Abstract
Previously, we showed that the internal rotenone-insensitive nicotinamide adenine dinucleotide (NADH)-quinone oxidoreductase (NDI1) gene from Saccharomyces cerevisiae (baker's yeast) can be successfully inserted into the mitochondria of mice and rats and the expressed enzyme was found to be fully functional. In this study, we investigated the ability of the Ndi1 enzyme to protect the dopaminergic neurons in a chronic mouse model of Parkinson disorder. After expression of the NDI1 gene in the unilateral substantia nigra of male C57BL/6 mice for 8 months, a chronic Parkinsonian model was created by administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) with probenecid and evaluated using neurochemical and behavioral responses 1–4 weeks post-MPTP/probenecid injection. We showed that expression of Ndi1 was able to significantly prevent the loss of dopamine and tyrosine hydroxylase as well as the dopaminergic transporters in the striatum of the chronic Parkinsonian mice. Behavioral assessment based on a methamphetamine-induced rotation test and spontaneous swing test further supported neurological preservation in the NDI1-treated Parkinsonian mice. The data presented in this study demonstrate a protective effect of the NDI1 gene in dopaminergic neurons, suggesting its therapeutic potential for Parkinson-like disorders.
Introduction
The idea of exploring gene therapy for treating Parkinson disease (PD) is a novel and rapidly growing field of research. A number of gene therapeutic targets involving the dopamine (DA)-producing enzymes, neurotrophic factors, and the inhibitory γ-aminobutyric acid-secreting or -transmitting (GABAergic) pathway have been investigated and reported.1–5 In addition to the plausible discoveries that certain familial forms of PD are associated with genetic mutations of known proteins and enzymes, recent evidence suggests that defects in the mitochondrial respiratory chain may play a role in the progression of PD.6, 7 Dysfunction of the mitochondrial respiratory chain, specifically the nicotinamide adenine dinucleotide (NADH)–quinone oxidoreductase or complex I, is characterized by an increase in the generation of reactive oxygen species (ROS), which is considered to be linked to neuronal damage and cell death.8–10 Our group has previously shown that the internal rotenone-insensitive NADH-quinone oxidoreductase (NDI1) gene from Saccharomyces cerevisiae (baker's yeast) can be expressed successfully and can fully sustain the function of mammalian mitochondria in cultured cells and in the central nervous system (CNS) of rodents.11–19 Preliminary human trials have shown promising results with regard to the safety of administering the gene delivery vector, recombinant adeno-associated virus (rAAV), as well as the incorporation of specific genes into CNS targets.2,4,5
To develop and characterize a novel gene therapy successfully and to make it eventually feasible for clinical trials, it is essential not only to confirm the viability and functional expression of the gene product in a selected target system but also to demonstrate its delivery and efficacy in vivo using an appropriate animal disease model. Genetic models of PD have presented a wide range of results and problems in animal survival and may not resemble a large population of PD with nongenetic or sporadic etiology.20–25 Several rodent models for PD that are induced by neurotoxins such as 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), 6-hydroxydopamine (6-OHDA), or rotenone have been used widely.21,26–29 However, a great deal of discussion and debate have surrounded the pros and cons of each model and whether or not the model closely resembles the symptomology and disease progression of human PD.24,26,30 The chemical lesion of the dopaminergic pathway in the substantia nigra with 6-OHDA leads to pathological features that differ from PD progression in humans; however, this model remains valuable for creating a unilateral lesion that allows for comparison between the normal and lesioned side of the brain within the same animal for the purpose of drug testing.21,26
The use of rotenone in preparation of an animal model for PD was developed more recently, and we have successfully employed it in our laboratory.31 However, rotenone is not effective in animals other than rats at the present time. In contrast, MPTP, which selectively destroys the nigrostriatal dopaminergic neurons has been commonly used for inducing Parkinsonism in primates and mice but not in rats.32 In fact, early discovery of the toxicity of this chemical in humans came from incidents in which people who used drugs contaminated with MPTP developed Parkinson-like symptoms. Several MPTP models for PD have been tested in mice that include acute injections of four doses of MPTP within 6 hours, subacute/chronic injections of a single dose of MPTP daily for a few days to several weeks, as well as chronic injections of MPTP with an adjuvant drug, probenecid, twice a week for 5 weeks.33–36 One of the main drawbacks of the acute and subacute MPTP models is the spontaneous recovery of neurons and motor symptoms shortly after MPTP treatment, which will not allow for long-term assessment of therapeutic outcomes in drug studies.35,36 The chronic mouse MPTP/probenecid model has been characterized to show a sustained loss of dopamine (DA) neurons in the nigrostriatal system along with motor deficit for at least 6 months after the induction of Parkinsonism.35
In this study, we investigated the expression and protective potential of the NDI1 gene on dopaminergic neurons and animal behaviors in the chronic mouse MPTP/probenecid model of PD. Furthermore, the length of incubation time for Ndi1 expression was increased to 8 months after gene inoculation, and our behavioral and neurological examinations were conducted 4–5 weeks after chronic Parkinsonism was established.
Materials and Methods
Animals
Twelve-week-old male (25–30 grams) C57BL/6 mice (obtained from our in-house breeding colony) were housed four per cage in a temperature-controlled environment under a 12-h light/dark cycle, with free access to food and water. The housing and care for the animals were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All the animal protocols described in this study were approved by the Institutional Animal Care and Use Committee at The Scripps Research Institute.
Injection of rAAV-NDI1
The rAAV serotype 2 (rAAV2) carrying the NDI1 gene (designated rAAV2-NDI1) was produced by The Powell Gene Therapy Center (University of Florida, Gainsville, FL).11,12 The final viral particle titer was determined to be 1.88 × 1013 viral particles per milliliter, as estimated by dot blot assay. Surgical procedures were performed as described previously by Seo et al.12 Briefly, anesthesia was induced with 3% isoflurane in oxygen, and mice were secured in a stereotaxic frame (Kopf Instruments, Tujunga, Ca). Anesthesia was maintained for the duration of the surgical procedure with 1.5–2% isoflurane in oxygen through a nose-tip fixed to the stereotaxic frame. All rAAV2-NDI1 injections were made using a 5-μL Hamilton microsyringe with a 30-gauge beveled needle. Two injections of 2-μL each were made in the right hemisphere (total of 4 μL suspended in phosphate-buffered saline [PBS] containing 0.1% fluorescent beads) at the following coordinates (measured from bregma/dura): AP (anterior-posterior), −3.3 mm; ML (medial-lateral), 1.5 mm; DL (dorsalvetral), −3.9 mm; and AP, −3.3 mm, ML, 1.0 mm; DL, −4.1 mm, at a rate of 0.2 μL/min. The syringe was left in place for an additional 5 min before removing slowly.
