Abstract
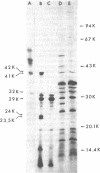
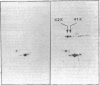
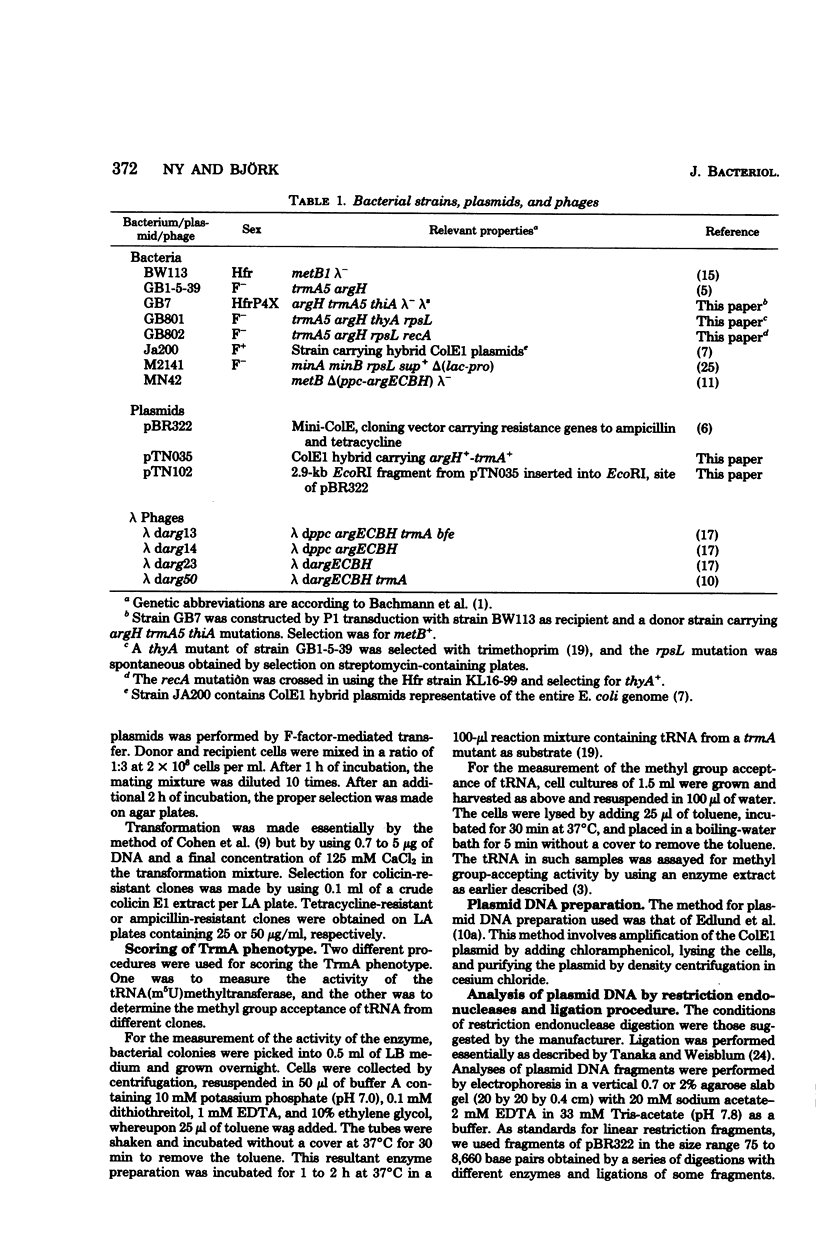
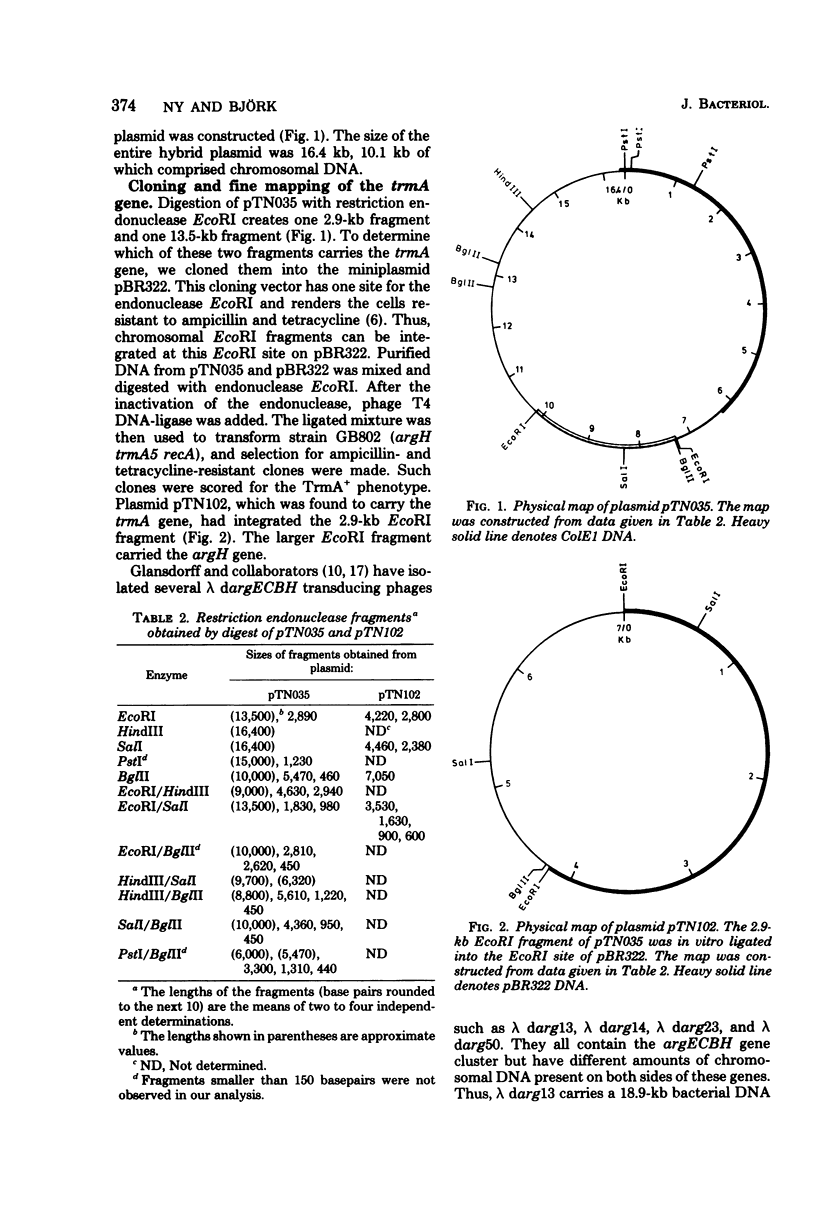
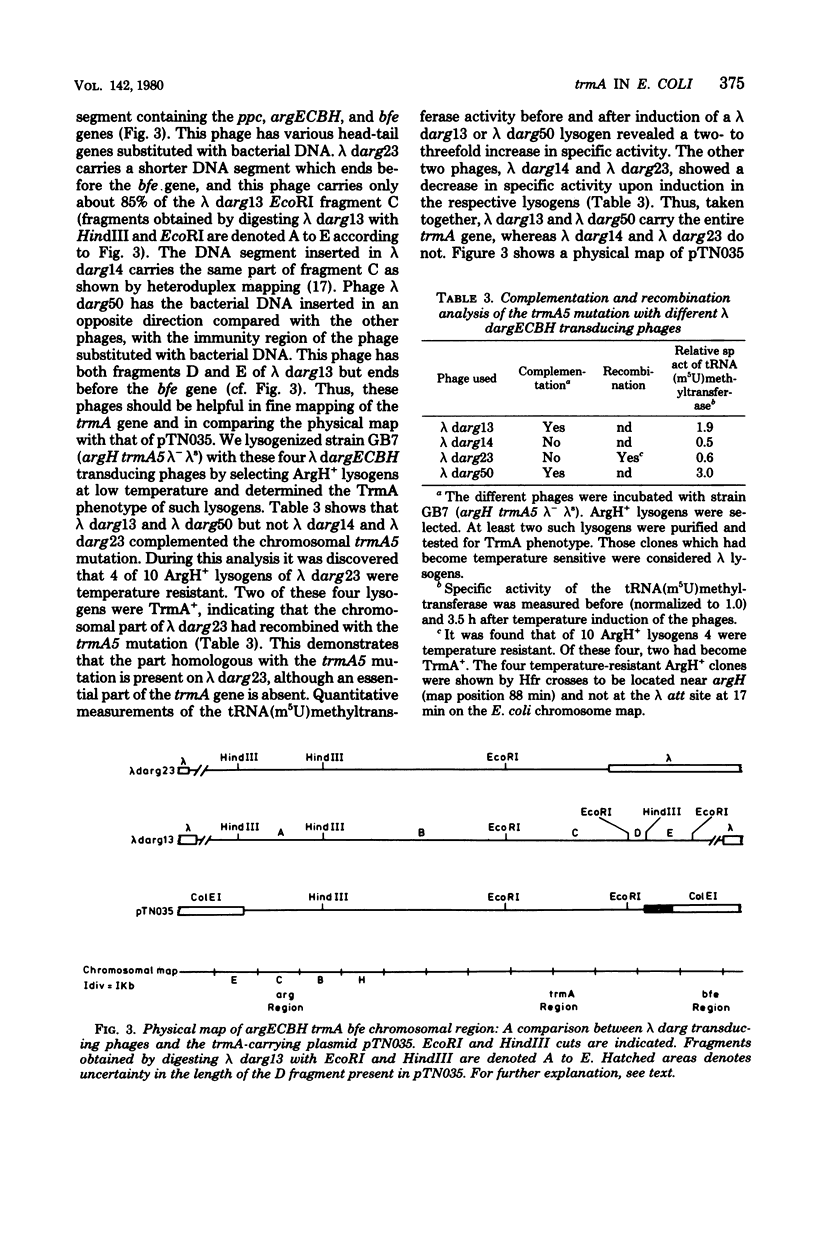
A hybrid plasmid from the Clarke and Carbon collection has been isolated. This plasmid carries the trmA gene of E. coli, which is necessary for the formation of 5-methyluridine (m5U,ribothymidine) present in all transfer ribonucleic acid (tRNA) chains of the organism so far sequenced. A restriction map of the argCBH-trmA regions is presented. By using cloning in vitro, the trmA gene was located on a 2.9-kilobase pair deoxyribonucleic acid (DNA) fragment. These results and comparison with lambda dargECBH transducing phages established the gene order: argECBH trmA bfe in the 88-min region of the E. coli chromosomal map. Plasmids carrying this 2.9-kilobase pair DNA fragment overproduce the enzyme tRNA(m5U)methyltransferase (EC 2.1.1.35) 20 to 40 times. When this 2.9-kilobase pair chromosomal DNA fragment was expressed in a minicell system, a polypeptide of a molecular weight of 42,000 was synthesized. This polypeptide was tentatively identified as the tRNA(m5U)methyltransferase. These results support the earlier suggestion that the trmA gene is the structural gene for the tRNA(m5U)methyltransferase.