Abstract
Introduction and hypothesis
We sought to define changes in vaginal distensibility (VD) induced by pregnancy and vaginal delivery using a novel in vivo biomechanical testing protocol.
Methods
Under sedation, a balloon was inserted into the vagina of 27 virgin, pregnant and 4-week postpartum Long–Evans rats and incrementally distended. Pressure–volume curves were generated with slopes characterizing VD (higher slope = less distensible). One-way ANOVA with a Bonferroni post-hoc test were used for statistical analyses.
Results
Mean pressures at an infusion volume of 1 cc were lower in pregnant and postpartum rats than in virgins (P<0.001). VD was increased in pregnant vs. virgin rats (P<0.001) and did not recover to virgin levels post partum (P<0.001).
Conclusions
We have developed a test that measures VD in vivo under clinically relevant loading conditions. The increased VD in the late postpartum period defines a persistent change in biomechanical behavior of the vagina related to pregnancy and vaginal delivery.
Keywords: Vaginal distensibility, Postpartum, Vaginal delivery, Rat
Introduction
Prolapse of pelvic organs is a common condition affecting the lives of millions of women; however, very little is known of its pathogenesis. Direct support to the pelvic organs (urethra, bladder, uterus, and rectum) is provided by the vagina and indirectly by the structures involved in vaginal support—the levator ani muscles and the connective tissue attachments between the vagina and the pelvic sidewall [1, 2]. Damage to any component of this complex can result in loss of vaginal support and prolapse of the pelvic organs. Epidemiologic studies suggest that vaginal birth is a major risk factor for pelvic organ prolapse (POP) and its associated disruptions in urinary, bowel, and sexual function [3, 4]. However, most women do not develop symptoms of prolapse until years, often decades, following vaginal delivery. Besides an epidemiologic link between parity, vaginal delivery, and pelvic floor dysfunction, the exact mechanism connecting these events is not clear. Thus, the initial events surrounding childbirth that predispose women to develop pelvic organ prolapse later in life remain undefined. We hypothesize that pregnancy induces specific adaptations in the vagina and its supportive tissues that afford increased vaginal distensibility to facilitate passage of the fetus, but occur at the expense of increased susceptibility to injury in the event that maximal distensibility is exceeded. Consequently, at the time of vaginal delivery, overt or subclinical injury results in damage, followed by incomplete recovery. Such permanent alterations in vaginal tissue likely modify its response to mechanical loads throughout subsequent life events, leading to further tissue deterioration, failure of supportive capacity, and increased risk for pelvic organ prolapse.
In this study, we sought to determine the impact of vaginal delivery on the biomechanical behavior of the vagina by comparing vaginal distensibility in pregnancy (to define the adaptation) and in the late postpartum period (to define recovery) relative to virgin controls, using an in vivo mechanical testing protocol in a primigravid rodent model. Our in vivo design allows us to define changes in the mechanical behavior of the vagina that afford distension during pregnancy, as well as the impact of delivery on vagina and the surrounding tissues. By utilizing an in vivo testing protocol, we have measured the collective effect of pregnancy and vaginal delivery on the vagina and all the tissues that support and maintain vaginal structure. In this way, the data obtained from this in vivo study will improve our knowledge of the events that define the switch from physiological to pathological remodeling of the vagina and its supportive tissues.
Materials and methods
Approval for this study was received from the Institutional Animal Care and Use Committee from the University of Pittsburgh (IACUC). A total of twenty seven Long–Evans rats were utilized to measure vaginal distensibility (VD) in vivo: virgin (N=7), late pregnant (17–20 days; N=10) and 4 week postpartum (N=10). Measurements of the animals' weight, genital hiatus (GH), and total vaginal length (TVL) were recorded. Animals were placed on the scale to obtain their weight in grams. Prior to the introduction of the balloon, with a measuring device that had millimeter markings, GH was measured from the middle of the external urethral meatus to the posterior midline introitus. TVL was measured as the greatest depth of the vagina from the posterior fornix to the introitus. Based on the measured TVL and volume needed to acquire the pressure of 10 mmHg, the initial vaginal diameter of each animal was estimated assuming a simple cylindrical shape. The calculation was made using the following formula for volume of the cylinder: Volume = TVL × π × diameter. Virgin and postpartum animals were in similar parts of the menstrual cycle (estrous and metestrous) as determined by vaginal smears.
