalloading dose in reducing 30-day major adverse cardiac events. Major bleeding was also similar between the two treatment groups. The authors concluded that although 600 mg was clinically safe, it was not associated with fewer periprocedural events or improved 30-day outcomes compared to 300 mg loading dose.9 Nevertheless, it should be noted that in this study clopidogrel loading dose was administered immediately before the procedure, which might be inadequate for providing protective antiplatelet aggregation in either treatment group.
In a meta-analysis of 10 studies (7 randomized, 3 nonrandomized) consisting of over 1,500 patients (712 loaded with 300 mg, 11 with 450 mg, 790 with 600 mg, and 54 with 900 mg), Lotrionte et al10 demonstrated that a high loading dose was significantly superior to a standard loading dose in preventing cardiac death or nonfatal MI (odds ratio 0.54, 95% CI 0.32 to 0.90, P = 0.02) without increasing the risk of major or minor bleeding (P = 0.55 and P = 0.98, respectively). Notably, meta-regression analysis suggested that the greatest benefits of a high loading dose were seen in the highest risk participants. Although most systematic reviews have some inherent limitations and the results may not be applicable to various patient populations, the authors concluded that the robustness of the study was supported by the magnitude of statistical significance, even in sensitivity analysis restricted to randomized trials.
In concert with the ARMYDA-2 study, results gained in the CURRENT-OASIS 7 study showed reduced composite end points of cardiovascular death, MI, and stroke as well as reduced stent thrombosis through 30 days with high-dose clopidogrel (600 mg loading followed by 150 mg for one week) therapy in ACS patients undergoing PCI. The reduction reached statistical significance but with an increased CURRENT major and severe bleeding. There was no significant difference in TIMI major, intracranial hemorrhage, or CABG-related bleeding.
The ISAR-REACT study investigators were among the first to demonstrate that the duration of high-dose clopidogrel pretreatment beyond 2 hours conferred no additional benefits among low- to intermediate-risk patients undergoing PCI.11 In this study over 2,000 patients with CAD who underwent PCI were randomized to receive adjunctive therapy with either abciximab or placebo. All patients were treated with 600 mg oral loading dose of clopidogrel at least 2 hours before elective PCI. Subgroup analysis of patients based on the duration of clopidogrel pretreatment (2 to 3 h, 3 to 6 h, 6 to 12 h, or >12 h) showed no significant differences in the 30-day composite end points of death, MI, or urgent revascularization or its individual components between patient groups irrespective of assignment to abciximab or placebo (P = 0.79 across groups). In the ISAR-CHOICE study, the same group of investigators demonstrated that a single dose of clopidogrel higher than 600 mg was not associated with additional suppression of platelet function (P = NS).12 In their single-center study, 60 patients with suspected or documented CAD were randomly assigned to one of the three clopidogrel loading dose (300, 600, or 900 mg). Pharmacokinetic studies demonstrated that increasing clopidogrel loading dose from 600 mg to 900 mg resulted in no further increase in plasma concentrations of the active metabolite and the unchanged form of the drug (P > 0.38) and no further suppression of ADP-induced platelet aggregation compared with the 600 mg clopidogrel dose. It is speculated that the lack of further beneficial response with doses higher than 600 mg may be due to limited intestinal absorption.12
Clopidogrel trials and outcomes are summarized in Table 2.
Table 2.
Clopidogrel trials and outcomes
| Trial | Study drug | N | 10 endpoints | Outcomes/comments |
|---|---|---|---|---|
| PCI-CURE | 300 mg Clopidogrel loading (Clopidogrel given median 10 days before PCI) Placebo |
1,313 1,345 |
Composite endpoints of CV death, MI, or urgent revascularization by 30 days after PCI | Compared with placebo, clopidogrel ↓ the risks of composite endpoints by nearly 1/3 (RR = 0.70, P = 0.03) |
| CREDO | 300 mg Clopidogrel loading Placebo |
1,053 1,063 |
Combined risks of death, MI or urgent revascularization @ day 28 | No difference between the 2 treatment groups Post-hoc: Clopidogrel better if given >15 hrs before PCI |
| ARMYDA-2 | 600 mg clopidogrel loading 300 mg clopidogrel loading |
126 129 |
Combined risks of death, MI, or target vessel revascularization @ 30 days | 600 mg versus 300 mg, no difference (P = 0.041) Multivariate analysis: 50% ↓ peri-procedure MI with 600 mg clopidogrel Comparable safety |
| CURRENT-OASIS 7* | Clopidogrel analysis: 600 mg loading, followed by 150 mg × 7 days, then 75 mg daily 300 mg loading, followed by 75 mg daily | 12,508 12,579 |
Composite endpoints of CV death, MI, or stroke @ 30 days | 600 mg versus 300 mg, no difference (P = 0.37) PCI subgroup: ↓ Composite endpoints (HR 0.85, P = 0.036) and ↓ Definite stent thrombosis with high dose (P = 0.001) ↓ Current major and severe bleeding with high dose (P = 0.01) |
| ISAR-REACT | Duration of 600 mg clopidogrel treatment before PCI with or without abciximab (2–3 h, 3–6 h, 6–12 h, or >12 h) | 2,159 | Composite endpoints of death, MI, or urgent revascularization @ 30 days | No incremental benefit with clopidogrel pretreatment >2–3 h (P = 0.79) with or without abciximab |
| ISAR-CHOICE | Clopidogrel 300 mg, 600 mg, or 900 mg loading | 60 | Plasma concentrations of active and inactive clopidogrel metabolites, and unchanged clopidogrel; values for ADP-induced platelet aggregation 4 h after clopidogrel | 600 mg versus 900 mg No further increase in concentrations of metabolites (P = 0.59) or ADP-induced platelet aggregation (P = 0.39) |
Within each clopidogrel group (600 mg versus 300 mg loading), patients were randomized to receive high-dose or low-dose aspirin (300–325 mg or 75–100 mg).
Abbreviation: N, number of patients.
Prasugrel
Prasugrel is a potent third generation thienopyridine with a more rapid onset of action than clopidogrel. Studies involving healthy participants suggest that orally administered prasugrel provides faster, higher, and more consistent inhibition of platelet aggregation compared to clopidogrel.13 Phase III clinical trials involving moderate- to high-risk patients with ACS undergoing PCI have demonstrated that prasugrel was superior to clopidogrel in reducing major adverse cardiac events albeit with increased risk of TIMI major hemorrhage.
TRITON-TIMI 38 was the first large-scale clinical events trial in which it was assessed whether the higher level of inhibition of platelet aggregation achieved by prasugrel resulted in an improvement in clinical outcomes compared with standard clopidogrel pretreatment. In this study, 13,608 patients with moderate- to high-risk ACS (10,074 with unstable angina/NSTEMI and 3,534 with STEMI) and with scheduled PCI were randomly assigned to receive prasugrel (60 mg loading dose followed by 10 mg daily maintenance dose) or clopidogrel (300 mg loading dose followed by 75 mg daily maintenance dose for 6 to 15 months).14 The primary efficacy end point (death from cardiovascular causes, nonfatal MI, or nonfatal stroke) occurred in 9.9% and 12.1% of prasugrel- vs clopidogrel-treated patients (HR 0.81, P < 0.001). Prasugrel-treated patients also showed a significant reduction in the incidence of MI (7.4% vs 9.7%, respectively, P < 0.001), urgent target-vessel revascularization (2.5% vs 3.7%, respectively, P < 0.001), and stent thrombosis (1.1% vs 2.4%, respectively, P < 0.001). However, major bleeding occurred in 2.4% of patients receiving prasugrel compared to 1.8% of those receiving clopidogrel (HR, 1.32, P = 0.03). Life-threatening bleeding including nonfatal and fatal bleeding was also significantly higher in the prasugrel-treated groups. However, there were no significant differences between treatment groups in the overall mortality rate (ie, cardiovascular death or death from any cause). Post hoc analysis demonstrated that patients with a history of stroke or transient ischemic attack (TIA) had a net harmful effect from prasugrel whereas those older than 75 years of age, and those weighing less than 60 kg had no net clinical benefit from prasugrel treatment. It should also be noted that in this study nearly 75% of patients received the study drug during PCI whereas only 25% to 26% of patients in each arm received study drug before PCI, which might not be relevant to current practice guidelines.
In an analysis involving STEMI patients in the TRITON-TIMI 38 study (n = 3,534), Montalescot et al15 demonstrated that prasugrel was associated with a significant reduction in the primary composite end points of cardiovascular death, non-fatal MI, or non-fatal stroke at 30 days and 15 months follow-up and there was no significant increase in major bleeding risk between the treatment groups during the study period. However, TIMI major bleeding after coronary artery bypass graft surgery was significantly increased in prasugrel-compared to clopidogrel-treated groups (OR 8.19, P = 0.0033).
In summary, the TRITON-TIMI 38 demonstrated that prasugrel therapy was associated with significantly reduced rates of ischemic events including stent thrombosis, but with an increased risk of major bleeding in a subset of patients. The drug was approved by the US Food and Drug Administration (FDA) in July 2009 for use in patients with ACS undergoing PCI. The clinical use of prasugrel will most likely be in the setting of STEMI. There are no clinical trials as of yet supporting the safety of “upstream” prasugrel use in the setting of NSTEMI. Patients with NSTEMI in the TRITON-TIMI 38 trial were not randomized until after definition of the coronary anatomy was made by coronary angiography. Prasugrel is not yet recommended for routine use in elective PCI unless patients are felt to be at higher risk of thrombosis by their interventional cardiologists. Prasugrel is contraindicated in patients with a prior history of stroke or TIA. The package insert recommends decreasing the daily maintenance dosage from 10 mg to 5 mg in patients weighing less than 60 kg although there is no clinical evidence supporting its safety.
