Abstract
Epidermal integrity is a complex process established during embryogenesis and maintained throughout the organism lifespan by epithelial stem cells. While Wnt regulates normal epithelial stem cell renewal, aberrant Wnt signaling can contribute to cancerous growth. Here, we explored the consequences of persistent expressing Wnt1 in an epidermal compartment that includes the epithelial stem cells. Surprisingly, Wnt caused the rapid growth of the hair follicles, but this was followed by epithelial cell senescence, disappearance of the epidermal stem cell compartment, and progressive hair loss. While Wnt1 induced the activation of β-catenin and the mTOR pathway, both hair follicle hyperproliferation and stem cell exhaustion were strictly dependent on mTOR function. These findings suggest that whereas activation of β-catenin contributes to tumor growth, epithelial stem cells may be endowed with a protective mechanism that results in cell senescence upon the persistent stimulation of proliferative pathways that activate mTOR, ultimately suppressing tumor formation.
Keywords: Stem cell, mTOR, Wnt, aging, cancer, signal transduction
Introduction
The Wnt family consists of 19 secreted cysteine-rich glycoproteins that initiate signaling by interacting with the N-terminal extracellular cysteine-rich region of the Frizzled family of seven-span transmembrane receptors, and with either LRP5 or LRP6, two members of the low-density-lipoprotein receptor-related (LDL-R) protein family (Moon et al., 2004; Nusse, 2008). Wnt can stimulate several major signaling cascades including the canonical Wnt/β-catenin pathway wherein β-catenin is stabilized and translocates to the nucleus, and the non-canonical Wnt pathways, which include the PCP (Planar Cell Polarity), c-Jun amino-terminal kinase (JNK), Rho, and calcium signaling pathways (reviewed in Moon et al., 2004; Reya and Clevers, 2005). Inappropriate activation of the Wnt/β-catenin pathway has been implicated in a large number of highly prevalent tumors supporting an important role of this pathway in promoting cell growth (Brennan and Brown, 2004; Klaus and Birchmeier, 2008). In addition, a large body of data shows that Wnt function is required for embryonic development and tissue maintenance and regeneration in adults (Liu et al., 2008) Specifically, Wnt controls a variety of processes ranging from gastrulation to organogenesis, including the heart, mammary gland branching, and the development of blood vessels, kidneys, and lung, often contributing to developmental events that involve epithelial-mesenchymal interactions (Klaus and Birchmeier, 2008; Moon et al., 2004; Nusse, 2008; Reya and Clevers, 2005).
In the epidermis, hair follicle (HF) development is initiated when mesenchymal cells populate the skin. During this process, signals emanating from the dermis induce epithelium thickening, elongation of the epithelial cells, and the formation of placodes containing Wnt-responsive cells (Andl et al., 2002) (reviewed in Alonso and Fuchs, 2003; Blanpain and Fuchs, 2009; Maretto et al., 2003). In response, placodes signal dermal cells to condense forming the dermal papilla component of the HF, which is also responsive to Wnt signaling (Maretto et al., 2003). The fully formed HF then retains detectable levels of β-catenin in the precortex, dermal papilla, and matrix cells (Maretto et al., 2003; Reddy et al., 2001), where it contributes to the maintenance of HF structures.
The ability to self-renew and generate differentiated progeny is what defines a stem cell. Embryonic stem cells are pluripotent, and thus capable to generate any tissues of the body, however the maintenance of most adult tissues is dependent on the presence of tissue-specific self-renewing somatic stem cells, which have a more restricted differentiation potential, and can regenerate only some of the cell types of the tissues from which they are derived (reviewed in Blau et al., 2001). Among a limited number of signaling networks controlling stem cell function, the canonical Wnt/β-catenin pathway has emerged as a key regulator of stem cell self-renewal (Alonso and Fuchs, 2003; Nusse, 2008). For example, activation of β-catenin by Wnt contributes to the inhibition of keratinocytes differentiation (Zhu and Watt, 1999), induction of hair follicle formation (Gat et al., 1998), and maintenance of proliferation of neuronal progenitors (Zechner et al., 2003). Wnt3A and 5A are also able to modulate the self-renewal of haematopoietic stem cells and increase the number of their progenitors (Nusse, 2008). However, expression of dominant active form of β-catenin may exert a negative effect on hematopoietic stem cells, leading to multilineage differentiation impairment, and the consequent loss of stem cell activity (Kirstetter et al., 2006; Scheller et al., 2006). Wnt may also play a role in activating cell senescence programs, as we have recently shown in the klotho animal model of aging (Liu et al., 2007).
Here, we investigate the long-term consequences of ectopic Wnt1-induced signaling in the epithelial compartment of the skin. Surprisingly, we found that Wnt causes the rapid growth of the HF, but this was followed by the disappearance of the epidermal stem cell compartment and progressive, premature hair loss. While Wnt1 expression induced the activation of both β-catenin and mTOR in the epithelial compartment of the skin, both HF growth and stem cell exhaustion were uniquely associated with mTOR activation. These findings suggest that epidermal stem cells may be endowed with a protective mechanism resulting in cell senescence upon the persistent stimulation of growth promoting pathways that activate mTOR, thereby helping to maintain the genetic integrity of the stem cell population, which may ultimately contribute to suppress tumor formation.
Results
Wnt1 induces the persistent maintenance of growth phase, anagen-like HF structures
To evaluate the effects of Wnt1 in the epidermis, we conditionally expressed the Wnt1 gene by crossing mice carrying the cytokeratin 5 promoter (K5) expressing the reverse tetracycline transactivator (rtTA) (Vitale-Cross et al., 2004) with FVB/N mice expressing the murine Wnt1 gene containing an IRES-firefly luciferase under the control of seven tet-responsive elements (tet-Wnt1) (Gunther et al., 2003). The offspring from this cross followed a normal Mendelian distribution, and although slightly smaller, no lethality was observed upon tet-on system activation during development by the administration of doxycycline to pregnant mice (not shown). Thus, we focused our attention on the effects of Wnt1 during the postnatal HF morphogenesis. The development of HF can be divided into eight distinct stages that are initiated during embryogenesis and culminate soon after animal birth (Paus et al., 1999). The last stage of HF morphogenesis (stage 8) involves a growth phase often referred to as anagen (Greco et al., 2009; Paus et al., 1999) that is followed by the nearly synchronous entrance into the catagen and telogen phases, following a well defined pattern during this first hair cycle (Figure 1A). During the growth phase (anagen), the lower cycling part of the HF is shaped by the differentiation of the keratinocytes into the outer root sheet (ORS) and the inner root sheet (IRS). The IRS is composed of the outer Henley’s layer, followed by the Huxley’s layer, and then the cuticle that separates the IRS from the hair shaft (Ito, 1986). During catagen, the HF undergoes an involution process mainly driven by apoptosis of the lower half of the HF. Telogen is the last stage of the hair cycle in which quiescent epithelial cells from the upper half of the HF remain quiescent until receiving new stimulation for growth (Morris et al., 2004). In order to analyze the consequences of Wnt1 expression during this first hair cycle, we selected four time points, 7, 14, 17, and 21 days of age (Figure 1B). We observed that upon doxycycline treatment, all K5rtTA/tet-Wnt1 transgenic mice entered prematurely into a rapid growth phase when compared to control littermates. This induction resulted in accelerated, yet controlled, HF growth with formation of all HF sheaths (ORS and IRS). However, unlike control littermates, HF from mutant mice failed to enter catagen and telogen. Consistent with this, K5rtTA/tet-Wnt1 mice maintained the skin thickness found during anagen, preserving the lower half of the HF in the subcutis at day 17 and 21, which was quite distinct from control littermates that were in catagen and telogen, respectively (Figure 1B). Similar findings were observed during the second hair cycle (Supplementary Figure 1), suggesting that Wnt1 promotes the persistence of HF in a growth phase, instead of undergoing consecutive cycles of growth and involution.
Figure 1.