Chronic MPTP/probenecid treatment
MPTP handling and safety measures were carried out in accordance with the Chemical Hygiene Plan developed at The Scripps Research Institute. Approximately 8 months after the rAAV2-NDI1 injection, mice were subjected to chronic MPTP/probenecid treatment, as reported by Lau et al.37 Briefly, each treated animal was injected with 250 mg/kg probenecid (dissolved in dimethylsulfoxide [DMSO]), intraperitoneally (i.p.), 30 min prior to 15 mg/kg MPTP (dissolved in saline) administration, subcutaneously (s.c.), twice a week for a total of 10 doses over 5 weeks. Control animals (N = 10) were injected with sterile saline. In a separate experiment, animals were treated with varying amounts of probenecid (0, 75, 150, or 250 mg/kg in DMSO) to determine whether probenecid alone had any chronic effects on mice. The chronic MPTP/probenecid-treated mice were divided into two groups: One group was pretreated with NDI1 (N = 14) and one without (N = 8).
Behavioral testing
One week and 4 weeks postchronic MPTP/probenecid treatment, animals were assessed behaviorally with the elevated body swing and methamphetamine-induced rotation tests.21 Rotation and swing test trials were videotaped and analyzed at a later time by an unbiased observer. For the rotation test, animals were first allowed 5 min to acclimate to the testing bowl environment. Evaluation of body rotational activity was initiated 15 min after the administration of methamphetamine (1.5 mg/kg, i.p.) and monitored for 40 min. The number of quarter turns and direction around the testing bowl were recorded for determining the level of protection provided by Ndi1 against chronic MPTP/probenecid-induced Parkinsonism. For the swing test, each animal was held 1 cm from the base of the tail and suspended approximately 1 cm above the table for 60 sec. Movement greater than 30° from vertical was counted as a swing, and the next swing was counted only after the animal returned to the neutral position.
High-performance liquid chromatography analysis
The mice from each chronic treatment group and one-half of the mice in the probenecid trials were sacrificed for high-performance liquid chromatography (HPLC) analysis of striatal DA and its metabolite levels. The mice were perfused with saline, after which brains were quickly removed and frozen on dry ice, and maintained at −80°C until chemical analysis was performed. The method used was similar to that outlined by Seo et al.12 Brains were dissected with a razor blade into ∼2-mm thick sections. Striatal regions from each hemisphere were isolated separately and weighed, while the remainder of the brain was frozen in OCT compound for immunohistochemical analysis (see below). Each sample was homogenized by sonication in 5 volumes of ice-cold 0.2 M perchloric acid and deproteinized by centrifugation at 14,000 rpm for 15 min at 4°C.
Aromatic monoamines and their metabolites were separated using ion-paired reversed-phase HPLC coupled with electrochemical detection. Samples (6 μL) kept on ice were injected into an HPLC system equipped with an SC-3ODS column (3 μm, 3 × 100 mm; Eicom) with a flow rate of 0.4 mL/min, at room temperature. The mobile phase was composed of 0.1 M citrate-acetate buffer, 1 mM sodium octane sulfate, and 13 μM EDTA · 2Na, with a final pH adjusted to 3.5 prior to adding 20% (vol/vol) methanol. The signal was detected on a graphite-working electrode set at +750 mV versus a Ag/AgCl reference electrode. The data were collected using an EPC-500 processor (Eicom); peak areas were calculated using the PowerChrom software and quantified from a calibration curve of standards.
Immunohistochemistry
The remainder of the unfixed brain was frozen in OCT compound (Sakura, Torrance, CA) and stored at −20°C until further processing. The other half of the animals in the probenecid group were sacrificed for immunohistochemistry by first perfusing with sterile saline, followed by fixation with 4% paraformaldehyde. Brains were removed and stored in paraformaldehyde overnight prior to mounting in OCT compound for sectioning. Next, 30-μm sections were collected using a cryostat (Microm, Germany), directly mounted onto slides, and stored at −20°C. Immunohistochemistry using an antibody against tyrosine hydroxylase (TH; 1:500, EMD Bioscience/Calbiochem, La Jolla, CA) was carried out on slide sections as previously described by Seo et al.12 Briefly, sections not fixed prior to sectioning were first incubated in 4% paraformaldehyde for 15 min at 4°C followed by rinsing in PBS. All sections were then incubated in boiling 50 mM Tris-buffered saline (TBS), pH 8.0, to remove excess fixation, for 10 min, cooled, and rinsed in PBS. This was followed by incubation in 3% hydrogen peroxidase solution for 30 min to quench native peroxidases and subsequent permeabilization and blocking for nonspecific binding with 10% goat serum, 5% horse serum, and 0.1% Triton X-100/PBS at room temperature for 1 h. Sections were then incubated with primary antibody overnight at 4°C. Next, they were incubated with biotinylated secondary antibody for 1 h at room temperature followed by revelation with the ABC elite kit (Vector Laboratories, Burlingame,CA) and 3,3′-diaminoben-zidine tetrachloride (DAB; Sigma-Aldrich, St. Louis, MO).
Western blotting
Samples used for HPLC analysis were further processed for western blotting according to the following protocol. Samples were thoroughly mixed and neutralized with 1 M Tris, pH 11, after which 2 μL of DNase (50 mg/mL; Roche, Indianapolis, IN) was added along with a protease inhibitor cocktail (Complete Mini, Roche). Sodium dodecyl sulfate (SDS) at a final concentration of 5% was added to the mixture, and the samples were incubated at room temperature for 1 h prior to determining the protein levels in each sample using the Bradford reagent (Bio-Rad, Hercules, CA). Each sample was diluted with sample buffer to a concentration of 4 μg/μL, and a total of 60 μg of protein was loaded and separated on a 10% SDS-polyacrylamide gel. The proteins were then transferred to a 0.22-μm nitrocellulose membrane (Fisher Scientific, Pittsburgh, PA). Detection of the protein expression of interest was performed using the following antibodies: Monoclonal mouse anti-TH, 1:1000 (EMD Bioscience/Calbiochem); polyclonal rabbit anti-VMAT2, 1:1000 (Chemicon, Temecula, CA); monoclonal rabbit-anti-dopamine transporter (DAT), 1:5000 (Chemicon); polyclonal ratanti-NDI1 1:5000 (prepared in-house;) and monoclonal mouse anti-GAPDH 1:2000 (Chemicon). Detection of the protein bands was accomplished using the appropriate secondary, either goat anti-mouse (1:1000, Pierce, Rockford, IL), goat anti-rat (1:5000 for NDI1 or 1:10,000 for DAT, Chemicon), or goat anti-rabbit (1:5000, GE Healthcare, United Kingdom) horseradish peroxidase followed by revelation with the SuperSignal West Pico kit (Pierce).
