Abstract
Hypoxia induced factor-1α (HIF-1α) is a master transcription factor that is critical for the regulation of various cellular functions. HIF-1α is rapidly degraded via a von Hippel-Lindau tumor suppressor gene product (VHL)-mediated ubiquitin-proteasome pathway in normoxia conditions. Most recent studies reveal that heat-shock proteins (Hsp) can regulate HIF-1α protein degradation via a VHL-independent pathway. Here we demonstrate that c-Jun N-terminal kinase 1 (JNK1) is required for such an Hsp-dependent HIF-1α regulation. HIF-1α stabilization was impaired in JNK1−/− cells, and could be rescued by reconstitutional expression of JNK1 in either hypoxia or chemical-mimicked hypoxia conditions. This phenomena was confirmed in different cell lines using the shRNA-JNK1 knockdown approach. Accordingly, HIF-1-dependent transcriptional activity and its downstream gene’s expression were dramatically reduced in JNK1-deficient cells. We further found that in JNK1−/− cells the low expression level of Hsp90/Hsp70 proteins affected the protective roles of these chaperones in maintaining newly synthesized HIF-1α stabilization, and forced expression of Hsp90 or Hsp70 in JNK1−/− cells showed a notable increase in HIF-1α stability compared with that of parental cells. Furthermore, our studies found that defective HDAC6 expression and subsequently increased Hsp90 acetylation could account for the reduction of Hsp90 chaperon activity in JNK1−/− cells. Taken together, our studies provide strong evidence for a novel function of JNK1 in regulating VHL-independent HIF-1α degradation.
Keywords: HIF-1α, JNKs, Hsp90, Hsp70, nickel
Introduction
Hypoxia-inducible factor-1 (HIF-1) is a heterodimeric transcription factor that plays a key role in cellular adaptations to hypoxia (deficiency of oxygen supply) by controlling the expression of a series of genes involved in angiogenesis, oxygen transport and glucose metabolism (1). Hypoxia occurs in many pathological processes, including ischemic heart disease, stroke, cancer, chronic lung disease, and congestive heart failure (2). Thus, HIF-1 has attracted a great deal of attention in the past few decades and serves as a pharmaceutical and gene therapeutic target for many diseases (3). HIF-1 consists of an oxygen-regulated HIF-1α subunit and a constitutively expressed hydrocarbon receptor nuclear translocator (also called HIF-1β or ARNT). While both HIF-1α and HIF-1β are required for formation of HIF heterodimer, HIF-1α is a key regulatory subunit responsible for HIF transcriptional function (4). In normoxia, HIF-1α is a very unstable protein with a half-life of less than 5 min. This rapid turnover is mediated by the ubiquitin-proteasome protein degradation system and requires hydroxylation of prolyl residues (P402 and P564) in the conserved oxygen-dependent degradation domain of HIF-1α. Such hydroxylation facilitates HIF-1α binding to VHL, which leads to the recognition of HIF-1α by the E3 ligase complex for proteasomal degradation (5). This hydroxylation of HIF-1α is executed by the mammalian prolyl hydroxylase domain enzymes (PHD1-3), which require oxygen, ferrous ion and 2-oxoglutarate for their activity (6). Therefore, deprivation of oxygen or treatment of cells with transition metals (cobalt and nickel), iron chelators (deferoxamine) or the 2-oxoglutarate inhibitor dimethyloxalylglycine (DMOG), could impair the activity of PHDs leading to inhibition of HIF-1α hydroxylation and subsequently result in HIF-1α protein accumulation (7). In the present studies, we used hypoxia and several chemical mimicked hypoxia conditions to increase HIF-1α protein levels by blocking the PHDs/VHL-dependent degradation pathway, and found that under the above conditions, the HIF-1α protein accumulation was impaired when JNK1 expression was deficient. Therefore, our results revealed that JNK1 participated in the VHL-independent HIF-1α regulating mechanism.
The JNKs are implicated in several physiological processes, including proliferation, apoptosis and differentiation (8). Although JNK1 or JNK2 mutations are not prevalent in cancer, many tumor cell lines have been reported to possess constitutively active JNKs (9, 10). The expression of JNK1 was increased markedly in breast cancer tissue compared to normal samples (11). Recent studies from Chen’s lab also indicated that JNK1 played pivotal role in the expression of the key signature genes and the prognostic outcomes of human hepatocellular carcinoma (12). The molecular mechanisms underlying the oncogenic role of JNK1 need to be further exploited. Our previous studies have demonstrated that JNK1 is responsible for the nickel-related COX-2 induction, a key molecule involved in inflammatory response as well as tumor development (13). Our present study further definitely shows that JNK1 is also implicated in HIF-1α stabilization in nickel mimicked hypoxia conditions, which adds another evidence for the oncogenic role of JNK1 in nickel-related carcinogenesis.
Materials and methods
Cell Culture and reagents
Beas-2B cells, HaCat cells, 293T cells, and MEFs were maintained at 37°C in a 5% CO2 incubator with DMEM supplemented with 10% FBS (14); A549 cells were cultured with Ham’sF-12K medium supplemented with10% FBS; Hela and Cl41 cells were cultured in MEM with 10% FBS and 5% FBS, respectively. Cycloheximide (CHX), trichostatin A (TSA), leupeptin, rapamycin, MG132, Sp600125 and novobiocin were purchased from Calbiochem (San Diego, CA). Nickel chloride was from Sigma-Aldrich (St. Louis, MO). DMOG was from Frontier Scientific (Logan, UT). Topotecan, UBEI-41 and tamoxifen were from Alexis Biochemicals Corporation (San Diego, CA). Antibodies against HIF-1α, PHD1 or PHD3 were purchased from Novus Biologicals, Inc. (Littleton, CO); Anti-VHL antibody was from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA); Antibody against Hsp90 was from Stressgene (Ann Arbor, MC); Anti-JNK1 antibody was from Invitrogen (Carlsbad, California); Anti-pan lysine acetylation, Hsp70, ATF2, total p70S6 kinase, phosph-p70S6K(Thr389, Thr421) and HDAC6 antibodies were from Cell Signaling Technology (Beverly, MA), α-Tubulin andβ-Actin antibodies were purchased from Sigma.