Expression of Wnt1 leads to a persistent proliferative state of the hair follicles (HF). A) The HFs grow in a cyclic fashion, involving a growth phase or anagen, an involution phase (catagen), and a resting phase (telogen). B) H&E sections of the first synchronous hair cycle from K5rtTA control animals showing the histological characteristic of each phase at the indicated days of age (upper panel). Typical examples of K5rtTA/tet-Wnt1 mice treated with doxycycline showing early entry into a growth phase followed by failure to undergo catagen and telogen (lower panel).
To further characterize the HF structure from mutant mice, we used specific differentiation markers for the spinous (cytokeratin 1 and 10) and cornified (fillagrin) layers (Fuchs, 1995). We also assessed HF markers such as the type II keratin 6, which is present in the innermost cell layer of the ORS (Fuchs, 1995); the AE13 Type I hair keratin that is present in the hair cortex (Lynch et al., 1986); and mIRSa, as a marker for the IRS (Porter et al., 2004) (Figure 2A and B). There were no evident alterations in the differentiation pattern of the interfollicular epidermis of K5rtTA/tet-Wnt1 mice (Figure 2A). However, when examining the structure of the hair follicles by day 21, we found aberrant expression of cytokeratin 10 in the ORS layer of the hair follicle, which was not present in the control littermates that were in telogen (Figure 2A). The 21 days old mutant mice maintained the expression of HF markers, such as AE13 and mIRSa, which were present in control littermates during late stage morphogenesis/anagen (P14) but not during telogen (P21) (Figure 2B). Thus, the expression of Wnt1 causes mice to persistently retain fully developed HF rather than entering in catagen and telogen.
Figure 2.
Alterations in HF structures in K5rtTA/tet-Wnt1 mice. A) Upper panel: Frozen sections from skin of 21 days old (P21) K5rtTA control mice show typical localization of the terminal differentiation marker fillagrin in the upper most layers of epidermis. The normal localization of cytokeratin 1 (K1) and 10 (K10) in the spinous layer of the interfollicular compartment and the normal expression of cytokeratin 6 (K6) in the HF are shown. Expression of α6 integrin (α6) delimitates the epidermal from the dermal compartment. DAPI was used for nuclear DNA staining (blue). Lower panel: K5rtTA/tet-Wnt1 mice fed with doxycycline show normal localization of fillagrin, cytokeratin 1, 10 and absence of cytokeratin 6 in the interfollicular epidermis (IF). HFs of mutant mice do not express fillagrin and cytokeratin 1, however an abnormal expression of cytokeratin 10 is present in the IRS compartment, co-localized with normal expression of cytokeratin 6. B) During late stage morphogenesis (P14), K5rtTA control mice express Type I hair keratins AE13 and the IRS specific marker (mIRSa). Expression of these markers cease during telogen (P21). K5rtTA/tet-Wnt1 mice fed with doxycycline instead maintain the expression of both markers through P21, consistent with the failure to entering in a resting state. Cytokeratin 5 (K5) staining reveals the basal epithelial layer. High magnification (63x) shows details of AE13 and mIRSa expressing structures in K5rtTA/tet-Wnt1 mice.
Wnt1 activates the β-catenin pathway in HF: prolonged exposure to Wnt1 leads to hair loss
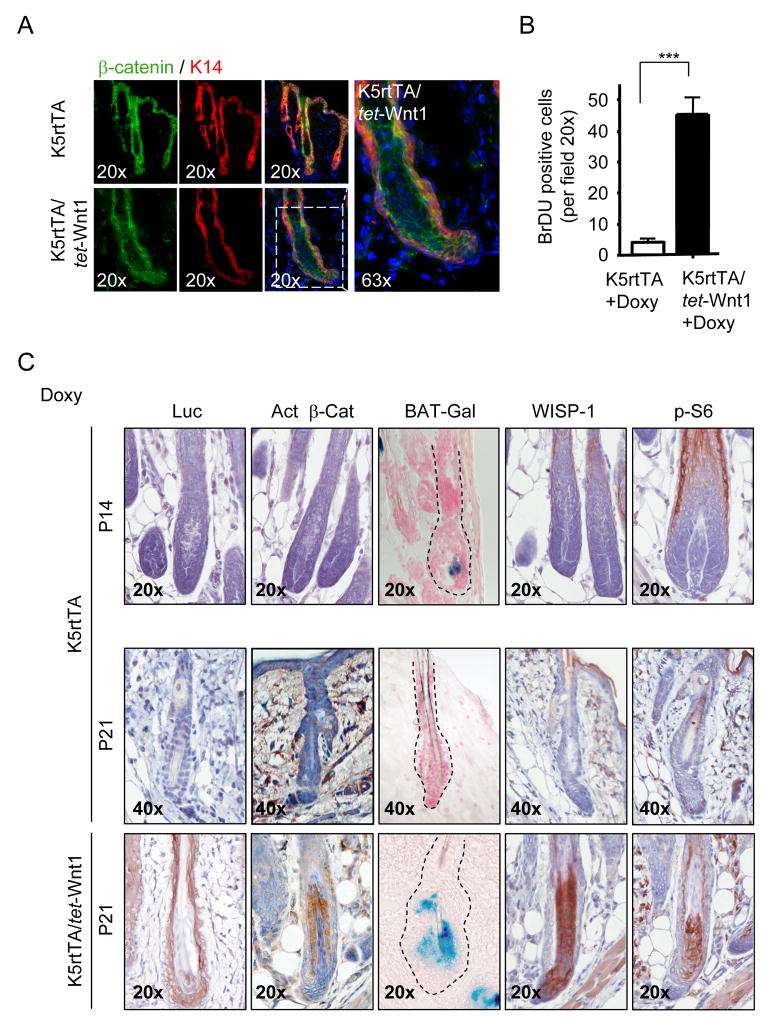
We next explored the expression and activity of the Wnt1 downstream molecule β-catenin. Both mutant and control mice exhibited widespread expression of β-catenin in the interfollicular region of the skin as well as in the follicular compartment including the bulge and bulb anatomical areas (Figure 3A). Although membrane and cytoplasmatic localization of β-catenin was readily detectable, no nuclear β-catenin staining could be observed, possibly due to the strong signal from membrane staining or the limited presence of nuclear β-catenin. However, K5-promoter driven expression of Wnt1 induced proliferation primarily of the cells within the basal K5-expressing layer of the HF, as assessed by BrDU incorporation and cyclin D1 expression (Figure 3B, Supplementary Figure 2).
Figure 3.
Activation of Wnt downstream targets in the epidermis and HF of doxycycline-treated K5rtTA/tet-Wnt1 mice. A) Presence of total β-catenin in control and mutant mice. Representative immunofluorescence analysis for total β-catenin (green), cytokeratin 14 (K14) (red), and DAPI (blue) in skins from control and K5rtTA/tet-Wnt1 fed with doxycycline are shown. High magnifications show membrane and cytoplasmatic localization of β-catenin. B) Hyperproliferation of epithelial HF cells in K5rtTA/tet-Wnt1 mice. Graphic bar represents number of BrDU-positive cells as the average ± S.E.M. of 10 fields at 20× of magnification in representative animals of the indicated genotype fed with doxycycline (***P<0.001). C) IHC for luciferase (Luc) shows negative staining in control littermates on P14 and P21, and positive staining in the ORS of K5rtTA/tet-Wnt1 mice aligned with the expression of the transgenic construct. Accumulation of the unphosphorylated, active form of β-catenin (Act β-Cat) in the HF IRS of mutant mice was noticeable. However, the active form of β-catenin was not detected by IHC in P14 and P21 control littermates. Using a reporter mouse line for β-catenin (BAT-Gal mouse) bred into our mouse lines, we were able to detect β-catenin activity at P14 (growth phase) but not at P21 (telogen) in control mice. K5rtTA/tet-Wnt1 mice crossed with BAT-Gal mice however, showed a strong activation of the β-catenin pathway visualized by the expression of β-Gal staining in the IRS. IHC reaction for WISP-1 (Wnt1 induced secreted protein 1) was observed in the IRS of the HF of K5rtTA/tet-Wnt1 mice but not in the K5rtTA control mice. Accumulation of high levels of the phosphorylated form of S6 (p-S6) was readily detectable in the IRS compartment of the HF upon Wnt-1 expression, and during late stage morphogenesis in control littermates (P14), but absent during telogen (P21).