The TRILOGY ACS is an ongoing trial comparing the relative efficacy and safety of prasugrel and clopidogrel in medically treated unstable angina/NSTEMI ACS patients. This trial has an expected completion date of October 2011.16
Thienopyridines current status
Currently available data support the use of high-dose clopidogrel loading (600 mg) if given > 2–6 hours pre-PCI, and standard-dose (300 mg) if given > 6–12 hours pre-PCI. Pharmacokinetic study results suggest that clopidogrel loading doses higher than 600 mg offer no additional beneficial effects on platelet function suppression due to limited clopidogrel absorption. However, large randomized trials evaluating clinical end points are needed. Clopidogrel 75 mg daily should be continued for a variable period depending in part on stent type (eg, one month for bare metal stent, one year with drug eluting stent except in patients at high risk for bleeding – or three months for Cypher, and six months for Taxus stent). For patients with ACS, clopidogrel should be continued for at least one year regardless of stent type.
In STEMI patients undergoing primary PCI, the ACC/ AHA guidelines recommend administration of clopidogrel 300 mg or 600 mg, favoring the 600 mg dose, or prasugrel 60 mg as soon as possible (Class I; Level of Evidence C for clopidogrel and B for prasugrel). If stents are implanted, BMS or DES, clopidogrel 75 mg daily or prasugrel 10 mg daily should be continued for at least 12 months unless the risk of bleeding outweighs the benefit (Class I). Prasugrel should not be given as part of the dual antiplatelet therapy in patients with a prior history of TIA or stroke (Class III).
Selected UA and NSTEMI patients undergoing invasive therapy should also receive dual antiplatelet therapy including aspirin with clopidogrel before or at the time of PCI or prasugrel at the time of PCI (Class I; Level of Evidence A for clopidogrel and B for prasugrel).17
Nonthienopyridines
Ticagrelor
Ticagrelor is the first reversible oral P2Y12 receptor antagonist of the nonthienopyridine class. It has a rapid onset of action and a half-life of approximately 12 hours. Phase II trial conducted in stable atherosclerosis patients demonstrated a dose-dependent increase in the level of inhibition with ticagrelor, with levels significantly higher than those achieved with clopidrogel.18 Similarly, in the phase-2 DISPERSE trial involving NSTEMI patients who were randomized to receive either ticagrelor 90 or 180 mg twice daily, or clopidrogel 300-mg loading dose, ticagrelor has been shown to exhibit greater mean inhibition of platelet aggregation than a standard regimen of clopidogrel.19 Safety data profile showed an increase in minor bleeding at the higher dose but no difference in major bleeding was observed. The rates of MI were lower in the ticagrelor compared to the clopidrogel groups, although this did not reach statistical significance.19
The phase III PLATO trial was a multicenter, double-blind, randomized trial comparing ticagrelor (180 mg loading dose, 90 mg twice daily thereafter) with clopidogrel (300 mg to 600 mg loading dose, 75 mg daily thereafter) for the prevention of cardiovascular events in over 18,000 patients admitted with ACS, with or without ST-segment elevation. 20 At 12 months follow-up, ticagrelor as compared with clopidogrel significantly reduced the primary composite end point of death from vascular causes, MI, or stroke (9.8% vs 11.7% in the ticagrelor vs clopidrogel group, respectively, P < 0.001) without an increase in the rate of overall major bleeding but with an increase in the rate of non-procedural related bleeding and a trend towards more hemorrhagic strokes in the ticagrelor- vs the clopidogrel-group (0.2% vs 0.1% respectively; P = 0.10). Other ticagrelor-related adverse events include dypsnea, ventricular pauses, and slightly increased creatinine and uric acid levels. Notably, discontinuation of the study drug due to adverse events occurred in 7.4% of ticagrelor- vs 6.0% of clopidrogel-treated patients (P < 0.01).
The advantages of ticagrelor vs clopidogrel were seen in ACS patients with or without ST-segment elevation, in patients undergoing invasive or non-invasive treatment, and in patients who had or had not received the currently recommended pretreatment of higher loading dose of clopidogrel. The only exception was the attenuated benefit of ticagrelor in patients weighing less than the median weight for their gender in the study, in patients not taking lipid-lowering drugs at randomization, and in patients enrolled in North America. Ticagrelor has not been approved by the FDA in the US.
Cangrelor
Similar to ticagrelor, cangrelor is a nonthienopyridine that binds reversibly to the platelet P2Y12 receptor. It has a rapid onset of action (within seconds if a bolus dose is administered), a rapid offset, and a half-life of a few minutes with complete recovery of platelet function within 1 hour.21 The CHAMPION PLATFORM trial was a double-blind, placebocontrolled study randomizing over 5000 patients into the cangrelor or placebo group at the time of PCI, followed by administration of 600 mg clopidogrel.22 Enrollment was stopped prematurely because an interim analysis failed to demonstrate the superiority of cangrelor over placebo in reducing the primary composite end points of death, MI, or ischemia-driven revascularization at 48 hours (P = 0.17). The pre-specified secondary end points, rate of stent thrombosis, and rate of death from any cause at 48 hours, were significantly reduced in the cangrelor group but should be interpreted with caution in the absence of a positive primary end point finding. In addition, the rate of major bleeding in the study group was higher mainly due to more groin hematomas based on the GUSTO bleeding scale.
The CHAMPION PCI trial compared cangrelor with 600 mg of clopidogrel administered before PCI in nearly 9,000 patients with ACS.23 The primary efficacy end point, a composite of death from any cause, MI, or ischemia-driven revascularization at 48 hours occurred in 7.5% of the cangrelor group and 7.1% of the clopidogrel group (odds ratio 1.05; 95% CI 0.88–1.24; P = 0.59). Cangrelor was also not superior to clopidogrel at 30 days. The secondary end points of death from any cause, Q-wave MI, or ischemia-driven revascularization showed a reduction trend favoring cangrelor but were not statistically significant. As seen in the CHAMPION PLATFORM trial, there was also a trend towards more major bleeding in the cangrelor group based on the GUSTO bleeding scale.
Despite the negative primary efficacy end points and safety concerns with cangrelor from both CHAMPION trials, cangrelor may be an attractive drug for “bridging therapy” in the perioperative setting due to its rapid onset and offset of action, and its reversibility. The BRIDGE study is an ongoing study designed to demonstrate that patients receiving cangrelor infusion before coronary artery bypass grafting have an acceptable safety profile and can undergo surgery without excessive bleeding perioperatively. This study has an expected completion date of July 2010.24
Glycoprotein IIB/IIIA inhibitors
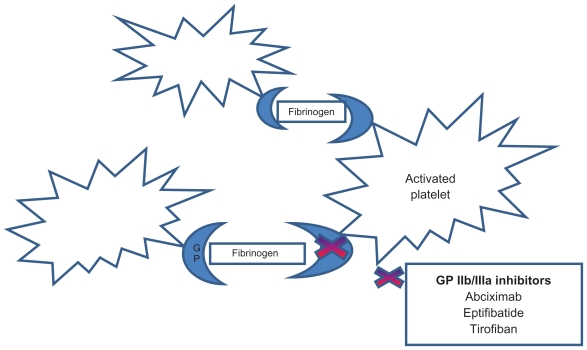
Inhibition of glycoprotein IIb/IIIa receptors – the final common pathway of platelet-thrombus formation (Figure 1) – has been shown to reduce thrombotic complications associated with PCI.
Figure 1.
Site of action of glycoprotein IIb/IIa inhibitors.
Aggregation requires activated platelets, glycoprotein receptor IIb/IIIa (GP), and fibrinogen. The latter acts as a bridge that attaches platelet to each other forming the initial hemostatic plug. The current ACC/AHA guidelines recommend the use of GP IIb/IIa antagonists in high-risk patients with STEMI-ACS and planned percutaneous coronary intervention.
Results gained in early studies demonstrated no clinical benefit from routine early invasive management compared with conservative ischemia-guided management in patients with NSTEMI (eg, the TIMI IIIB trial, the VANQWISH trial, and the MATE trial).28 However, with the advent of coronary stents and glycoprotein IIb/IIIa inhibitors, independent investigators have sought to evaluate the short- and long-term outcomes of a routine invasive strategy compared with a selective strategy in patients with UA and NSTEMI in the pre- compared with the post-glycoprotein IIb/IIIa and coronary stenting era. The TIMI IIIB and the TACTICS-TIMI 18 were both trials of an early invasive strategy in patients with UA/NSTEMI with nearly identical study design and patient enrollment criteria.25,29 However, the two studies were conducted 10 years apart, and the use of upstream GP IIb/IIIa was mandated and coronary stenting was routinely used in TACTICS-TIMI 18. Among patients matched for similar degrees of baseline risks, patients in TACTICS-TIMI 18 had significantly lower rates of the composite end points of death, MI, or rehospitalization for ACS through 6 months compared with patients in TIMI IIIB, after adjusting for differences in baseline risk. Although the favorable outcomes in the TACTICS-TIMI 18 compared with the TIMI IIIB trials were likely multifactorial, including advances in the care of patients with UA/ NSTEMI, GP IIb/IIIa inhibition, and coronary stenting, the investigators speculated that the differences in outcomes most likely reflect the use of GP IIb/IIIa inhibitors and coronary stenting in TACTICS-TIMI 18.
