Abstract
Wnt proteins are secreted post-translationally modified proteins that signal locally to regulate development and proliferation. The production of bioactive Wnts requires a number of dedicated factors in the secreting cell whose coordinated functions are not fully understood. A screen for small molecules identified inhibitors of vacuolar acidification as potent inhibitors of Wnt secretion. Inhibition of the V-ATPase or disruption of vacuolar pH gradients by diverse drugs potently inhibited Wnt/β-catenin signaling both in cultured human cells and in vivo, and impaired Wnt-regulated convergent extension movements in Xenopus embryos. WNT secretion requires its binding to the carrier protein wntless (WLS); we find that WLS is ER-resident in human cells and WNT3A binding to WLS requires PORCN-dependent lipid modification of WNT3A at serine 209. Inhibition of vacuolar acidification results in accumulation of the WNT3A–WLS complex both in cells and at the plasma membrane. Modeling predictions suggest that WLS has a lipid-binding β-barrel that is similar to the lipocalin-family fold. We propose that WLS binds Wnts in part through a lipid-binding domain, and that vacuolar acidification is required to release palmitoylated WNT3A from WLS in secretory vesicles, possibly to facilitate transfer of WNT3A to a soluble carrier protein.
Keywords: Wnt, Acidification, Palmitate, Secretion, WLS
Introduction
Wnt proteins (Wnts) are a conserved family of secreted, acylated and glycosylated protein hormones that act at short range to regulate diverse signaling pathways. Wnts are crucial in embryonic development, where they regulate cell proliferation, fate determination, morphology, polarity and motility. In adult life, Wnt signaling has a role in the homeostasis of rapidly proliferating tissues, including skin and gut. Wnt proteins initiate signaling through β-catenin stabilization, as well as through activation of Rho, Rap1, Ca2+ and multiple other pathways (for a review, see Chien et al., 2009; Tsai et al., 2007). Although activation by downstream mutations within Wnt/β-catenin signaling pathways occurs through multiple mechanisms in diverse human malignances (for a review, see Coombs et al., 2008), the continued secretion of various Wnts appears to be important even when the β-catenin pathway is activated (Caldwell et al., 2004; Suzuki et al., 2004; Wissmann et al., 2003). As Wnt secretion is important in both normal and cancer biology, a better understanding of the secretory process may provide insights and new tools for investigating the roles of Wnts and the diverse signaling pathways they regulate.
There are a number of distinct steps in Wnt production and secretion. Wnts are synthesized in the rough endoplasmic reticulum (ER) and post-translationally modified by several processes. Correct disulfide bond formation is guided by the ER-resident chaperone grp78 (Verras et al., 2008). The membrane-bound acyltransferase porcupine (PORCN) (Takada et al., 2006; Willert et al., 2003) is required to add both a palmitate and a palmitoleate to WNT3A; similar modification is thought to occur on most, but not all, Wnts. Secreted Wnts must also be glycosylated in the ER (Kurayoshi et al., 2007; Smolich et al., 1993) and the ER-resident protein post-GPI-attachment-to-proteins 1 (PGAP1) may regulate release of Wnts from the ER (Zoltewicz et al., 2009). Modified Wnts are escorted to the plasma membrane in complex with a specific integral membrane carrier protein, known as wntless (WLS) (Banziger et al., 2006; Bartscherer et al., 2006; Goodman et al., 2006; Hausmann et al., 2007). How WLS binds to Wnts is not understood. The interaction does not require glycosylation or palmitoylation of WNT3A at cysteine 77 (Cys77) (Komekado et al., 2007). To complete secretion, Wnt must somehow be released from WLS. WLS then recycles to the Golgi complex through endocytosis and recruitment of the retromer complex.
Wnts are released from cells in high molecular weight complexes that are likely to dictate their ability to travel locally. Wnts in cultured cell medium are associated with lipoprotein particles that – at least in Drosophila – have been suggested to be either lipophorins or lipocalins (for a review, see Bartscherer and Boutros, 2008; Neumann et al., 2009; Nusse et al., 2008; Panakova et al., 2005). These lipoprotein partners might be the primary vehicle that permits longer-range signaling through diffusion of soluble Wnt away from producing cells. Whether acylation is required for lipoprotein binding is not known, nor is it clear how Wnts are transferred from secreting cells to these soluble complexes. Both acylation and specific transport of WNT to the cell surface are clearly crucial to signaling, because mutation of acylation sites on Wnt, genetic ablation of WLS, or inhibition of PORCN impairs Wnt secretion and signaling (Chen et al., 2009; Fu et al., 2009; Galli et al., 2007; Kim et al., 2009; Komekado et al., 2007; Kurayoshi et al., 2007; Takada et al., 2006; Willert et al., 2003; Zhai et al., 2004).
To better understand how functional Wnt molecules are processed for secretion, we screened a marine natural-products library for small molecules that could block production of active Wnt proteins. The screen identified two closely related chondropsins as potent inhibitors of Wnt secretion. Chondropsins are known to inhibit the vacuolar ATPase (V-ATPase). We found that inhibition of vacuolar acidification by a variety of small molecules inhibited the ability of cells to secrete active WNT3A into culture medium. This inhibition has physiologic relevance, as we found that V-ATPase inhibitors affected both β-catenin-dependent and -independent Wnt signaling in vertebrate development. We investigated further why a low pH is required for the production of functional WNT3A. Specific palmitoylation of WNT3A at serine residue 209 (Ser209) was required for WLS binding. Acidification inhibitors did not disrupt glycosylation or lipidation of WNT, nor did they block binding of WNT3A to WLS; however, the release of WNT3A from WLS was blocked by acidification inhibitors. A WNT3A–WLS complex was found at the cell surface and accumulated in whole-cell lysates, unable to either dissociate or properly recycle. These findings support a model for Wnt secretion in which WLS is a lipid-binding protein and acidification of secretory vesicles is an essential event in the transfer of WNT3A from WLS to its downstream carrier proteins.
Results
New inhibitors of Wnt signaling
To better characterize specific steps in Wnt secretion, we developed a multi-step cell-based assay using human HEK293 cells containing a stably integrated luciferase reporter under the control of eight tandem repeats of the superTOPFlash promoter (STF cells) (McCulloch et al., 2009; Xu et al., 2004). The initial screen used STF cells that, in addition, stably overexpress murine WNT3A and, therefore, have high levels of active β-catenin readily assessible by firefly luciferase activity. Using these STF3A cells, we screened a 5632-fraction library of pre-fractionated marine organism extracts (supplementary material Table S1, Fig. S1). To identify inhibitors of Wnt secretion, fractions were chosen that inhibited autocrine signaling but did not inhibit signaling initiated by WNT3A-conditioned medium. The screen identified two potent adjacent fractions – C4 and C5 – that met these criteria (Fig. 1B). Fraction C5 had a half maximal inhibitory concentration (IC50) of 14 ng/ml for inhibition of Wnt signaling, suggesting low to sub-nanomolar efficacy. The active compounds within C4 and C5 were further fractionated and identified as chondropsin A and 73-deoxychondropsin A (supplementary material Fig. S2).
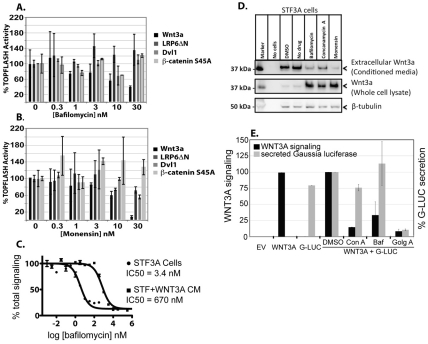
Fig. 1.
