Abstract
Background
In this study we sought to validate urinary biomarkers for diabetes and two common complications, coronary artery disease (CAD) and diabetic nephropathy (DN).
Methods
A CAD score calculated by summing the product of a classification coefficient and signal amplitude of 15 urinary polypeptides was previously developed. Five sequences of biomarkers in the panel were identified as fragments of collagen Α-1(I) and Α-1(III). Prospectively collected urine samples available for analysis from 19 out of 20 individuals with CAD (15 with type 1 diabetes [T1D] and four without diabetes) and age-, sex-, and diabetes-matched controls enrolled in the Coronary Artery Calcification in Type 1 Diabetes study were analyzed for the CAD score using capillary electrophoresis and electrospray ionization mass spectrometry. Two panels of biomarkers that were previously defined to distinguish diabetes status were analyzed to determine their relationship to T1D. Three biomarker panels developed to distinguish DN (DNS) and two biomarker panels developed to distinguish renal disease (RDS) were examined to determine their relationship with renal function.
Results
The CAD score was associated with CAD (odds ratio with 95% confidence interval, 2.2 [1.3–5.2]; P = 0.0016) and remained significant when adjusted individually for age, albumin excretion rate (AER), blood pressure, waist circumference, intraabdominal fat, glycosylated hemoglobin, and lipids. DNS and RDS were significantly correlated with AER, cystatin C, and serum creatinine. The biomarker panels for diabetes were both significantly associated with T1D status (P < 0.05 for both).
Conclusions
We validated a urinary proteome pattern associated with CAD and urinary proteome patterns associated with T1D and DN.
Introduction
Coronary artery disease (CAD) is the leading cause of morbidity and mortality in people with type 1 diabetes (T1D),1 and historically diabetic kidney disease has heralded the rapid progression of CAD.2–5 Current clinical methods to diagnose both early diabetic kidney disease and subclinical CAD are subject to measurement variability (urinary albumin excretion rate [AER]) are invasive (iothalamate or other methods for directly measuring glomerular filtration rate [GFR], angiography for CAD), or deliver radiation (coronary perfusion testing, coronary angiography, coronary artery calcification). Improved methods to detect early diabetic kidney disease and CAD are needed.
Proteome analysis has recently emerged as a potentially powerful tool to define biomarkers that enable diagnosis6–8 but also prognosis9 and assessment of therapeutic intervention.10 The different technological considerations, with respect to both samples and technological platform, have recently been discussed and reviewed.11–15 We have focused on urinary proteome analysis as urine has been found to be quite stable16,17 and contains an array of low-molecular-weight proteins and peptides that can be analyzed without the need for additional manipulation such as proteolytic digests.12
In several recent studies, it has been shown that urinary proteome analysis enables the definition of biomarkers specific for diabetes,6,18 for diabetic nephropathy (DN),10,18,19 and also for cardiovascular disease.20 Consistent with recently published guidelines for clinical proteome analysis,21 in this study we aimed to validate these biomarkers and biomarker-based models in an independent blinded set of samples, collected prospectively in a center that has not been involved in the original identification of biomarkers to rule out any center-based bias.
Research Design and Methods
Patients, procedures, and demographics
Thirty-eight individuals participated in this study: 19 were CAD cases (15 with T1D, four without diabetes) who were enrolled into the Coronary Artery Calcification in Type I Diabetes (CACTI) study, were asymptomatic for CAD at enrollment, and subsequently developed clinical CAD (defined as myocardial infarction [MI] [n = 4], coronary artery bypass graft [CABG] [n = 6], or angioplasty [n = 9]). There were 20 participants with events, but one participant did not have a stored urine sample and was not included in the analysis. Nineteen controls were matched for age, diabetes status, duration of diabetes (for those participants with T1D), and gender. Controls were selected to have the lowest possible coronary calcium score at baseline of all potential matching participants (16 had scores of 0, two had scores between 0 and 10, and one had a score of 51.7) and had a follow-up visit on average 2.4 ± 0.3 years later with no significant progression of coronary artery calcium (CAC) from baseline to follow-up. The CACTI study is a prospective cohort study that includes 1,416 individuals who were asymptomatic for CAD at baseline (652 with T1D, 764 controls without diabetes) and who are being followed up longitudinally for both progression of CAC measured by electron beam computed tomography and the development of clinical CAD.22 Significant progression was defined as an increase of 2.5 or greater in the square root transformed CAC volume from baseline to follow-up.23
The local ethics committee approved the study, and all subjects gave their informed consent to participate. The study was performed in accordance with the Helsinki Declaration.