Similar to the TACTICS-TIMI 18 trial, analysis of the CRUSADE community-based registry database consisting of over 55,000 NSTE MI patients demonstrated a beneficial effect of early GP IIb/IIIa inhibition in reducing the risk of in-hospital mortality in patients at all risk levels. Notably, among troponin-positive patients, GP IIb/IIIa inhibition was associated with an even lower adjusted mortality risk.30
Currently available GP IIb/IIIa inhibitors include abciximab, tirofiban, and eptifibatide. Selected trials involving the use of each individual GP IIb/IIIa inhibitor in patients undergoing PCI are discussed.
Abciximab
Abciximab is a chimeric monoclonal antibody with a high affinity receptor binding and a long biological half-life. Unlike eptifibatide or tirofiban which are selective for the GP IIb/IIIa receptor, abciximab receptor binding is nonspecific. In the early EPIC trial consisting of patients at high risk for thrombotic complications after coronary intervention, abciximab has been shown to reduce ischemic complications of coronary intervention albeit with an increased risk of bleeding.31 Subsequent large randomized controlled trials extend the findings of the EPIC trial on the beneficial role of GP IIb/IIIa inhibitors in patients undergoing PCI.
In a European meta-analysis of three randomized trials (ISAR-2, ADMIRAL, and ACE) in which patients under-going primary PCI and stenting for ST-elevation MI were randomized to receive abciximab (n = 550) or placebo (n = 551), Montalescot et al32 demonstrated a 37% relative risk reduction in death or reinfarction in abciximab-compared to placebo-treated groups (RR 0.633, P = 0.008). Similarly, mortality rates were reduced in the abciximab-compared to placebo-treated arms although this did not reach statistical significance (P = 0.05). The impact of abciximab on the primary outcomes was observed up to 3 years of follow-up. Major bleeding occurred in 2.5% and 2.0% of abciximab and placebo-treated groups, respectively (P = NS). Notably, there was a 47.5% relative risk reduction in the primary endpoint of death or MI, and an 85% relative risk reduction in reinfarction in diabetics compared to their non-diabetic counterparts (P = 0.022).
Eptifibatide
Eptifibatide is a small cyclic heptapeptide that is highly specific for the GP IIb/IIIa receptor. It has a relatively low binding affinity and rapidly dissociates from its receptor after cessation of therapy, leading to an early restoration of platelet function after discontinuation of infusion. Its time to restoration of normal platelet aggregation after cessation of therapy is 4 hours, compared to 72 hours for abciximab.
In the IMPACT-II trial consisting of 4,010 patients undergoing elective, urgent, or emergency PCI who were randomized to receive placebo or bolus eptifibatide followed by two different dosing regimen of continuous infusion therapy (135 μg/kg eptifibatide followed by an infusion of 0.5 μg/kg/min for 20–24 h or 0.75 μg/kg/min infusion), a significant reduction in the primary composite end points of death, MI, or urgent target vessel revascularization at 30 days was demonstrated in the 135/0.5 treated-group (11.6% vs 9.1%, eptifibatide vs placebo, respectively, P = 0.035), with a less substantial reduction in the 135/0.75 treated- group (11.6% vs 10.0%, eptifibatide vs placebo, respectively, P = 0.18). There was no significant increase in the primary safety end point of major bleeding in eptifibatide-treated groups.33
In a subsequent randomized, double-blind, placebo-controlled, crossover-permitted ESPRIT trial (n = 2,064) using double-bolus eptifibatide therapy followed by infusion therapy at a dose that was 4-fold higher than that used in the IMPACT-II study, O’Shea et al34 demonstrated that in patients undergoing non-urgent PCI with stent implantation, eptifibatide significantly reduced the primary composite end points of death, MI, and urgent target vessel revascularization within 48 h compared with placebo (0.3% vs 0.4%, P = 0.027). The beneficial effect of adjunctive eptifibatide therapy was maintained through six months of follow up.
Tirofiban
Tirofiban is a small nonpeptide tyrosine derivative GP IIb/IIIa antagonist. Similar to eptifibatide, tirofiban is a selective inhibitor of the GP IIb/IIIa receptor with a rapid onset of action and a short half-life of 2–4 hours. Its action is rapidly reversible upon cessation of therapy. Such reversibility may have important clinical implications such as in the setting of emergent coronary artery bypass graft surgery when rapid reversal of antithrombotic effects is desirable.
Early studies in which lower-dose bolus tirofiban was used failed to demonstrate the non-inferiority of tirofiban as compared with abciximab in patients undergoing percutaneous coronary revascularization with the intent to perform stenting of a newly stenotic or restenotic atherosclerotic lesions of a native vessel or bypass graft.35 However, subsequent trials using high-dose bolus tirofiban support its efficacy in patients with STEMI undergoing PCI. The ON-TIME 2 trial was the first randomized, placebo-controlled trial evaluating whether pre-hospital initiation of high-bolus dose tirofiban in addition to dual antiplatelet therapy improved ST-segment resolution and clinical outcome after PCI.36 A total of 984 patients with STEMI who were candidates to undergo PCI were randomly assigned to either high-bolus dose tirofiban (n = 491) or placebo (n = 493) in addition to aspirin (500 mg), heparin (5000 IU), and clopidogrel (600 mg). The study drug was initiated at a median of 76 minutes after symptom onset and 55 minutes prior to angiography/PCI. The cumulative residual ST-segment deviation was significantly lower in the tirofiban compared to placebo groups 1 hour post-PCI (3.6 mm [SD 4.6] vs 4.8 mm [SD 6.3], P = 0.003). There was no significant increase in the rates of major bleeding between the two groups (4% vs 3%; P = 0.36). At 30 days there was a significant reduction in the combined incidence of death, recurrent MI, urgent target vessel revascularization, or thrombotic bailout in the tirofiban compared to placebo groups (26% vs placebo 32.9%, P = 0.02). Further analysis suggested an association between the level of residual ST-segment deviation and mortality.
Similarly, the MULTISTRATEGY trial involving 745 patients presenting with STEMI or new left bundle-branch block demonstrated that high-dose bolus regimen of tirofiban was superior to standard dose abciximab in ST-segment recovery at 90 minutes following coronary intervention.37 With regard to stent type, sirolimus-eluting stent implantation was associated with a significantly lower risk of major adverse cardiac events compared to uncoated stents within eight months after intervention owing primarily to a reduction of revascularization rates (10.2% vs 3.2%). The incidence of ischemic and hemorrhagic outcomes was similar between the tirofiban and abciximab groups.
Abciximab, eptifibatide, and tirofiban in the setting of PCI: meta-analysis
In a meta-analyis of 21 randomized trials comparing GP IIb/IIIa inhibitors with controls (in which 15 used abciximab, four used eptifibatide, and two used tirofiban), Labinaz et al38 demonstrated that treatment with GP IIb/IIIa in the setting of PCI significantly reduced 30-day mortality rates, MI, and revascularization procedures. At 30 days there was a significant 0.38% absolute reduction in the primary outcome of all-cause mortality. The reduction in mortality rate was seen as early as 7 days but the benefit appeared to dissipate by six months and was of borderline significance at one year. Similarly, the beneficial effect of GP IIb/IIIa inhibitors in reducing both MI and the need for repeat revascularization was observed as early as seven days and persisted to 30 days and six months. The early reduction in clinical events associated with GP IIb/IIIa inhibition was thought to be consistent with the pharmacodynamic effects of these agents whereby potent GP IIb/IIIa antagonism reduces local platelet deposition at the site of PCI. This results in a reduction in the local platelet-mediated vasoconstriction, distal vessel platelet-thrombin microembolization and abrupt vessel closure – all of which may contribute to the reduction in reinfarction risk and the subsequent need for repeat revascularization procedures.38 The beneficial effects of GP IIb/IIIa inhibitors are achieved at an increased risk of thrombocytopenia (OR 1.41, 95% CI 1.10 to 1.81) and minor bleeding (OR 1.80, 95% CI 1.47 to 2.21), but not major bleeding (OR 1.29, 95% CI 0.98 to 1.68).
GP IIb/IIIa trials and outcomes are summarized in Table 3.
Table 3.