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERTANI G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J Bacteriol. 1951 Sep;62(3):293–300. doi: 10.1128/jb.62.3.293-300.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J., Low K. B., Taylor A. L. Recalibrated linkage map of Escherichia coli K-12. Bacteriol Rev. 1976 Mar;40(1):116–167. doi: 10.1128/br.40.1.116-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björk G. R., Isaksson L. A. Isolation of mutants of Escherichia coli lac king 5-methyluracil in transfer ribonucleic acid or 1-methylguanine in ribosomal RNA. J Mol Biol. 1970 Jul 14;51(1):83–100. doi: 10.1016/0022-2836(70)90272-x. [DOI] [PubMed] [Google Scholar]
- Björk G. R., Neidhardt F. C. Physiological and biochemical studies on the function of 5-methyluridine in the transfer ribonucleic acid of Escherichia coli. J Bacteriol. 1975 Oct;124(1):99–111. doi: 10.1128/jb.124.1.99-111.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björk G. R. Transductional mapping of gene trmA responsible for the production of 5-methyluridine in transfer ribonucleic acid of Escherichia coli. J Bacteriol. 1975 Oct;124(1):92–98. doi: 10.1128/jb.124.1.92-98.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Clarke L., Carbon J. A colony bank containing synthetic Col El hybrid plasmids representative of the entire E. coli genome. Cell. 1976 Sep;9(1):91–99. doi: 10.1016/0092-8674(76)90055-6. [DOI] [PubMed] [Google Scholar]
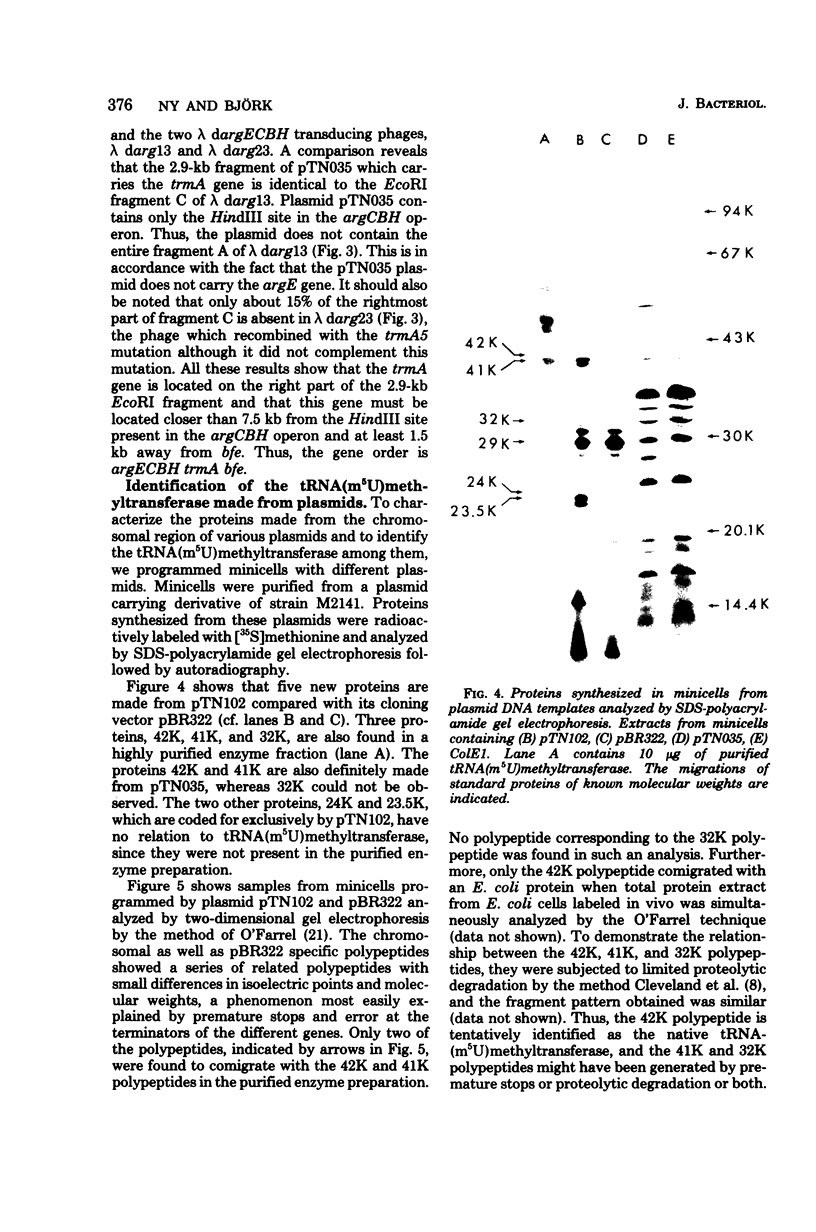
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabeel M., Charlier D., Glansdorff N. Studies on the bipolar argECBH operon of E. coli: characterization of restriction endonuclease fragments obtained from gammadargECBH transducing phages and a ColE1 argECBH plasmid. Mol Gen Genet. 1977 Mar 7;151(2):161–168. doi: 10.1007/BF00338690. [DOI] [PubMed] [Google Scholar]
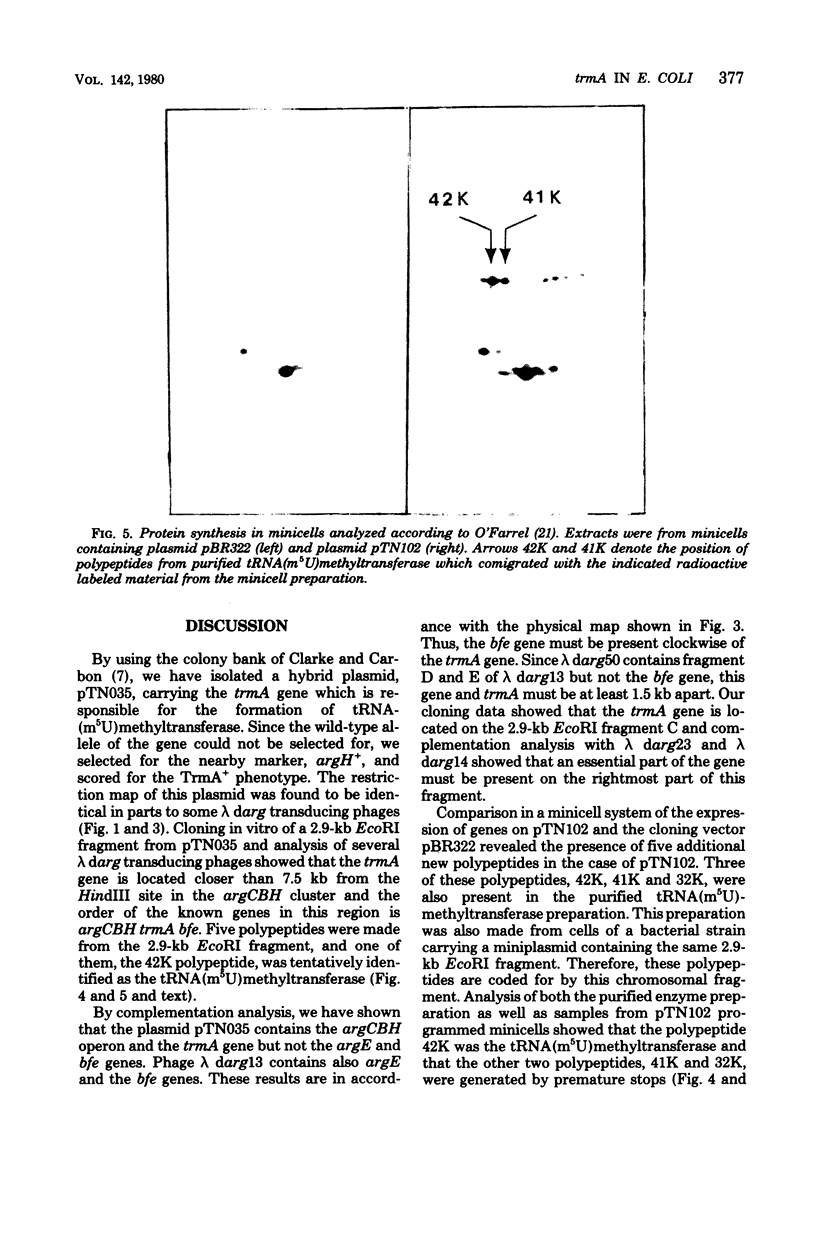
- Edlund T., Grundström T., Normark S. Isolation and characterization of DNA repetitions carrying the chromosomal beta-lactamase gene of Escherichia coli K-12. Mol Gen Genet. 1979 Jun 7;173(2):115–125. doi: 10.1007/BF00330301. [DOI] [PubMed] [Google Scholar]