Constructs and Transfections
HRE and VEGF-luciferase reporters were described as previously (15, 16), and transfected in WT MEFs and JNK1−/− cells. Hsp90 expression vector (17) or Hsp70 expression vector (18) was transfected into JNK1−/− cells, and the transfectants were established and named as JNK1−/−(Hsp90) or JNK1−/−(Hsp70). The dominant-negative mutant of HIF-1α (DN-HIF-1α) (19, 20) was transfected into HaCat cells. A catalytically inactive mutant of HDAC6 construct (HDAC6-DC, H216/611A) (21), was transfected into WT MEFs. The shRNA-JNK1 set (RHS4531) was purchased from Open Biosystems (Thermo Fisher Scientific., Huntsville, AL).
Reverse transcription polymerase chain reaction (RT-PCR)
Total RNA was extracted using Trizol reagent (Invitrogen) following various treatment. Total cDNAs were synthesized by ThermoScript ™ RT-PCR system (Invitrogen). The mRNA amount present in the cells was measured by semi-quantitative RT-PCR. The primers for mouse hif-1α were 5′-AGC CCT AGA TGG CTT TGT GA-3′ and 5′-TAT CGA GGC TGT GTC GAC TG-3′, for mouse hsp90 were 5′-GTG TGC AAC AGC TGA AGG AA-3′ and 5′-ACA GCA GCA CTG GTG TCA TC-3′, for mouse hsp70 were 5′-CGA CCT GAA CAA GAG CAT CA-3′ and 5′-ATG ACC TCC TGG CAC TTG TC-3′, and for mouse hdac6 were 5′-CCC CAA TCT AGC GGA GGT AAA-3′ and 5′-CAT GAG TGC ATC TAC CAG CCG-3′. The mouse β-actin was used as control (22). The PCR products were separated on 2% agarose gels, stained with EB. The results were imagined with Alpha Innotech SP image system (Alpha Innotech Corporation., San Leandro, CA).
Luciferase reporter assay
The cells stably transfected with HRE or VEGF luciferase reporters were seeded into 96-well plates (8×103/well) and subjected to the various treatments. Luciferase activities were determined using a luminometer (Wallac 1420 Victor 2multilabel counter system) as described previously (23).
Cell transformation protocols
Cells were exposed to 1 mM NiCl2 for 24 h. Nickel containing medium was then removed by rinsing each well with PBS three times. Nickel-treated cells were cultured in fresh 10% FBS DMEM medium for 2 days. The cultures were split and subjected to another round of treatment. In total, nickel exposure was repeated twice a week for 8 weeks. For the Sp600125 or topotecan co-exposure groups, cells were pretreated with either Sp600125 (50 μM) or topotecan (1 μM) for 4 h before each of nickel exposure. After completion of 8-week nickel exposure, nickel-induced anchorage-independent growth capability was evaluated in the soft agar assay as described previously (24). The colonies were scored under microscopy.
Nuclear extraction
WT and JNK1−/− MEFs were seeded into 10-cm dishes and treated with 0.5 mM NiCl2 for 12 h. The nuclear proteins were extracted according to the protocol of Nuclear/Cytosol Fractionation Kit (Biovision Incorporated, Mountain View, CA).
Western blotting
After various treatments, cells were extracted in a cell lysis buffer and total protein was quantified with a Dc protein assay kit (Bio-Rad, Hercules, CA). Western Blotting was carried out as described previously (25).
Pulse and Pulse-chase assays
Cells (1 × 106) were seeded into 10 cm dishes and cultured for 24 h. Cells were exposed to nickel (0.5 mM) for 12 h and then incubated with methionine-cysteine-free DMEM (Gibco-BRL) containing2% FBS for 1 h. In the pulse assay, [35S]-labelled methionine/cysteine (Trans 35S-Label; ICN, 250 μCi/dish) was added and cultured for indicated time periods and the cells were collected for immunoprecipitation. In the pulse-chase assay, after the cells were pulsed for 45 min, cells were washed three times in PBS, and cold DMEM with 2% FBS (containing L-methionine 100 μg/mL and L-cystein 500 μg/mL) was then added. Cells were harvested at different time points in cell lysis buffer (1%Triton X-100, 150 mM NaCl, 10 mM Tris, pH 7.4, 1 mM EDTA, 1mM EGTA, 0.2 mM Na3VO4, 0.5% NP-40, and complete protein inhibitorsmixture tablet) on ice. 0.5 mg total lysate was incubated with 2 μg of anti-HIF-1α monoclonal antibody(Sigma) for 2 h at 4°C. Then Protein A/G plus-agarose beads (Santa Cruz Biotechnology, Inc.) that were pre-cleared by 20 mg/ml BSA for 2 h was added into the mixture and incubated with agitation for an additional 2 h at 4°C. The immunoprecipitated samples were washed with the cell lysis buffer and heated at 100°C for 5 min. Radiolabeled HIF-1α protein as well as the input cell lysate were assessed using SDS-PAGE analysis.
Immunoprecipitation
0.5 mg total lysate from WT and JNK1−/− MEFs was incubated with 2 μg anti-Hsp90 antibody for 2 h at 4°C. 40 μl Protein A/G plus-agarose were added into the mixture and incubated with agitation for an additional 4 h at 4°C. The immunoprecipitated samples were washed with the cell lysis buffer and subjected to the Western blot assay with the anti-pan-lysine-acetylation and anti-Hsp90 antibodies.
Results
Knockout of JNK1 gene impairs HIF-1α stabilization and the transactivation activity of HIF-1
Exposure of WT MEFs to nickel chloride at concentrations ranging from 0.125 mM to 0.5 mM induced an accumulation of HIF-1α protein in a dose-dependent manner, and the induction of HIF-1α was sustained at all time points tested. The stabilization of HIF-1α protein by nickel was reproducible in different cell lines, including mouse epidermal cell JB6 Cl41, human normal keratinocyte HaCat, human bronchial epithelial cell Beas-2B, and human cervix adenocarcinoma Hela cells (Fig. 1A), indicating that HIF-1α induction by nickel exposure is not cell-type specific. Nickel exposure elevated HIF-1-dependent transcriptional activity significantly in various cell types by using HRE luciferase reporter. Accordingly, VEGF, a HIF-1α downstream target gene, was also induced by nickel exposure in a VEGF-luciferase reporter assay (Fig. 1B). In addition, a HIF-1α chemical inhibitor, topotecan (26, 27), and a HIF-1α dominant negative mutant plasmid, DN-HIF-1α (19, 20), were used to further confirm that VEGF induction by nickel was directly dependent on HIF-1α transactivation (Fig. 1C). Then, we detected the involvement of HIF-1α in nickel carcinogenesis by evaluating anchorage-independent growth capability in response to nickel exposures. As shown in Fig. 1D, repeated exposure of cells to nickel for 8 weeks led to cell transformation in soft agar assay. Furthermore, co-incubation of cells with topotecan significantly reduced colony formation induced by nickel, strongly suggesting the involvement of HIF-1α in the carcinogenic effect of nickel exposure.