To ensure the specificity of our K5-driven tet-Wnt1-IRES-firefly luciferase mice we analyzed the expression of luciferase by immunohistochemistry, which showed a strong reactivity in the K5-expressing layers comprising the ORS of mutant mice (Figure 3C). Next, we performed a detailed analysis of the β-catenin activation status. Using a specific antibody that recognizes the dephosphorylated form of β-catenin on Ser37 or Thr41-the active form of β-catenin (van Noort et al., 2002), we found cytoplasmatic localization of active β-catenin confined to the IRS, opposing the compartment expressing luciferase in the K5 layer (Figure 3C). Thus, Wnt1 expression may cause the paracrine activation of β-catenin in the adjacent HF epithelial layers. Whether this involves the contribution of particular Wnt1 receptors in the HF matrix that might be absent in the ORS, as recently reported (Reddy et al., 2004), warrants further investigation. To further examine this paracrine effect, we made use of the β-catenin reporter mouse line BAT-Gal, which expresses the lacZ gene under the control of β-catenin/T cell factor responsive elements (Maretto et al., 2003), and crossed these mice with the K5rtTA/tet-Wnt1 mouse line. Indeed, LacZ detection of β-Gal activity was detected during the growth phase of the first hair cycle in control littermates (P14) and in 21 days old mutant mice, at a time in which no β-Gal activity could be observed in control mice that were already in telogen (P21) (Figure 3C). Furthermore, we also examined the expression of endogenous Wnt-regulated molecules in this HF compartment. For example, by IHC analysis, we were able to detect the expression of WISP-1 (Wnt1 induced secreted protein 1), a β-catenin-regulated molecule (Xu et al., 2000), in the IRS compartment of hair follicles in mutant mice (P21) (Figure 3C), consistent with the analysis of β-catenin-activated gene expression by the β-Gal reporter system. Based on recent studies supporting that the unconventional protein kinase mTOR represents a direct target of the Wnt pathway (Inoki et al., 2006), we also characterized the levels of the phosphorylated form of the mTOR downstream molecule, S6 (Wendel et al., 2004). Transgenic Wnt1 expression provoked a very strong accumulation of pS6 at the IRS when compared with control littermates (Figure 3C), with a pattern similar to that of WISP-1 staining, active β-catenin accumulation, and β-Gal reporter activity. Overall, these observations suggest that Wnt1-secreting cells localized at the ORS induce paracrine activation of Wnt-downstream targets in the IRS compartment of HF.
The prolonged effects of Wnt1 were analyzed by following a cohort of mutant and wild-type littermates (n=25) for >1 year. Although transgenic expression of active β-catenin from the K14 promoter leads to the tumoral growth of HF (pilomatricomas) (Gat et al., 1998), prolonged exposure to Wnt1 caused a quite distinct biological outcome. The persistent proliferative anagen-like phenotype observed in K5rtTA/tet-Wnt1 mice evolved into a progressive hair loss (Figure 4A) reflected microscopically by the expansion of all sheaths, which was quite evident during the second hair cycle (Supplemental Figure 1) when compared to control mice. Doxycycline-induced Wnt1 expression caused the terminal differentiation of HF after 90 days (Figure 4B). Subsequently, the ORS, bulge, and sebaceous glands differentiate into cyst-like structures after prolonged Wnt1 expression (Figure 4B, Supplemental Figure 3A). Secondary periappendageal dermal inflammation with multinuclear giant cells was also observed after prolonged doxycycline treatment, accompanied by a progressive decrease on HF density (Figure 4B–C and Supplementary Figure 3B). However, no major alterations other than a moderate acanthosis (thickening of the epithelial compartment) was observed in the IF epidermis (Supplementary Figure 3C).
Figure 4.
Progressive premature hair loss in K5rtTA/tet-Wnt1 mice. A) K5rtTA/tet-Wnt1 transgenic mice fed with doxycycline show complete loss of their fur by 1 year of age when compared with K5rtTA control littermates. B) H&E histological sections of K5rtTA and K5rtTA/tet-Wnt1 by 21, 90, and 360 days of age showing progressive HF degeneration when compared to normal K5rtTA control mice. K5rtTA/tet-Wnt1 mice present a growth phase, anagen-like phenotype by 21 days of age, which evolves into altered HF structures and subsequent HF loss. C) Quantification of hair follicles was performed using histological sections cut in parallel to the surface of the epidermis. Graphic bar represent the number of hair follicles at the indicated age as the average ± S.E.M. of 10 fields at 10X magnification from representative animals of the indicated genotype (***P<0.001; **P<0.01; ns: P > 0.05).
Wnt1 induces senescence and impairs the regenerative capacity of HF by causing stem cell ablation
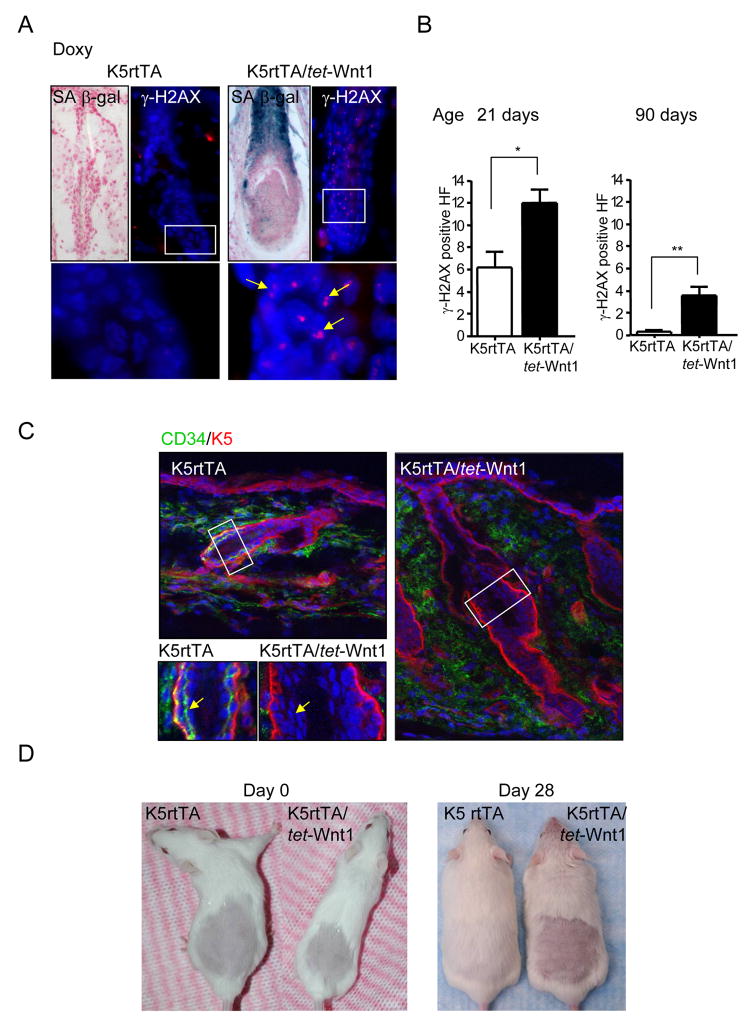
The progressive changes in the HF structures and subsequent hair loss led us to explore whether prolonged exposure to Wnt1 induces cell apoptosis or senescence. While we could not detect the accumulation of apoptotic cells in response to Wnt1 expression (not shown), we observed that Wnt1 can promote cell senescence as judged by the detection of DNA-double strand breaks revealed by the presence of γ-H2AX foci in cell nuclei (Motoyama and Naka, 2004) and the expression of endogenous β-Gal activity at pH 6, a known characteristic of senescent cells (SAβ-Gal) (Dimri et al., 1995) (Figures 5A–B). Surprisingly, this correlated with the complete absence of CD34+ HF stem cells when compared to littermates (Figure 5C). To confirm these results we analyzed the impact of Wnt1 expression on CD34+ cells expressing high and low levels of α6 integrin, each of which represent a distinct subpopulation of hair follicle stem cells harboring self renewal capacity (Blanpain et al., 2004). FACS analysis of 7 weeks old K5rtTA/tet-Wnt1 and control littermate double labeled for CD34 and α6 integrin (CD49f) shown a dramatic reduction in the number of both populations of stem cells (CD34hiα6hi, and CD34hiα6low) in the K5rtTA/tet-Wnt1 mice (Supplementary Figure 4A and B), supporting the emerging notion that Wnt1 promotes the ablation of the hair follicle stem cell compartment. We next ran a functional assay to assess the ability of the stem cell population of our K5rtTA/tet-Wnt1 mice to reactivate a new hair cycle after shaving the dorsal hair of the mice (Sarin et al., 2005). Control and mutant mice received doxycycline pellets 2 days prior to shaving, and hair re-growth was monitored daily for four weeks. Control mice regrew hair in the shaved area within 28 days, while mutant mice failed to regenerate the fur when treated with doxycycline (Figure 5D), even after a follow up of more than 65 days (not shown). Thus, taken together, these data suggest that Wnt1 may induce HF stem cells to exit their quiescent state, which might contribute to the initial proliferative burst of epithelial cells in the HF, followed by the exhaustion of the HF stem cell population.