Statistical analysis
Statistical analysis of the data was performed using the Student t-test. Results are expressed as the mean ± standard error of the mean (SEM). Statistical significance is described in the legend for each figure.
Results
Evaluation of the effect of probenecid on nigrostriatal system in the mouse brain
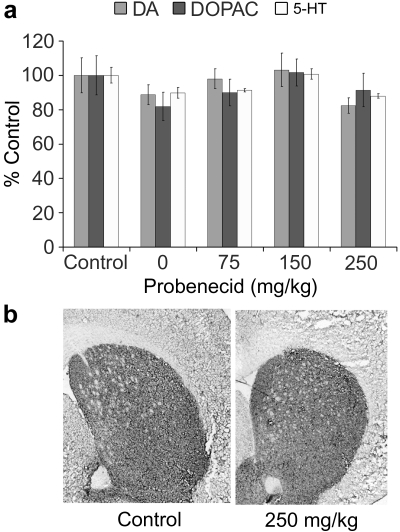
Because the chronic mouse MPTP/probenecid model of PD involves a coadministration of an adjuvant drug, probenecid, which is known to inhibit the clearance of MPTP and its metabolite, the 1-methyl-4-phenylpyridinium ion (MPP+), from the kidneys and brain, resulting in potentiating MPTP neurotoxicity,37,38 we wanted to first confirm that probenecid does not directly produce significant effects to the nigrostriatal system in the mouse brain. In a dose–response study, probenecid alone (0, 75, 150, and 250 mg/kg, i.p., twice a week for 5 weeks) did not change the striatal levels of DA, 3,4-dihydroxyphenylacetic acid (DOPAC), and 5-hydroxytryptamine (5-HT, serotonin), when compared to the saline-injected control animals 4 weeks after treatment (Fig. 1a). The mean DA content for the saline control group (N = 6) and 250 mg/kg probenecid-treated group (N = 5) were 6.37 ± 0.65 and 5.25 ± 0.28 ng/mg tissue, respectively. It should be noted that in the 0 mg/kg probenecid group, the animals were injected only with DMSO, a solvent in which probenecid was dissolved. Obviously, DMSO alone did not cause any neurochemical changes either (Fig. 1a). In addition, the immunohistochemical analysis did not show any significant changes in TH reactivity in the neostriatal region at all concentrations of probenecid that we tested (data not shown). A representative photomicrograph is shown in Fig. 1b, which demonstrates that the mean density (in arbitrary units) of TH immunoreactivity for the saline control group (n = 1, N = 10) and 250 mg/kg probenecid-treated group (N = 2, n = 32) was 96.4 ± 5.4 and 86.9 ± 5.3, respectively.
FIG. 1.
Effect of probenecid on striatal DA system in mice. A control study was performed to evaluate the effect of probenecid alone on the levels of DA, DOPAC, and 5-HT (a) and TH immunoreactivity (b). Animals (N = 25) were separated into five groups and injected with various concentrations of probenecid, i.p., twice a week for 5 weeks. Following an additional 4 weeks, one-half of the animals from each group were sacrificed for HPLC analysis and the other half were used for immunohistochemical analysis. (a) Compared to saline-injected controls, probenecid (0, 75, 150, 250 mg/kg/injection) did not alter the striatal contents of DA, DOPAC, and 5-HT. At 0 mg/kg level, only DMSO (10 mL/kg), the vehicle in which probenecid was dissolved, was injected. The 100% values (in ng/mg tissue) were 6.37 (DA), 0.53 (DOPAC), and 0.72 (5-HT). (b) A representative photomicrograph showing that chronic probenecid (250 mg/kg per injection) did not change the density of TH-immunoreactivity in the neostriatum. Probenecid at concentrations lower than 250 mg/kg per injection and DMSO alone also produced no effect on striatal TH-immunoreactivity (pictures not shown). Data are stated as mean ± SEM. DA, Dopamine; DOPAC, 3,4-dihydroxyphenylacetic acid; 5-HT, 5-hydroxytryptamine; HPLC, high-performance liquid chromatography; DMSO, dimethylsulfoxide; TH, tyrosine hydroxylase; SEM, standard error of the mean.
Effect of Ndi1 treatment on behavioral deficits in the MPTP/probenecid-treated mice
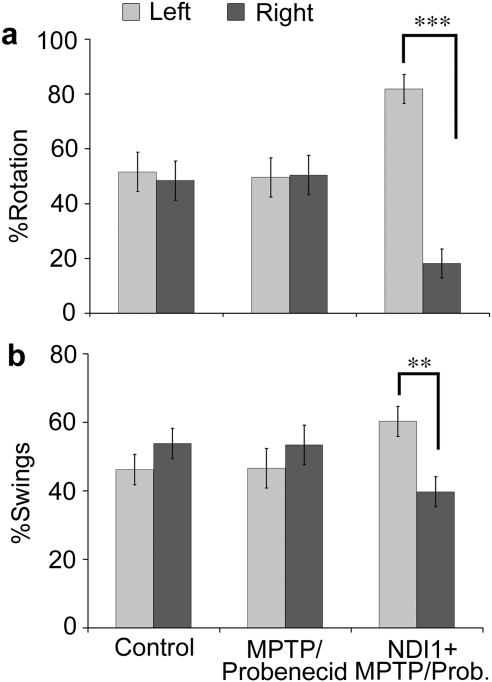
We first evaluated the Parkinsonian-related behavioral phenotypes in control, chronic MPTP/probenecid-treated mice 1 week (data not shown) and 4 weeks after treatment. Two behavioral tests, methamphetamine-induced rotation39 and elevated body swing,40 were used in our study to evaluate Parkinson-like abnormalities in mice (Fig. 2). Both rotation and swing tests showed no significant body movement with preferential turning toward one or the other direction for the control group (N = 9) and the chronic MPTP/probenecid-treated group (N = 6), signifying that there was no neurological imbalance between either side of the brain. Interestingly, a significant bias was observed for both behavioral tests in the chronic Parkinsonian mice (N = 8), which had been unilaterally pretreated with rAAV-NDI1 8 months before the chronic MPTP/probenecid treatment (Fig. 2). With the rotation test, the chronic Parkinsonian mice had a significantly higher preference (82%) toward the left (Fig. 2a). With the swing test, the chronic Parkinsonian mice showed a slightly less distinct, but still clear, preference (60%) toward the left (Fig. 2b). These results suggest that Ndi1 expression in the right substantia nigra prevented the neurodegenerative toxicity of chronic MPTP ipsilaterally, which leads to dopaminergic dominancy in the right striatum (see Fig. 3) and causes counterlateral movements.