Figure 1. Nickel induces HIF-1α protein accumulation and enhances HIF-1-dependent transcriptional activity and VEGF transcription.
(A), Different cells were exposed to various doses of NiCl2 for 24 h or 0.5 mM nickel for the indicated time periods. The cells were extracted and subjected to Western Blotting assay. (B), HRE or VEGF luciferase reporter stable transfectants were used to determine HRE transactivation activities or VEGF transcription induction. The asterisk (*) indicates a significant increase compared with medium control (p< 0.05). (C), HRE or VEGF luciferase reporter transfectants were pretreated with HIF-1α inhibitor, topotecan, for 30 min and then exposed to nickel (0.5 mM) for 24 h. The asterisk (♣) indicates a significant decrease compared with nickel treatment (p< 0.05) (left panel). DN-HIF-1α was stably transfected into HaCat cells in combination with HRE or VEGF luciferase reporter. The luciferase activities were determined after the cells were exposed to NiCl2 for 24 h. The asterisk (♣) indicates a significant decrease compared with vector control cells (p< 0.05) (right panel). (D), MEFs cells were treated with 1 mM NiCl2 for 8 weeks. In the topotecan group, the cells were pretreated with 1 μM topotecan for 4 h before each exposure to nickel. The anchorage-independent growth capability of nickel-treated cells was evaluated in soft agar assay.
More importantly, HIF-1α protein accumulation was impaired in JNK1−/− cells at both time points tested in comparison to that in WT cells (Fig. 2A). Moreover, the deficiency of HIF-1α accumulation in JNK1−/− cells was confirmed under the treatments with either hypoxia or PHD enzyme inhibitors, deferoxamine (28) and DMOG (29) (Fig. 2A). In addition, HIF-1-dependent transcriptional activity as well as VEGF transcription upon nickel exposure was both impaired in JNK1−/− cells (Fig. 2A). Accordingly, nickel-induced cell transformation was also blocked by co-incubation of cells with JNK inhibitor, Sp600125 (Fig. 2B). And ectopic expression of JNK1 in JNK1−/− cells restored HIF-1α protein accumulation and HIF-1-dependent transcriptional activity in response to nickel exposure compared with those in parental JNK1−/− cells (Fig. 2C). To confirm the contribution of JNK1 to nickel-induced HIF-1α protein accumulation in other types of cells, shRNA-JNK1 was introduced into Hela and A549 cells to knock down endogenous JNK1 expression. In agreement with above studies, HIF-1α accumulation by nickel exposure was reduced in these JNK1 knockdown cells (Fig. 2D). Taken together, our results demonstrate that JNK1 is an important player in the regulation of HIF-1α protein accumulation, HIF-1-dependent transactivation, and its downstream target gene transcription in cellular response to either hypoxia or chemical-mimicked hypoxia conditions.
Figure 2. JNK1 deficiency impairs HIF-1α stabilization and HIF-1 transcriptional activity and VEGF transcription.
(A), The cell extracts from WT and JNK1−/− cells exposed to nickel, hypoxia, deferoxamine, or DMOG as indicated were subjected to Western Blotting assay. HRE transactivation and VEGF transcription induction in response to nickel exposure were compared between WT and JNK1−/− cells. The asterisk (*) indicates a significant increase compared with medium control (p< 0.05). The spade (♣) indicates a significant decrease compared with WT cells (p< 0.05). (B), MEFs cells were treated with 1 mM NiCl2 in combination with or without 50 μM Sp600125 for 8 weeks. Then the cells were plated in soft agar to evaluate capability of anchorage-independent growth. (C), The cell extracts from WT, JNK1−/− and JNK1−/−(JNK1) cells exposed to NiCl2 were subjected to Western Blotting (left panel). HRE-luciferase reporter stable transfectants of JNK1−/− and JNK1−/−(JNK1) were used to determine HRE transactivation in response to NiCl2 exposure. The asterisk (*) indicates a significant increase compared with medium control (p< 0.05). The spade (♣) indicates a significant decrease compared with JNK1−/− cells (p< 0.05). (D), ShRNA-JNK1 stable transfectants of Hela or A549 cells were exposed to NiCl2 and the cell extracts was subjected to Western Blotting for determination of HIF-1α protein induction.