Figure 5.
Induction of senescence and loss of CD34+ epithelial stem cells in K5rtTA/tet-Wnt1 mice. A) Frozen skin sections of K5rtTA and K5rtTA/tet-Wnt1 mice fed with doxycycline (Doxy) showing SA β-Gal reactivity as a marker of senescence. Mutant HF are positive for this senescence marker while control sample present no staining for SAβ-Gal. Foci of IF staining for the double strand DNA-damage marker γ-H2AX was observed in the nuclei of HF from K5rtTA/tet-Wnt1 mice (γ-H2AX-red, and nuclear staining with DAPI). Higher magnifications are shown below. B) Quantification of γ-H2AX foci in HF from mutant and K5rtTA control mice. Bar graphs represent the quantitative analysis of positive HF, as the average ± S.E.M. of the number of HF presenting γ-H2AX positive cells in 10 fields at 20x magnification from representative animals of the indicated genotype and treatment (**P<0.01; *P <0.05). C) Frozen skin samples were sectioned and stained for keratin 5 (K5) (red) and CD34 (green) markers. K5rtTA mice display strong and well demarked staining for CD34 at the bulge compartment of the HF while no CD34+ cells were observed in the HF of K5rtTA/tet-Wnt1 mice fed with doxycyline. Higher magnifications (lower left) show the presence of CD34+ cells (Arrow) in K5rtTA control mice but absence of CD34+ cells in the K5rtTA/tet-Wnt1 mice (Arrow). D) A cohort of 6 mice per group was shaved simultaneously with the administration of doxycycline diet. By day 28, K5rtTA mice have completely recovered dorsal fur while K5rtTA/tet-Wnt1 doxycycline treated mice failed to recover the shaved area. Representative mice are shown.
A role for mTOR in Wnt-induced cell senescence
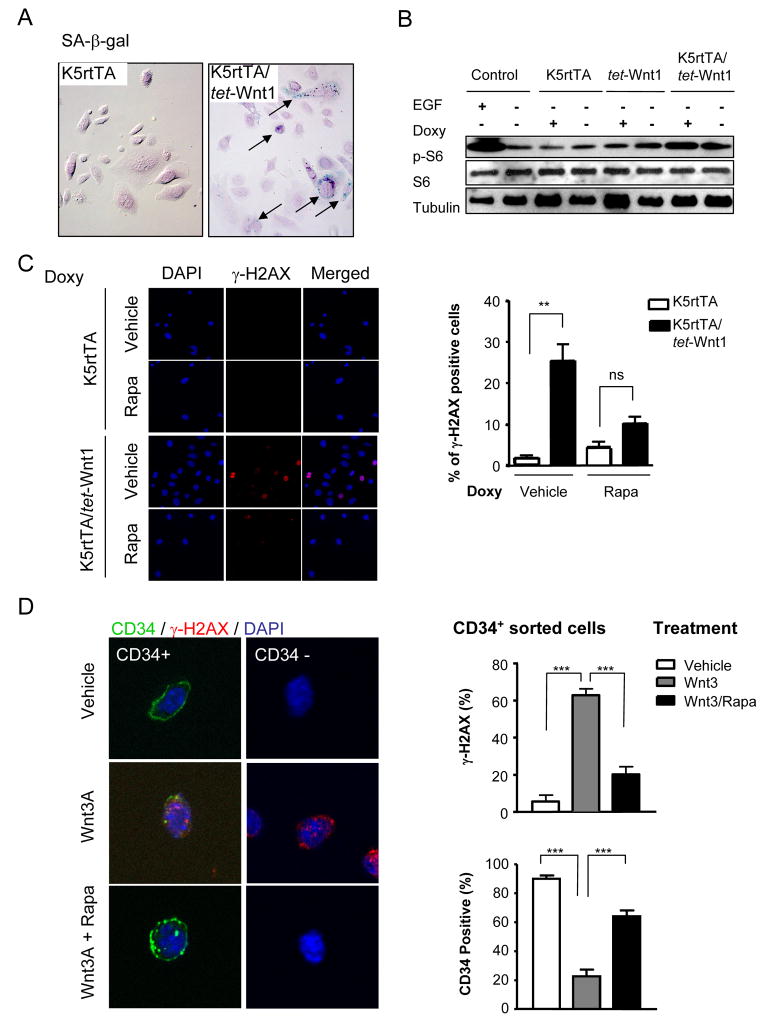
We next explored if the remarkable effects induced by Wnt on the HF were dependent on cell senescence cues generated by the stroma and the surrounding tissues. For that, we cultured in vitro primary keratinocytes from control and K5rtTA/tet-Wnt1 mice and controls. The expression of Wnt1 in vitro could be achieved within the first 24 h of doxycycline treatment, as assessed by luciferase activity (Supplementary Figure 5A). Concomitantly, primary keratinocytes cells from K5rtTA/tet-Wnt1 mice showed early evidence of senescence as judge by SAβ-Gal staining (Figure 6A). We also observed that Wnt1 expression caused increased levels of pS6 (Figure 6B), similar to that of control cells stimulated with EGF, suggesting that Wnt1 can regulate mTOR activity in epithelial cells in vitro and in vivo. This observation prompted us to explore whether mTOR contributes to the senescence phenotype triggered by Wnt1 activation. For this, we took advantage of the ability of rapamycin to inhibit the mTOR pathway (Abraham, 2002). As shown in Figure 6C, exposure of primary keratinocytes from K5rtTA/tet-Wnt1 mice to doxycycline led to the appearance of cells exhibiting nuclear γ-H2AX foci. Surprisingly, however, rapamycin caused a reduction of the number of cells displaying γ-H2AX foci (Figure 6C). We next took advantage of the observation that hair follicle epithelial stem cells express CD34 in their cell surface (Blanpain et al., 2004) to isolate CD34+ and CD34− keratinocytes and explore the consequences of stimulating these cell populations with Wnt Figure 6D). When Wnt was exogenously added to their culture medium, it caused the appearance of nuclear γ-H2AX foci in both CD34− and CD34+ cells, concomitant with the reduction in the number of cells expressing CD34 in CD34+ sorted cell populations (Figure 6D). While rapamycin did not affect the basal expression of CD34 and γ-H2AX (not shown), this mTOR inhibitor prevented the accumulation of nuclear γ-H2AX foci and the decreased CD34 expression caused by exposing sorted CD34+ keratinocytes to Wnt (Figure 6D, right panels).