- Elseviers D., Cunin R., Glansdorff N. Control regions within the argECBH gene cluster of Escherichia coli K12. Mol Gen Genet. 1972;117(4):349–366. doi: 10.1007/BF00333028. [DOI] [PubMed] [Google Scholar]
- Jorgensen R. A., Rothstein S. J., Reznikoff W. S. A restriction enzyme cleavage map of Tn5 and location of a region encoding neomycin resistance. Mol Gen Genet. 1979;177(1):65–72. doi: 10.1007/BF00267254. [DOI] [PubMed] [Google Scholar]
- Kennedy N., Beutin L., Achtman M., Skurray R., Rahmsdorf U., Herrlich P. Conjugation proteins encoded by the F sex factor. Nature. 1977 Dec 15;270(5638):580–585. doi: 10.1038/270580a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Low B. Rapid mapping of conditional and auxotrophic mutations in Escherichia coli K-12. J Bacteriol. 1973 Feb;113(2):798–812. doi: 10.1128/jb.113.2.798-812.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazaitis A. J., Palchaudhuri S., Glansdorff N., Maas W. K. Isolation and characterization of lambdadargECBH transducing phages and heteroduplex analysis of the argECBH cluster. Mol Gen Genet. 1976 Jan 16;143(2):185–196. doi: 10.1007/BF00266921. [DOI] [PubMed] [Google Scholar]
- Ny T., Björk G. R. Growth rate-dependent regulation of transfer ribonucleic acid (5-methyluridine) methyltransferase in Escherichia coli B/r. J Bacteriol. 1980 Jan;141(1):67–73. doi: 10.1128/jb.141.1.67-73.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ny T., Björk G. R. Stringent regulation of the synthesis of a transfer ribonucleic acid biosynthetic enzyme: transfer ribonucleic acid(m5U)methyltransferase from Escherichia coli. J Bacteriol. 1977 May;130(2):635–641. doi: 10.1128/jb.130.2.635-641.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe J. G. pBR322 restriction map derived from the DNA sequence: accurate DNA size markers up to 4361 nucleotide pairs long. Nucleic Acids Res. 1978 Aug;5(8):2721–2728. doi: 10.1093/nar/5.8.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Weisblum B. Construction of a colicin E1-R factor composite plasmid in vitro: means for amplification of deoxyribonucleic acid. J Bacteriol. 1975 Jan;121(1):354–362. doi: 10.1128/jb.121.1.354-362.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R., Achtman M. The control region of the F sex factor DNA transfer cistrons: restriction mapping and DNA cloning. Mol Gen Genet. 1978 Oct 24;165(3):295–304. doi: 10.1007/BF00332530. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]