JNK1 protects HIF-1α from degradation through VHL-independent pathway
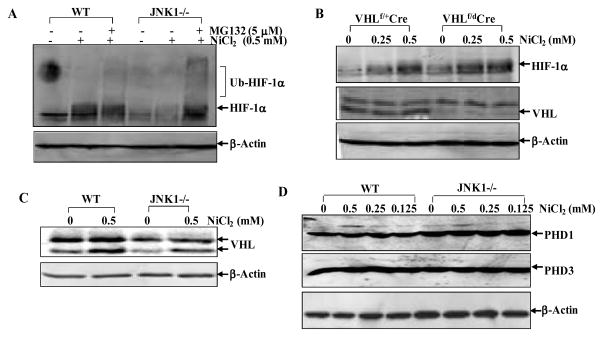
Our RT-PCR results showed no observable difference on HIF-1α mRNA expression between WT and JNK1−/− cells (Fig. 3A). These results indicate that JNK1-mediated HIF-1α accumulation upon nickel exposure might occur at the post-transcriptional level. We then compared HIF-1α cellular distribution between WT and JNK1−/− cells. As shown in Fig. 3B, HIF-1α protein was mostly present in the nuclear fraction in both types of MEFs, and only a small part of HIF-1α protein resided in the cytosolic compartment, suggesting that JNK1 did not affect HIF-1α intracellular distribution. Thus, we determined the possible involvement of JNK1 in regulation of HIF-1α protein translation using [35S]-labelled methionine and cysteine pulse analysis. As shown in Fig. 3C, HIF-1α protein synthesis rates were almost identical between WT and JNK1−/− cells. Therefore, the potential role of JNK1 in regulation of HIF-1α protein turnover was taken into consideration. By applying the protein translation inhibitor CHX to prevent de novo HIF-1α protein synthesis after nickel exposure, we compared HIF-1α protein degradation rate between WT and JNK1−/− cells. As shown in Fig. 3D, in presence of CHX, nickel-stabilized HIF-1α protein underwent a gradual degradation from 2 h to 5 h in WT cells, however in JNK1−/− cells HIF-1α protein degradation was augmented and HIF-1α protein was almost absent 4 h after CHX treatment. We further performed pulse-chase experiment and found that HIF-1α degradation rate was higher in JNK1−/− cells compared with that in WT cells (Fig. 3D). A similar finding was observed when JNK1 expression was knocked down in Hela cells, although HIF-1α protein degradation rate was slower in parental Hela cells (Fig. 3D). Interestingly, nickel-induced HIF-1α accumulation was enhanced in JNK1−/− cells by co-treatment of cells with MG132 and nickel, and the ubiquitination of HIF-1α was more abundant in JNK1−/− cells as indicated by the multiple retarded migration bands (Fig. 4A). These results suggest that JNK1 might be responsible for ubiquitin-mediated stabilization of HIF-1α protein in cellular response to nickel exposure. In order to investigate the involvement of VHL in HIF-1α accumulation, VHL conditional knockout MEFs, in which the expression level of VHL can be depleted by treatment of tamoxifen (30), were employed. As shown in Fig. 4B, VHL expression was almost absent in VHLf/dCre cells post tamoxifen treatment in comparison to that in VHLf/+Cre cells. Knockout of VHL led to the increment in both basal and nickel-induced HIF-1α protein levels in VHLf/dCre cells. These results suggest that VHL is an important player in mediation of HIF-1α protein accumulation. To further determine whether the reduced HIF-1α protein accumulation in JNK1−/− MEFs is due to the up-regulation of VHL protein expression, we compared the protein levels of VHL in these two cell lines. However, our results indicated that the VHL protein expression level in JNK1−/− MEFs was not higher, but lower than that in WT cells, revealing that VHL was not responsible for the reduced HIF-1α protein accumulation in JNK1−/− MEFs (Fig. 4C). Furthermore, PHD proteins (PHD1 and PHD3) show comparable expression levels in WT and JNK1−/− cells (Fig. 4D). Therefore, we anticipate that JNK1 regulates HIF-1α stability in a VHL-independent manner in cellular response to nickel exposure.
Figure 3. JNK1 modulates HIF-1α protein degradation, but not mRNA expression or translation in cellular response to nickel exposure.
(A), Total RNA was extracted from WT and JNK1−/− MEFs exposed to NiCl2 as indicated using Trizol reagent. RT-PCR was carried out for detection of hif-1α mRNA expression. (B), The cytosolic and nuclear proteins were isolated from WT and JNK1−/− MEFs exposed to NiCl2, and then subjected to Western Blotting. (C), WT and JNK1−/− MEFs were exposed to nickel (0.5 mM) for 12 h. Cells were then incubated with methionine-and cysteine-free DMEM for 1 h. Pulse assay was performed using 35S-labeled methionine and cysteine. (D), HIF-1α protein degradation rates were compared between WT and JNK1−/− MEFs exposed to NiCl2 for 12 h followed by addition of CHX (50 μg/ml) for different time periods or in the pulse-chase assay using 35S-labeled methionine and cysteine (upper three panels). shRNA JNK1 was also used to carry out the pulse-chase assay in Hela cells (bottom panel).
Figure 4. JNK1 prevents HIF-1α from VHL-independent degradation in cellular response to nickel exposure.
(A), WT and JNK1−/− MEFs were pretreated with MG132 (5 μM) for 0.5 h and then exposed to NiCl2 for 12 h. The cell extracts were subjected to Western Blotting. (B), VHL conditional knockout MEFs, VHLf/dCre, and its wild-type control VHLf/+Cre MEFs were pretreated with tamoxifen (0.1 μM) for 2 days, and then exposed to NiCl2 for 12 h. HIF-1α induction was detected by Western Blotting assay. (C and D), Cells extracts from WT and JNK1−/− MEFs exposed to NiCl2 were subjected to Western Blotting.
JNK1 regulates HIF-1α stability through modulation of Hsp90 and Hsp70 expression
Hsp90 and Hsp70 are molecular chaperones required for the stability and function of a number of proteins implicated in cancer cell growth, survival or both (31–34). When we pretreated cells with novobiocin, a Hsp90/Hsp70 inhibitor that can alter the interaction of Hsp90/Hsp70 with their clients (35, 36), we found that novobiocin reduced nickel-induced HIF-1α accumulation in wild type MEFs (Fig. 5A). More importantly, expression levels of Hsp90 and Hsp70 in JNK1−/− cells were found much lower than those in WT cells (Fig. 5B), and β-Actin loading controls were at the similar levels between the two cell lines (Fig. 2A). To provide evidence that the reduction of Hsp90/Hsp70 expression is responsible for the attenuated HIF-1α accumulation in JNK1−/− cells, exogenous Hsp90 and Hsp70 were ectopically expressed in JNK1−/− cells. We found that this intervention increased the accumulation of HIF-1α protein in response to nickel as compared with that in the parental JNK1−/− cells (Fig. 5B). Our results strongly indicate that Hsp90 and Hsp70 are JNK1 downstream mediators for the regulation of HIF-1α stability in cellular response to nickel exposure.
Figure 5. JNK1 mediates HIF-1α protein accumulation through regulation of Hsp90/Hsp70 protein expression.
(A), WT MEFs were pretreated with novobiocine for 30 min, and then exposed to NiCl2 for 12 h. The cells extracts were subjected to Western Blotting. (B), WT, JNK1−/− MEFs or JNK1−/− transfectants JNK1−/−(Hsp90) or JNK1−/−(Hsp70) were treated with NiCl2, and cell extracts were subjected to Western Blotting. The loading controls of the upper panel were the same as shown in Fig. 2A. (C), RT-PCR was carried out for detection of hsp90 and hsp70 mRNA expressions. (D), WT and JNK1−/− MEFs were treated with 50 μg/ml CHX (left panel), or 10 μM UBEI-41 (right panel) for different time periods. The cells extracts were subjected to Western blotting assays.