Glycoprotein IIb/IIa trials and outcomes
| Trial | Study drug | N | 10 endpoints | Outcomes/comments |
|---|---|---|---|---|
| European Meta-analysis (ISAR-2, ACE, ADMIRAL) | Abciximab Placebo |
550 551 |
Composite of death or re-infarction; up to 3 years of follow-up | Compared with placebo, abciximab ↓ the composite endpoints by 37% (RR = 0.633, P = 0.008) Diabetics versus non-diabetics: ↓ composite endpoints and re-infarction (P = 0.022) No difference in major bleeding |
| IMPACT-II | Eptifibatide bolus followed by two continous infusions (Bolus 135 ug/kg, infusion 0.5 or 0.75 ug/kg/min ×20–24 h) Placebo |
4,010 (total) 1,349 (infusion @ 50) 1,333 (infusion @ 75) 1,328 (placebo) |
Composite of death, MI, or urgent target vessel revascularization @ 30 days | Eptifibatide 135/0.5 versus placebo 11.6% versus 9.1%, respectively (P = 0.035) Eptifibatide 135/0.75 versus placebo 11.6% versus 10.0%, respectively (P = NS) No difference in major bleeding in eptifibatide-treated groups |
| ESPRIT | Double-bolus eptifibatide followed by high-dose infusion Placebo |
1,040 (eptifibatide) 1,024 (placebo) |
Composite of death, MI, or urgent target vessel revascularization through 6 months | Eptifibatide versus placebo (0.3% versus 0.4%, P = 0.027) |
| ON-TIME 2 | Pre-hospital high-bolus dose tirofiban + DAT + heparin Placebo + DAT + heparin |
491 493 |
Combined incidence of death, recurrent MI, urgent target vessel revascularization, or thrombotic bailout @ 30 days; ST-segment resolution | Tirofiban versus placebo 26% versus 32.9%, respectively (P = 0.02) in STEMI with PCI Lower residual ST-segment deviation 1 h after PCI (P = 0.003) No difference in rates of major bleeding between groups |
Abbreviation: N, number of patients.
GP IIb/IIa inhibitors current status
In selected STEMI patients, the ACC/AHA guidelines recommend treatment with a GP IIb/IIIa receptor antagonist at the time of primary PCI with or without stenting (Class IIa; Level of Evidence A for abciximab and B for eptifibatide and tirofiban). The usefulness of these agents as routine treatment in STEMI patients is uncertain (Class IIb) but its beneficial effect may be greater in the presence of inadequate thienopyridine loading or large thrombus burden.
Patients with UA or NSTEMI undergoing early invasive treatment may be administered GP IIb/IIIa receptor antagonist or clopidogrel before angiography (Class I; LOE A). In the setting of recurrent ischemia or high risk features including positive troponin, both GP IIb/IIIa receptor antagonist and clopidogrel may be given in addition to aspirin before angiography (Class IIa; LOE B). Abciximab should not be administered if PCI is delayed for over 24 hours (Class III).
Antithrombins
Indirect thrombin inhibitors
Unfractionated heparin
The heparins, Unfractionated heparin (UFH) are indirect thrombin inhibitors that complex with antithrombin (AT) and convert AT from a slow to a rapid inactivator of thrombin, factor Xa, and to a lesser extent, factors XIIa, XIa, and IXa. UFH has been the standard adjunctive antithrombin therapy during PCI for nearly three decades. However, there are several intrinsic limitations to heparin therapy including its inability to bind clot-bound thrombin. More importantly, such clot-bound thrombin remains enzymatically active, amplifying its own generation leading to further thrombus formation. Other disadvantages of UFH include its dependence on AT for inhibition of thrombin activity, sensitivity to platelet factor 4, and marked interindividual variability in therapeutic response. To overcome some of the limitations of the heparins, newer agents with more predictable pharmacokinetics and anticoagulant effect are being evaluated for their safety and efficacy in the setting of PCI. These include enoxaparin, fondaparinux, and bivalirudin.
Enoxaparin
Enoxaparin is a low molecular weight heparin with a longer half-life and greater anti-Xa activity than UFH, and less plasma and protein binding. It has a high bioavailability with significantly less interindividual variation in therapeutic response than UFH. In patients with normal renal function the drug can be given subcutaneously without the need for laboratory monitoring or dose adjustment. Enoxaparin has been shown to be superior to UFH in reducing ischemic events in patients treated conservatively for NSTEMI. However, studies of high-risk patients undergoing early coronary intervention failed to demonstrate the superior effectiveness of enoxaparin over UFH. In the SYNERGY trial involving 4,687 high-risk patients with non-ST-segment elevation ACS undergoing PCI who were randomized to receive enoxaparin or UFH, White et al39 demonstrated similar rates of death and MI at 30 days between the two treatment groups (enoxaparin vs UFH: 13/1% vs 14.2%, respectively, P = 0.289). The incidence of GUSTO severe bleeding was comparable between the two treatment groups (P = 0.289) whereas TIMI major bleeding was significantly higher in the enoxaparin group (enoxaparin vs UFH: 3.7% vs 2.5%, respectively, P = 0.28). The authors concluded that in high-risk patients undergoing PCI for ACS, enoxaparin avoids the need for monitoring and achieves similar effectiveness to UFH but is associated with more bleeding. The routine recommendation of enoxaparin use in high-risk patients undergoing PCI awaits further study.
The ATOLL trial is an ongoing randomized trial evaluating enoxaparin vs unfractionated heparin in lowering ischemic and bleeding events in patients with acute STEMI treated with primary angioplasty. The trial has an expected completion date of January 2012.40
Fondaparinux
Fondaparinux is an indirect synthetic factor Xa inhibitor that binds to AT with a higher affinity than UFH or LMWH, and causes a conformational change in AT that significantly increases the ability of AT to inactivate factor Xa. The binding of fondaparinux to AT causes rapid and predictable inhibition of factor Xa. It has a half-life of 15 hours, with linear pharmacokinetics and low inter- and intra-individual variability obviating the need for laboratory monitoring. However, it should be noted that unlike heparin, fondaparinux is not inactivated by protamine and currently has no known antidote.
In a pilot, phase II, randomized, multicenter, blinded ASPIRE trial comparing two dosing regimen of fondaparinux with UFH in patients undergoing PCI, Mehta et al41 demonstrated similar bleeding complications and composite efficacy outcome of all-cause mortality, myocardial infarction, urgent revascularization, or need for a bailout GP IIb/IIIa antagonist between UFH and the combined fondaparinux groups. Bleeding was less common in the 2.5 mg-compared to the 5 mg-fondaparinux group although this did not reach statistical significance (P = 0.06). In a subsequent OASIS-6 trial involving 3,788 patients with STEMI undergoing primary PCI who were randomized to receive UFH for 4–48 hours vs fondaparinux 2.5 mg SQ daily for up to 8 days in a placebo-controlled double-blind trial, the 30-day composite end points of death or reinfarction and bleeding complications were comparable between UFH and fondaparinux groups (P = NS).42 However, there were higher rates of coronary complications in the fondaparinux-treated groups largely due to guiding catheter thrombosis. It should be noted that in patients undergoing primary PCI, intravenous heparin was used in all patients in the control group (by protocol design) whereas only 21% in the fondaparinux group received heparin. Further analysis demonstrated that among 496 patients who received UFH prior to primary PCI, the rates of clinical complications (coronary complications, catheter thrombus, and severe bleeding) were similar between fondaparinux and control groups. The results of both OASIS-5 (comparing fondaparinux vs enoxaparin in ACS)43 and OASIS-6 trials suggest that using UFH with fondaparinux during PCI substantially reduces the risk of catheter thrombosis and related complications without an increase in clinical complications or major bleeds. Nonetheless, currently available data do not support a role for fondaparinux during PCI for stable or unstable coronary artery disease.
Indirect thrombin inhibitor trials and outcomes are summarized in Table 4.
Table 4.
Indirect thrombin inhibitor trials and outcomes
| Trial | Study drug | N | 10 endpoints | Outcomes/comments |
|---|---|---|---|---|
| SYNERGY | Enoxaparin UFH |
2,323 2,364 |
Composite of death and MI @ 30 days | No difference between 2 groups Trend towards ↑ TIMI major bleeding with enoxaparin (P = 0.28) No difference in GUSTO severe bleeding between 2 groups |
| OASIS-6 | Fondaparinux 2.5 mg UFH for 4–48 h |
3,788 (total)* 1,890 (Fondaparinux) 1,898 (UFH) |
Composite of death or reinfarction | No difference in composite endpoints between 2 groups No difference in bleeding complications Increased catheter thrombosis with fondaparinux (P < 0.001) Of the 21% in the fondaparinux group receiving UFH before 10 PCI, rates of coronary complications, catheter thrombosis, and severe bleeding were similar to control group |
Notes: Patients with STEMI undergoing primary PCI.
Abbreviation: N, number of patients.
Direct thrombin inhibitors
Bivalirudin
Bivalirudin is a bivalent synthetic, reversible direct thrombin inhibitor that overcomes several limitations of heparin including its ability to inhibit clot-bound thrombin. Unlike heparin, bivalirudin does not require cofactors and is not neutralized by platelet product. In addition, it has a short half-life (25 minutes) that allows for a rapid return to hemostasis. Table 5 summarizes the advantages of bivalirudin over heparin.
Table 5.
Advantages of bivalirudin over heparin
| Heparin | Bivalirudin |
|---|---|
|
|
Abbreviations: AT, antithrombin; PK-PD, pharmacokinetics-pharmacodynamics; HIT, heparin-induced thrombocytopenia; PF4, platelet factor4; PK, pharmacokinetics.