In vivo vaginal distensibility measurements
A modification of a protocol developed to measure left ventricular distensibility was utilized to determine vaginal distensibility [5]. After the induction of anesthesia with ketamine (30 mg/kg), a thin custom-made latex balloon containing a catheter tip pressure transducer (MPC-500, Millar Instruments, Houston TX), connected to a computer system, was gently inserted into the rat's vagina (Fig. 1). Prior to the insertion into the rat vagina, the balloon was inflated outside of the body and the balloon pressure at each volume was recorded. The pressures resulting from balloon inflation up to 1 cc were negligible. At higher volumes, the reported pressures reflect the vaginal wall pressure after the baseline pressure of the balloon was subtracted at each volume. Only the pressure created by the vaginal wall was considered in all of the distensibility calculations. After insertion into the vagina, the balloon was distended with water at a constant rate, controlled by the infusion pump (Harvard Apparatus), at 0.2-cc increments to a maximum volume of 1 cc for seven virgin animals. This volume had been shown in preliminary studies to be appropriate for assessing biomechanical behavior of the vagina without producing tissue damage. For all tests, the pressure was allowed to equilibrate for 4 min after each incremental increase in volume before a measurement was recorded. This procedure was repeated for each animal a minimum of three times until pressures at each volume were repeatable (Fig. 2). Study animals were monitored for the signs of discomfort/distress during the entire experiment. If an increase in heart rate, respiratory rate, or movement of the whiskers was noted, additional ketamine was administered. The mean maximum pressure at 1 cc volume in the virgin group was then calculated. The mean maximum pressure at a volume of 1 cc was also assessed in six pregnant and five postpartum animals. In an additional four pregnant and five postpartum animals, the balloon was distended at 0.2 cc increments until the vaginal pressure reached the same mean maximum pressure observed in the virgin group. At these increased volumes, pressure values were adjusted to account for the intrinsic pressure of the balloon. Pressure–volume curves were generated by plotting the pressure vs. volume and the slope of the curves was used to characterize vaginal distensibility (higher slope = less distensible). The pressures were then normalized to the maximum pressure so the slope could be repeatedly calculated from the 40–100% region of the maximum pressure (Fig. 3). This was done to ensure that slopes from similar regions of the curve were being compared for all groups.
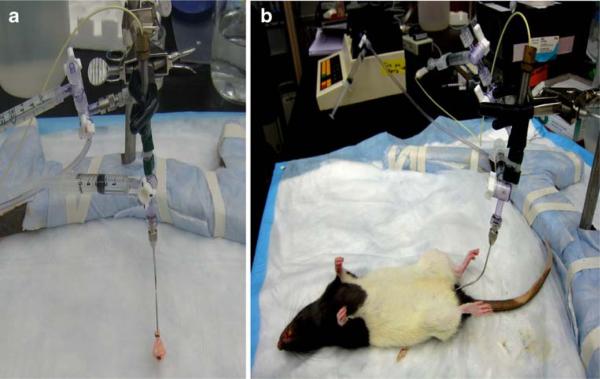
Fig. 1.
Experimental set-up for in vivo measurement of vaginal distensibility: a thin custom-made latex balloon contiguous with a pressure transducer (a), connected to a computer system, is inserted into the rat vagina (b)
Fig. 2.
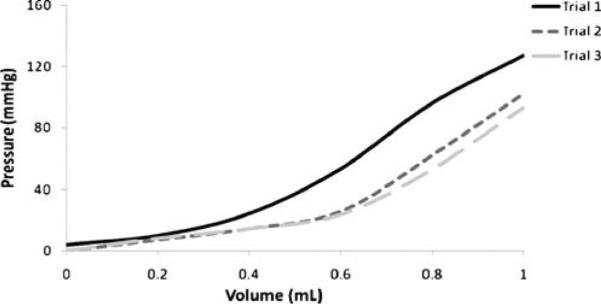
Example of preconditioning the tissue via cyclical loading in order to achieve repeatable pressure–volume curves which slope represents vaginal distensibility
Fig. 3.