To investigate the molecular mechanisms involved in JNK1 modulation of Hsp90 and Hsp70 expressions, Hsp90 and Hsp70 mRNA levels were assessed by RT-PCR. As shown in Fig. 5C, Hsp90 mRNA levels were comparable between these two cells, while Hsp70 mRNA was more abundant in JNK1−/− cells than that in WT cells, indicating that JNK1-mediated regulation of Hsp90/Hsp70 expression occurs at the post-transcriptional levels. CHX was then used to inhibit de novo protein synthesis and to compare the degradation rate of Hsp90/Hsp70 in both cell types. As shown in Fig. 5D, in WT cells CHX treatment for up to 24 h did not induce a noticeable reduction of Hsp90 and Hsp70 proteins, consistent with the notion that chaperon molecules are always long-life proteins. In contrast, in JNK1−/− cells CHX treatment for 20 h and 24 h led to obvious reduction of Hsp90 and Hsp70 proteins, clearly indicating that the degradation rate of Hsp90/Hsp70 proteins in JNK1−/− cells was more rapid than that in WT cells. Moreover, this rapid degradation of Hsp90/Hsp70 in JNK1−/− was dependent on the 26S proteasome pathway since ubiquitin E1 inhibitor (UBEI-41) treatment caused the accumulation of Hsp90/Hsp70 proteins in JNK1−/− cells (Fig. 5D). Collectively, our results demonstrate that JNK1 protects Hsp90/Hsp70 from proteasome-dependent degradation, which subsequently contributes to HIF-1α stabilization in cellular response to nickel exposure.
JNK1 maintains Hsp90 chaperon function by regulating HDAC6 expression
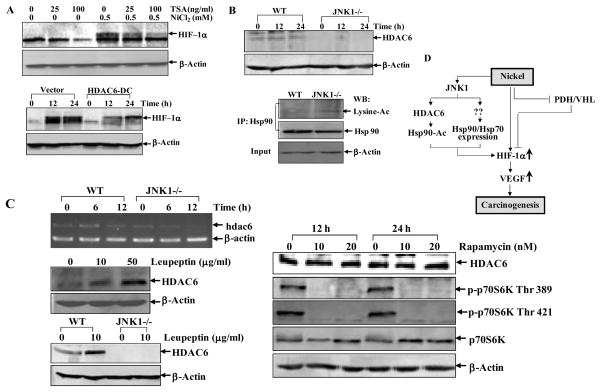
Posttranslational modifications such as reversible acetylation can affect function of Hsp90 on ligand-dependent activation of glucocorticoid receptor, a nuclear receptor client of Hsp90 (37, 38), and histone deacetylase 6 (HDAC6) was identified as a regulator of Hsp90 acetylation (37, 38). Therefore we tested whether JNK1 could affect Hsp90 activity by regulating HDAC6-mediated Hsp90 acetylation. To this end, we used TSA, a pan HDAC inhibitor, and found that TSA pretreatment decreased nickel induced HIF-1α accumulation (Fig. 6A), suggesting HDACs were associated with HIF-1α protein accumulation. To further verify role of HDAC6 in this process, functional deficient HDAC6 (HDAC6-DC, H216/611A catalytically inactive mutant) (37) was employed. As shown in Fig. 6A, the HIF-1α protein induction by nickel exposure was markedly attenuated following overexpression of HDAC6-DC, indicating the requirement of HDAC6 activity for HIF-1α stabilization upon nickel exposure. We further compared HDAC6 expression levels in WT and JNK1−/− cells. We found that HDAC6 protein was almost absent in JNK1−/− cells, and consequently, the acetylation of Hsp90 was much higher in JNK1−/− cells (Fig. 6B). The RT-PCR results indicated that mRNA expression of hdac6 did not parallel with protein level in JNK1−/− cells. Although nickel treatment reduced HDAC6 mRNA in JNK1−/− cells, the basal level of HDAC6 mRNA was comparable between these two cells (Fig. 6C, left upper panel). Therefore, the investigation of post-transcriptional regulation of HDAC6 was performed. Our results showed that HDAC6 degradation was not through MG132-sensitive proteasome pathway, because MG132 could not accumulate HDAC6 protein in WT MEFs (data not shown). Rather, we found that pretreatment of cells with leupeptin, a lysosome inhibitor (39), led to a strong accumulation of HDAC6 in WT MEFs (Fig. 6C, left middle panel), suggesting HDAC6 was decayed in a lysosome-dependent manner. However, leupeptin failed to accumulate HDAC6 in JNK1−/− cells (Fig. 6C, left bottom panel). Therefore, we anticipate that the translation machinery of HDAC6 was defective in JNK1−/− cells. Previous studies show that nearly 90% of cellular proteins are translated in eukaryotes in a manner that depends on the m7GpppN 5′-cap structure of mRNA, which recruits cap-binding protein eIF4E along with the ribosomal preinitiation complex (40). Functional disruption of mTOR by rapamycin could inhibit those cap-dependent protein translation (41). Therefore, we used rapamycin (10 nM and 20 nM) to treat wild type MEFs for 12 h and 24 h, in which conditions the activation of p70S6K was markedly inhibited as evidenced by the disappearance of the phosphorylated form of the p70S6K on T389/421 (Fig. 6C, right panel). However, rapamycin did not show any inhibition on HDAC6 protein expression, revealing that HDAC6 is translated in a cap-independent mechanism. It has been reported that some key proteins that were indispensable for cell survival, differentiation, or apoptosis, possesses a cap-independent, internal ribosomal entry site (IRES)-dependent translation capability (40). Thus, we presumably anticipate that HDAC6 might be translated in an IRES-dependent mechanism, which is likely mediated by JNK1. The details about JNK1-mediated HDAC6 translation are under investigation in our lab. Taken together, our results suggest that the deficiency of HDAC6 in JNK1−/−cells leads to hyperacetylation of Hsp90 protein, which alters Hsp90 activity, and in turn results in HIF-1α protein degradation. Based on these results, we conclude that JNK1 not only stabilizes Hsp90/Hsp70 from proteasome-dependent degradation, but also maintains Hsp90 activity by removing acetylation of Hsp90 through regulating HDAC6 expression as summarized in Fig. 6D.
Figure 6. Hsp90 hyperacetylation results from deficiency of HDAC6 expression in JNK1−/− cells.