FIG. 2.
Effect of NDI1 treatment on animal behaviors in chronic MPTP/probenecid-treated mice. Mice were tested for behavioral deficits at 1 and 4 weeks after chronic MPTP/probenecid treatment. (a) For the methamphetamine-induced rotation test, animals were injected with 1.5 mg/kg methamphetamine and behavior was recorded for 40 min, following a 15-min acclimation period. There was no preference in turn direction for the control (N = 9) or chronic MPTP/probenecid (N = 6) group. However, a preferential contralateral body turning was found in chronic MPTP/probenecid-treated mice 4 weeks after treatment, which had been pretreated with NDI1 gene for 8 months (right hemisphere) (N = 8). (b) For the elevated body swing test, the number of body swings to the left or right, greater than 30° from the vertical position were counted. Similar observations were obtained with the elevated body swing test as that of the methamphetamine-induced rotation test. Data are presented as the mean percentage ± SEM. ***p < 0.001; **p < 0.01. MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; Prob., probenecid.
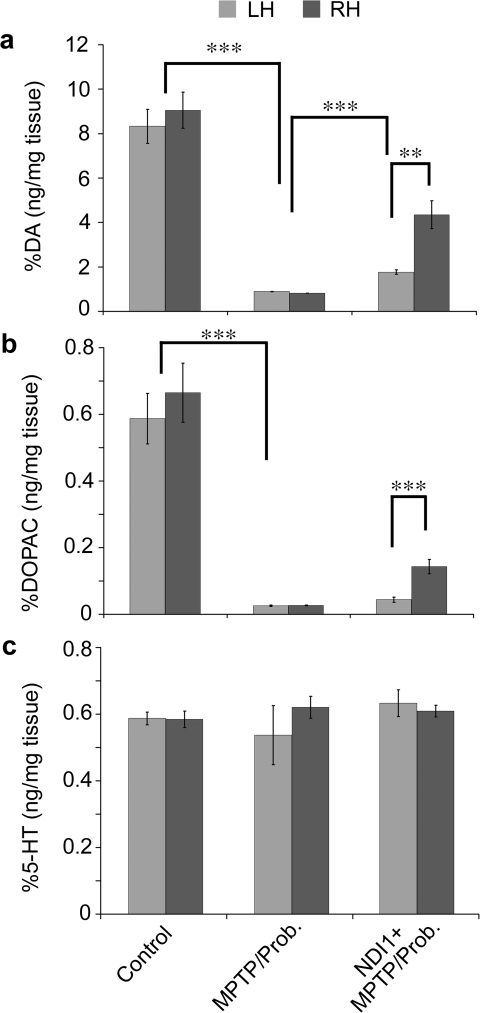
FIG. 3.
Effect of NDI1 on biogenic amine levels in the striatum of chronic MPTP/probenecid-treated mice. The levels of DA(a), the major metabolite of DA, DOPAC (b), and serotonin or 5-HT (c) were measured in each side of neostriatum from control (N = 5) and chronic MPTP/probenecid-treated mice 5 weeks after treatment, which had been pretreated without (N = 5) or with (N = 7) NDI1 gene for 8 months. Bilateral loss of over 90% of DA and DOPAC was detected in the striatum of chronic parkinsonian mice 5 weeks after systemic injections with MPTP/probenecid (a and b). The striatal content of 5-HT was not affected by the chronic MPTP/probenecid treatment (c). A partial prevention of DA and DOPAC loss was detected in the right striatum of the chronic parkinsonian mice, which had been pretreated (right hemisphere) ipsilaterally with rAAV-Ndi1 8 months before the chronic MPTP/probenecid treatment (a and b). The striatal content of 5-HT was not affected by the rAAV-NDI1 pretreatment in chronic parkinsonian mice (c). Results are expressed as mean ± SEM. ***p < 0.001; **p < 0.01. MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; DA, Dopamine; DOPAC, 3,4-dihydroxyphenylacetic acid; 5-HT, 5-hydroxytryptamine; SEM, standard error of the mean; LH, left hemisphere; RH, right hemisphere; Prob., probenecid.
Assessment of striatal biogenic amine levels in the MPTP/probenecid-treated mice
To correlate the animal behaviors with dopaminergic activities in the neostriatum, we measured and compared the levels of DA, DOPAC, and 5-HT in the left and right striatum between the control and chronic MPTP/probenecid-treated group of mice. Consistent with our previous observations, we again detected over 90% loss of DA and DOPAC bilaterally in the striatum of chronic Parkinsonian mice 5 weeks after systemic injections with MPTP/probenecid (Fig. 3a,b). The striatal content of 5-HT was not affected by the chronic MPTP/probenecid treatment (Fig. 3c). Notably, a partial prevention of DA and DOPAC loss was detected in the right striatum of the chronic Parkinsonian mice, which had been pretreated ipsilaterally with rAAV-Ndi1 8 months before the chronic MPTP/probenecid treatment (Fig. 3a,b). The striatal content of 5-HT was not affected by the rAAV-NDI1 pretreatment in chronic Parkinsonian mice (Fig. 3c). These findings suggest that the chronic MPTP-induced lesion and Ndi1-induced partial protection are confined to the target site and selective to the dopaminergic system.