Figure 6.
Expression of Wnt1 in isolated primary epidermal keratinocytes causes cell senescence through mTOR. A) Primary cultures of keratinocytes derived from K5rtTA, and K5rtTA/tet-Wnt1 mice were treated with doxycycline for 5 days. Detection of senescence was observed in K5rtTA/tet-Wnt1 cells by SA-βgal staining (arrows). B) Monolayers of keratinocytes from K5rtTA, tet-Wnt1, and K5rtTA/tet-Wnt1 mice were starved overnight and treated with doxycycline (24 h). Cells were lysed and analyzed by Western Blotting for phosphorylated S6 (p-S6), total S6 (S6), and tubulin content as a loading control. Total cell lysates from each mouse genotype without doxycycline treatment were used as controls. Cells from K5rtTA mice starved overnight and pretreated for 30 min with EGF (30ng/ml) served as additional controls. C) Primary culture of keratinocytes from transgenic mice were plated onto cover slips and treated with doxycycline (1μg/ml) with or without rapamycin (50nM) for 24 h, as indicated. DAPI nuclear staining and IF for γ-H2AX, were performed as indicated, and images merged in the right panel. Quantification of γ-H2AX staining in K5rtTA, and K5rtTA/tet-Wnt1 derived cells treated with doxycycline plus vehicle or rapamycin. Data are represented as the percentage of γ-H2AX+ cells, expressed as the average ± S.E.M. of 10 fields at 20x magnification from representative cells of the indicated genotype and treatment (**P<0.001, ns: P >0.05). D) Keratinocytes from 7 weeks old mice were sorted into two distinct cellular populations (CD34+ and CD34−). Sorted cells were plated in collagen IV coated dishes and treated as indicated. Cells sorted for CD34 show membrane CD34 staining in vehicle treated cells, which was lost concomitant with the nuclear accumulation of γ-H2AX foci when treated with Wnt3 for 6 days. CD34− cells also accumulated nuclear γ-H2AX foci in response to Wnt3. Rapamycin inhibited γ-H2AX accumulation in both CD34+ and CD34− cells, and prevented the decreased expression of CD34 in CD34+ sorted keratinocytes caused by Wnt3. Quantitative analysis of CD34 and γ-H2AX staining of treated CD34+ sorted keratinocytes is shown in the right. Data are represented as the percentage of positive cells expressed as the average ± S.E.M. in triplicate cultures for each indicated treatment (***P<0.001; **P<0.01; ns: P >0.05).
Based on these results, we asked whether rapamycin administration would affect any of the biological responses provoked by Wnt1 expression in the skin of the K5rtTA/tet-Wnt1 mice in vivo. We observed that the inhibition of mTOR by rapamycin prevented the gross changes caused by Wnt1 in the fur of the transgenic mice (Figure 7A-left). Rapamycin administration also abolished pS6 accumulation in the upper layers of the interfollicular skin and in the IRS region of K5rtTA/tet-Wnt1 treated with doxycycline, and the hyperproliferative response to Wnt1 in the hair follicles and skin of these mice (Figure 7A-right). While rapamycin caused a reduction in cell proliferation in both K5rtTA and K5rtTA/tet-Wnt1 mice (Supplementary Figure 5B), it did not impair the expression of the transgene (Supplementary Figure 5C) and the expression of the β-catenin reporter when stimulated by Wnt1 in K5rtTA/tet-Wnt1/Bat-gal mice (Figure 7B).
Figure 7.
Blockade of mTOR with rapamycin protects from hair follicle alterations, cell senescence, and the depletion of CD34+ epithelial stem cells caused by Wnt1. A) Twenty one days old mice fed with doxycycline pellets received daily doses of rapamycin from 3 days of age, while control littermates received only the vehicle. Rapamycin treatment prevented the development of changes in the fur, a phenotype that can be readily observed in of K5rtTA/tet-Wnt1 treated with doxycycline and vehicle control. Frozen sections were stained for p-S6 immunoreactivity. Rapamycin treatment prevented the activation of p-S6 in the IRS compartment and the histological changes caused by doxycycline in K5rtTA/tet-Wnt1 mice, as observed in H&E histological sections. Note the failure of the lower half of the HF to regress in K5rtTA/tet-Wnt1 mice fed with doxycycline and the re-establishment of a normal hair cycle (telogen) in these mice upon rapamycin treatment. B) Rapamycin did not prevent the expression from the β-catenin reporter system, as judged by the detection of β-Gal in 14 days old K5rtTA/tet-Wnt1/BAT-Gal mice treated with doxycycline and rapamycin. C) K5rtTA/tet-Wnt1 mice were fed with doxycycline by 3 days of age and received daily IP injections of rapamycin or vehicle and sacrificed at 21 days of age. Frozen sections of K5rtTA control mice show the presence of CD34+ epithelial stem cells in the bulge region of the HF, and absence of nuclear γ-H2AX foci. Instead, K5rtTA/tet-Wnt1 mice fed with doxycycline present high number of cells displaying γ-H2AX staining, and lack of CD34+, but both were prevented by the rapamycin treatment. D) Quantification of CD34+ and γ-H2AX staining in K5rtTA/tet-Wnt1 mice fed with doxycycline treated with vehicle control or rapamycin. Data are represented as the percentage of HF displaying CD34+ cells, expressed as the average ± S.E.M. of 10 fields at 20× magnification from representative animals of the indicated genotype and treatment (***P<0.001, *P<0.05), and as the percentage of nuclei exhibiting γ-H2AX positive foci, expressed as the average ± S.E.M. after counting 300 HF cells at 20× magnification from representative animals of the indicated genotype and treatment (**P<0.01; ns: P >0.05).
Rapamycin treatment prevented the accumulation of hair follicles displaying nuclear γ-H2AX foci upon Wnt1 expression in vivo (Figure 7C–D), thus paralleling the results obtained in vitro. Remarkably, rapamycin rescued the loss of CD34+ cells in the bulge region of the hair follicles of Wnt1 expressing mice (Figure 7C–D). Indeed, although K5rtTA/tet-Wnt1 mice treated with rapamycin still had fewer hair follicles, the vast majority of them retained an intact bulge stem cell compartment. This suggests that mTOR function is critical in mediating CD34+ epithelial cell loss in response to Wnt1, while additional rapamycin-insensitive mechanisms might also exist, which may play a more limited role. In fact, rapamycin prevented the long term Wnt1-induced hair loss: K5rtTA/tet-Wnt1 mice treated with doxycycline and rapamycin retained their hair for more than 40 days, a time at which most of these mice had a substantial hair loss if treated with vehicle control instead of rapamycin (not shown). Thus, the mTOR pathway appears to mediate the hyperproliferative response and subsequent aging-like phenotype provoked by Wnt-1 expression in the skin, including the exhaustion of its stem cell compartment.
Discussion
Cells from embryonic tissues are able to divide for a restricted number of times, and then undergo cell division arrest until their death. This phenomenon, known as replicative senescence (Hayflick and Moorhead, 1961), is an irreversible process which can be also triggered by a variety of conditions that result in cellular stress, such as DNA-damage, exposure to cytotoxic drugs and oxidative stress, telomerase dysfunction, and aberrant oncogene-induced proliferative signals, the latter known as oncogene-induced senescence (OIS) (reviewed in Serrano and Blasco, 2001). In normal (non-malignant) cells, OIS or premature activation of senescence can be observed independently of telomeric shortening (Serrano and Blasco, 2001). When initiated by the activation of oncogenic signals, this irreversible process leads to cell cycle arrest, although cells remain alive and functional. Here, we observed that expression of Wnt1 induces the hyperproliferation of HF cells and the rapid exhaustion of their stem cells, as reflected by the ablation of CD34+ cells in the HF stem cell niche, concomitant with the activation of cell senescence pathways. Activation of OIS in our animal model was reflected by the accumulation of the senescence marker γ-H2AX and SAβ-gal and the absence of regrowth of hair after shaving, a functional assay for hair follicle stem activity in vivo (Sarin et al., 2005). We can speculate that the demise of the stem cell reservoir upon persistent mitogenic stimulation may act as a preventive strategy to avoid the incorporation of genetic alterations into self-renewing multipotent cells, and thus its devastating consequences, including tumor formation.