(A), WT MEFs were pretreated with TSA for 30 min and then exposed to nickel for 12 h. Cell extracts were subjected to Western Blotting assays (upper panel). WT MEFs transfected with HDAC6-DC were exposed to 0.5 mM nickel and cell extracts were subjected to Western Blotting assays (lower panel). (B), WT and JNK1−/− cells were exposed to 0.5 mM nickel and cell extracts were subjected to Western Blotting assays (upper panel). Whole cell extracts from WT and JNK1−/− MEFs were immunoprecipitated with anti-Hsp90 antibody. Acetylated Hsp90 was detected with anti-pan-lysine-acetylation antibody. Input of the proteins subjected to immunoprecipitation was also shown (lower panel). (C), RT-PCR was carried out for detection of hdac6 mRNA expressions in WT MEFs and JNK1−/− cells 6 h and 12 h following nickel exposure. β-actin was used as control (left upper panel). Wild type MEFs were treated with leupeptin for 12 h (left middle panel), or with rapamycin for 12 h and 24 h (right panel) and the cells were extracted with SDS-sample buffer and detected by Western blotting. Wild type and JNK1−/− MEFs were exposed to leupeptin for 12 h and collected for Western Blotting assay (left bottom panel). (D), Model for JNK1-mediated HIF-1α stability through Hsp90/Hsp70-dependent, VHL-independent pathway.
Discussion
Epidemiological studies have associated occupational exposure to nickel compounds to elevated incidences of human cancers, such as lung and nasal cancers (42). Nickel has been proposed to contribute to human carcinogenesis by multiple mechanisms, including activation or silencing of certain genes and transcription factors like HIF-1α (19). The molecular mechanism investigation in the current studies further revealed that HIF-1-dependent transcription activity as well as expression of the downstream target VEGF can be regulated by JNK1 through modulating HIF-1α stability in VHL-independent, Hsp90/Hsp70-dependent manner.
It has been reported that a group of positive regulators such as coactivator proteins and molecular chaperones contribute to HIF-1α transcriptional activity and stability under hypoxia and normoxia conditions (6). Hsp90 can interact with HIF-1α in vivo and in vitro by binding to HIF-1α PAS domain, and such binding is crucial for rapid HIF-1α protein accumulation (17, 32). Hsp90 inhibitors, such as geldanamycin, promote HIF-1α ubiqitination and proteasomal-dependent degradation (34). Our present studies clearly show that although nickel exposure reduces VHL-dependent HIF-1α degradation due to the inactivation of PHDs, HIF-1α still undergoes a VHL-independent degradation, the efficiency of which is compromised by the presence of JNK1. JNK1 protects HIF-1α from this inefficient turnover following nickel exposure through facilitating the expression of Hsp90/Hsp70, the molecular chaperons known for regulating proper folding, maturation as well as stabilization of about 100 client proteins.
Although JNKs are known to phosphorylate heat shock transcription factor-1, which controls Hsp90/Hsp70 transcription (43), our RT-PCR results strongly indicate that the Hsp90/Hsp70 mRNA is unchanged in JNK1−/− cells, implying that JNK1 does not affect their transcription. Actually we have demonstrated that JNK1 prevents Hsp90/Hsp70 from proteasome-dependent degradation. This conclusion is based on these facts: (a) following CHX treatment, the pre-existing Hsp90/Hsp70 degraded faster upon JNK1 deficiency; (b) the ubiquitin E1 inhibitor, UBEI-41, could restore Hsp90/Hsp70 expression in JNK1−/− cells. Based on these results, we anticipate that certain unspecified E3 ligase functions as a mediator for Hsp90/Hsp70 protein degradation.
Our present studies further demonstrate that JNK1 is able to sustain HDAC6 expression, which is responsible for removing Hsp90 acetylation and consequently facilitating its chaperon activity. The chaperon activity of Hsp90 protein is regulated by its expression level (under heat shock conditions) (44), the interactions with the co-chaperones (45) as well as post-translational modifications, such as acetylation (37, 46). Although a specific acetyl-transferase for Hsp90 still needs to be identified, the deacetylase of Hsp90 protein investigated by Yao et al is attributable to HDAC6 in the case of ligand-dependent activation of the glucocorticoid receptor (37). HDAC6 belongs to the class II histone deacetylases, and possess unique structural modules so that it may have special biological functions such as regulation of acetylation of non-histone proteins, microtubulin (21) and Hsp90 (47). In our present study, we also demonstrate Hsp90 acetylation is elevated in JNK1−/− cells resulting from the total absence of HDAC6 expression.
In summary, our present studies for the first time disclose a novel function of JNK1 in the modulation of HIF-1α stabilization under nickel mimicked chemical hypoxia conditions through regulation of Hsp90/Hsp70 expression as well as HDAC6-mediated Hsp90 acetylation modification. This novel function may contribute to JNK1-mediated carcinogenic effects in response to nickel exposure.
Acknowledgments
We thank Dr. Laura S Schmidt (SAIC-Frederick, Inc., NCI-Frederick, Frederick, MD) for providing VHL conditional knockout cells, Dr. Dörthe M. Katschinski1 (Martin-Luther-University Halle, Germany) for the generous gift of Hsp90 expression construct, Dr. Hector R. Wong (Children’s Hospital Medical Center, Cincinnati, OH) for providing Hsp70 expression construct, Dr. Tso-Pang Yao (Duke University, NC) for providing catalytically inactive mutant of HDAC6 construct. We thank Dr. JaWanda Grant for her critical reading of this manuscript. This work was supported in part by grants from NIH CA112557 (CH), and ES012451 (CH), ES010344 (MC), ES005512 (MC), ES014454 (MC) and ES000260 (MC).