Estimation of the expression of functional proteins in the neostriatum of the chronic Parkinsonian mice
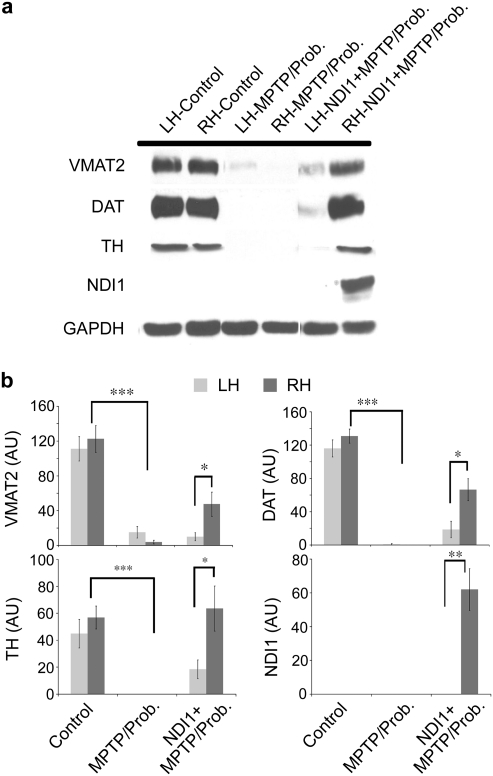
We also examined and compared the expression of several functional proteins, which are known biomarkers indicating the viability and integrity of dopaminergic neurons in the left and right striatum between the control and chronic MPTP/probenecid-treated group of mice. As expected, the protein expression of vesicular monoamine transporter 2 (VMAT2), DAT, and TH on both sides of the striatum was markedly reduced in the chronic Parkinsonian mice 5 weeks after systemic injections with MPTP/probenecid (Fig. 4a,b), suggesting significant loss of striatal dopaminergic terminals associated with chronic MPTP-induced neurodegeneration. An appreciable amount of Ndi1 expression could be detected in the injected side of the striatum from the chronic Parkinsonian mice (Fig. 4a,b), suggesting successful NDI1 gene delivery and long-lasting gene expression, which are desirable prerequisites for gene therapy. Importantly, the sustained Ndi1 expression in the injected side of the striatum resulted in significant retention of VMAT2, DAT, and TH protein expression ipsilaterally in the chronic Parkinsonian mice (Fig. 4a,b). These data indicate that NDI1 gene administration protects against degeneration of striatal dopaminergic terminals in the chronic mouse model of PD.
FIG. 4.
Effect of Ndi1 treatment on striatal dopaminergic proteins and Ndi1 in the chronic parkinsonian mice. The expression of several dopaminergic functional proteins was analyzed in each side of neostriatum from control (N = 8) and chronic MPTP/probenecid-treated mice 5 weeks after treatment, which had been pretreated without (N = 6) or with (N = 8) NDI1 gene for 8 months. The protein expression of VMAT2, DAT, and TH on the left (LH) and right hemispheres (RH) in the chronic parkinsonian mice 5 weeks after systemic injections with MPTP/probenecid was markedly reduced (a and b). Marked Ndi1 expression could be detected in the injected side of the striatum from the chronic parkinsonian mice pretreated with NDI1 (a and b). Sustained expression of VMAT2, DAT, and TH proteins was observed in the right striatum of the chronic parkinsonian mice, which had been pretreated ipsilaterally with rAAV-NDI1 8 months the chronic MPTP/probenecid treatment (a and b). Results are expressed as mean ± SEM. ***p 0.001; **p < 0.01; *p < 0.05. MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; VMAT2, vesicular monoamine transporter 2; DAT, dopamine transporter; TH, tyrosine hydroxylase; SEM, standard error of the mean; Prob., probenecid.
In addition, for the first time the presence of the Ndi1 protein was detected in the protected side of mouse striatum, which substantiates the protective action of Ndi1 in sustaining DA, TH, DAT, and VMAT2 levels, suggesting normal dopaminergic terminal plasticity. It may be noticed that the nontransduced hemisphere also showed a slight recovery in the levels of both transporters and TH. This is likely due to some spread of the NDI1 gene expression into the substantia nigra of the opposite side, which was observed in a small number of animals. The amount of Ndi1 in the contralateral striatum, however, was too small to be detected in the western blotting experiment using the anti-Ndi1 antibody.
Evaluation of Ndi1 treatment on the TH-positive cells in the substantia nigra of the chronic Parkinsonian mice
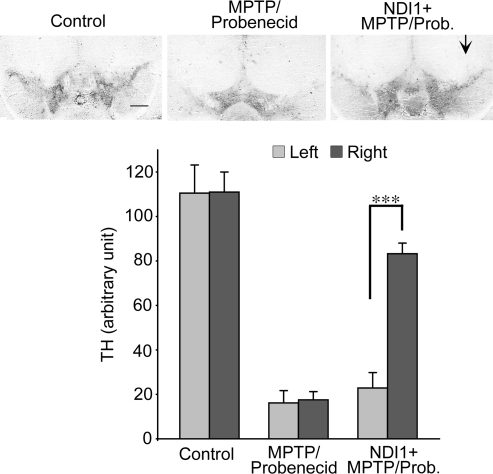
To further substantiate the neuroprotective effect of Ndi1 in chronic Parkinsonism, we investigated and qualitatively compared the density of TH-positive cells in the left and right substantia nigra between the control and chronic MPTP/probenecid-treated mice with or without the 8-month rAAV-NDI1 pretreatment. Chronic systemic MPTP/probenecid treatment resulted in a substantial loss of TH-immunore-activity bilaterally in the substantia nigra 5 weeks posttreatment when compared with the control animals (Fig. 5). When mice were preinjected with rAAV-NDI1 prior to MPTP/probenecid treatment, we observed a healthy level of TH-immunoreactivity on the Ndi1-pretreated side of the substantia nigra after chronic Parkinsonism was induced (Fig. 5).
FIG. 5.
Effect of Ndi1 treatment on the TH-positive cells in the substantia nigra of the chronic parkinsonian mice. Representative photomicrographs of TH staining of brain sections illustrate the density of TH-positive cells in the left and right substantia nigra between the control and chronic MPTP/probenecid-treated mice without or with 8-month rAAV-NDI1 pretreatment (right hemisphere, shown by arrow). Scale bar, 500 μm. The results are compared in the histograms for the control group (N = 6), the chronic MPTP/probenecid-treated group (N = 8), and the rAAV-NDI1-treated group (N = 10). Chronic systemic MPTP/probenecid treatment resulted in a substantial loss of TH-immunoreactivity bilaterally in the substantia nigra 5 weeks posttreatment when compared with the control animals. When mice were preinjected with rAAV-NDI1 in the right substantia nigra (arrow) 8 months prior to MPTP/probenecid treatment, near-normal TH-immunoreactivity was observed in the Ndi1-pretreated side of substantia nigra 5 weeks after the induction of chronic Parkinsonism. ***p < 0.001. TH, Tyrosine hydroxylase; MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; Prob., probenecid.