Early clinical trials conducted for patients with unstable angina during PCI have shown that bivalirudin reduces ischemic complications and bleeding after angioplasty compared with high-dose heparin.44 Subsequent pilot studies suggested that bivalirudin with planned or provisional abciximab may be at least as safe and effective as low-dose heparin plus abciximab during percutaneous coronary intervention.45,46 Similarly, in a randomized, open-labeled, phase III study (ACUITY) involving 13,819 patients with ACS in whom urgent or early intervention was planned, both bivalirudin plus a GP IIb/IIIa inhibitor and bivalirudin monotherapy were found to be superior to heparin plus GP IIb/IIIa inhibitor for the primary composite end points of MI, unplanned revascularization or death from any cause at 30 days.47 The primary end point of major bleeding unrelated to coronary artery bypass graft surgery was significantly lower in patients receiving bivalirudin monotherapy compared to those receiving heparin plus GP IIb/IIIa inhibitor after 30 days. In patients receiving bivalirudin plus a GP IIb/IIIa inhibitor, bleeding complications were superior to a regimen of heparin plus planned GP IIb/IIIa. At the 1-year follow-up, the composite end points of ischemia and all-cause mortality was comparable among bivalirudin plus a GP IIb/ IIIa inhibitor-, bivalirudin monotherapy-, and heparin plus a GP IIb/IIIa inhibitor-treated groups.
The Randomized Evaluation in PCI Linking Angiomax to Reduced Clinical events (REPLACE)-2 trial was among the f irst large-scale randomized, double-blind, active controlled trial designed to test the safety and efficacy of bivalirudin and provisional GP IIb/IIIa blockade during PCI compared with the standard practice of low-dose heparin plus planned GP IIb/IIIa blockade.48 The study comprised over 6,000 patients who were randomly assigned to receive intravenous bivalirudin (0.75 mg/kg followed by 1.75 mg/kg per hour for the duration of PCI; n = 2,999), or heparin (65 U/kg) with planned GP IIb/IIIa inhibition (abciximab or eptifibatide; n = 3,011). All patients received daily aspirin and a thienopyridine for at least 30 days after PCI. There was no statistically significant difference in the primary end points of 30-day incidence of death, MI, urgent repeat revascularization, or in-hospital major bleeding between bivalirudin- and heparin-treated groups (9.2% vs 10.0%, respectively, P = 0.32). Similarly, the secondary composite end point was comparable between the two treatment groups. Provisional GP IIb/IIIa was administered in 7.2% of patients in the bivalirudin group. Notably, in-hospital major bleeding rates were significantly reduced in bivalirudin-treated groups (2.4% vs 4.1%, P < 0.01).
The encouraging results on the safety and efficacy of bivalirudin alone (as compared with heparin plus GP IIb/IIIa) observed in the REPLACE-2 trial have sparked interest in the use of this antithrombotic regimen in the setting of primary PCI in patients with ST-elevation acute MI. Results from the HORIZONS-AMI consisting of 3,602 patients with STEMI who were randomized to receive bivalirudin monotherapy and provisional GP IIb/IIIa inhibitors or heparin plus GP IIb/IIIa inhibitors demonstrated that bilivarudin monotherapy resulted in significantly reduced 30-day rates of major bleeding and net adverse clinical events (9.2% vs 12.1%; relative risk, 0.76, P = 0.005), compared to heparin plus GP IIb/IIIa inhibitors (abciximab or eptifibatide) owing to a lower rate of major bleeding (4.9% vs 8.3%, P < 0.001).49 Treatment with bivalirudin alone, as compared with heparin plus GP IIb/IIIa inhibitors resulted in significantly lower 30-day rates of death from cardiac causes (1.8% vs 2.9%; relative risk, 0.62; 95% CI, 0.40 to 0.95; P = 0.03) and death from all causes (2.1% vs 3.1%; relative risk, 0.66; 95% CI, 0.44 to 1.00; P = 0.047). There was an increased risk of acute stent thrombosis within 24 hours in the bivalirudin group but no significant increase was pre after 30 days. Glycoprotein IIb/IIIa inhibitors were administered in 7.2% of patients (129 patients) who were assigned to bivalirudin, in 47 because of a sustained absence of reflow after PCI, in 32 because of a giant thrombus after PCI, and in the rest for various other indications. It is speculated that the early increase in stent thrombosis with bivalirudin alone may be explained in part by adenosine diphosphate-induced platelet activation before maximal thienopyridine blockade by the P2Y12 receptor or by residual thrombin activity after the discontinuation of bivalirudin. Indeed, a clopidogrel loading dose administered before the procedure might be inadequate to provide protective antiplatelet aggregation, particularly in a subset of patients with clopidogrel resistance. Approximately one third of patients in each arm received clopidogrel 300 mg and two thirds received a 600 mg loading dose. Whether pretreatment with more rapidly acting and potent thienopyridine agent (or higher loading dose regimen of clopidogrel), or a longer course of bivalirudin, or both, may reduce the incidence of early stent thrombosis without increasing the risk of bleeding is unknown and remains to be studied. Nonetheless, it should be noted that there is currently no agent to reverse the antithrombotic effect of bivalirudin.
Analysis evaluating the predictive factors of acute, subacute, and late stent thrombosis after acute MI primary angioplasty in the HORIZONS-AMI trial revealed that the rates of acute stent thrombosis at 24 hours were significantly lower in those who received bivalirudin and pre-randomization heparin (bivalirudin + pre-randomization heparin) compared to those who did not receive pre-randomization heparin (bivalirudin, no pre-randomization heparin) [0.9% vs 2.6%, respectively, P = 0.006].
Bivalirudin trials and outcomes are summarized in Table 6.
Table 6.
Direct thrombin inhibitor trials and outcomes
| Trial | Study drug | N | 10 endpoints | Outcomes/comments |
|---|---|---|---|---|
| ACUITY | Bivalirudin+GP IIb/IIa Bivalirudin monotherapy Heparin (or enoxaparin)+ GP IIb/IIa | 2,609 2,619 2,561 |
Composite of death from any cause, MI, or unplanned revascularization @ 30 days; major bleeding unrelated to CABG | Both bivalirudin regimen were non-inferior to heparin (or enoxaparin) + GP IIb/IIa Lower major bleeding with bivalirudin monotherapy Comparable composite of ischemia and all-cause mortality among 3 groups at 1 yr |
| REPLACE-2 | Bivalirudin+provisional GP IIb/IIa Low-dose heparin+ planned GP IIb/IIa |
2,999 3,011 |
Composite of death, MI, urgent repeat revascularization, or in-hospital major bleeding @ 30 days | No difference between 2 groups ↓ in-hospital bleeding in 7% of bivalirudin group not given GP IIb/IIa (P < 0.01) |
| HORIZONS-AMI | Bivalirudin monotherapy+ provisional GP IIb/IIa Heparin+GP IIb/IIa |
1,800 1,802 |
Major bleeding; combined adverse CV events (combination of major bleeding & MACE*) within 30 days or net adverse clinical events | Bivalirudin monotherapy versus Heparin GP IIb/IIa (9.2% versus 12.1%, respectively, P = 0.005) (↓ major bleeding in bivalirudin group, P < 0.001) ↓ death from cardiac and non-cardiac causes (P = 0.03 and P = 0.047 respectively) ↑ stent thrombosis with bivalirudin <24 h (P = 0.0007) but not by 30 days |
MACE: Major Adverse Cardiac Events defined as death, re-infarction, target vessel revascularization for ischemia and stroke.
Bivalirudin: current status
Currently available data suggest that bivalirudin may be considered as an alternative strategy to heparin plus GP IIb/IIIa inhibitors, particularly in patients at high risk for bleeding complications. However, its use is relatively contraindicated in patients with chronic total occlusion because there is currently no agent to reverse its antithrombotic effects. For elective PCI, bivalirudin monotherapy (with provisional GP IIb/IIIa for procedural complications) has emerged as the antithrombin of choice, providing similar protection from ischemic events as low dose UFH plus routine GP IIb/IIIa (or high dose UFH alone) with markedly less bleeding.
Antithrombins current status
In STEMI patients undergoing primary PCI, the ACC/AHA guidelines recommend supportive anticoagulant therapy with unfractionated heparin (UFH) (Class I, LOE C) or bivalirudin (Class I, LOE B) in addition to aspirin and thienopyridine. Patients who have already been on UFH should receive additional boluses to maintain therapeutic activated clotting time, taking into account whether a GP IIb/IIIa receptor antagonist has been given. Patients who have not been given a GP IIb/IIIa should have a target ACT 250–300 seconds using a Hemotec device or target ACT 300–350 seconds using a Hemochron device. Patients who have been given a GP IIb/IIIa should have a target ACT of 200–250 seconds. Enoxaparin may be considered as an alternative to UFH (Class IIB). Bivalirudin may be administered with or without UFH and may be preferable in patients with high bleeding risk (Class IIA; LOE B).
In NSTEMI patients selected for early invasive strategy, addition of UFH, enoxaparin, or bivalirudin may also be considered. Both UFH and enoxaparin have a Class I, LOE A indications. Bivalirudin have a Class I, LOE B indications. Despite the updated AHA/ ACC guidelines regarding enoxaparin, it is not widely used for procedural anticoagulation during PCI due to the lack of a rapid bedside assay for monitoring its anti-coagulative effects. Current data do not support a role for fondaparinux during PCI. For patients undergoing PCI with prior fondaparinux treatment, additional intravenous therapy with an anticoagulant with anti-IIa activity should be administered.