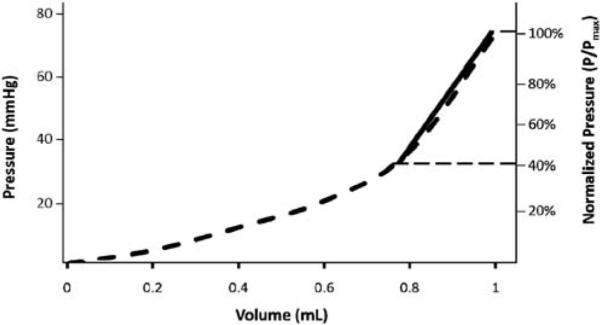
Calculation of the vaginal distensibility in a virgin sample. Pressure (mmHg, left axis) was normalized to the maximum pressure of the curve (%, right axis). The curve was isolated from 40–100% of the maximum pressure as noted by the horizontal dashed lines. This area was fit with a line (solid line) whose slope represents vaginal distensibility
Data analysis and statistics
No preliminary data exists for the vaginal distensibility experiment; therefore, the number of animals per group was based on a previous study examining the biomechanical properties of the rat model. Based on this study, five animals per group would be required to achieve statistical significance with a power of at least 80% [6]. Results are represented as mean±SD. Statistical analyses were performed using one-way ANOVA with a Bonferroni post-hoc with significance set to 0.05. No significant differences in the variances was found on Levene's test for the homogeneity of the variances (P=0.15).
Results
Baseline characteristics obtained for the animals used in the study are listed in Table 1. As expected, pregnant rats were heavier than the virgin animals. Genital hiatus and total vaginal length did not differ between the groups (P=0.09 and P=0.14, respectively) (Table 1). Vaginal diameters were based on TVL and the volumes required to achieve 10 mmHg of vaginal pressure. By this method, initial vaginal diameters were measured and were determined to be similar between the three groups with the mean of 0.39±0.05 cm in virgin, 0.45±0.01 cm in pregnant, and 0.41±0.08 cm in postpartum rats (P=0.19).
Table 1.
Baseline characteristics
| Group | N | Weighta (gm) | TVLa (cm) | GHa (cm) | Vaginal diametera (cm) |
|---|---|---|---|---|---|
| Virgin | 7 | 245.00±20.31 | 2.38±0.08 | 0.22±0.04 | 0.39±0.05 |
| Pregnant | 10 | 291.20±22.95 | 2.48±0.13 | 0.25±0.04 | 0.45±0.01 |
| Postpartum | 10 | 269.00±10.54 | 2.30±0.22 | 0.28±0.08 | 0.41±0.08 |
| Overall P value† | 0.01 | 0.14 | 0.09 | 0.19 |
TVL total vaginal length, GH genital hiatus
Data presented as a mean±standard deviation
b P value obtained from one-way analysis
The results of the pressure–volume testing to measure mechanical adaptation with the maximum volume of 1 cc are depicted in Fig. 4. The slope of the vaginal distensibility curve of virgin animals had a bilinear appearance, yielding two distinct regions: one linear region at low volumes and a second linear region, with an increased slope, at high volumes. The curve representing pregnant animals, on the other hand, demonstrated a single uniform linear behavior throughout the range of volumes. This is related to the fact that pregnant vagina can accommodate a significantly larger volume without an increase in pressure as compared to the virgin animals. The slope of the vaginal distensibility curve of postpartum animals was almost identical to the pregnant curves to volumes of 1 cc, also demonstrating a linear behavior throughout a wide range of volumes and increased volumetric capacity without impacting pressure. A mean maximum pressure of 106 mmHg was measured at a volume of 1 cc in the virgin vagina, while pregnant and postpartum rats demonstrated a respective 68% and 63% lower mean pressure at the same volume (P<0.001; Fig. 4).
Fig. 4.
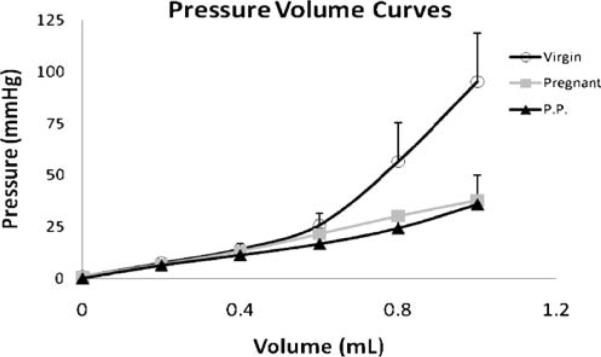
Pressure–volume curves demonstrating a steep rise in pressure at higher volumes in the virgin animals and a linear behavior throughout the range of volumes in pregnant and postpartum rats, secondary to the last two groups being able to accommodate larger volumes without significant increase in pressure
Since virgins generated higher pressures at smaller volumes than the pregnant and postpartum rats, an additional four pregnant and five postpartum rats were utilized to determine the volume necessary to generate the mean maximum pressure observed in virgins (106 mmHg). As can be observed from Fig. 5, postpartum rats required mean instillation of more than 2 cc, while pressures in the pregnant group remained significantly lower, despite instillation up to 4 cc (Fig. 5). Thus, vaginal distensibility, derived from the slope of the pressure–volume curves, was markedly increased in pregnant vs. virgin rats as demonstrated by the lower slope value: 11.4±7.12 mmHg/mL vs. 195.36±50.22 mmHg/mL (P<0.001; Fig. 5). Postpartum rats had decreased vaginal distensibility when compared to the pregnant animals; however, they did not recover to the pre-pregnancy state, as demonstrated by significantly higher distensibility corresponding to a much lower slope of 72.0±20.82 mmHg/mL when compared to the slope of virgin rats (P<0.001; Fig. 5) indicating alteration of the tissues.