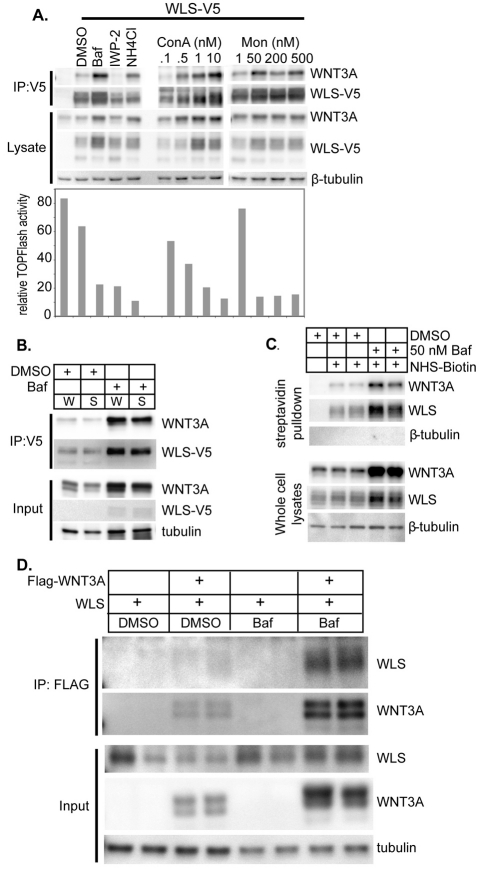
Inhibition of vesicular acidification reduces WNT3A secretion. (A,B) Diverse acidification inhibitors inhibit Wnt/β-catenin signaling upstream of the membrane. β-catenin-dependent luciferase expression was stimulated by transient expression of the indicated proteins in STF cells. Renilla luciferase plasmid was included as a transfection control. Twenty-four hours after transfection, cells were incubated with the indicated concentrations of (A) bafilomycin or (B) monensin for an additional 14 hours, and then firefly and Renilla luciferase activity, and lactate dehydrogenase activity were measured. Data are reported as maximal activation for each upstream activator in percent (mean ± s.d.). (C) Bafilomycin inhibits Wnt/β-catenin signaling downstream of WNT3A synthesis and upstream of the membrane. STF3A cells or STF cells in WNT3A-conditioned medium were exposed to bafilomycin (1 pM to 316 nM for STF3A, and 1 nM to 316 μM for STF in WNT3A-conditioned medium) overnight. Wnt/β-catenin signaling was assessed as above and normalized for clarity. Each bafilomycin concentration was assayed in triplicate. Data are representative of two independent experiments. (D) Acidification inhibitors decrease secreted and increase cell-associated WNT3A. STF3A cells were grown to 90% confluence and then incubated with bafilomycin (50 nM), concanamycin A (50 nM), monensin (5 μM) or vehicle in DMEM with 1% FBS for 16 hours. Lysates and conditioned medium were analyzed by SDS-PAGE and immunoblotting. Top panel: inhibitors of vacuolar acidification reduced secretion of WNT3A levels into the conditioned medium. Middle panel: vacuolar acidification inhibitors caused a concomitant increase in cell-associated WNT3A. Bottom panel: β-tubulin as a control for equal loading of gel lanes. Marker, molecular mass. (E) V-ATPase inhibitors are not general inhibitors of bulk secretion. STF cells were transfected with empty vector, or plasmids expressing WNT3A and/or Gaussia luciferase. After 24 hours, cells were treated with DMSO, 50 nM concanamycin A, 100 nM bafilomycin or the general secretion inhibitor golgicide A (Gu and Gruenberg, 2000) (25 μM) as indicated. After 12 hours of treatment, Gaussia luciferase activity was measured in 100 μl of conditioned medium. Cells were then lysed and firefly luciferase activity (WNT3A signaling reporter) was measured as above. Final values are reported as percent of control secretion and signaling.
Chondropsins regulate WNT3A signaling by inhibition of the V-ATPase
Chondropsins are recently characterized marine natural products with potent anti-tumor activity that inhibit V-ATPases (Bowman et al., 2003; Qi et al., 2007), which suggests that the effect of chondropsins on autocrine Wnt signaling also occurs through inhibition of V-ATPase. To further determine whether this is, in fact, owing to inhibition of the V-ATPase rather than an unrelated target, we tested a panel of chemically dissimilar V-ATPase inhibitors, including bafilomycin, concanamycin and lobatamide. All V-ATPase inhibitors tested blocked WNT3A autocrine signaling with IC50 values similar to their reported binding affinity (Ki) values towards V-ATPase (Bowman et al., 1988; Bowman et al., 2003; Muroi et al., 1993; Shen et al., 2002) (Table 1). Inhibition of Wnt signaling by bafilomycin has also been reported by others (Cruciat et al., 2010; George et al., 2007).
V-ATPases regulate pH in acidic subcellular compartments including the Golgi complex, endo- and exocytic vesicles, and lysosomes. To test whether vacuolar acidification itself is required in Wnt signaling, we tested additional compounds that disrupt vacuolar acidification by mechanisms other than V-ATPase inhibition (Table 1). The ionophore monensin blocked acidification by transporting monovalent cations across membranes, thus dissipating proton gradients. Monensin inhibited WNT3A signaling with an IC50 of 3 nM, similar to the potency of the V-ATPase inhibitors. Chloroquine, an antimalarial drug that is taken up by and prevents acidification of vesicles, also inhibited Wnt signaling, with an IC50 of 240 nM. Finally, autocrine Wnt signaling was disrupted efficiently by treatment of cells with NH4Cl (IC50=1.1 mM), which raises the pH of intracellular vesicles by overwhelming cellular buffering capacity. We conclude that autocrine Wnt signaling requires vacuolar acidification.
Diverse acidification inhibitors block WNT3A signaling upstream of the membrane receptors
Our screen identified compounds that blocked autocrine signaling but were much less potent at blocking signaling from WNT3A-conditioned medium, suggesting that V-ATPase functions in WNT3A secretion. Additionally, we repeated this screen using a terrestrial natural-products library and identified monensin-like ionophores, as well as concanamycins and bafilomycins (V-ATPase inhibitors), as potent inhibitors of autocrine WNT3A signaling. This differs from a recent report that showed that V-ATPases function as Wnt co-receptors with low-density lipoprotein receptor-related protein 6 (LRP6) at the membrane and are required during the endocytosis of LRP6 (Cruciat et al., 2010). To test whether inhibition of vacuolar acidification by agents with diverse mechanisms block WNT3A/β-catenin signaling primarily at the level of WNT3A secretion, we tested the effect of bafilomycin and monensin on signaling initiated by expression of various Wnt/β-catenin pathway activators, including WNT3A, LRP6ΔN, Dvl1 and stabilized β-catenin (S45A). Bafilomycin was used in these and subsequent assays in place of chondropsins because bafilomycin is commercially available, was identified in our second screen and has a similar mechanism of action. Bafilomycin and monensin inhibited signaling due to forced expression of Wnt ligand (Fig. 1A,B), but had significantly less of an effect on signaling stimulated by expression of downstream activators, including the membrane co-receptor LRP6. This confirms and extends a prior report (George et al., 2007) on the effects of bafilomycin on Wnt signaling and shows a distinct effect from the small interfering RNA (siRNA)-mediated knockdown of V-ATPase subunits recently reported (Cruciat et al., 2010). Thus, vacuolar acidification inhibitors with diverse modes of action all block production of active WNT3A.
In our initial screen, chondropsins did not inhibit β-catenin signaling stimulated by WNT3A-conditioned medium. We confirmed that bafilomycin acted upstream of WNT3A production in a similar manner, because it inhibited autocrine signaling in our test cell line ~200-fold more potently than it inhibited signaling from WNT3A-conditioned medium (Fig. 1C). Hence, bafilomycin may act at more than one site in the Wnt/β-catenin signaling pathway, with secretion being more sensitive than downstream events to inhibitors of vacuolar acidification.
We directly examined whether the secretion of WNT3A protein into the medium was dependent on vacuolar pH (Fig. 1D). Bafilomycin, concanamycin A and the ionophore monensin all effectively inhibited secretion of WNT3A into the conditioned medium. The inhibition of secretion was not due to decreased production or increased degradation, because there was a corresponding increase in the amount of WNT3A retained in the cells. The inhibition of Wnt secretion was specific; the V-ATPase inhibitors did not block the secretion of Gaussia luciferase (G-Luc), which contains a native secretion signal sequence and is not thought to require a carrier protein (Fig. 1E) (Tannous et al., 2005; Verhaegent and Christopoulos, 2002). These findings indicate that acidification of one or more compartments of the secretory pathway is specifically required for Wnt secretion.
Bafilomycin and concanamycin A antagonize Wnt/β-catenin signaling in chick neural tube explants
We next asked whether acidification inhibitors alter Wnt signaling in vivo. A number of canonically acting Wnt ligands are expressed in the vertebrate midbrain region, including WNT1, WNT3 and WNT3A (Hollyday et al., 1995). Fgf8 expression is a useful marker of Wnt/β-catenin pathway activity. Wnt1−/− mice display a loss of midbrain and anterior hindbrain by embryonic day 9.5 (E9.5) (Thomas and Capecchi, 1990). Although Fgf8 expression is initially induced in these Wnt1−/− mice, it is subsequently lost. Conditional inactivation of β-catenin in the mouse neural tube results in a similar phenotype. Wnt signaling from the chick midbrain regulates expression of Fgf8 in adjacent regions in a similar manner, and forced expression of SFRP2 – a secreted negative regulator of the Wnt pathway – results in loss of Fgf8 expression from the midbrain to rhombomere 2 in explanted tissue (Canning et al., 2007). In the presence of bafilomycin (Fig. 2B), expression of Fgf8 at the midhindbrain region was abolished compared with control explants (Fig. 2A). Similar results were observed for neural tissue cultured in the presence of concanamycin A (compare Fig. 2C and Fig. 2D). These explants were probed for expression of Fgf8 (red) and Wnt1 (blue) to assess the integrity of explants post culture. Consistent with an effect on WNT1 secretion, both bafilomycin and concanamycin A caused a reduction of Fgf8 expression without loss of Wnt1 transcripts.
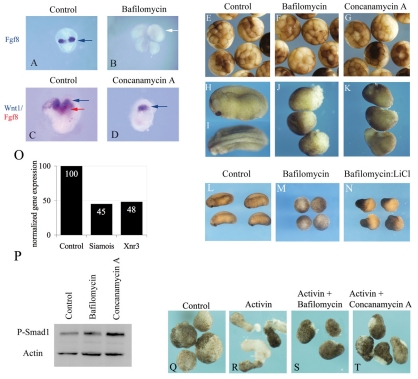
Fig. 2.