Antithrombotic dosing and bleeding risks
The advent of potent antiplatelet and antithrombin agents over the past decade has resulted in significant improvement in reducing ischemic events in ACS patients. However, the use of antiplatelet and antithrombotic combination therapy, often in the settings of PCI, has led to an increase in the risk of bleeding. More importantly, such complications have been reported to be associated with increased mortality, myocardial infarction, and stroke risks.50–52 The CRUSADE registry database revealed that over 42% of patients with NSTEMI received one or more antithrombotic agents above the recommended dosage range. Excessive dosing and the number of agents administered in excess were found to be directly related to the risks of bleeding. Suggested factors associated with elevated bleeding risks included older age defined as age > 65 years, (particularly age > 75), female gender, chronic kidney disease, low body weight with increasing risk for every 5 kg decrease in weight, diabetes, and congestive heart failure. Proper dosing requires adjustments based on body weight and renal function.53 Similar to the CRUSADE registry study results, numerous researchers have demonstrated that older age, female gender, low body mass weight, and chronic kidney disease are powerful predictors of bleeding complications.51,52 Other suggested risk factors include invasive procedures and baseline hemoglobin and hematocrit values. In addition to the patient’s baseline characteristics, the type, degree, and rapidity of anticoagulation and platelet inhibition may play a contributory role in bleeding complications.52 Assessment of bleeding risks should be an integral part of risk stratification for ACS. The judicious balance between antithrombotic effect and risk of bleeding may further improve clinical outcomes of patients with ACS.
Summary
Percutaneous coronary intervention (PCI), including angioplasty and coronary stent placement, is currently the treatment of choice for patients with ACS and STEMI. In such patients, aspirin and clopidogrel dual therapy has remained the cornerstone of oral antiplatelet therapy. Currently available data support the use of high-dose clopidogrel loading (600 mg) if given > 2–6 hours pre-PCI, and standard loading dose (300 mg) if given > 6–12 hours pre PCI. For elective PCI, clopidogrel 75 mg daily should be continued for a variable period depending in part on stent type. For patients with ACS, clopidogrel should be continued at least one year regardless of the stent type. Studies in which the safety and efficacy of higher loading and maintenance dose regimen of clopidogrel versus standard dose regimen and high-dose (>300 mg) vs low-dose aspirin (<100 mg) were evaluated favor administration of high dose aspirin and high loading and maintenance dose of clopidogrel in ACS patients undergoing PCI. There was no statistically significant difference in cardiovascular end point among patients taking high- vs low-dose aspirin with the exception of the high-dose aspirin and high-dose clopidogrel PCI supgroup. Due to the wide interindividual variability in the response to clopidogrel, a newer third generation thienopyridine drug was developed. The TRITON-TIMI 38 trial involving moderate- to high-risk patients with ACS undergoing PCI demonstrated that prasugrel was superior to clopidogrel in reducing major adverse cardiac events although its use was associated with an increased risk of TIMI major hemorrhage in a subset of patients. Prasugrel was recently approved by the FDA for use in ACS patients undergoing PCI as an alternative to clopidogrel. Its use may be preferable to clopidogrel in patients with high thrombus burden or high risk for thrombus formation. Results from the PLATO trial demonstrated that ticagrelor was also superior to clopidogrel in reducing ischemic events but without an increased risk of bleeding. Ticagrelor, however, has not been approved by the FDA for use in ACS patients.
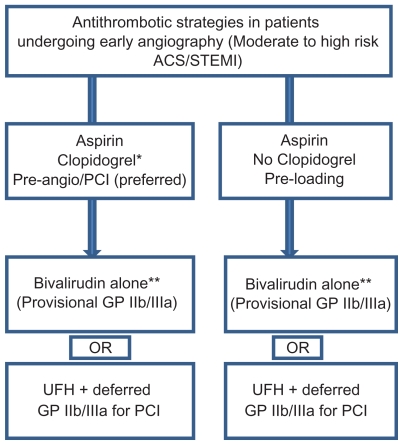
In addition to dual antiplatelet therapy, patients with ACS should be given supportive anticoagulant therapy in the early periprocedural period. Administration of UFH is still regarded as the gold standard antithrombotic therapy. Bivalirudin overcomes several limitations of heparin and may be considered as an alternative strategy to UFH plus GP IIb/IIIa inhibitors particularly in patients at risk for bleeding complications. However, its use is relatively contraindicated in patients with chronic total occlusion because there is currently no agent to reverse the antithrombotic effect of bivalirudin. Bivalirudin should be used with caution in patients without UFH or clopidrogel pretreatment due to increased risk of acute stent thrombosis. Although enoxaparin has been suggested to be superior to UFH and its use obviates the need for laboratory monitoring, enoxaparin is associated with increased bleeding complications. The routine recommendation of enoxaparin use in high-risk patients undergoing PCI awaits further studies. Similar to enoxaparin, fondaparinux causes less interindividual variation in therapeutic response than UFH and appears to be an attractive therapeutic option in patients undergoing PCI. Nevertheless, fondaparinux has been shown to result in higher rates of coronary complications compared to UFH. Currently available data does not support a role for fondaparinux during PCI. The use of fondaparinux and adjunctive UFH during PCI has been suggested to provide the beneficial effect of reducing the risk of catheter thrombosis and related complications without an increase in clinical complications or major bleeds. However, further studies are needed. Suggested antithrombotic strategies in patients undergoing PCI for ACS or STEMI in the contemporary era of early invasive coronary intervention are summarized in Figure 2.
Figure 2.
Antithrombotic strategies in patients undergoing early angiography.
Notes: *Clopidogrel 600 mg loading dose is preferred. Prasugrel may also be considered in selected patients with STEMI undergoing PCI (see text).
**Bivalirudin is relatively contraindicated in patients with chronic total occlusion.
Table 1.
Study acronyms and their respective clinical trial full names (in alphabetical order)
| Acronyms | Clinical trial full names |
|---|---|
| ACE | Abciximab and Carbostent Evaluation |
| ACUITY | Acute Catheterization and Urgent Intervention Triage Strategy |
| ADMIRAL | Abciximab Before Direct angioplasty and stenting in Myocardial Infarction Regarding Acute and Long-term Follow-up |
| ARMYDA-2 | The Antiplatelet Therapy for Reduction of Myocardial Damage during Angioplasty – 2nd edition |
| ASPIRE | Arixtra Study in Percutaneous Coronary Intervention |
| ATOLL | Acute STEMI Treated With Primary Angioplasty and Intravenous Lovenox or Unfractionated Heparin |
| BRIDGE | Maintenance of Platelet inhibition with cangreLor after discontinuation of Thienopyridines in Patients Undergoing surgery |
| CHAMPION-PCI | Cangrelor versus Standard Therapy to Achieve Optimal Management of Platelet Inhibition-percutaneous Coronary Intervention |
| CHAMPION-PLATFORM | Cangrelor versus Standard Therapy to Achieve Optimal Management of Platelet Inhibition (Platform) |
| CREDO | Reduction of Events during Observation |
| CURRENT/OASIS-7 | Clopidogrel Optimal Loading Dose Usage to Reduce Recurrent EveNTs/Optimal Antiplatelet Strategy for Interventions |
| DISPERSE | Dose Confirmation Study Assessing Anti-platelet Effects |
| EPIC | Evaluation of 7E3 for the Prevention of Ischemic Complications |
| ESPRIT | Enhanced Suppression of the Platelet IIb/IIa Receptor with Integrilin Therapy |
| HORIZONS-AMI | Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction |
| IMPACT-II | Integrilin to Minimize Platelet Aggregation and Coronary Thrombosis |
| ISAR | Intracoronary Stenting and Antithrombotic Regimen |
| ISAR-CHOICE | Intracoronary Stenting and Antithrombotic Regimen: Choose Between 3 High Oral Doses for Immediate Clopidogrel Effect |
| ISAR-REACT | Intracoronary Stenting and Antithrombotic Regimen-Rapid Early Action for Coronary Treatment |
| MATE | A prospective randomized trial of triage angiography in acute coronary syndromes ineligible for thrombolytic therapy |
| MULTISTRATEGY | MULTIcenter Evaluation of Single High-dose Bolus Tirofiban versus Abciximab with Sirolimus Eluting STEnt or Bare Metal Stent in Acute Myocardial Infacrtion |