References
- 1.Maxwell PH, Pugh CW, Ratcliffe PJ. Activation of the HIF pathway in cancer. Current Opinion in Genetics & Development. 2001;11:293–9. doi: 10.1016/s0959-437x(00)00193-3. [DOI] [PubMed] [Google Scholar]
- 2.Semenza G, Agani F, Feldser D, et al. Hypoxia, HIF-1, and the pathophysiology of common human diseases. Adv Exp Med Biol. 2000;475:123–30. doi: 10.1007/0-306-46825-5_12. [DOI] [PubMed] [Google Scholar]
- 3.Semenza GL. HIF-1: mediator of physiological and pathophysiological responses to hypoxia. J Appl Physiol. 2000;88:1474–80. doi: 10.1152/jappl.2000.88.4.1474. [DOI] [PubMed] [Google Scholar]
- 4.Ji-Won Lee S-HB, Jeong Joo-Won, Kim Se-Hee, Kim Kyu-Won. Hypoxia-inducible factor (HIF-1)alpha: its protein stability and biological functions. Exp Mol Med. 2004;36 doi: 10.1038/emm.2004.1. [DOI] [PubMed] [Google Scholar]
- 5.Maxwell P, Pugh C, Ratcliffe P. Insights into the Role of the von Hippel-Lindau Gene Product. Exp Nephrol. 2001;9:235–40. doi: 10.1159/000052617. [DOI] [PubMed] [Google Scholar]
- 6.Bardos JI, Ashcroft M. Negative and positive regulation of HIF-1: A complex network. Biochimica et Biophysica Acta (BBA) - Reviews on Cancer. 2005;1755:107–20. doi: 10.1016/j.bbcan.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 7.Tan C, de Noronha RG, Roecker AJ, et al. Identification of a Novel Small-Molecule Inhibitor of the Hypoxia-Inducible Factor 1 Pathway. Cancer Res. 2005;65:605–12. [PubMed] [Google Scholar]
- 8.Karin M, Gallagher E. From JNK to Pay Dirt: Jun Kinases, their Biochemistry, Physiology and Clinical Importance. IUBMB Life. 2005;57:283–95. doi: 10.1080/15216540500097111. [DOI] [PubMed] [Google Scholar]
- 9.Ann MB, Zigang D. The functional contrariety of JNK. Molecular Carcinogenesis. 2007;46:591–8. doi: 10.1002/mc.20348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cui J, Han S-Y, Wang C, et al. c-Jun NH2-Terminal Kinase 2{alpha}2 Promotes the Tumorigenicity of Human Glioblastoma Cells. Cancer Res. 2006;66:10024–31. doi: 10.1158/0008-5472.CAN-06-0136. [DOI] [PubMed] [Google Scholar]
- 11.Sivaraman V, Wang H, Nuovo G, Malbon C. Hyperexpression of mitogen-activated protein kinase in human breast cancer. J Clin Invest. 1997;99:1478–83. doi: 10.1172/JCI119309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen F, Castranova V. Beyond apoptosis of JNK1 in liver cancer. Cell Cycle. 2009;8:1145–7. doi: 10.4161/cc.8.8.8200. [DOI] [PubMed] [Google Scholar]
- 13.Zhang D, Li J, Wu K, et al. JNK1, but not JNK2, is required for COX-2 induction by nickel compounds. Carcinogenesis. 2007;28:883–91. doi: 10.1093/carcin/bgl186. [DOI] [PubMed] [Google Scholar]
- 14.Sabapathy K, Kallunki T, David J-P, Graef I, Karin M, Wagner EF. c-Jun NH2-Terminal Kinase (JNK)1 and JNK2 Have Similar and Stage-dependent Roles in Regulating T Cell Apoptosis and Proliferation. J Exp Med. 2001;193:317–28. doi: 10.1084/jem.193.3.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ding J, Li J, Chen J, et al. Effects of Polycyclic Aromatic Hydrocarbons (PAHs) on Vascular Endothelial Growth Factor Induction through Phosphatidylinositol 3-Kinase/AP-1-dependent, HIF-1{alpha}-independent Pathway. J Biol Chem. 2006;281:9093–100. doi: 10.1074/jbc.M510537200. [DOI] [PubMed] [Google Scholar]
- 16.Jiang B-H, Jiang G, Zheng JZ, Lu Z, Hunter T, Vogt PK. Phosphatidylinositol 3-Kinase Signaling Controls Levels of Hypoxia-inducible Factor 1. Cell Growth Differ. 2001;12:363–9. [PubMed] [Google Scholar]
- 17.Katschinski DM, Le L, Schindler SG, Thomas T, Voss AK, Wenger RH. Interaction of the PAS B domain with HSP90 accelerates hypoxia-inducible factor-1Α stabilization. Cellular Physiology and Biochemistry. 2004;14:351–60. doi: 10.1159/000080345. [DOI] [PubMed] [Google Scholar]
- 18.Wong HR, Menendez IY, Ryan MA, Denenberg AG, Wispe JR. Increased expression of heat shock protein-70 protects A549 cells against hyperoxia. Am J Physiol Lung Cell Mol Physiol. 1998;275:L836–41. doi: 10.1152/ajplung.1998.275.4.L836. [DOI] [PubMed] [Google Scholar]
- 19.Li J, Davidson G, Huang Y, et al. Nickel Compounds Act through Phosphatidylinositol-3-kinase/Akt-Dependent, p70S6k-Independent Pathway to Induce Hypoxia Inducible Factor Transactivation and Cap43 Expression in Mouse Epidermal Cl41 Cells. Cancer Res. 2004;64:94–101. doi: 10.1158/0008-5472.can-03-0737. [DOI] [PubMed] [Google Scholar]
- 20.Forsythe JA, Jiang BH, Iyer NV, et al. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604–13. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hubbert C, Guardiola A, Shao R, et al. HDAC6 is a microtubule-associated deacetylase. Nature. 2002;417:455–8. doi: 10.1038/417455a. [DOI] [PubMed] [Google Scholar]
- 22.Zhang D, Song L, Li J, Wu K, Huang C. Coordination of JNK1 and JNK2 is critical for GADD45alpha induction and its mediated cell apoptosis in arsenite responses. J Biol Chem. 2006:M602821200. doi: 10.1074/jbc.M602821200. [DOI] [PubMed] [Google Scholar]
- 23.Song L, Li J, Ye J, et al. p85{alpha} Acts as a Novel Signal Transducer for Mediation of Cellular Apoptotic Response to UV Radiation. Mol Cell Biol. 2007;27:2713–31. doi: 10.1128/MCB.00657-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang D, Li J, Gao J, Huang C. c-Jun/AP-1 pathway-mediated cyclin D1 expression participates in low dose arsenite-induced transformation in mouse epidermal JB6 Cl41 cells. Toxicology and Applied Pharmacology. 2009;235:18–24. doi: 10.1016/j.taap.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang D, Song L, Li J, Wu K, Huang C. Coordination of JNK1 and JNK2 Is Critical for GADD45{alpha} Induction and Its Mediated Cell Apoptosis in Arsenite Responses. J Biol Chem. 2006;281:34113–23. doi: 10.1074/jbc.M602821200. [DOI] [PubMed] [Google Scholar]