Wnt signaling is vital for axial patterning and progenitor cell fate, as part of a regulatory signaling system conserved from flies to humans (Nusse, 2008). Indeed, Wnts function in a wide range of developmental processes (Chang et al., 2004; Olivera-Martinez et al., 2001) as well as in the control of proliferation and differentiation (Moon et al., 2004; Nusse, 2008; Reya and Clevers, 2005). Aberrant activation of Wnt1 is associated with tumor development in many organs including the mammary gland (reviewed in Lindvall et al., 2007). In our animal model, we were surprised to find that epidermal activation of the Wnt1-initiated pathway did not lead to tumor formation as expected based on prior studies utilizing a stable, active form of β-catenin (Gat et al., 1998). Instead, Wnt1 triggered HF to grow and maintain a persistent proliferative state rather than entering consecutive cyclic programs of proliferation, regression, and resting states. Most importantly, we did not find any signs of dysplasia, metaplasia, or neoplasia, even after prolonged (>1 year) observation. A plausible explanation for the difference between the epidermal phenotypes of the Wnt1 and β-catenin animal models is that the emerging complexity of the Wnt-induced pathways (Moon et al., 2004; Reya and Clevers, 2005) can only be partially mimicked by the expression of truncated forms of β-catenin. In this scenario, whereas active forms of β-catenin can promote cancer progression, Wnt stimulates β-catenin but it may also initiate the concomitant activation of cell senescence programs thereby protecting from tumoral growth.
Each new hair cycle is initiated when epidermal stem cells located in the bulge region migrate to the bulb area of the HF where they proliferate and give raise to all cell lineages present in the HF (Alonso and Fuchs, 2003; Morris et al., 2004). In this regard, the appropriate balance between the proliferative and quiescent states is critical to maintaining a functional stem cell compartment (Cheng et al., 2000; Kobielak et al., 2007). Thus, the proper regulation of cycles of growth and rest may enhance the lifespan of HF stem cells and lengthen the overall time that hair is present as the animals age. Indeed, the fact that maintaining a persistent HF growth phase results in the disappearance of its stem cell compartment suggests that this cyclic growth and rest may be required to preserve the self renewal capacity of epidermal stem cells, which under normal circumstances proliferate only rarely (Alonso and Fuchs, 2003; Kobielak et al., 2007; Morris et al., 2004). We interpret our results to show that constant proliferation of the HF structures results in the exhaustion of their stem cell pool, ultimately leading to premature hair loss.
The impact of Wnt expression on the stem cells is likely highly tissue specific. For example, when Wnt1 is overexpressed in the mammary, a rapid expansion of its stem cell compartment is observed (Cho et al., 2008; Li et al., 2000), which may contribute to tumor formation upon acquisition of genetic alterations in tumor suppressor pathways, including p53 (Debies et al., 2008). In the case of the HF, however, their stem cells may be particularly prone to senescence upon aberrant activation of pro-proliferative pathways. This may represent an overriding protective mechanism considering that epithelial cells in the hair follicles and skin have to withstand multiple environmental DNA-damaging agents (Cang et al., 2007). We can hypothesize that under oncogenic stress or upon persistent growth promotion, the stem cell compartment of HF might be transiently expanded, but eventually becomes exhausted therefore halting the carcinogenic process by limiting the life-span and self-renewing capacity of their stem cells. Indeed, this scenario may in turn explain the tissue specific nature of the response to Wnt1 expression, which can cause either cell senescence or tumors formation, depending on the presence or not of specific protective antitumoral mechanism in each particular stem cell population.
On the other hand, while ectopic activation of the mTOR pathway has been implicated in tumor progression (Sabatini, 2006; Shaw and Cantley, 2006), mTOR also controls the life span in C. elegans, yeast, worms, and Drosophila (reviewed in Schieke and Finkel, 2006), with decreased function of mTOR leading to an increase in the life span (reviewed in Powers et al., 2006). Here, we provide evidence that mTOR represents a key downstream component of the pathway by which Wnt1 activation can lead to cell growth and tissue aging. Wnt activates mTOR by a recently elucidated mechanism that involves the inhibition of GSK3 (Inoki et al., 2006). In this case, GSK3 enhances the activity of the tumor suppressor protein TSC2, a GTPase activating protein that diminishes the GTP-bound levels of RheB1, a small GTPase that activates mTOR. Thus, by inhibiting GSK3, Wnt reduces the inhibitory effect of TSC2 on mTOR, resulting in enhanced mTOR function (Inoki et al., 2006). Indeed, activation of mTOR, as judged by pS6 accumulation, occurred in the same cell compartment in which we observed the increased expression from a β-catenin reporter system and the enhanced expression of a Wnt-regulated protein, WISP-1, supporting that the stimulation of mTOR is a direct consequence of Wnt1 expression. This activation of mTOR by Wnt1 contributes to the aberrant growth of mammary gland cells (Inoki et al., 2006). In the skin, however, the mTOR-dependent persistent proliferation of epithelial cells within the HF in response to Wnt1, or the direct activation of mTOR in HF stem cells, may result in the exhaustion of the HF stem cells compartment. In this scenario, excessive proliferation and activation of mTOR in stem cells may increase their turnover, prematurely exhausting the ability of these stem cells to repopulate the hair follicle. Consistent with this, pharmacological inhibition of mTOR with rapamycin was sufficient to prevent stem cell ablation upon Wnt1 expression, as reflected the maintenance of the population of CD34+ bulge stem cells. mTOR inhibition also impaired the acquisition of a premature senescence phenotype caused by Wnt1 expression in mice and by the exposure of CD34+ cells to Wnt, thus raising the possibility that mTOR may contribute to OIS when triggered by the aberrant activity of other oncogenes, an area that will warrant further investigation.
The emerging picture from our study and recent published reports is that Wnt1 may promote the proliferation and regenerative capacity of tissue-specific stem cells (Katoh and Katoh, 2007), and that mTOR may play a key role as part of the growth promoting pathway by which Wnt acts (Inoki et al., 2006). Long term activation of Wnt, however, may cause cell senescence and the exhaustion or demise of the stem cell compartment by the persistent activation of mTOR. While the exhaustion of the stem cells may act as a protective mechanism against oncogenic perturbation of this particular self-renewing cell population, the persistent activation of mTOR may also contribute to accelerating aging, hence providing a novel molecular target for pharmacological intervention in multiple diseases that are characterized by the pathological depletion of stem cells, loss of tissue regenerative capacity, and tissue aging.
Materials and Methods
Experimental mice
All animal studies were carried out according to NIH approved protocols, in compliance with the Guide for the Care and Use of Laboratory Animals. FVB/N mice carrying the cytokeratin 5 promoter in the reverse tetracycline transactivator (rTA) (k5-tet-on) and Wnt1-transgenic mouse expressing Wnt1 and IRES-firefly luciferase downstream of the seven tet-responsive elements have been previously described (Gunther et al., 2003; Vitale-Cross et al., 2004). Doxycycline grain-based pellets (Bioserv N.J., USA) were administered in a concentration of 6 g/kg. To determine the canonical Wnt activation status during the HF cycle, we used the β-catenin-activated transgene driving expression of nuclear β-galactosidase reporter (BAT-Gal) transgenic mice (Maretto et al., 2003). All transgenic lines were maintained on an FVB/N background. See Supplemental information for additional procedures.
BrDU, cell proliferation assay, and primary culture of murine keratinocyte
Cell proliferation study was conducted after injection of BrDU (100μg/g body weight) 2 h before sacrificing the animals. Primary culture of keratinocytes and [3H]-thymidine incorporation was performed as previously described (Castilho et al., 2007), with the administration of doxycycline (1μg/ml) concomitant or not with the administration of rapamycin (50 nM) for 48 h. Sorted cells were assessed for viability with trypan blue (Sigma) staining and cultured on collagen coated dishes with 15% serum and 0.3 mM of calcium. For immunofluorescence staining, cells were plated in collagen coated glass slides.