Discussion
In the present study, the chronic mouse MPTP/probenecid model of PD was chosen for evaluating the effectiveness of the NDI1 gene treatment to protect dopaminergic neurons in the nigrostriatal system. In addition, this study differs from previous reports using the same NDI1 gene in that the expression time has been greatly extended from 4 months to approximately 8 months before the animals are treated with MPTP/probenecid.12 Of concern during the preparation of the chronic MPTP/probenecid model is what influence, if any, the adjuvant drug probenecid might have on the model. A parallel experiment was performed to establish the levels of monoamines in the striatum after chronic exposure to probenecid. Both through HPLC and immunohistochemical analysis, it was determined that probenecid alone had no significant effect on the nigrostriatal dopaminergic system, when compared to saline-treated animals as previously reported by Lau et al.41
The underlying mechanism for dopaminergic neuroprotection by the NDI1 gene treatment in the chronic MPTP/probenecid model has not been clarified; however, a neurotoxic mechanism for MPTP has been proposed.28,42,43 Briefly, following systemic administration, MPTP enters the brain and is converted by the monoamine oxidase in the astrocytes to MPP+, which is the active neurotoxic species that initiates striatal neuronal degeneration after being taken up by the DAT transporters. Within the striatal dopaminergic terminals, MPP+ may either be taken up by the VMAT2 transporter and sequestered within the dopamine-containing vesicles or passively enter the mitochondria causing complex I inhibition and possibly an overproduction of ROS or depletion of adenosine triphosphate (ATP), or both, and consequential neuronal death.23,25–27 Pretreatment with the NDI1 gene in Parkinsonism would presumably rejuvenate the mitochondrial integrity and function and prevent the deleterious effects of MPP+ on neuronal mitochondria and neuronal loss. This mechanistic hypothesis requires further investigation.
The initial evaluation of Ndi1 expression and chronic MPTP/probenecid treatment on dopaminergic function was accomplished through behavioral assessment. The use of behavioral testing in a unilateral Parkinson model has been widely adopted in a number of different toxin-induced models.21,36,39,40 The most commonly selected test is drug-induced rotation; it has a well-defined pattern of behavior that matches with the neurochemical degradation typically found with lesions of the nigrostriatal network.21,39 We have chosen this as our primary method for evaluating our model, using methamphetamine as the stimulant. The test results for the methamphetamine-induced rotation at 1 and 4 weeks posttreatment showed a significant bias in rotation only in the Ndi1 pretreated chronic Parkinsonian mice, demonstrating a clear difference in DA levels in the two hemispheres. In addition to the drug-induced behavior, a nondrug-challenged swing test further confirmed that this chronic MPTP/probenecid model progressively loses its motor function in association with striatal DA depletion, as previously reported.35 Initial evaluation of motor skills using the swing test resulted in no preference in swing direction at 1 week post-MPTP/probenecid treatment. However, subsequent swing testing at 4 weeks post-MPTP/probenecid treatment revealed a significant behavioral bias in the Ndi1 pretreated chronic Parkinsonian mice, showing greater preference of body movement toward the direction of the Ndi1 nontransduced hemisphere and confirming the same observations as obtained from the rotation test.
Following behavioral assessment, we further examined the gene-transducing effects of NDI1 on the levels of DA, DAT, TH, and Ndi1 within the nigrostriatal system. Analysis of striatal tissues clearly demonstrates drastic bilateral losses of DA content, TH, VMAT2, and DAT expression in the striatum of chronic MPTP/probenecid-treated mice 5 weeks after treatment. Strikingly, significant retention of DA, TH, VMAT2, and DAT levels could be demonstrated in the hemisphere where the NDI1 gene was administered, and its expression persisted for 8 months, in contrast to that in the nonNdi1–transduced hemisphere. This report demonstrates for the first time that the NDI1 gene treatment remains effective for at least 8 months and its long-lasting expression provides nigrostriatal neuroprotection in the chronic Parkinsonian mouse model. The primary mechanism for idiopathic PD is not clear and could be due to multiple factors, including mitochondrial oxidative stress and dysfunction. Thus, developing therapeutic strategies to protect or restore mitochondrial integrity could in part protect or restore neuronal functions.
In this study, we have demonstrated that NDI1 treatment is neuroprotective in the chronic mouse model of PD, which could potentially slow the neurodegenerative process in PD. The clinical efficacy and safety of using NDI1 therapy for treating PD needs to be investigated and validated; these studies are being planned in our laboratory.
Acknowledgments
We thank Dr. Mathieu Marella of our laboratory for discussion and Dr. Terrence Flotte of the University of Massachusetts for construction of rAAV-NDI1. This work was supported by National Institutes of Health (NIH) R01DK053244 and NIH R01NS048441 (to T.Y. and A.M.-Y.) and NIH R01NS047920 (to Y.-S.L.).