Inhibition of vesicular acidification mimics loss of function of canonical and non-canonical mediated Wnt effects in vivo. (A–D) Bafilomycin and concanamycin A treatment results in a loss of Fgf8 transcripts in chick mb–r2 explants. These explants were isolated from HH10 chick embryos, embedded in collagen and treated with drug for 24 hours. Explants were probed for the expression of Fgf8 mRNA (A and B, blue; C and D, red) and Wnt1 mRNA (C and D, blue). Explants A and B were processed simultaneously, as were explants C and D. The differences in explant size are consistent with drug-induced inhibition of Wnt secretion and Wnt-dependent loss of fibroblast growth factor (FGF), as these are well-established mitogens in neural development (Canning et al., 2007). (E–K) Bafilomycin and concanamycin lead to a loss of axial patterning in Xenopus laevis embryos. Two-cell embryos were cultured for 24 hours alone (E) or in 1 μM bafilomycin (F,J) or 2 μM concanamycin (G,K). Drug treatment had no effects on embryos assessed at the 32-cell stage (E–G), demonstrating they are healthy and viable during the early stages of development. Drug-induced Wnt-dependent defects are seen as the embryos undergo gastrulation, as seen at the 24-hour time point (H–K). Control embryos (H,I) develop normally, whereas those cultured in the presence of bafilomycin or concanamycin A have disrupted axial development. (L–N) Treatment of whole embryos with 20 mM LiCl rescues the effect of treatment (as above) with 1 μM bafilomycin. (O) The Wnt targets Siamois and Xnr3 are induced by Xenopus Wnt8 but are reduced to 45% and 48%, respectively, in the presence of 1 μM bafilomycin. Animal caps were injected with control (not shown) or Xenopus Wnt8. Gene expression is measured relative to that of ODC, an internal control. (P) Treatment of whole embryos with vesicular acidification inhibitors does not abrogate all signaling events in the early embryo, as assayed by phosphorylation of Smad-1, a read-out of activation of BMP signaling. Actin serves as a loading control. (Q–T) Bafilomycin and concanamycin A treatment inhibits non-canonical-mediated convergent extension movements in Xenopus embryos. (Q) Animal caps dissected from control embryos do not undergo convergent extension movements compared with those treated with activin alone (R). Bafilomycin (S) and concanamycin A (T) treatment inhibits activin-mediated convergence and extension.
Bafilomycin and concanamycin A antagonize Wnt/β-catenin-mediated events in early Xenopus embryos
Mesoderm induction is a major signaling event that occurs in the early Xenopus laevis embryo and serves to establish both dorso-ventral and anterior-posterior axes. Activation of Wnt/β-catenin signaling drives the formation of dorsal structures at the expense of ventral tissue and induces secondary axes (Sokol et al., 1991). By contrast, depletion of maternal β-catenin leads to embryos that lack visible axes or dorsal structures and are, therefore, referred to as being ventralized (Heasman et al., 1994). To address whether inhibition of vacuolar acidification mimicked maternal β-catenin depletion, two-cell embryos were cultured in the presence of bafilomycin and concanamycin A (Fig. 2E–K). Embryos cultured in bafilomycin or concanamycin resembled untreated siblings until stage 9 (just prior to gastrulation). However, in treated embryos, gastrulation was markedly delayed and, in some cases, the dorsal lip did not appear at all. When assessed 24 hours after fertilization, embryos displayed specific defects consistent with a delay or disruption in gastrulation events. Embryos treated with acidification inhibitors completely lacked dorsal structures (Fig. 2J,K). These defects are identical to the ventralizing defect observed in embryos formed after depletion of maternal β-catenin. Control embryos underwent considerable morphological movements, with establishment of three primary germ layers, formation of dorso-ventral and anterior-posterior axes, development of neural plates and completed neural tube closure (Fig. 2H,I). To test whether the bafilomycin-induced ventralized phenotype results from upstream inhibition of β-catenin signaling, bafilomycin-treated embryos were cultured in the presence of LiCl, an activator of Wnt/β-catenin signaling. LiCl was able to convert ventralized embryos to strongly dorsalized embryos, indicating that restoration of β-catenin signaling reverses the morphological phenotypes induced by acidification inhibitors [Fig. 2N, Dorso-Anterior Index score of 10 (Kao and Elinson, 1988)] (Klein and Melton, 1996; Slack et al., 1988).
To address whether acidification inhibitors block the expression of Wnt/β-catenin target genes, we assessed the effect of bafilomycin on the Wnt8-induced expression of Siamois and Xnr3 in the animal caps of Xenopus embryos. Bafilomycin treatment lead to a >50% reduction in the Wnt8-induced expression of both genes (Fig. 2O), indicating that these inhibitors effectively inhibit Wnt/β-catenin signaling. To assess non-specific effects of acidification inhibitors, we examined the effects of bafilomycin and concanamycin A on the activity of another pathway regulated by a secreted protein – bone morphogenetic protein (BMP) signaling – as assayed by the phosphorylation of SMAD1. Whereas acidification inhibitors decreased the expression of Xenopus Wnt8 target genes, BMP signaling was not inhibited relative to control embryos (Fig. 2P). Thus, bafilomycin and concanamycin are not acting as non-specific inhibitors of signaling pathways.
Inhibitors of V-ATPase block activin-mediated convergent extension movements in Xenopus embryos
Vacuolar acidification inhibitors should, by blocking Wnt secretion, inhibit both β-catenin dependent and independent pathways. To test this in an animal model, we examined β-catenin-independent morphogenetic movements. The mesoderm-inducing factor activin instructs presumptive ectodermal cells to undergo convergent extension movements and initiate the expression of mesoderm-specific markers such as brachyury (Symes and Smith, 1987). In Xenopus, a key target gene of brachyury, Wnt11, is required for activin-induced elongation of animal caps. Xenopus WNT11 activates non-canonical Wnt signaling to mediate convergent extension movements (Tada and Smith, 2000). As expected, animal caps dissected from X. laevis embryos and cultured in the presence of activin underwent characteristic convergent extension movements when compared with control animal caps, which did not undergo any elongation or constriction movements (Fig. 2R versus Fig. 2Q). In comparison to activin-treated animal caps, those cultured in bafilomycin plus activin, or concanamycin A plus activin did not display typical convergent extension movements (Fig. 2S,T). These results suggest that V-ATPase inhibitors also inhibit non-canonical Wnt-mediated events in vivo.
Acylation-dependent interaction of Wnts and WLS
We next investigated how acidification inhibitors prevented Wnt secretion. Many processes in the secretory pathway, including glycosylation, protein folding, pro-protein proteolysis and protein transport, require a specific acidic pH. Because nonglycosylated WNT3A has been reported to be retained in the ER (Komekado et al., 2007), we first tested whether bafilomycin alters WNT3A glycosylation. As predicted, both bafilomycin and tunicamycin (a glycosylation inhibitor) blocked WNT3A signaling, and a WNT3A glycosylation site mutant (Asn residue changed to Glu; WNT3A NQ) was unable to signal. Unlike tunicamycin, however, bafilomycin did not significantly change the glycosylation of WNT3A as assessed by protein mobility by SDS-PAGE (Fig. 3A).
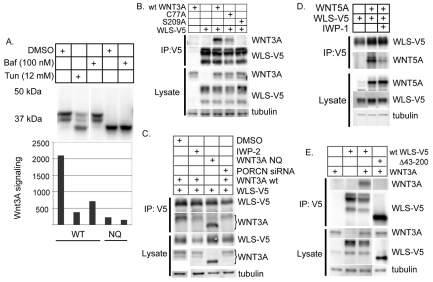
Fig. 3.
WLS-mediated export of WNT3A requires WNT3A palmitoylation. (A) Bafilomycin does not cause major changes in WNT3A glycosylation. STF cells were transfected with plasmids expressing wild type (WT; lanes 1–3 [lanes 1 to 5 from left to right]) or glycosylation site mutant N87/298Q (NQ; lanes 4–5) WNT3A. Cells were treated with DMSO, tunicamycin (Tun) or bafilomycin (Baf) for 14 hours as indicated, following which Wnt/β-catenin signaling and glycosylation were assessed by SDS-PAGE and immunoblotting (top), and luciferase assay (bottom). (B,C) WNT3A S209 is required for WNT3A–WLS interaction. Wild-type or acylation-site mutant WNT3A were co-expressed with WLS-V5-His6 in HEK293 cells as indicated. WNT3A–WLS interaction was assessed by immunoprecipitation with anti-V5 antibody followed by SDS-PAGE and immunoblotting. Mutation of the palmitoylated serine to alanine (Ser209Ala) in WNT3A, treating cells with the PORCN inhibitor IWP-2 or knockdown of PORCN by siRNA all abrogate binding of WNT3A to WLS. By contrast, mutation of the asparagine glycosylation sites N87 and N298 has no deleterious effect on the interaction between WNT3A and WLS. (D) WNT5A interaction with WLS is blocked by the PORCN inhibitor IWP-1. Wild-type WNT5A was co-expressed with WLS-V5-His as above. (E) The first intracellular domain of WLS is required for WNT3A binding. Wild-type or Δ43-200 WLS-V5-His6 were co-expressed with WNT3A as indicated, and their interaction was assessed by co-immunoprecipitation and immunoblotting as above.