Fig. 5.
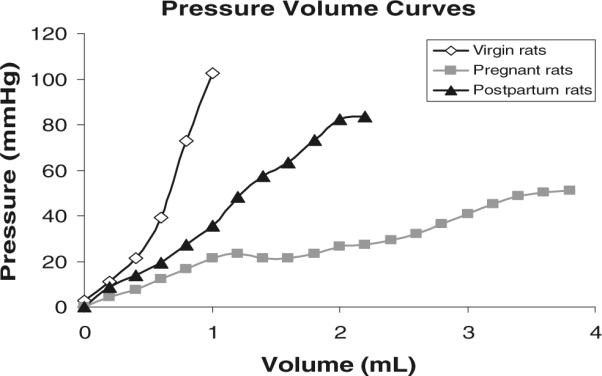
Pressure–volume curves demonstrating significantly increased vaginal distensibility in pregnant rats as demonstrated by the lower slope. Postpartum rats did not recover to the pre-pregnancy state, as demonstrated by higher distensibility, compared to virgin controls
Discussion
In this study, we developed a testing protocol to quantify mechanical adaptations of the vagina in vivo using a rodent model. We use the mechanical measure of distensibility as an indication of changes in the collective function of the vagina and its supportive tissues in maintaining vaginal shape. In this way, we compare vaginal distensibility in pregnant and late postpartum rats with that of virgin rats in order to provide quantitative data on the increase in distensibility of the vagina; which facilitates vaginal delivery but perhaps at the expense of permanent tissue alterations. Our experimental set-up simulated the passage of a fetus by circumferentially distending the vagina; thus, allowing us to measure vaginal distensibility using clinically relevant loading conditions.
One of the primary findings of this paper was that vaginal distensibility increased during pregnancy. This finding characterizes an important maternal adaptation. The ability of the vagina to distend with minimal increase in pressure during pregnancy, as marked by a significantly lower slope of the pressure–volume curve, likely has evolved to reduce the risk of injury to both mother and fetus during vaginal delivery. Indeed, increased distensibility (i.e., compliance) of the vaginal tissue would minimize pressure exerted on the vaginal walls during the passage of the fetus. However, as we have shown previously using a structural test that mimics downward distention, increased compliance during pregnancy is also associated with a weaker structure [7]. Thus, in the event that maximal distensibility is exceeded as in the case of a large fetus, the protective adaptations are exceeded, and injury to the vagina and surrounding tissues ensues to the extent at which effective recovery may not be possible.
The second major finding of the study was that vaginal distensibility did not return to pre-pregnancy state in late postpartum period, demonstrating a persistent increase relative to virgin controls. These in vivo findings delineate a persistent change in biomechanical behavior of the vagina as a consequence of pregnancy and spontaneous vaginal delivery. We suspect that such persistent changes are potentially even more pronounced in women as the maximal dimensions of the birth canal of a modern human female approximate the size of the head of their newborns, making birth in humans a more difficult and more traumatic process than in other species, whose pelvic dimensions are larger than neonatal head sizes [8]. Indeed, in non-diabetic rodents, the fetal head size is significantly smaller relative to the maximal distensibility of the vagina making labor dystocias and overt injury uncommon. However, rodents have large litter sizes thereby, increasing the risk for injury. The persistent change in vaginal distensibility observed in this study in the late postpartum period likely defines a persistent consequence of injury acquired during vaginal delivery. Alternatively, it may represent an adaptation in the tissues that persists to facilitate subsequent deliveries. Additional studies, with longer time points after delivery are needed to establish whether or not this change is permanent. It has been previously shown that prolonged vaginal distention resulting in an injury in a rat model is associated with a lower leak point pressures and increased severity of stress urinary incontinence [9, 10]. Additional studies are necessary to induce injury at the time of delivery to understand its impact on recovery of the vaginal tissue and to correlate findings to the studies focusing on the urinary dysfunction after simulated birth injury.