References
- 1.During MJ. Leone P. Targets for gene therapy of Parkinson's disease: growth factors, signal transduction, and promoters. Exp Neurol. 1997;144:74–81. doi: 10.1006/exnr.1996.6391. [DOI] [PubMed] [Google Scholar]
- 2.Eberling JL. Jagust WJ. Christine CW. Starr P. Larson P. Bankiewicz KS. Aminoff MJ. Results from a phase I safety trial of hAADC gene therapy for Parkinson disease. Neurology. 2008;70:1980–1983. doi: 10.1212/01.wnl.0000312381.29287.ff. [DOI] [PubMed] [Google Scholar]
- 3.Fiandaca M. Forsayeth J. Bankiewicz K. Current status of gene therapy trials for Parkinson's disease. Exp Neurol. 2007;209:51–57. doi: 10.1016/j.expneurol.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 4.Kaplitt MG. Feigin A. Tang C. Fitzsimons HL. Mattis P. Lawlor PA. Bland RJ. Young D. Strybing K. Eidelberg D. During MJ. Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson's disease: an open label, phase I trial. Lancet. 2007;369:2097–2105. doi: 10.1016/S0140-6736(07)60982-9. [DOI] [PubMed] [Google Scholar]
- 5.Marks WJ., Jr. Ostrem JL. Verhagen L. Starr PA. Larson PS. Bakay RA. Taylor R. Cahn-Weiner DA. Stoessl AJ. Olanow CW. Bartus RT. Safety and tolerability of intraputaminal delivery of CERE-120 (adeno-associated virus serotype 2-neurturin) to patients with idiopathic Parkinson's disease: an open-label, phase I trial. Lancet Neurol. 2008;7:400–408. doi: 10.1016/S1474-4422(08)70065-6. [DOI] [PubMed] [Google Scholar]
- 6.Abou-Sleiman PM. Muqit MM. Wood NW. Expanding insights of mitochondrial dysfunction in Parkinson's disease. Nat Rev Neurosci. 2006;7:207–219. doi: 10.1038/nrn1868. [DOI] [PubMed] [Google Scholar]
- 7.Schapira AH. Evidence for mitochondrial dysfunction in Parkinson's disease—a critical appraisal. Mov Disord. 1994;9:125–138. doi: 10.1002/mds.870090202. [DOI] [PubMed] [Google Scholar]
- 8.Parker WD., Jr. Boyson SJ. Parks JK. Abnormalities of the electron transport chain in idiopathic Parkinson's disease. Ann Neurol. 1989;26:719–723. doi: 10.1002/ana.410260606. [DOI] [PubMed] [Google Scholar]
- 9.Betarbet R. Canet-Aviles RM. Sherer TB. Mastroberardino PG. McLendon C. Kim JH. Lund S. Na HM. Taylor G. Bence NF. Kopito R. Seo BB. Yagi T. Yagi A. Klinefelter G. Cookson MR. Greenamyre JT. Intersecting pathways to neurodegeneration in Parkinson's disease: Effects of the pesticide rotenone on DJ-1, α-synudein, and the ubiquitin-proteasome system. Neurobiol Dis. 2006;22:404–420. doi: 10.1016/j.nbd.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Sherer TB. Betarbet R. Testa CM. Seo BB. Richardson JR. Kim JH. Miller GW. Yagi T. Matsuno-Yagi A. Greenamyre JT. Mechanism of toxicity in rotenone models of Parkinson's disease. J Neurosci. 2003;23:10756–10764. doi: 10.1523/JNEUROSCI.23-34-10756.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seo BB. Nakamaru-Ogiso E. Cruz P. Flotte TR. Yagi T. Matsuno-Yagi A. Functional expression of the single subunit NADH dehydrogenase in mitochondria in vivo: A potential therapy for complex I deficiencies. Hum Gene Ther. 2004;15:887–895. doi: 10.1089/hum.2004.15.887. [DOI] [PubMed] [Google Scholar]
- 12.Seo BB. Nakamaru-Ogiso E. Flotte TR. Matsuno-Yagi A. Yagi T. In vivo complementation of complex I by the yeast Ndi1 enzyme: possible application for treatment of Parkinson disease. J Biol Chem. 2006;281:14250–14255. doi: 10.1074/jbc.M600922200. [DOI] [PubMed] [Google Scholar]
- 13.Yagi T. Seo BB. Nakamaru-Ogiso E. Marella M. Barber-Singh J. Yamashita T. Matsuno-Yagi A. Possibility of transkingdom gene therapy for Complex I diseases. Biochim Biophys Acta. 2006;1757:708–714. doi: 10.1016/j.bbabio.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 14.Yagi T. Seo BB. Nakamaru-Ogiso E. Marella M. Barber-Singh J. Yamashita T. Kao MC. Matsuno-Yagi A. Can a single subunit yeast NADH dehydrogenase (Ndi1) remedy diseases caused by respiratory complex I defects? Rejuvenation Res. 2006;9:191–197. doi: 10.1089/rej.2006.9.191. [DOI] [PubMed] [Google Scholar]
- 15.Seo BB. Kitajima-Ihara T. Chan EK. Scheffler IE. Matsuno-Yagi A. Yagi T. Molecular remedy of complex I defects: Rotenone-insensitive internal NADH-quinone oxidoreductase of Saccharomyces cerevisiae mitochondria restores the NADH oxidase activity of complex I-deficient mammalian cells. Proc Natl Acad Sci USA. 1998;95:9167–9171. doi: 10.1073/pnas.95.16.9167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seo BB. Matsuno-Yagi A. Yagi T. Modulation of oxidative phosphorylation of human kidney 293 cells by transfection with the internal rotenone-insensitive NADH-quinone oxidoreductase (NDI1) gene of Saccharomyces cerevisiae. Biochim Biophys Acta. 1999;1412:56–65. doi: 10.1016/s0005-2728(99)00051-1. [DOI] [PubMed] [Google Scholar]
- 17.Seo BB. Wang J. Flotte TR. Yagi T. Matsuno-Yagi A. Use of the NADH-quinone oxidoreductase (NDI1) gene of Saccharomyces cerevisiae as a possible cure for complex I defects in human cells. J Biol Chem. 2000;275:37774–37778. doi: 10.1074/jbc.M007033200. [DOI] [PubMed] [Google Scholar]
- 18.Seo BB. Nakamaru-Ogiso E. Flotte TR. Yagi T. Matsuno-Yagi A. A single-subunit NADH-quinone oxidoreductase renders resistance to mammalian nerve cells against complex I inhibition. Mol Ther. 2002;6:336–341. doi: 10.1006/mthe.2002.0674. [DOI] [PubMed] [Google Scholar]
- 19.Bai Y. Hajek P. Chomyn A. Chan E. Seo BB. Matsuno-Yagi A. Yagi T. Attardi G. Lack of complex I activity in human cells carrying a mutation in mtDNA-encoded ND4 subunit is corrected by the Saccharomyces cerevisiae NADH-quinone oxidoreductase (NDI1) gene. J Biol Chem. 2001;276:38808–38813. doi: 10.1074/jbc.M106363200. [DOI] [PubMed] [Google Scholar]