WNT3A secretion requires both acylation and the Wnt transporter protein WLS. The pH of the ER – where Wnts are acylated by PORCN – is not regulated by V-ATPases and, not surprisingly, acidification inhibitors did not alter the partitioning of WNT3A into TX-114-soluble fractions, suggesting no major change in lipid modification (data not shown). We next tested whether acidification inhibition alters the interaction between WNT3A and WLS. WNT3A secretion requires acylation at Ser209 – but not at Cys77 – by the ER-resident acyltransferase PORCN (Komekado et al., 2007; Takada et al., 2006; Willert et al., 2003). We found that mutation of Ser209 but not Cys77 of WNT3A abolished its binding to WLS (Fig. 3B, compare lane 5 with lanes 3 and 4). This indicates that palmitoylation of WNT3A at Ser209 is absolutely required for binding to WLS. Notably, the glycosylation site mutant WNT3A NQ still bound to WLS, suggesting that Ser209 can be palmitoylated in the absence of WNT3A glycosylation.
The requirements for acylation-dependent WNT3A binding to WLS were further characterized. Consistent with the crucial role of acylation in the Wnt–WLS interaction, siRNA against the ER-resident O-acyl transferase PORCN (Fig. 3C, lane 4) and addition of the PORCN inhibitor IWP-2 (Fig. 3C, lane 2) also effectively inhibited binding of WNT3A to WLS. In a separate experiment, the related inhibitor IWP-1 blocked the binding of the non-canonical WNT5A to WLS (Fig. 3D). We conclude that PORCN-dependent acylation of Wnts at the highly conserved serine (Ser209 in WNT3A) is required for interaction with WLS.
The primary sequence of WLS was inspected for a potential Wnt binding domain. There is a predicted extended intralumenal domain between the first and second predicted transmembrane domains (supplementary material Table S1). Fu et al. have recently demonstrated that a glutathione S-transferase (GST) fusion protein containing this region was sufficient to bind to WNT3A in cell lysates (Fu et al., 2009). The fact that the WLS mutant that lacks amino acids 43–200 (WLS mutant D43–200) was unable to bind to WNT3A (Fig. 3E) further supports that this region is necessary for the interaction between WLS and WNT3A. This suggests that this region contains the palmitoleate-interacting domain.
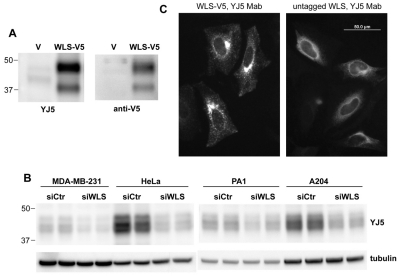
How Wnts are transferred after acylation by PORCN in the ER to WLS is not clear. The most parsimonious route is for WLS to bind Wnts directly upon their palmitoylation. However, in overexpression studies, WLS with C-terminal epitope tags has been visualized to be primarily localized at the plasma membrane, the Golgi complex and in cytoplasmic vesicles, rather than in the ER (Bartscherer et al., 2006; Belenkaya et al., 2008; Yang et al., 2008). We also found that His-tagged WLS-V5 (WLS-V5-His) and GFP-tagged WLS (WLS–GFP) localized predominantly to the Golgi complex (Fig. 4C, left panel and data not shown). However, most of the previously reported localization studies have used WLS expression constructs with various epitope tags at the C terminus of the protein, a region that may determine subcellular localization. To examine the localization of untagged WLS, we generated a monoclonal antibody (YJ5) that recognizes untagged WLS (Fig. 4A,B). Although the antibody did not clearly identify endogenous WLS in cultured cells, indirect immunofluorescence microscopy revealed that transiently expressed untagged WLS had a strong ER distribution (note the strong perinuclear staining), indicating it is in the same compartment in which Wnt is synthesized and palmitoylated (Fig. 4C). No obvious changes in localization of WLS were seen upon treatment with acidification inhibitors (data not shown).
Fig. 4.
Localization of WLS to the ER. (A) Western blot using monoclonal antibody YJ5 recognizes transiently expressed WLS. WLS-V5-His was expressed in HEK293 cells and lysates were probed with mAb YJ5 or anti-V5 as indicated. V, lysates from cells transfected with vector alone. (B) WLS was expressed in multiple cancer cell lines. Whole-cell lysates from indicated cell lines (25 μg) transfected with control (SiCtr) or specific siRNA directed against WLS were probed with YJ5 Mab. (C) WLS was present in the ER. HeLa cells that transiently expressed C-terminally V5-His tagged or untagged WLS were stained with YJ5. Strong perinuclear staining, indicative of ER localization, was seen when untagged WLS was expressed. YJ5 immunoreactivity was seen very weakly in non-transfected cells (data not shown).
Vacuolar acidification is required for WNT3A release
Having determined that WNT3A requires appropriate acylation to interact with WLS, and that WNT3A–WLS association can take place in the ER, we then tested the effect of acidification inhibitors on the interaction between WNT3A and WLS. It has previously been found that bafilomycin treatment increases the abundance of WLS, presumably owing to inhibition of lysosomal degradation (Yang et al., 2008). We found that WNT3A, WLS and the WNT3A–WLS complex all increased in abundance in response to several acidification inhibitors (Fig. 5A), but not to the control PORCN inhibitor IWP-2. The increase in co-immunoprecipitation was not simply due to sequestration into vesicles because the complexes remained soluble in the S100 fraction (Fig. 5B). Because PORCN function is required for binding of WNT3A and WNT5A to WLS, we also infer that acidification inhibitors do not impair PORCN activity. More importantly, acidification inhibitors increase the abundance of signaling-incompetent WNT3A in complex with WLS.
Fig. 5.
Acidification inhibitors increased the abundance of cellular WNT3A, WLS and WNT3A–WLS. (A) Acidification inhibitors increased the abundance of WNT3A–WLS complexes. WLS-V5 was expressed in STF3A cells that were then treated with the indicated compounds for 22 hours. The WNT3A–WLS interaction was assessed by co-immunoprecipitation (top) as before, and Wnt/β-catenin signaling (bottom) by luciferase assay. Concentrations were as follows or as indicated: bafilomycin (Baf), 50 nM; the porcupine inhibitor IWP-2, 2 μM; NH4Cl, 40 mM. ConA, concanamycin A; Mon, monensin. (B) Bafilomycin-enhanced WNT3A–WLS complexes remained in the S100 fraction. WLS-V5 and WNT3A were co-expressed in HEK293 cells. Cells were treated with or without 100 nM bafilomycin for 25 hours and then WLS complexes were isolated by immunoprecipitation by using anti-V5 antibody, either from whole-cell lysates (W) as before, or from supernatants (S) after centrifugation at 100,000 g for 60 minutes. (C) Bafilomycin increased WNT3A and WLS abundance in cells and at the cell surface. WNT3A and WLS abundance in cells and at the cell surface was analyzed using cell-surface biotinylation. STF3A cells were treated with DMSO (lanes 2–3) or 100 nM bafilomycin (lanes 4–5) overnight and then were surface-labeled with 1 mg/ml sulfo-NHS-SS-biotin (Pierce/Thermo) in PBS for 1 hour at 4°C. Cells in lane 1 were unlabeled. Upper panel: lysates were subjected to precipitation with streptavidin beads followed by analysis of the pellet by SDS-PAGE and immunoblotting. Intracellular tubulin was not labeled by the biotinylation reagent. Lower panel: whole-cell lysates were analyzed by SDS-PAGE and immunoblotting. (D) Bafilomycin increased WNT3A–WLS complex at the cell surface. WNT3A–WLS complexes at the cell surface were assessed with cell-surface immunoprecipitation. WLS with or without FLAG-WNT3A was expressed in HEK293 cells. Cells were treated with DMSO (lanes 1–4) or 20 nM bafilomycin (lanes 5–8) overnight and then were surface-labeled with 2 μg anti-FLAG M2 antibody (Sigma) in PBS for 1 hour at 4°C. Unbound antibody was removed by PBS washes, and then 500 μg of lysates were subjected to precipitation with protein A/G plus agarose followed by analysis of the pellet by SDS-PAGE and immunoblotting. Whole-cell lysates (25 μg) were analyzed by SDS-PAGE and immunoblotting.
Accumulation of WNT3A–WLS complexes could be because the complex fails to reach the cell surface or release WNT3A from the cell surface. In both the endocytic and exocytic pathways, acidification can promote the dissociation of soluble ligands from their membrane-bound receptors. We tested whether acidification inhibitors allow WNT3A–WLS complexes to reach the cell surface without the release of WNT3A. Proteins at the cell surface were biotinylated by brief incubation of control or bafilomycin-treated cells with a cell-impermeable N-hydroxysuccinimide-biotin reagent. Biotinylated proteins were isolated by streptavidin precipitation and analyzed by immunoblotting. Bafilomycin treatment led to an increase in WNT3A and endogenous WLS both in whole-cell lysates and, notably, at the cell surface (Fig. 5C). By performing cell-surface immunoprecipitation, we confirmed that the cell-surface WNT3A was in fact bound to WLS (Fig. 5D). Together with the finding that bafilomycin prevents WNT3A release into the medium, this demonstrates that acidification inhibitors do not prevent WNT3A from reaching the cell surface but they do prevent its release.