| OASIS-5 | The Fifth Organization to Assess Strategies in Acute Ischemic Syndromes |
| OASIS-6 | The Sixth Organization to Assess Strategies in Acute Ischemic Syndromes |
| ON-TIME-2 | Ongoing Tirofiban in Myocardial Infaction Evaluation 2 |
| PCI-CURE | Percutaneous coronary intervention-Clopidogrel in Unstable angina to prevent Recurrent Events |
| PLATO | A Study of Platelet Inhibition and Patient Outcomes |
| REPLACE-2 | Randomized Evaluation in Percutaneous Coronary Intervention Linking Angiomax to Reduced Clinical Events-2 |
| SYNERGY | Safety and efficacy of enoxaparin versus unfractionated heparin in patients with non-ST segment elevation acute coronary syndromes who receive tirofiban and aspirin |
| TACTICS-TIMI 18 | Treat Angina with Aggrastat and Determine Cost of Therapy with an Invasive or Conservative Strategy-Thrombolysis in Myocardial Infarction 18 |
| TIMI IIIB | Thrombolysis in Myocardial Ischemia |
| TRILOGY | A Comparison of Prasugrel and Clopidogrel in Acute Coronary Syndrome Subjects With Unstable Angina/ Non-ST-Elevation Myocardial Infarction Who Are Medically Managed |
| TRITON-TIMI 38 | Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel-Thrombolysis in Myocardial Infarction |
| VANQWISH | Veterans Affairs Non-Q-Wave Infarction Strategies In-Hospital |
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Jolly SS, Pogue J, Haladyn K, et al. Effcets of asapirin dose on ischemic events and bleeding after percutaneous coronary intervention: insights from the PCI-CURE Study. Eur Heart J. 2009;30:900–907. doi: 10.1093/eurheartj/ehn417. [DOI] [PubMed] [Google Scholar]
- 2.Mehta SR, Bassand JP, Chrolavicius S, et al. Design and rationale of CURRENT-OASIS 7: a randomized, 2 × 2 factorial trial evaluation optimal dosing strategies for clopidogrel and aspirin in patients with ST and non-ST-elevation acute coronary syndromes managed with an early invasive strategy. Am Heart J. 2008;156:1080–1088. doi: 10.1016/j.ahj.2008.07.026. [DOI] [PubMed] [Google Scholar]
- 3.Krumholz HM, Anderson JL, Bachelder BL, et al. ACC/AHA 2008 Performance Measures for Adults With ST-Elevation and Non-ST-Elevation Myocardial Infarction A Report of the American College of Cardiology/American Heart Association Task Force on Performance Measures. J Am Coll Cardiol. 2008;52:2046–2099. doi: 10.1016/j.jacc.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 4.Mehta SR, Yusuf S, Peters RJ, et al. Effects of pretreatment with clopidrogel and aspririn followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet. 2001;358:527–533. doi: 10.1016/s0140-6736(01)05701-4. [DOI] [PubMed] [Google Scholar]
- 5.Steinhubl SR, Berger PB, Mann JT, et al. Early and sustained dula oral antiplatelet therapy following percutaneous coronary intervention. JAMA. 2002;288:2411–2420. doi: 10.1001/jama.288.19.2411. [DOI] [PubMed] [Google Scholar]
- 6.Steinhubl SR, Berger PB, Brennan DM, et al. Optimal timing for the initiation of pretreatment with 300 mg clopidrogel before percutaneous coronary intervention. J Am Coll Cardiol. 2006;47:939–943. doi: 10.1016/j.jacc.2005.10.047. [DOI] [PubMed] [Google Scholar]
- 7.Patti G, Colonna G, Pasceri V, et al. Randomized trial of high loading dose of clopidrogel for reduction of periprocedural myocardial infarction in patients undergoing coronary intervention: results from the ARMYDA-2 (Antiplatelet therapy for Reduction of Myocardial Damage during angioplasty) Study. Circulation. 2005;111:2099–2106. doi: 10.1161/01.CIR.0000161383.06692.D4. [DOI] [PubMed] [Google Scholar]
- 8.Triocco P, Harrington RA. Letters regarding Article by Patti et al. “Randomized trial of high loading dose of clopidrogel for reduction of periprocedural myocardial infarction in patients undergoing coronary intervention: results from the ARMYDA-2 (Antiplatelet therapy for Reduction of Myocardial Damage during angioplasty) Study”. Circulation. 2005;112:e282. doi: 10.1161/CIRCULATIONAHA.105.568139. [DOI] [PubMed] [Google Scholar]
- 9.Wolfram R, Torguson RL, Hassani S-E, et al. Clopidrogel loading dose (300 versus 600 mg) strategies for patients with stable angina pectoris subjected to percutaneous coronary intervention. Am J Cardiol. 2006;97:984–989. doi: 10.1016/j.amjcard.2005.10.064. [DOI] [PubMed] [Google Scholar]
- 10.Lotrionte M, Biondi-Zoccai GGL, Agostoni P, et al. Meta-analysis appraising high Clopidrogel loading in patients undergoing percutaneous coronary intervention. Am J Cardiol. 2007;100:1199–1206. doi: 10.1016/j.amjcard.2007.05.048. [DOI] [PubMed] [Google Scholar]
- 11.Kandzari DE, Berger PB, Kastrati A, et al. Influence of treatment duration with a 600-mg dose of clopidogrel before percutaneous coronary revascularization. J Am Coll Cardiol. 2004;44:2133–2136. doi: 10.1016/j.jacc.2004.08.060. [DOI] [PubMed] [Google Scholar]
- 12.von Beckerath N, Taubert D, Pogatsa-Murray G, et al. Absorption, metabolism, and antiplatelet effects of 300-, 600-, and 900-mg loading doses of clopidogrel. Results of the ISAR-CHOICE (Intracoronary Stenting and Antithrombic Regimen: Choose Between 3 High Oral Doses for Immediate Clopidogrel Effect) Trial. 2005;112:2946–2950. doi: 10.1161/CIRCULATIONAHA.105.559088. [DOI] [PubMed] [Google Scholar]
- 13.Brandt JT, Payne CD, Wiviott SD, et al. A comparison of prasugrel and clopidrogel loading dose on platelet function: Magnitude of platelet inhibition is related to active metabolite formation. Am Heart J. 2007;153:66–69. doi: 10.1016/j.ahj.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 14.Wiviott SD, Braunwald E, McCabe C, et al. Prasugrel versus clopidrogel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–2015. doi: 10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]
- 15.Montalescot G, Wiviott SD, Braunwald E, et al. Prasugrel compared with clopidrogel in patients undergoing percutaneous coronary intervention for ST-elevation myocardial infarction (TRITON-TIMI 38): double-blind, randomized controlled trial. Lancet. 2009;373:723–731. doi: 10.1016/S0140-6736(09)60441-4. [DOI] [PubMed] [Google Scholar]
- 16.A Comparison of Prasugrel and Clopidogrel in Acute Coronary Syndrome Subjects (TRILOGY ACS). This study is currently recruiting participants. ClinicalTrials.gov.
- 17.Kushner FG, Hand M, Smith SC, Jr, et al. Focused Updates: ACC/ AHA Guidelines for the Management of Patients With ST-Elevation Myocardial Infarction (Updating the 2004 Guideline and 2007 Focused Update) and ACC/AHA/SCAI Guidelines on Percutaneous Coronary Intervention (Updating the 2005 Guideline and 2007 Focused Update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2009;2009;120:2271–2306. doi: 10.1161/CIRCULATIONAHA.109.192663. [DOI] [PubMed] [Google Scholar]
- 18.Husted S, Emanuelsson H, Heptinstall S, et al. Parmacodynamics, pharmacokinetics, and safety of the oral reversible P2Y12 antagonoist AZD6140 with aspirin in patients with atherosclerosis: a double-blind comparison to clopidrogel with aspirin. Eur Heart J. 2006;27:1038–1047. doi: 10.1093/eurheartj/ehi754. [DOI] [PubMed] [Google Scholar]
- 19.Cannon CP, Hudsted S, Harrington A, et al. Safety, tolerability, and initial efficacy of AZD6140, the first reversible oral adenosine diphosphate receptor antagonist, compared with clopidrogel, in patients with non-ST segment elevation acute coronary syndrome: primary results of the DISPERSE-2 trial. J Am Coll Cardiol. 2007;50:1844–1851. doi: 10.1016/j.jacc.2007.07.053. [DOI] [PubMed] [Google Scholar]
- 20.Wallentin L, Becker RC, Budaj A, et al. for the PLATO Investigators. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl Med J. 2009;361:1045–1057. doi: 10.1056/NEJMoa0904327. [DOI] [PubMed] [Google Scholar]
- 21.Angiolillo DJ, Bhatt DL, Gurbel PA, et al. Advances in antiplatelet therapy: agents in clinical development. Am J Cardiol. 2009;103:40A–51A. doi: 10.1016/j.amjcard.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 22.Bhatt DL, Lincoff AM, Gibson CM, et al. for the CHAMPION PLATFORM Investigators. Intravenous platelet blockade with cangrelor during PCI. N Eng J Med . 2009;361:2330–2341. doi: 10.1056/NEJMoa0908629. [DOI] [PubMed] [Google Scholar]
- 23.Harrington RA, Stone GW, McNulty S, et al. Platelet inhibition with cangrelor in patients undergoing PCI. N Engl J Med. 2009;361:2318–2329. doi: 10.1056/NEJMoa0908628. [DOI] [PubMed] [Google Scholar]
- 24.Maintenance of Platelet Inhibition with Cangrelor (Bridge). This study is currently recruiting participants. ClinicalTrials.gov.