FACS analysis and cell Sorting
Seven week old K5rtTA/Wnt1 mice and control littermates where used for each experiment, as previously described (Blanpain et al., 2004). Briefly, the subcutis underlying the dorsal skin was removed using a scalpel, and the skin was trypsinized for 2 hours at 37°C and filtered through a 70 μm mesh filter (BD Bioscience) to achieve single cell suspension. Cells were then incubated with 2%FCS in PBS on ice for 30 minutes followed by the primary antibody (CD34 1:100, CD49f 1:50) coupled with FITC and PE/Cye5 fluorochrome, respectively, for 30 min. FACS analysis was performed in a FACSCalibur flow cytometer (BD Bioscience). Cell sorting was performed in a DAKO-Cytomation MoFlo High Speed Sorter.
IHC, IF, antibodies, reagents, and immunobloting
Please find detailed information in the supplemental data.
Rapamycin administration
Rapamycin (LC Laboratories) was administered through intraperitoneal injections at a dose of 4 mg/kg per day for the indicated time.
Supplementary Material
Acknowledgments
We are grateful to Dr. R. M. Porter (Wales College of Medicine) for the polyclonal antibody against IRS, and to Dr. T. Finkel (National Heart, Lung, and Blood Institute/NIH) for critical reading of the manuscript. This research was supported by the Intramural Research Program and the Craniofacial Tissue Remodeling Initiative of the NIH, NIDCR.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham RT. Identification of TOR signaling complexes: more TORC for the cell growth engine. Cell. 2002;111:9–12. doi: 10.1016/s0092-8674(02)01009-7. [DOI] [PubMed] [Google Scholar]
- Alonso L, Fuchs E. Stem cells in the skin: waste not, Wnt not. Genes Dev. 2003;17:1189–1200. doi: 10.1101/gad.1086903. [DOI] [PubMed] [Google Scholar]
- Andl T, Reddy ST, Gaddapara T, Millar SE. WNT signals are required for the initiation of hair follicle development. Dev Cell. 2002;2:643–653. doi: 10.1016/s1534-5807(02)00167-3. [DOI] [PubMed] [Google Scholar]
- Blanpain C, Fuchs E. Epidermal homeostasis: a balancing act of stem cells in the skin. Nature reviews. 2009;10:207–217. doi: 10.1038/nrm2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004;118:635–648. doi: 10.1016/j.cell.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Blau HM, Brazelton TR, Weimann JM. The evolving concept of a stem cell: entity or function? Cell. 2001;105:829–841. doi: 10.1016/s0092-8674(01)00409-3. [DOI] [PubMed] [Google Scholar]
- Brennan KR, Brown AM. Wnt proteins in mammary development and cancer. J Mammary Gland Biol Neoplasia. 2004;9:119–131. doi: 10.1023/B:JOMG.0000037157.94207.33. [DOI] [PubMed] [Google Scholar]
- Cang Y, Zhang J, Nicholas SA, Kim AL, Zhou P, Goff SP. DDB1 is essential for genomic stability in developing epidermis. Proc Natl Acad Sci U S A. 2007;104:2733–2737. doi: 10.1073/pnas.0611311104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castilho RM, Squarize CH, Patel V, Millar SE, Zheng Y, Molinolo A, Gutkind JS. Requirement of Rac1 distinguishes follicular from interfollicular epithelial stem cells. Oncogene. 2007;26:5078–5085. doi: 10.1038/sj.onc.1210322. [DOI] [PubMed] [Google Scholar]
- Chang CH, Jiang TX, Lin CM, Burrus LW, Chuong CM, Widelitz R. Distinct Wnt members regulate the hierarchical morphogenesis of skin regions (spinal tract) and individual feathers. Mech Dev. 2004;121:157–171. doi: 10.1016/j.mod.2003.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng T, Rodrigues N, Shen H, Yang Y, Dombkowski D, Sykes M, Scadden DT. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science. 2000;287:1804–1808. doi: 10.1126/science.287.5459.1804. [DOI] [PubMed] [Google Scholar]
- Cho RW, Wang X, Diehn M, Shedden K, Chen GY, Sherlock G, Gurney A, Lewicki J, Clarke MF. Isolation and molecular characterization of cancer stem cells in MMTV-Wnt-1 murine breast tumors. Stem Cells. 2008;26:364–371. doi: 10.1634/stemcells.2007-0440. [DOI] [PubMed] [Google Scholar]
- Debies MT, Gestl SA, Mathers JL, Mikse OR, Leonard TL, Moody SE, Chodosh LA, Cardiff RD, Gunther EJ. Tumor escape in a Wnt1-dependent mouse breast cancer model is enabled by p19Arf/p53 pathway lesions but not p16 Ink4a loss. J Clin Invest. 2008;118:51–63. doi: 10.1172/JCI33320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E. Keratins and the skin. Annu Rev Cell Dev Biol. 1995;11:123–153. doi: 10.1146/annurev.cb.11.110195.001011. [DOI] [PubMed] [Google Scholar]
- Gat U, DasGupta R, Degenstein L, Fuchs E. De Novo hair follicle morphogenesis and hair tumors in mice expressing a truncated beta-catenin in skin. Cell. 1998;95:605–614. doi: 10.1016/s0092-8674(00)81631-1. [DOI] [PubMed] [Google Scholar]
- Greco V, Chen T, Rendl M, Schober M, Pasolli HA, Stokes N, Dela Cruz-Racelis J, Fuchs E. A two-step mechanism for stem cell activation during hair regeneration. Cell Stem Cell. 2009;4:155–169. doi: 10.1016/j.stem.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunther EJ, Moody SE, Belka GK, Hahn KT, Innocent N, Dugan KD, Cardiff RD, Chodosh LA. Impact of p53 loss on reversal and recurrence of conditional Wnt-induced tumorigenesis. Genes Dev. 2003;17:488–501. doi: 10.1101/gad.1051603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- Inoki K, Ouyang H, Zhu T, Lindvall C, Wang Y, Zhang X, Yang Q, Bennett C, Harada Y, Stankunas K, et al. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell. 2006;126:955–968. doi: 10.1016/j.cell.2006.06.055. [DOI] [PubMed] [Google Scholar]
- Ito M. The innermost cell layer of the outer root sheath in anagen hair follicle: light and electron microscopic study. Arch Dermatol Res. 1986;279:112–119. doi: 10.1007/BF00417531. [DOI] [PubMed] [Google Scholar]
- Katoh M, Katoh M. WNT signaling pathway and stem cell signaling network. Clin Cancer Res. 2007;13:4042–4045. doi: 10.1158/1078-0432.CCR-06-2316. [DOI] [PubMed] [Google Scholar]
- Kirstetter P, Anderson K, Porse BT, Jacobsen SE, Nerlov C. Activation of the canonical Wnt pathway leads to loss of hematopoietic stem cell repopulation and multilineage differentiation block. Nat Immunol. 2006;7:1048–1056. doi: 10.1038/ni1381. [DOI] [PubMed] [Google Scholar]
- Klaus A, Birchmeier W. Wnt signalling and its impact on development and cancer. Nat Rev Cancer. 2008;8:387–398. doi: 10.1038/nrc2389. [DOI] [PubMed] [Google Scholar]
- Kobielak K, Stokes N, de la Cruz J, Polak L, Fuchs E. Loss of a quiescent niche but not follicle stem cells in the absence of bone morphogenetic protein signaling. Proc Natl Acad Sci U S A. 2007;104:10063–10068. doi: 10.1073/pnas.0703004104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Hively WP, Varmus HE. Use of MMTV-Wnt-1 transgenic mice for studying the genetic basis of breast cancer. Oncogene. 2000;19:1002–1009. doi: 10.1038/sj.onc.1203273. [DOI] [PubMed] [Google Scholar]