We considered two models for how pH might release WNT3A from WLS. In a simple model, low pH alone might lead to a conformational change in WLS and allow WNT3A to dissociate. Alternatively, low pH might facilitate transfer of palmitoylated WNT3A from membrane-bound WLS to a soluble carrier protein in a reaction that requires additional components. When testing the simple model, multiple attempts were made to dissociate WNT3A from WLS in immunoprecipitates, including incubation at low pH in the presence of 200 μM palmitate or palmitoleate, 10% serum and non-ionic detergents. We also tested a wide range of osmolarities from 9 to 150 mM NaCl at neutral and low pH values. However, 1% SDS was the only reliable way to dissociate the WNT3A–WLS complex in vitro. Thus, we postulate that, in the cell, low pH is necessary but not sufficient to release WNT3A from WLS.
Discussion
Here we report several findings regarding the pathway of WNT3A secretion. First, the results of multiple assays showed that vacuolar acidification inhibitors with diverse mechanisms of action act upstream of both WNT3A-conditioned medium and activated LRP6 in order to inhibit autocrine signaling. Investigating the mechanism, we found that early in WNT3A biosynthesis, PORCN-dependent modification of WNT3A at Ser209 is essential for its interaction with WLS. In the presence of acidification inhibitors, the WNT3A–WLS complex is able to reach the cell surface but the release of WNT3A from WLS is hindered. Hence, we speculate that, in the Wnt secretory pathway, acidification of a secretory vesicle is required for transfer of WNT3A from WLS to a soluble lipoprotein carrier.
These findings differ from recent reports that have shown that the V-ATPase is required downstream of WNT3A engagement of membrane receptors (Cruciat et al., 2010; George et al., 2007). Using a two-stage cell-based Wnt/β-catenin signaling assay that differentiated between the activity of autocrine WNT3A and WNT3A supplied from an exogenous source, we identified vacuolar acidification as a crucial step in the secretion of active Wnts. Multiple distinct acidification inhibitors with different mechanisms of action were all effective inhibitors of Wnt secretion. George and colleagues reported that bafilomycin blocked signaling from WNT3A-conditioned medium (George et al., 2007). More recently, Cruciat et al. identified a V-ATPase interacting protein (prorenin receptor [PRR]) in a screen aimed at finding modifiers of Wnt receptors on receiving cells (Cruciat et al., 2010). Depletion of PRR also blocked signaling from WNT3A-conditioned medium, an effect that appears to occur through failure to activate LRP6. Our study illustrates a distinct additional role for V-ATPase function, namely, facilitating the ability of cells to release active WNT3A. These results are not incompatible. V-ATPases have multiple roles within the cell by acidification of compartments and vesicles within the secretory pathway, clathrin- and caveolin-dependent endocytic pathways, and lysosomes. Our findings suggest that one function of vacuolar acidification is to facilitate release of WNT3A from the producing cells. The most plausible mechanism is by facilitating either the dissociation of WNT3A from WLS or the transfer of WNT3A from WLS to a soluble carrier protein. Cruciat et al. have identified a different function of the V-ATPase: to physically facilitate internalization and enzymatically facilitate activation of LRP6 (Cruciat et al., 2010). Although, in our study, acidification inhibitors blocked signaling due to WNT3A-conditioned medium, it took 100-fold more inhibitor than it took to block autocrine signaling. Therefore, we suspect that there is a physical or structural requirement for PRR and the associated V-ATPase in the formation of the LRP6-containing endocytic vesicles, whereas the enzymatic function of the V-ATPase may be more crucial in WNT3A secretion.
WNT3A binding to WLS requires its lipid modification because it is inhibited by the WNT3A mutant S209A, siRNA knockdown of the acyltransferase PORCN, and the small molecule PORCN inhibitors IWP-1 and IWP-2 (Chen et al., 2009). The need for Wnt palmitoylation to bind to WLS may be a general requirement of Wnts, because PORCN inhibitors also blocked the interaction between WNT5A and WLS. Because we demonstrated that WLS is prominently localized to the ER, where PORCN resides, the WNT3A–WLS interaction may happen as early in Wnt biosynthesis as WNT3A palmitoylation. WNT3A (S209A), which cannot interact with WLS, has been found to accumulate in the ER (Takada et al., 2006). The inability of non-palmitoylated WNT3A to bind to WLS probably explains why inhibition of PORCN function, either genetically or chemically, prevents Wnt secretion. It is also consistent with a recent report that a lipid-independent Wnt in Drosophila is also wntless-independent (Ching et al., 2008).
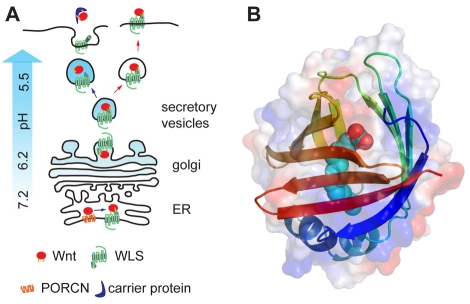
Although addition of saturated fatty acids, such as the palmitate on WNT3A Cys77, can direct proteins to lipid rafts, proteins with unsaturated fatty acids such as palmitoleate (the modification of WNT3A S209) do not enter rafts well (cf. Moffett et al., 2000). Hence, as first suggested by Takada et al., the monounsaturated palmitoleate attached to Ser209 may instead facilitate the formation of lipid–protein complexes (Takada et al., 2006). How the palmitoleate moiety on Wnts physically interacts with WLS is not yet known. We speculate that WLS contains a lipid-binding fold, probably in the first intralumenal loop, because this domain is both necessary and sufficient to interact with WNT3A (Fu et al., 2009) (Fig. 3E). Various algorithms for secondary-structure prediction agree that this domain of WLS consists predominantly of β-strand-forming stretches of sequence (Fig. 6 and supplementary material Table S1). Intriguingly, most of the proteins known to bind palmitate and that contain the comparable β-strand content belong to the lipocalin-fold family (supplementary material Tables S2 and S3). Solved lipocalin structures show domains of lengths very close to that of the region delineated by the experiments of Fu et al. (Fu et al., 2009). Furthermore, the β-barrel of a lipocalin fold is an attractive model for the palmitoleate-interaction domain of WLS, because lipocalins have also been shown to release bound lipids through a complex pH-dependent mechanism that also requires additional protein–protein interactions – in the form of dimerization – to drive the ligand-releasing conformational change (Arnoux et al., 2009). Calculated electrostatic surface potential of the first intralumenal domain sequence of WLS modeled onto the structure of the fatty-acid-binding protein of rat intestine suggests a pH-dependent polarity in the electrostatic potential between the two surfaces of the WLS intralumenal domain (supplementary material Fig. S3), consistent with a peripheral-membrane-associated protein. This is also consistent with a report from the Nusse lab that both Wg and WNT3A solubility in medium is facilitated by binding to a different lipocalin-fold-containing molecule that they have named soluble Wnt interacting molecule (SWIM) (Nusse et al., 2008).
Fig. 6.
Model: the role of Wnt palmitoylation, WLS and vesicular acidification in Wnt secretion. (A) During Wnt biogenesis, ER-localized PORCN (orange) adds a palmitoleic acid to ser209 in WNT3A. Palmitoylation is required for subsequent binding to WLS (green) in the ER. A pH gradient (white to cyan) exists in the secretory apparatus of the cell, ranging from pH 7.2 in the ER to 5.5 in the secretory vesicles. The low pH in secretory vesicles appears to be required for dissociation of WNT3A from WLS, allowing WNT3A bound to a lipoprotein carrier to be released into the medium. Cells treated with acidification inhibitors do not form this pH gradient (red arrows, right); therefore, WNT3A remains associated with WLS as it reaches the cell surface. (B) Model of the first intralumenal loop of WLS based on the solved structure of a palmitate-binding lipocalin. Amino acids 46–213 of human WLS are modeled onto the 2ifb structure (Sacchettini et al., 1989).