- 25.The TIMI IIIB Investigators. Effects of tissue plasminogen activator and a comparison of early invasive and conservative strategies in unstable angina and non-Q-wave myocardial infarction: results of the TIMI IIIB trial. Circulation. 1994;89:1545–1556. doi: 10.1161/01.cir.89.4.1545. [DOI] [PubMed] [Google Scholar]
- 26.Boden WE, O’Rourke RA, Crawford MH, et al. Outcomes in patients with acute non-Q-wave myocardial infarction randomly assigned to an invasive as compared with a conservative management strategy. N Engl J Med. 1998;338:1785–1792. doi: 10.1056/NEJM199806183382501. [DOI] [PubMed] [Google Scholar]
- 27.McCullough PA, O’Neil WW, Graham M, et al. A prospective randomized trial of triage angiography in acute coronary syndromes ineligible for thrombotic therapy: results of the Medicine versus Angiography in Thrombolytic Exclusion (MATE) trial. J Am Coll Cardiol. 1998;32:596–605. doi: 10.1016/s0735-1097(98)00284-8. [DOI] [PubMed] [Google Scholar]
- 28.Arora RR, Rai F, Rai F. Antiplatelet intervention in acute coronary syndrome. Am J Therapeutics. 2009;16:e29–e40. doi: 10.1097/MJT.0b013e31804c7238. [DOI] [PubMed] [Google Scholar]
- 29.Cannon CP, Weintraub WS, Demopoulos LA, et al. Comparison of early invasive and conservative strategies in patients with unstable coronary syndromes treated with the glycoprotein GIIb/IIIa inhibitor tirofiban. N Engl J Med. 2001;344:1879–1887. doi: 10.1056/NEJM200106213442501. [DOI] [PubMed] [Google Scholar]
- 30.Hoekstra JW, Roe MT, Peterson ED, et al. Early glycoprotein IIb/IIIa inhibitor use for non-ST-segment elevation acute coronary syndrome: patient selection and associated treatment patterns. Acad Emerg Med. 2005;12:431–437. doi: 10.1197/j.aem.2004.11.029. [DOI] [PubMed] [Google Scholar]
- 31.Topol GJ, Califf RM, Weissman HF, et al. Randomized trial of coronary intervention with antibody against platelet glycoprotein IIb/IIIa integrin for reduction of clinical restenosis: results at 6 months. The EPIC investigators. Lancet. 1994;343:1430–1435. doi: 10.1016/s0140-6736(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 32.Montalescot G, Antoniucci D, Kastrati A, et al. Abciximab in primary coronary stenting of ST-elevation myocardial infarction: a European meta-analysis on individual patients’ data with long-term follow up. European Heart J. 2007;28:443–449. doi: 10.1093/eurheartj/ehl472. [DOI] [PubMed] [Google Scholar]
- 33.The Impact-II investigators. Randomized, placebo-controlled trial of effect of eptifibatide on complications of percutaneous coronary intervention. Impact-II. Lancet. 1997;349:1422–1428. [PubMed] [Google Scholar]
- 34.O’Shea JC, Hafley GE, Greenberg S, et al. ESPRIT Investigators. Platelet GP IIb/IIIa integrin blockade with eptif ibatide in coronary stent intervention: the ESPRIT trial. JAMA. 2001;285:2468–2473. doi: 10.1001/jama.285.19.2468. [DOI] [PubMed] [Google Scholar]
- 35.Topol EJ, Moliterno DJ, Herrmann HC, et al. Comparison of two platelet glycoprotein IIb/IIIa inhibitors, tirofiban and abciximab, for the prevention of ischemic events with percutaneous coronary revascularization. N Engl Med J. 2001;344:1888–1894. doi: 10.1056/NEJM200106213442502. [DOI] [PubMed] [Google Scholar]
- 36.Van’t Hof AW, Ten Berg J, Heetermans T, et al. Prehospital intiation of tirofiban in patients with ST-elevation myocardial infarction undergoing primary angioplasty (On-TIME) 2: a multicentre, double-blind, randomized controlled trial. Lancet. 2008;16372:537–546. doi: 10.1016/S0140-6736(08)61235-0. [DOI] [PubMed] [Google Scholar]
- 37.Valgimigli M, Campo G, Arcozzi C, et al. Comparison of angioplasty with infusion of tirofiban or abciximab and with implantation of sirolimus-eluting or uncoated stents for acute myocardioal infarction. The MULTISTRATEGY randomized trial. JAMA. 2008 doi: 10.1001/ jama.299.15.joc80026. [DOI] [PubMed] [Google Scholar]
- 38.Labinaz M, Ho C, Banerjee S, et al. Meta-analysis of clinical efficacy and bleeding risk with intravenous glycoprotein IIb/IIIa antagonists for percutaneous coronary intervention. Can J Cardiol. 2007;23:963–970. doi: 10.1016/s0828-282x(07)70858-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.White HD, Kleiman NS, Mahaffey KW, et al. Efficacy and safety of enoxaparin compared with unfractionated heparin in high-risk patients with non-ST-segment elevation acute coronary syndrome undergoing percutaneous coronary intervention in the Superior Yield of the New Strategy of Enoxaparin, Revascularization and glycoprotein IIb/III ainhibitors (SYNERGY) trial. Am J Heart. 2006;152:1042–1050. doi: 10.1016/j.ahj.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 40.STEMI Treated With Primary Angioplasty and Intravenous Lovenox or Unfractionated Heparin (UFH) (ATOLL). This study is currently recruiting participants. ClinicalTrials.gov.
- 41.Mehta SR, Steg PG, Granger CB, et al. Randomized, blinded trial comparing fondaparinux with unfractionated heparin in patients undergoing contemporary percutaneous coronary intervention. Arixtra Study in Percutaneous Coronary Intervention: a randomized evaluation (ASPIRE) pilot trial. Circulation. 2005;111:1390–1397. doi: 10.1161/01.CIR.0000158485.70761.67. [DOI] [PubMed] [Google Scholar]
- 42.The Oasis-6 Trial. Effects of fondaparinux on mortality and reinfarction in patients with acute ST-segment elevation myocardial infarction. The OASIS-6 Randomized Trial. JAMA. 2006;295(13):1519–1530. doi: 10.1001/jama.295.13.joc60038. [DOI] [PubMed] [Google Scholar]
- 43.Mehta SR, Granger CB, Eikelboom JW, et al. Efficacy and safety of fondaparinux versus enoxaparin in patients with acute coronary syndromes undergoing percutaneous coronary intervention: results from the OASIS-5 trial. J Am Coll Cardiol. 2009;50:742–1751. doi: 10.1016/j.jacc.2007.07.042. [DOI] [PubMed] [Google Scholar]
- 44.Bittl JA, Chaitman BR, Feit F, et al. for the Bivalirubin Angioplasty Study Investigators. Bivalirubin versus heparin during coronary angioplasty for unstable for postinfarction angina: final report reanalysis of the Bivalirubin Angioplasty Study. Am J Heart. 2001;142:952–959. doi: 10.1067/mhj.2001.119374. [DOI] [PubMed] [Google Scholar]
- 45.Lincoff AM, Kleiman NS, Kottke-Marchant K, et al. Bivalirubin with planned or provisional abciximab versus low-dose heparin and abciximab during percutaneous coronary revascularization: results of the Comaprison of Abciximab Complications with Hirulog for Ischemic Events Trial (CACHET) Am J Heart. 2002;143:847–853. doi: 10.1067/mhj.2002.122173. [DOI] [PubMed] [Google Scholar]
- 46.Lincoff AM, Bittl JA, Kleiman NS, et al. The REPLACE-1 Trial: comparison of bivalirubin versus heparin during percutaneous coronary intervention (The Randomized Evaluation of Percutaneous Coronary Intervention Linking Angiomax to Reduced Clinical Events [REPLACE]-1 trial. Am J Cardiol. 2004;93:1092–1096. doi: 10.1016/j.amjcard.2004.01.033. [DOI] [PubMed] [Google Scholar]
- 47.White HD, Ohman EM, Lincoff AM, et al. Safety and efficacy of bivalirubin with and without glycoprotein IIb/IIIa inhibitors in patients with acute coronary syndromes undergoing percutaneous coronary intervention: 1-year results from the ACUITY (Acute Catheterization and Urgent Intervention Triage StartegY) trial. J Am Coll Cardiol. 2008;52:807–814. doi: 10.1016/j.jacc.2008.05.036. [DOI] [PubMed] [Google Scholar]
- 48.Lincoff AM, Bittl JA, Harrington RA, et al. Bivalirubin and provisional glycoprotein IIb/IIIa blockade compared with heparin and planned glycoprotein IIb/IIIa blockade during percutaneous coronary intervention: REPLACE-2 Randomized Trial. JAMA. 2003;289(7):853–863. doi: 10.1001/jama.289.7.853. [DOI] [PubMed] [Google Scholar]
- 49.Stone GW, Witzenbichler B, Guagliumi G, et al. Bivalirubin during primary PCI in acute myocardial infarction. N Engl Med J. 2008;358:2218–2230. doi: 10.1056/NEJMoa0708191. [DOI] [PubMed] [Google Scholar]
- 50.Angiolillo DJ, Guzman LA, Bass TA. Current antiplatelet therapies: benefits and limitations. Am Heart J. 2008;156:S3–S9. doi: 10.1016/j.ahj.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 51.Bassand JP. Bleeding and transfusion in acute coronary syndromes: a shift in the paradigm. Heart. 2008;94:661–666. doi: 10.1136/hrt.2007.125047. [DOI] [PubMed] [Google Scholar]
- 52.de Luca L, Casella G, Lettino M, et al. Clinical implications and management of bleeding events in patients with acute coronary syndromes. J Cardiovasc Med. 2009;10(9):677–686. doi: 10.2459/JCM.0b013e3283299808. [DOI] [PubMed] [Google Scholar]
- 53.Alexander KP, Chen AY, Roe MT, et al. CRUSADE investigators. Excess dosing of antiplatelet and antithrombin agents in the treatment of non- ST-segment elevation acute coronary syndromes. JAMA. 2005;294:3108–3116. doi: 10.1001/jama.294.24.3108. [DOI] [PubMed] [Google Scholar]