- 20.Ekstrand MI. Terzioglu M. Galter D. Zhu S. Hofstetter C. Lindqvist E. Thams S. Bergstrand A. Hansson FS. Trifunovic A. Hoffer B. Cullheim S. Mohammed AH. Olson L. Larsson NG. Progressive parkinsonism in mice with respiratory-chain-deficient dopamine neurons. Proc Natl Acad Sci USA. 2007;104:1325–1330. doi: 10.1073/pnas.0605208103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iancu R. Mohapel P. Brundin P. Paul G. Behavioral characterization of a unilateral 6-OHDA-lesion model of Parkinson's disease in mice. Behav Brain Res. 2005;162:1–10. doi: 10.1016/j.bbr.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 22.Melrose HL. Lincoln SJ. Tyndall GM. Farrer MJ. Parkinson's disease: A rethink of rodent models. Exp Brain Res. 2006;173:196–204. doi: 10.1007/s00221-006-0461-3. [DOI] [PubMed] [Google Scholar]
- 23.Przedborski S. Tieu K. Perier C. Vila M. MPTP as a mitochondrial neurotoxic model of Parkinson's disease. J Bioenerg Biomembr. 2004;36:375–379. doi: 10.1023/B:JOBB.0000041771.66775.d5. [DOI] [PubMed] [Google Scholar]
- 24.Terzioglu M. Galter D. Parkinson's disease: Genetic versus toxin-induced rodent models. FEBS J. 2008;275:1384–1391. doi: 10.1111/j.1742-4658.2008.06302.x. [DOI] [PubMed] [Google Scholar]
- 25.Richardson JR. Claudle WM. Guillot TS. Watson JL. Nakamaru-Ogiso E. Seo BB. Sherer TB. Greenamyre JT. Yagi T. Matsuno-Yagi A. Miller GW. Obligatory role for complex I inhibition in the dopaminergic neurotoxicity of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) Toxicol Sci. 2007;95:196–204. doi: 10.1093/toxsci/kfl133. [DOI] [PubMed] [Google Scholar]
- 26.Dauer W. Przedborski S. Parkinson's disease: Mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 27.Gerlach M. Riederer P. Przuntek H. Youdim MBH. MPTP mechanisms of neurotoxicity and their implications for Parkinson's disease. Eur J Pharmacol Mol Pharmacol. 1991;208:273–286. doi: 10.1016/0922-4106(91)90073-q. [DOI] [PubMed] [Google Scholar]
- 28.Przedborski S. Vila M. The 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model: a tool to explore the pathogenesis of Parkinson's disease. Ann NY Acad Sci. 2003;991:189–198. [PubMed] [Google Scholar]
- 29.Betarbet R. Sherer TB. MacKenzie G. Garcia-Osuna M. Panov AV. Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nat Neurosci. 2000;3:1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt WJ. Alam M. Controversies on new animal models of Parkinson's disease pro and con: the rotenone model of Parkinson's disease (PD) J Neural Transm Suppl. 2006:273–276. [PubMed] [Google Scholar]
- 31.Marella M. Seo BB. Nakamaru-Ogiso E. Greenamyre JT. Matsuno-Yagi A. Yagi T. Protection by the NDI1 gene against neurodegeneration in a rotenone rat model of Parkinson's disease. PLoS ONE. 2008;3:e1433. doi: 10.1371/journal.pone.0001433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Langston JW. Ballard P. Tetrud JW. Irwin I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983;219:979–980. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- 33.Anderson DW. Bradbury KA. Schneider JS. Neuroprotection in Parkinson models varies with toxin administration protocol. Eur J Neurosci. 2006;24:3174–3182. doi: 10.1111/j.1460-9568.2006.05192.x. [DOI] [PubMed] [Google Scholar]
- 34.Petroske E. Meredith GE. Callen S. Totterdell S. Lau YS. Mouse model of Parkinsonism: A comparison between subacute MPTP and chronic MPTP/probenecid treatment. Neuroscience. 2001;106:589–601. doi: 10.1016/s0306-4522(01)00295-0. [DOI] [PubMed] [Google Scholar]
- 35.Lau YS. Progressive neurodegeneration in the chronic MPTP/probenecid model of Parkinson's disease. In: Ebadi M, editor; Pfeiffer R, editor. Parkinson's Disease. Boca Raton: CRC Press; 2005. pp. 109–115. [Google Scholar]
- 36.Kurosaki R. Muramatsu Y. Kato H. Araki T. Biochemical, behavioral and immunohistochemical alterations in MPTP-treated mouse model of Parkinson's disease. Pharmacol Biochem Behav. 2004;78:143–153. doi: 10.1016/j.pbb.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 37.Lau YS. Trobough KL. Crampton JM. Wilson JA. Effects of probenecid on striatal dopamine depletion in acute and long-term 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated mice. Gen Pharmacol. 1990;21:181–187. doi: 10.1016/0306-3623(90)90898-v. [DOI] [PubMed] [Google Scholar]
- 38.Lau YS. Meredith GE. From drugs of abuse to parkinsonism. The MPTP mouse model of Parkinson's disease. Methods Mol Med. 2003;79:103–116. doi: 10.1385/1-59259-358-5:103. [DOI] [PubMed] [Google Scholar]
- 39.Pycock CJ. Turning behaviour in animals. Neuroscience. 1980;5:461–514. doi: 10.1016/0306-4522(80)90048-2. [DOI] [PubMed] [Google Scholar]
- 40.Borlongan CV. Sanberg PR. Elevated body swing test: A new behavioral parameter for rats with 6-hydroxydopamine-induced hemiparkinsonism. J Neurosci. 1995;15:5372–5378. doi: 10.1523/JNEUROSCI.15-07-05372.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meredith GE. Totterdell S. Petroske E. Santa CK. Callison RC., Jr. Lau YS. Lysosomal malfunction accompanies alpha-synuclein aggregation in a progressive mouse model of Parkinson's disease. Brain Res. 2002;956:156–165. doi: 10.1016/s0006-8993(02)03514-x. [DOI] [PubMed] [Google Scholar]
- 42.Jakowec MW. Nixon K. Hogg E. McNeill T. Petzinger GM. Tyrosine hydroxylase and dopamine transporter expression following 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurodegeneration of the mouse nigrostriatal pathway. J Neurosci Res. 2004;76:539–550. doi: 10.1002/jnr.20114. [DOI] [PubMed] [Google Scholar]
- 43.Speciale S. MPTP. Insights into parkinsonian neurodegeneration. Neurotoxicol Teratol. 2002;24:607–620. doi: 10.1016/s0892-0362(02)00222-2. [DOI] [PubMed] [Google Scholar]