If the role of WLS is to carry palmitoylated Wnts from the ER to sorting vesicles or the plasma membrane, then there must be a mechanism to release Wnts and transfer them to soluble lipoprotein carriers. Our data suggest that release of WNT3A from WLS requires an acidic pH, presumably in the secretory vesicles. However, washes of immunoprecipitates with low pH buffers under various conditions, including incubation with fatty acids and low osmolarity, were not sufficient to release WNT3A from WLS. We considered the possibility that low pH led to trapping of the complex in a high molecular weight form, such as an aggregate or detergent-resistant vesicle, but found that the complexes remained soluble after centrifugation at 100,000 g. Because low pH alone is not sufficient to dissociate the complex, this raises the possibility that additional co-factors are needed. The model described in Fig. 6 suggests a potential mechanism. Bänziger et al. have proposed that WLS is required for loading Wnts onto lipoprotein particles during secretion (Banziger et al., 2006). If the release of WNT3A, or the transfer of WNT3A from WLS to SWIM or an alternative carrier protein, is blocked by acidification inhibitors, the Wnt–WLS complex may ultimately accumulate in lysosomes, where – in the absence of low pH (again owing to the acidification inhibitors) – they are not degraded. Consistent with this, we saw an increased concentration of WNT3A–WLS complexes both in cell lysates and at the cell membrane. The increased concentration of complexes at the cell membranes could simply reflect an increase in total cellular WNT3A–WLS complexes but, at a minimum, shows that acidification inhibitors do not block WNT3A–WLS complexes from reaching the cell surface. An alternative model is that WLS has an enzymatic function to release WNT3A from the ER. On the basis of sequence considerations, WLS is unlikely to be the desaturase that converts palmitate to palmitoleate. Zoltewicz et al. proposed that Wnts can be retained in the ER by a glycosylphosphatidylinositol (GPI) anchor; a potential enzymatic function of ER-localized WLS could be to cleave this GPI anchor (Zoltewicz et al., 2009). However, this would not explain why WLS cycles to the membrane and acidification inhibitors increase the abundance of WLS.
Understanding the localization of WLS is important to understanding its function. A number of studies have used C-terminal epitope-tagged WLS and found Golgi, vesicular and membrane localization. Although we confirmed these results with tagged WLS, we found that untagged WLS occurred predominantly in the ER. This positions WLS appropriately to interact with Wnt as it is palmitoylated. It also suggests that the localization of WLS is regulated in part by its C-terminus, a finding that has implications for how WLS localization and recycling is controlled. The simplest explanation is that modification of the C-terminus by extra sequence alters the kinetics of WLS recycling, allowing it to accumulate in trans Golgi networks and Rab5-containing vesicles. Wnts are known to be differentially released from different surfaces of polarized cells. Specific modifications of its C-terminus may direct WLS and its cargo Wnt to traffic to alternative destinations in the cell, perhaps, for example, allowing differentiation between apical and basolateral Wnt release.
Materials and Methods
Cells, plasmids and reagents
The STF reporter cell line (HEK 293 cells with the firefly luciferase gene under the control of eight tandem repeats of the TOPFlash TCF/Lef responsive promoter element) was a gift from Kang Zhang (University of California San Diego, La Jolla, CA) (Xu et al., 2004). The STF3A cell line contains, in addition, stably integrated pPGK plus mWNT3A (a gift from Karl Willert, University of California San Diego, La Jolla, CA) and a co-transfected blasticidin resistance marker. STF LRP6ΔN cells had been modified in a similar manner, stably integrating pCS2+ plus VSVG-tagged LRP6ΔN linearized at NotI (Swiatek et al., 2006) into STF cells. STF β-catenin S45A cells were made using the vector pCS2MT plus β-catenin S45A linearized at SalI. WNT3A-NQ was a gift from Akira Kikuchi (Hiroshima University, Hiroshima, Japan) (Kurayoshi et al., 2007). pcDNA3 WLS-V5/His, encoding WLS with a C-terminal V5 epitope and a hexahistidine tag, was a gift from Xinhua Lin (Cincinatti Children's Hospital Medical Center, Cincinatti, OH) (Belenkaya et al., 2008) and was converted to express untagged WLS by reintroduction of the native stop codon by site-directed mutagenesis. IWP-1 and IWP-2 were gifts from Lawrence Lum (University of Texas Southwestern, Dallas, TX) (Chen et al., 2009). Purified 73 deoxy-chondropsin A and lobatamides B, E and F were obtained from the National Cancer Institute (Bethesda, MD). Concanamycin A, monensin, chloroquine and NH4Cl were from Sigma. Bafilomycin was from AG Scientific (San Diego, CA). Anti-V5 antibody was from Invitrogen, anti-MYC (clone 9E10) was from Santa Cruz Biotechnologies and anti-WNT3A was a generous gift from Shinji Takada (Okazaki Institute for Integrative Bioscience and National Institute for Basic Biology, Okazaki, Japan). Knockdown of PORCN was accomplished using the siRNA sequence 5′-CAGUGGAGUUCAUGGGCUA-3′. Mouse monoclonal antibody YJ5 was generated using recombinant WLS amino acids 69–202 expressed as a fusion with His-HBcAg with the assistance of Stefan Schuechner and Egon Ogris (Medical University of Vienna, Vienna, Austria).
Wnt inhibitor screen
A total of 2′104 STF3A cells per well were plated in white-walled, clear bottom 96-well tissue culture plates pretreated with poly-L-lysine. Plates were incubated overnight at 37°C in 5% CO2. Just prior to adding compounds, medium was replaced with 200 μl per well of fresh medium to remove existing Wnt ligands. One microliter of marine extract was added to each well and allowed to incubate overnight. Cells were washed once in 200 μl per well cold PBS and then lysed in 20 μl per well PBS containing 0.6% Igepal CA-630 and complete protease inhibitor cocktail without EDTA (Roche). Lysates were mixed with 60 μl 0.15 mg/ml D-luciferin in 25 mM gly-gly pH 7.8, 15 mM MgSO4 and 4 mM EGTA. Luminescence data were obtained using a Veritas microplate luminometer (Promega, Sunnyvale, CA). Confirmatory and secondary screens were performed by the same methods.
Measurement of endogenous lactate dehydrogenase activity
To control for cell number and detect cell toxicity, we measured endogenous enzymatic activity of lactate dehydrogenase in cell lysates. Lysates were mixed with 60 μl of substrate and light absorbance at 490 nm was read every 10 seconds for 3 minutes. Vmax values were used to normalize luminescence data. Substrate was stored as three components at −20°C. Component 1: 316 mM sodium L-lactate, 10 mM Tris pH 8.5; component 2: 40 mM iodonitrotetrazolium chloride in dimethyl sulfoxide (DMSO); component 3: 10 units/ml diaphorase, 325 μg/ml BSA, 12 mg/ml sucrose, 3 mg/ml NAD+ in PBS. Component 2 was diluted 1:10 in PBS and components were then mixed 1:1:1 prior to use.
Identification of chondropsin A and 73-deoxychondropsin A
A scaled-up extraction of an Ircinia sp. specimen (sample PSO04-1-010, collected from Sorgoson, Philippines) was conducted. The MeOH extract (1.4 g) of the sponge was chromatographed on HP20SS resin using a gradient of 100% H2O to 100% isopropanol in 25% steps. The second fraction (25% isopropanol/75% H2O) was determined to contain chondropsin A and 73-deoxychondropsin A by high-resolution mass spectrometry (LTQ-FT, Thermo Scientific) and chromatographed further on a Phenomenex Onyx Monolithic C18 (100′3.0 mm) column using a gradient of 10% acetonitrile/0.1% formic acid to 100% acetonitrile at 1 ml/minute to yield 20 fractions. Fractions containing the chondropsin mass cluster were combined to yield less than 1 mg of chondropsin A and 73-deoxychondropsin A.
Generation of WNT3A and parental media
Mouse L and L3a cells (American Type Culture Collection) were maintained in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS), 50 units/ml penicillin and 50 μg/ml streptomycin at 37°C, 5% CO2 and 100% relative humidity. WNT3A-conditioned and parental media were prepared according to ATCC instructions. For collection of WNT3A for immunoblot assays, cells were incubated in 1% FBS.
Chick neural tube explants
Fertilized white leghorn eggs were incubated at 38°C in a humid atmosphere until embryos reached Hamburger and Hamilton stage 10 (HH10, 2 days). The neural tube was dissected and treated with Dispase I (Boehringer Mannheim, 1 mg/ml) in L-15 medium (Life Technologies) containing 5 mg/ml DNase I (Boehringer Mannheim) for 5 minutes to separate the neural tube from surrounding mesenchymal cells. Explant tissues comprising midbrain to rhombomere 2 (mb–r2) were isolated using sharp tungsten needles. Collagen solution was made by adding 100 μl of 10′ MEM (Sigma) and 100 μl bicarbonate buffer (0.1 M NaOH, 240 mM NaHCO3) to 0.8 ml collagen (PurCol, Nutacon, The Netherlands). Explants were cultured alone in 75% (v/v) Optimem:25% (v/v) Leibovitz medium 15 (Invitrogen), or supplemented with bafilomycin (100 nM, AG Scientific) or concanamycin A (2 μM, Sigma). Double in situ hybridization using digoxygenin and fluorescein-labeled probes was carried out as previously described (Canning et al., 2007). In each experiment, all explants were cultured and processed simultaneously such that experimental conditions were comparable.