Previously, we examined the biomechanical properties of the vaginal supportive-tissue complex (VSTC) using an ex vivo mechanical testing protocol in a rodent model [7]. Ex vivo experiments demonstrated a significantly lower stiffness, defined as force per elongation, of the VSTC in pregnancy; however, the biomechanical properties returned to pre-pregnancy state in the late postpartum period. The divergence between these and our current findings in the late postpartum period may be explained by the different loading conditions in the two experiments: downward distension along the longitudinal axis in the uniaxial testing vs. a combination of circumferential (outward) and longitudinal distention as applied in this study. In addition, there are the differences inherent to the experimental design (ex vivo and in vivo). Despite restoration of the VSTC stiffness to pre-pregnancy levels in the ex vivo downward distention testing, permanent change in the biomechanical properties, manifested in persistent increase in vaginal distensibility, was observed using our in vivo experiment, which suggests that permanent changes resulting from pregnancy are likely more apparent in the circumferential direction and that factors not measureable in a nonviable ex vivo experiment (e.g., smooth muscle) may be playing a role.
It should be mentioned that failure to achieve increases in the pressure–volume relationship in pregnant animals despite the infusion of a volume of 4 cc may be in part the consequence of increased cervical compliance, thus allowing for the balloon to migrate into the uterus during testing. While it may be technically challenging, future experiments may want to consider suturing the cervix to ensure that the vagina is a closed system.
In the future, we will examine if abdominal delivery obviates permanent changes in vaginal distensibility seen in late postpartum period after vaginal delivery. Studies like this will aid us in breaching the gap in our current knowledge of the exact mechanism connecting vaginal delivery and pelvic floor dysfunction.
Acknowledgments
This research received financial support from AUGS Foundation Astellas Grant.
Footnotes
Conflicts of interest The authors have no conflicts of interest.
References
- 1.Norton PA. Pelvic floor disorders: the role of fascia and ligaments. Clin Obstet Gynecol. 1993;36:926–938. doi: 10.1097/00003081-199312000-00017. [DOI] [PubMed] [Google Scholar]
- 2.DeLancey JO. Pelvic organ prolapse: clinical management and scientific foundations. Clin Obstet Gynecol. 1993;36:895–896. [Google Scholar]
- 3.Jelovsek JE, Maher C, Barber MD. Pelvic organ prolapse. Lancet. 2007;24:1027–1038. doi: 10.1016/S0140-6736(07)60462-0. [DOI] [PubMed] [Google Scholar]
- 4.Bump RC, Norton PA. Epidemiology and natural history of pelvic floor dysfunction. Obstet Gynecol Clin Noth Am. 1998;25:723–746. doi: 10.1016/s0889-8545(05)70039-5. [DOI] [PubMed] [Google Scholar]
- 5.Campbell KB, Wu Y, Simpson AM, Kirkpatrick RD, Shroff SG, Granzier HL, et al. Dynamic myocardial contractile parameters from left ventricular pressure/volume measurements. Am J Physiol Heart Circ Physiol. 2005;289:H114–H130. doi: 10.1152/ajpheart.01045.2004. [DOI] [PubMed] [Google Scholar]
- 6.Moalli PA, Debes KM, Meyn LA, Howden NS, Abramowitch SD. Hormones restore biomechanical properties of the vagina and supportive tissues after surgical menopause in young rats. Am J Obstet and Gynecol. 2008;199(2):161–168. doi: 10.1016/j.ajog.2008.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lowder JL, Debes KM, Moon DK, Howden N, Abramowitch SD, Moalli PA. Biomechanical adaptations of the rat vagina and supportive tissues in pregnancy to accommodate delivery. Obstet Gynecol. 2007;109:136–143. doi: 10.1097/01.AOG.0000250472.96672.6c. [DOI] [PubMed] [Google Scholar]
- 8.Franciscus RG. When did the modern human pattern of childbirth arise? New insights from an old Neanderthal pelvis. PNAS. 2009;106:9125–9126. doi: 10.1073/pnas.0903384106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cannon TW, Wojcik EM, Ferguson CL, Saraga S, Thomas C, Damaser MS. Effects of vaginal distension on urethral anatomy and function. BJU Intern. 2007;90:403–407. doi: 10.1046/j.1464-410x.2002.02918.x. [DOI] [PubMed] [Google Scholar]
- 10.Damaser MS, Broxton-King C, Ferguson C, Kim FJ, Kerns JM. Functional and neuroanatomical effects of vaginal distention and pudendal nerve crush in the female rat. J Urol. 2003;170:1027–1031. doi: 10.1097/01.ju.0000079492.09716.43. [DOI] [PubMed] [Google Scholar]