- Lindvall C, Bu W, Williams BO, Li Y. Wnt signaling, stem cells, and the cellular origin of breast cancer. Stem Cell Rev. 2007;3:157–168. doi: 10.1007/s12015-007-0025-3. [DOI] [PubMed] [Google Scholar]
- Liu F, Kohlmeier S, Wang CY. Wnt signaling and skeletal development. Cell Signal. 2008;20:999–1009. doi: 10.1016/j.cellsig.2007.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Fergusson MM, Castilho RM, Liu J, Cao L, Chen J, Malide D, Rovira II, Schimel D, Kuo CJ, et al. Augmented Wnt signaling in a mammalian model of accelerated aging. Science. 2007;317:803–806. doi: 10.1126/science.1143578. [DOI] [PubMed] [Google Scholar]
- Lynch MH, O’Guin WM, Hardy C, Mak L, Sun TT. Acidic and basic hair/nail (“hard”) keratins: their colocalization in upper cortical and cuticle cells of the human hair follicle and their relationship to “soft” keratins. J Cell Biol. 1986;103:2593–2606. doi: 10.1083/jcb.103.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maretto S, Cordenonsi M, Dupont S, Braghetta P, Broccoli V, Hassan AB, Volpin D, Bressan GM, Piccolo S. Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proc Natl Acad Sci U S A. 2003;100:3299–3304. doi: 10.1073/pnas.0434590100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon RT, Kohn AD, De Ferrari GV, Kaykas A. WNT and beta-catenin signalling: diseases and therapies. Nat Rev Genet. 2004;5:691–701. doi: 10.1038/nrg1427. [DOI] [PubMed] [Google Scholar]
- Morris RJ, Liu Y, Marles L, Yang Z, Trempus C, Li S, Lin JS, Sawicki JA, Cotsarelis G. Capturing and profiling adult hair follicle stem cells. Nat Biotechnol. 2004;22:411–417. doi: 10.1038/nbt950. [DOI] [PubMed] [Google Scholar]
- Motoyama N, Naka K. DNA damage tumor suppressor genes and genomic instability. Curr Opin Genet Dev. 2004;14:11–16. doi: 10.1016/j.gde.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Nusse R. Wnt signaling and stem cell control. Cell Res. 2008;18:523–527. doi: 10.1038/cr.2008.47. [DOI] [PubMed] [Google Scholar]
- Olivera-Martinez I, Thelu J, Teillet MA, Dhouailly D. Dorsal dermis development depends on a signal from the dorsal neural tube, which can be substituted by Wnt-1. Mech Dev. 2001;100:233–244. doi: 10.1016/s0925-4773(00)00540-2. [DOI] [PubMed] [Google Scholar]
- Paus R, Muller-Rover S, Van Der Veen C, Maurer M, Eichmuller S, Ling G, Hofmann U, Foitzik K, Mecklenburg L, Handjiski B. A comprehensive guide for the recognition and classification of distinct stages of hair follicle morphogenesis. The Journal of investigative dermatology. 1999;113:523–532. doi: 10.1046/j.1523-1747.1999.00740.x. [DOI] [PubMed] [Google Scholar]
- Porter RM, Gandhi M, Wilson NJ, Wood P, McLean WH, Lane EB. Functional analysis of keratin components in the mouse hair follicle inner root sheath. Br J Dermatol. 2004;150:195–204. doi: 10.1111/j.1365-2133.2004.05720.x. [DOI] [PubMed] [Google Scholar]
- Powers RW, 3rd, Kaeberlein M, Caldwell SD, Kennedy BK, Fields S. Extension of chronological life span in yeast by decreased TOR pathway signaling. Genes Dev. 2006;20:174–184. doi: 10.1101/gad.1381406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy S, Andl T, Bagasra A, Lu MM, Epstein DJ, Morrisey EE, Millar SE. Characterization of Wnt gene expression in developing and postnatal hair follicles and identification of Wnt5a as a target of Sonic hedgehog in hair follicle morphogenesis. Mech Dev. 2001;107:69–82. doi: 10.1016/s0925-4773(01)00452-x. [DOI] [PubMed] [Google Scholar]
- Reddy ST, Andl T, Lu MM, Morrisey EE, Millar SE. Expression of Frizzled genes in developing and postnatal hair follicles. J Inves Dermatol. 2004;123:275–282. doi: 10.1111/j.0022-202X.2004.23215.x. [DOI] [PubMed] [Google Scholar]
- Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- Sabatini DM. mTOR and cancer: insights into a complex relationship. Nat Rev Cancer. 2006;6:729–734. doi: 10.1038/nrc1974. [DOI] [PubMed] [Google Scholar]
- Sarin KY, Cheung P, Gilison D, Lee E, Tennen RI, Wang E, Artandi MK, Oro AE, Artandi SE. Conditional telomerase induction causes proliferation of hair follicle stem cells. Nature. 2005;436:1048–1052. doi: 10.1038/nature03836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheller M, Huelsken J, Rosenbauer F, Taketo MM, Birchmeier W, Tenen DG, Leutz A. Hematopoietic stem cell and multilineage defects generated by constitutive beta-catenin activation. Nat Immunol. 2006;7:1037–1047. doi: 10.1038/ni1387. [DOI] [PubMed] [Google Scholar]
- Schieke SM, Finkel T. Mitochondrial signaling, TOR, and life span. Biol Chem. 2006;387:1357–1361. doi: 10.1515/BC.2006.170. [DOI] [PubMed] [Google Scholar]
- Serrano M, Blasco MA. Putting the stress on senescence. Curr Opin Cell Biol. 2001;13:748–753. doi: 10.1016/s0955-0674(00)00278-7. [DOI] [PubMed] [Google Scholar]
- Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441:424–430. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- Squarize CH, Castilho RM, Sriuranpong V, Pinto DS, Jr, Gutkind JS. Molecular cross-talk between the NFkappaB and STAT3 signaling pathways in head and neck squamous cell carcinoma. Neoplasia. 2006;8:733–746. doi: 10.1593/neo.06274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Noort M, Meeldijk J, van der Zee R, Destree O, Clevers H. Wnt signaling controls the phosphorylation status of beta-catenin. J Biol Chem. 2002;277:17901–17905. doi: 10.1074/jbc.M111635200. [DOI] [PubMed] [Google Scholar]
- Vitale-Cross L, Amornphimoltham P, Fisher G, Molinolo AA, Gutkind JS. Conditional expression of K-ras in an epithelial compartment that includes the stem cells is sufficient to promote squamous cell carcinogenesis. Cancer Res. 2004;64:8804–8807. doi: 10.1158/0008-5472.CAN-04-2623. [DOI] [PubMed] [Google Scholar]
- Wendel HG, De Stanchina E, Fridman JS, Malina A, Ray S, Kogan S, Cordon-Cardo C, Pelletier J, Lowe SW. Survival signalling by Akt and eIF4E in oncogenesis and cancer therapy. Nature. 2004;428:332–337. doi: 10.1038/nature02369. [DOI] [PubMed] [Google Scholar]
- Xu L, Corcoran RB, Welsh JW, Pennica D, Levine AJ. WISP-1 is a Wnt-1-and beta-catenin-responsive oncogene. Genes Dev. 2000;14:585–595. [PMC free article] [PubMed] [Google Scholar]
- Zechner D, Fujita Y, Hulsken J, Muller T, Walther I, Taketo MM, Crenshaw EB, 3rd, Birchmeier W, Birchmeier C. beta-Catenin signals regulate cell growth and the balance between progenitor cell expansion and differentiation in the nervous system. Dev Biol. 2003;258:406–418. doi: 10.1016/s0012-1606(03)00123-4. [DOI] [PubMed] [Google Scholar]
- Zhu AJ, Watt FM. beta-catenin signalling modulates proliferative potential of human epidermal keratinocytes independently of intercellular adhesion. Development. 1999;126:2285–2298. doi: 10.1242/dev.126.10.2285. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.