Xenopus laevis embryo culture and micromanipulation
Xenopus laevis oocytes were fertilized in vitro, chemically dejellied with 2.2% L-cysteine (pH 7.8–8.1) and maintained in 1/10 Marc's modified Ringer (MMR) solution as previously described (Niederlander et al., 2001). Whole embryos were cultured alone or in bafilomycin (1 μM), concanamycin (2 μM) or bafilomycin (1 μM) plus 0.3 M lithium chloride (LiCl). LiCl was added to whole embryos at the 32-cell stage for 6 minutes. Embryos were injected with XWnt8 RNA (10–20 pg) into both cells at the two-cell stage and cultured until they reached stage 8. Animal caps were manually dissected from midblastula-stage embryos and cultured alone in 1′ MMR or in the presence of activin (12 U/ml) plus 0.1% BSA or supplemented with bafilomycin (1 μM) or concanamycin (2 μM) until sibling embryos reached stage 10. Embryonic staging was according to Nieuwkoop and Faber (Nieuwkoop and Faber, 1994). Animal caps were snap-frozen on dry ice and RNA was extracted using Trizol as per the manufacturer's guidelines (Invitrogen). Primers used for RT-PCR were as follows: ornithine decarboxylase (ODC) forward 5′-CAGCTAGCTGTGGTGTGG-3′, ODC reverse 5′-CAACATGGAAACTCACAC-3′, Xnr3 forward 5′-CGAGTGCAAGAAGGTGGACA-3′, Xnr3 reverse 5′-ATCTTCATGGGGACACAGGA-3′, Siamois forward 5′-AAGATAACTGGCATTCCTGAGC-3′ and Siamois reverse 5′-GGTAGGGCTGTGTATTTGAAGG-3′.
Immunoprecipitation
Cells were lysed with 65 mM Tris HCl, 154 mM NaCl, 1% NP-40, 0.25% deoxycholate, 1 mM EDTA and protease inhibitor tablet (Complete, Roche). A total of 500 μg lysate was incubated with the indicated antibodies followed by addition of 20 μl of 50% slurry of protein A/G plus agarose. Pellets were washed four times with lysis buffer.
Western blot analysis
Xenopus laevis whole embryos cultured in the presence of V-ATPase inhibitors (concentration as described above) were used for protein extraction. Proteins were extracted with 20 mM Tris pH 8.0, 1 mM EDTA, 0.1% NP-40, 10% glycerol, 1 mM Na3VO4, 1 mM PMSF and 1′ Proteinase inhibitor cocktail (Roche). Samples were separated by SDS-PAGE on an 8% bis-acrylamide gel and transferred to PVDF membrane (Hybond, GE Healthcare). The membrane was blocked in 5% non-fat dry milk and incubated overnight in 1′ TBS/0.1% Tween 20/5% non-fat dry milk containing anti-phosphorylated Smad1 (Ser463/465; Cell Signaling) and anti-actin (1:2000; Chemicon), which served as a loading control. Peroxidase conjugated secondary antibodies (1:1000) were detected using chemiluminescence (ECL+, Amersham).
Supplementary Material
Acknowledgments
We thank Shinji Takada for providing the WNT3A mAb used in these studies. Simran Kaur and Ha Yin Lee provided excellent technical assistance. The Ircinia sp. sponge was collected as part of a National Cooperative Drug Discovery Groups program under the terms of a Memorandum of Understanding between the University of Utah and the University of the Philippines. Samples of 73-deoxychondropsin A and lobatamides B, E and F used for confirmation studies were a generous gift from Tawnya C. McKee, National Cancer Institute, Frederick, MD. Electrostatic calculations were performed by Kavitha Bharatham. This work was supported by a Singapore Translational Research Award (to D.M.V.) and by the National Institutes of Health (R01 CA 36622 to C.M.I.). Deposited in PMC for release after 12 months.
Footnotes
Supplementary material available online at http://jcs.biologists.org/cgi/content/full/123/19/3357/DC1
References
- Arnoux P., Morosinotto T., Saga G., Bassi R., Pignol D. (2009). A structural basis for the pH-dependent xanthophyll cycle in Arabidopsis thaliana. Plant Cell 21, 2036-2044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banziger C., Soldini D., Schutt C., Zipperlen P., Hausmann G., Basler K. (2006). Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell 125, 509-522 [DOI] [PubMed] [Google Scholar]
- Bartscherer K., Boutros M. (2008). Regulation of Wnt protein secretion and its role in gradient formation. EMBO Rep. 9, 977-982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartscherer K., Pelte N., Ingelfinger D., Boutros M. (2006). Secretion of Wnt ligands requires Evi, a conserved transmembrane protein. Cell 125, 523-533 [DOI] [PubMed] [Google Scholar]
- Belenkaya T. Y., Wu Y., Tang X., Zhou B., Cheng L., Sharma Y. V., Yan D., Selva E. M., Lin X. (2008). The retromer complex influences Wnt secretion by recycling wntless from endosomes to the trans-Golgi network. Dev. Cell 14, 120-131 [DOI] [PubMed] [Google Scholar]
- Bowman E. J., Siebers A., Altendorf K. (1988). Bafilomycins: a class of inhibitors of membrane ATPases from microorganisms, animal cells, and plant cells. Proc. Natl. Acad. Sci. USA 85, 7972-7976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman E. J., Gustafson K. R., Bowman B. J., Boyd M. R. (2003). Identification of a new chondropsin class of antitumor compound that selectively inhibits V-ATPases. J. Biol. Chem. 278, 44147-44152 [DOI] [PubMed] [Google Scholar]
- Caldwell G. M., Jones C., Gensberg K., Jan S., Hardy R. G., Byrd P., Chughtai S., Wallis Y., Matthews G. M., Morton D. G. (2004). The Wnt antagonist sFRP1 in colorectal tumorigenesis. Cancer Res. 64, 883-888 [DOI] [PubMed] [Google Scholar]
- Canning C. A., Lee L., Irving C., Mason I., Jones C. M. (2007). Sustained interactive Wnt and FGF signaling is required to maintain isthmic identity. Dev. Biol. 305, 276-286 [DOI] [PubMed] [Google Scholar]
- Chen B., Dodge M. E., Tang W., Lu J., Ma Z., Fan C. W., Wei S., Hao W., Kilgore J., Williams N. S., et al. (2009). Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat. Chem. Biol. 5, 100-107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien A. J., Conrad W. H., Moon R. T. (2009). A Wnt survival guide: from flies to human disease. J. Invest. Dermatol. 129, 1614-1627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching W., Hang H. C., Nusse R. (2008). Lipid-independent secretion of a Drosophila Wnt protein. J. Biol. Chem. 283, 17092-17098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs G. S., Covey T. M., Virshup D. M. (2008). Wnt signaling in development, disease and translational medicine. Curr. Drug Targets 9, 513-531 [DOI] [PubMed] [Google Scholar]
- Cruciat C.-M., Ohkawara B., Acebron S. P., Karaulanov E., Reinhard C., Ingelfinger D., Boutros M., Niehrs C. (2010). Requirement of prorenin receptor and vacuolar H+-ATPase-mediated acidification for Wnt signaling. Science 327, 459-463 [DOI] [PubMed] [Google Scholar]
- Fu J., Jiang M., Mirando A. J., Yu H. M., Hsu W. (2009). Reciprocal regulation of Wnt and Gpr177/mouse Wntless is required for embryonic axis formation. Proc. Natl. Acad. Sci. USA 106, 18598-18603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli L. M., Barnes T. L., Secrest S. S., Kadowaki T., Burrus L. W. (2007). Porcupine-mediated lipid-modification regulates the activity and distribution of Wnt proteins in the chick neural tube. Development 134, 3339-3348 [DOI] [PubMed] [Google Scholar]
- George A., Leahy H., Zhou J., Morin P. J. (2007). The vacuolar-ATPase inhibitor bafilomycin and mutant VPS35 inhibit canonical Wnt signaling. Neurobiol. Dis. 26, 125-133 [DOI] [PubMed] [Google Scholar]
- Goodman R. M., Thombre S., Firtina Z., Gray D., Betts D., Roebuck J., Spana E. P., Selva E. M. (2006). Sprinter: a novel transmembrane protein required for Wg secretion and signaling. Development 133, 4901-4911 [DOI] [PubMed] [Google Scholar]
- Gu F., Gruenberg J. (2000). ARF1 regulates pH-dependent COP functions in the early endocytic pathway. J. Biol. Chem. 275, 8154-8160 [DOI] [PubMed] [Google Scholar]
- Hausmann G., Bänziger C., Basler K. (2007). Helping Wingless take flight: how WNT proteins are secreted. Nat. Rev. Mol. Cell Biol. 8, 331-336 [DOI] [PubMed] [Google Scholar]
- Heasman J., Crawford A., Goldstone K., Garner-Hamrick P., Gumbiner B., McCrea P., Kintner C., Noro C. Y., Wylie C. (1994). Overexpression of cadherins and underexpression of beta-catenin inhibit dorsal mesoderm induction in early Xenopus embryos. Cell 79, 791-803 [DOI] [PubMed] [Google Scholar]