- 26.Beppu K, Nakamura K, Linehan WM, Rapisarda A, Thiele CJ. Topotecan Blocks Hypoxia-Inducible Factor-1{alpha} and Vascular Endothelial Growth Factor Expression Induced by Insulin-Like Growth Factor-I in Neuroblastoma Cells. Cancer Res. 2005;65:4775–81. doi: 10.1158/0008-5472.CAN-04-3332. [DOI] [PubMed] [Google Scholar]
- 27.Rapisarda A, Uranchimeg B, Scudiero DA, et al. Identification of Small Molecule Inhibitors of Hypoxia-inducible Factor 1 Transcriptional Activation Pathway. Cancer Res. 2002;62:4316–24. [PubMed] [Google Scholar]
- 28.Wang GL, Semenza GL. Desferrioxamine induces erythropoietin gene expression and hypoxia- inducible factor 1 DNA-binding activity: implications for models of hypoxia signal transduction. Blood. 1993;82:3610–5. [PubMed] [Google Scholar]
- 29.Hanson ES, Rawlins ML, Leibold EA. Oxygen and Iron Regulation of Iron Regulatory Protein 2. J Biol Chem. 2003;278:40337–42. doi: 10.1074/jbc.M302798200. [DOI] [PubMed] [Google Scholar]
- 30.Hong S-B, Furihata M, Baba M, Zbar B, Schmidt LS. Vascular defects and liver damage by the acute inactivation of the VHL gene during mouse embryogenesis. Lab Invest. 2006;86:664–75. doi: 10.1038/labinvest.3700431. [DOI] [PubMed] [Google Scholar]
- 31.Neckers L, Ivy S. shock protein 90. Curr Opin Oncol. 2003;15:419–24. doi: 10.1097/00001622-200311000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Gradin K, McGuire J, Wenger RH, et al. Functional interference between hypoxia and dioxin signal transduction pathways: competition for recruitment of the Arnt transcription factor. Molecular And Cellular Biology. 1996;16:5221–31. doi: 10.1128/mcb.16.10.5221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hur E, Kim H-H, Choi SM, et al. Reduction of Hypoxia-Induced Transcription through the Repression of Hypoxia-Inducible Factor-1alpha/Aryl Hydrocarbon Receptor Nuclear Translocator DNA Binding by the 90-kDa Heat-Shock Protein Inhibitor Radicicol. Mol Pharmacol. 2002;62:975–82. doi: 10.1124/mol.62.5.975. [DOI] [PubMed] [Google Scholar]
- 34.Isaacs JS, Jung Y-J, Mimnaugh EG, Martinez A, Cuttitta F, Neckers LM. Hsp90 Regulates a von Hippel Lindau-independent Hypoxia-inducible Factor-1alpha -degradative Pathway. J Biol Chem. 2002;277:29936–44. doi: 10.1074/jbc.M204733200. [DOI] [PubMed] [Google Scholar]
- 35.Yun BG, Huang W, Leach N, Hartson SD, Matts RL. Novobiocin Induces a Distinct Conformation of Hsp90 and Alters Hsp90-Cochaperone-Client Interactions. Biochemistry. 2004;43:8217–29. doi: 10.1021/bi0497998. [DOI] [PubMed] [Google Scholar]
- 36.Marcu MG, Schulte TW, Neckers L. Novobiocin and Related Coumarins and Depletion of Heat Shock Protein 90-Dependent Signaling Proteins. J Natl Cancer Inst. 2000;92:242–8. doi: 10.1093/jnci/92.3.242. [DOI] [PubMed] [Google Scholar]
- 37.Kovacs JJ, Murphy PJM, Gaillard S, et al. HDAC6 Regulates Hsp90 Acetylation and Chaperone-Dependent Activation of Glucocorticoid Receptor. Molecular Cell. 2005;18:601–7. doi: 10.1016/j.molcel.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 38.Calderwood SK, Khaleque MA, Sawyer DB, Ciocca DR. Heat shock proteins in cancer: chaperones of tumorigenesis. Trends in Biochemical Sciences. 2006;31:164–72. doi: 10.1016/j.tibs.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 39.Tatsumi Y, Cho Y-Y, He Z, et al. Involvement of the Paxillin Pathway in JB6 Cl41 Cell Transformation. Cancer Res. 2006;66:5968–74. doi: 10.1158/0008-5472.CAN-05-4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stoneley M, Anne WE. Cellular internal ribosome entry segments: structures, trans-acting factors and regulation of gene expression. Oncogene. 2004;23:3200–7. doi: 10.1038/sj.onc.1207551. [DOI] [PubMed] [Google Scholar]
- 41.Chang Q, Bhatia D, Zhang Y, et al. Incorporation of an Internal Ribosome Entry Site-Dependent Mechanism in Arsenic-Induced GADD45{alpha} Expression. Cancer Res. 2007;67:6146–54. doi: 10.1158/0008-5472.CAN-07-0867. [DOI] [PubMed] [Google Scholar]
- 42.Costa M. Molecular Mechanisms of Nickel Carcinogenesis. Biological Chemistry. 2002;383:961–7. doi: 10.1515/BC.2002.102. [DOI] [PubMed] [Google Scholar]
- 43.Dai R, Frejtag W, He B, Zhang Y, Mivechi NF. c-Jun NH2-terminal Kinase Targeting and Phosphorylation of Heat Shock Factor-1 Suppress Its Transcriptional Activity. J Biol Chem. 2000;275:18210–8. doi: 10.1074/jbc.M000958200. [DOI] [PubMed] [Google Scholar]
- 44.Catelli MG, Binart N, Jung-Testas I, et al. The common 90-kd protein component of non-transformed ‘8S’ steroid receptors is a heat-shock protein. EMBO J. 1985;4:3131–5. doi: 10.1002/j.1460-2075.1985.tb04055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Siligardi G, Panaretou B, Meyer P, et al. Regulation of Hsp90 ATPase Activity by the Co-chaperone Cdc37p/p50cdc37. J Biol Chem. 2002;277:20151–9. doi: 10.1074/jbc.M201287200. [DOI] [PubMed] [Google Scholar]
- 46.Aoyagi S, Archer TK. Modulating molecular chaperone Hsp90 functions through reversible acetylation. Trends in Cell Biology. 2005;15:565. doi: 10.1016/j.tcb.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 47.Murphy PJM, Morishima Y, Kovacs JJ, Yao T-P, Pratt WB. Regulation of the Dynamics of hsp90 Action on the Glucocorticoid Receptor by Acetylation/Deacetylation of the Chaperone. J Biol Chem. 2005;280:33792–9. doi: 10.1074/jbc.M506997200. [DOI] [PubMed] [Google Scholar]