- Hollyday M., McMahon J. A., McMahon A. P. (1995). Wnt expression patterns in chick embryo nervous system. Mech. Dev. 52, 9-25 [DOI] [PubMed] [Google Scholar]
- Kao K. R., Elinson R. P. (1988). The entire mesodermal mantle behaves as Spemann's organizer in dorsoanterior enhanced Xenopus laevis embryos. Dev. Biol. 127, 64-77 [DOI] [PubMed] [Google Scholar]
- Kim H., Cheong S. M., Ryu J., Jung H. J., Jho E. H., Han J. K. (2009). Xenopus Wntless and the retromer complex cooperate to regulate XWnt4 secretion. Mol. Cell. Biol. 29, 2118-2128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein P. S., Melton D. A. (1996). A molecular mechanism for the effect of lithium on development. Proc. Natl. Acad. Sci. USA 93, 8455-8459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komekado H., Yamamoto H., Chiba T., Kikuchi A. (2007). Glycosylation and palmitoylation of Wnt-3a are coupled to produce an active form of Wnt-3a. Genes Cells 12, 521-534 [DOI] [PubMed] [Google Scholar]
- Kurayoshi M., Yamamoto H., Izumi S., Kikuchi A. (2007). Post-translational palmitoylation and glycosylation of Wnt-5a are necessary for its signalling. Biochem. J. 402, 515-523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCulloch M. W., Coombs G. S., Banerjee N., Bugni T. S., Cannon K. M., Harper M. K., Veltri C. A., Virshup D. M., Ireland C. M. (2009). Psammaplin A as a general activator of cell-based signaling assays via HDAC inhibition and studies on some bromotyrosine derivatives. Bioorg. Med. Chem. 17, 2189-2198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffett S., Brown D. A., Linder M. E. (2000). Lipid-dependent targeting of G proteins into rafts. J. Biol. Chem. 275, 2191-2198 [DOI] [PubMed] [Google Scholar]
- Muroi M., Shiragami N., Nagao K., Yamasaki M., Takatsuki A. (1993). Folimycin (concanamycin A), a specific inhibitor of V-ATPase, blocks intracellular translocation of the glycoprotein of vesicular stomatitis virus before arrival to the Golgi apparatus. Cell Struct. Funct. 18, 139-149 [DOI] [PubMed] [Google Scholar]
- Neumann S., Coudreuse D. Y., van der Westhuyzen D. R., Eckhardt E. R., Korswagen H. C., Schmitz G., Sprong H. (2009). Mammalian Wnt3a is released on lipoprotein particles. Traffic 10, 334-343 [DOI] [PubMed] [Google Scholar]
- Niederlander C., Walsh J. J., Episkopou V., Jones C. M. (2001). Arkadia enhances nodal-related signalling to induce mesendoderm. Nature 410, 830-834 [DOI] [PubMed] [Google Scholar]
- Nieuwkoop P. D., Faber J. (1994). Normal table of Xenopus laevis (Daudin), 3rd edn.New York: Garland Publishing; [Google Scholar]
- Nusse R., Fuerer C., Ching W., Harnish K., Logan C., Zeng A., ten Berge D., Kalani Y. (2008). Wnt signaling and stem cell control. Cold Spring Harbor Symp. Quant. Biol. 73, 59-66 [DOI] [PubMed] [Google Scholar]
- Panakova D., Sprong H., Marois E., Thiele C., Eaton S. (2005). Lipoprotein particles are required for Hedgehog and Wingless signalling. Nature 435, 58-65 [DOI] [PubMed] [Google Scholar]
- Qi J., Wang Y., Forgac M. (2007). The vacuolar (H+)-ATPase: subunit arrangement and in vivo regulation. J. Bioenerg. Biomembr. 39, 423-426 [DOI] [PubMed] [Google Scholar]
- Sacchettini J. C., Gordon J. I., Banaszak L. J. (1989). Crystal structure of rat intestinal fatty-acid-binding protein. Refinement and analysis of the Escherichia coli-derived protein with bound palmitate. J. Mol. Biol. 208, 327-339 [DOI] [PubMed] [Google Scholar]
- Shen R., Lin C. T., Bowman E. J., Bowman B. J., Porco J. A., Jr (2002). Synthesis and V-ATPase inhibition of simplified lobatamide analogues. Org. Lett. 4, 3103-3106 [DOI] [PubMed] [Google Scholar]
- Slack J. M., Isaacs H. V., Darlington B. G. (1988). Inductive effects of fibroblast growth factor and lithium ion on Xenopus blastula ectoderm. Development 103, 581-590 [DOI] [PubMed] [Google Scholar]
- Smolich B. D., McMahon J. A., McMahon A. P., Papkoff J. (1993). Wnt family proteins are secreted and associated with the cell surface. Mol. Biol. Cell 4, 1267-1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol S., Christian J. L., Moon R. T., Melton D. A. (1991). Injected Wnt RNA induces a complete body axis in Xenopus embryos. Cell 67, 741-752 [DOI] [PubMed] [Google Scholar]
- Suzuki H., Watkins D. N., Jair K. W., Schuebel K. E., Markowitz S. D., Chen W. D., Pretlow T. P., Yang B., Akiyama Y., Van Engeland M., et al. (2004). Epigenetic inactivation of SFRP genes allows constitutive WNT signaling in colorectal cancer. Nat. Genet. 36, 417-422 [DOI] [PubMed] [Google Scholar]
- Swiatek W., Kang H., Garcia B. A., Shabanowitz J., Coombs G. S., Hunt D. F., Virshup D. M. (2006). Negative regulation of LRP6 function by casein kinase I epsilon phosphorylation. J. Biol. Chem. 281, 12233-12241 [DOI] [PubMed] [Google Scholar]
- Symes K., Smith J. C. (1987). Gastrulation movements provide and early marker of mesoderm induction in Xenopus laevis. Development 101, 339-349 [Google Scholar]
- Tada M., Smith J. C. (2000). Xwnt11 is a target of Xenopus Brachyury: regulation of gastrulation movements via Dishevelled, but not through the canonical Wnt pathway. Development 127, 2227-2238 [DOI] [PubMed] [Google Scholar]
- Takada R., Satomi Y., Kurata T., Ueno N., Norioka S., Kondoh H., Takao T., Takada S. (2006). Monounsaturated fatty acid modification of Wnt protein: its role in Wnt secretion. Dev. Cell 11, 791-801 [DOI] [PubMed] [Google Scholar]
- Tannous B. A., Kim D. E., Fernandez J. L., Weissleder R., Breakefield X. O. (2005). Codon-optimized Gaussia luciferase cDNA for mammalian gene expression in culture and in vivo. Mol. Ther. 11, 435-443 [DOI] [PubMed] [Google Scholar]
- Thomas K. R., Capecchi M. R. (1990). Targeted disruption of the murine int-1 proto-oncogene resulting in severe abnormalities in midbrain and cerebellar development. Nature 346, 847-850 [DOI] [PubMed] [Google Scholar]
- Tsai I. C., Amack J. D., Gao Z. H., Band V., Yost H. J., Virshup D. M. (2007). A Wnt-CKIvarepsilon-Rap1 pathway regulates gastrulation by modulating SIPA1L1, a Rap GTPase activating protein. Dev. Cell 12, 335-347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhaegent M., Christopoulos T. K. (2002). Recombinant Gaussia luciferase. Overexpression, purification, and analytical application of a bioluminescent reporter for DNA hybridization. Anal. Chem. 74, 4378-4385 [DOI] [PubMed] [Google Scholar]
- Verras M., Papandreou I., Lim A. L., Denko N. C. (2008). Tumor hypoxia blocks Wnt processing and secretion through the induction of endoplasmic reticulum stress. Mol. Cell. Biol. 28, 7212-7224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willert K., Brown J. D., Danenberg E., Duncan A. W., Weissman I. L., Reya T., Yates J. R., 3rd, Nusse R. (2003). Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature 423, 448-452 [DOI] [PubMed] [Google Scholar]
- Wissmann C., Wild P. J., Kaiser S., Roepcke S., Stoehr R., Woenckhaus M., Kristiansen G., Hsieh J. C., Hofstaedter F., Hartmann A., et al. (2003). WIF1, a component of the Wnt pathway, is down-regulated in prostate, breast, lung, and bladder cancer. J. Pathol. 201, 204-212 [DOI] [PubMed] [Google Scholar]
- Xu Q., Wang Y., Dabdoub A., Smallwood P. M., Williams J., Woods C., Kelley M. W., Jiang L., Tasman W., Zhang K., et al. (2004). Vascular development in the retina and inner ear: control by Norrin and Frizzled-4, a high-affinity ligand-receptor pair. Cell 116, 883-895 [DOI] [PubMed] [Google Scholar]
- Yang P., Lorenowicz M., Silhankova M., Coudreuse D., Betist M., Korswagen H. (2008). Wnt signaling requires retromer-dependent recycling of MIG-14/Wntless in Wnt-producing cells. Dev. Cell 14, 140-147 [DOI] [PubMed] [Google Scholar]
- Zhai L., Chaturvedi D., Cumberledge S. (2004). Drosophila wnt-1 undergoes a hydrophobic modification and is targeted to lipid rafts, a process that requires porcupine. J. Biol. Chem. 279, 33220-33227 [DOI] [PubMed] [Google Scholar]
- Zoltewicz J. S., Ashique A. M., Choe Y., Lee G., Taylor S., Phamluong K., Solloway M., Peterson A. S. (2009). Wnt signaling is regulated by endoplasmic reticulum retention. PLoS ONE 4, e6191 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.