Sample preparation and proteome analysis
All analysis and data processing was performed in accordance with the minimum information about a proteomics experiment guidelines24 and also in line with the suggested guidelines for clinical proteome analysis.21 All urine samples were from spontaneously voided urine collected as a timed overnight sample and were stored at −80°C until analysis (6 years on average). For proteomic analysis, a 0.7-mL aliquot of urine was thawed immediately before use and diluted with 0.7 mL of 2 M urea and 10 mM NH4OH containing 0.02% sodium dodecyl sulfate as previously described.17 In order to remove proteins of higher molecular mass, such as albumin and immunoglobulin G, the sample was filtered using Centrisart® ultracentrifugation filter devices (20 kDa molecular weight cutoff; Sartorius, Goettingen, Germany) at 1,700 g until 1.1 mL of filtrate was obtained. The filtrate was then applied onto a PD-10 desalting column (Amersham Bioscience, Uppsala, Sweden) and equilibrated in 0.01% NH4OH in high-performance liquid chromatography-grade H2O (Carl Roth GmbH & Co. KG, Karlsruhe, Germany) to decrease matrix effects by removing urea, electrolytes, and salts. Finally, all samples were lyophilized, stored at 4°C, and suspended in high-performance liquid chromatography-grade H2O shortly before capillary electrophoresis (CE)-coupled mass spectrometry (MS) analysis.
CE-MS was performed as described17,25 using a CE system (P/ACE MDQ, Beckman Coulter, Fullerton, CA) online-coupled to a time-of-flight mass spectrometer (micrOTOF™, Bruker Daltonic, Bremen, Germany). The electrospray ionization (ESI) interface (ESI sprayer, Agilent Technologies, Palo Alto, CA) was grounded, and the ion spray interface potential was set between −4 and −4.5 kV. Data acquisition and MS acquisition methods were automatically controlled by the CE via contact-close-relays. Spectra were accumulated every 3 s, over a range of m/z 350 to 3,000. Accuracy, precision, selectivity, sensitivity, reproducibility, and stability have been previously described.17
Data processing and analysis
Mass spectral ion peaks representing identical molecules at different charge states were deconvoluted into single masses using MosaiquesVisu software (Mosaiques Diagnostics and Therapeutics AG, Hannover, Germany).26 In addition, the migration time and ion signal intensity (amplitude) were normalized using internal polypeptide standards.27 The resulting peak list characterizes each polypeptide by its molecular mass (in kDa), normalized migration time (in min), and normalized signal intensity. All polypeptides detected were deposited, matched, and annotated in a Microsoft (Redmond, WA) SQL database, allowing further analysis and comparison of multiple samples (patient groups). Polypeptides within different samples were considered identical if the mass deviation was less than ±50 ppm for small (<1,000 Da) and linear increasing to ± 75 ppm (to 20,000 Da) for larger peptides, and the migration time deviation was linearly increased from ±0.4 to ±2.5 min in the range from 19 to 50 min.
Biomarkers of CAD
In a recent study20 a panel of 15 biomarkers associated with CAD confirmed by coronary angiography were identified and validated. Five sequences of biomarkers in the panel were identified as fragments of collagen α-1(I) and α-1(III). Here we sought to test if these biomarkers were associated with prospective CAD events. The 38 paired samples were therefore evaluated using the model of Zimmerli et al.20 A CAD score was calculated by using a linear classifier algorithm to sum the product of a classification coefficient and CE-MS signal for each of the 15 protein biomarkers as previously described.20
Biomarkers of diabetes
Two previously developed panels of biomarkers for diabetes18 were tested for validation. The first was a panel of 40 peptides (model diabetes 4c), nine of which have been sequenced and include collagen I and uromodulin fragments. A second model for diabetes was generated that resulted in the definition of 261 potential biomarkers, which were utilized to build a support vector machine-based model for diabetes (model diabetes 7). Both biomarker models were tested in the 19 CAD event samples from the CACTI study to examine the relationship between the subjects' diabetes status and the biomarkers of diabetes. Analysis was restricted to the CAD event cases as the controls were selected based on matching criteria and were therefore not representative of all controls.
Biomarkers of DN
As nephropathy in those with diabetes presents highly differently than in controls with normal renal status,6,10,11,19 we investigated several biomarker models for DN that have been previously reported.18 The diagnostic criteria of DN used were: albumin excretion ≥300 mg/24 h and the coexistence of DN or a renal biopsy specimen showing diabetic glomerulosclerosis. Model DN1 is based on 102 biomarkers recently described.18 Model DN2 is based on 65 biomarkers that were subsequently selected out of the 102 biomarkers. Model DN3 is based on 67 biomarkers that represent a subgroup of the 102 biomarkers. The models were additive, combined to calculate a single score for DN, the DNScore.
In addition, we have tested two biomarker models that have been shown to be indicative of chronic renal damage.18 Chronic kidney disease was defined clinically as biopsy-proven immunoglobulin A nephropathy, focal segmental glomerulosclerosis, membranous glomerulonephritis, and minimal-change disease. Model RD1 is based on 33 biomarkers that were found to be statistically significantly altered in cases with chronic renal diseases in comparison to controls.28 Model RD2 is based on 35 biomarkers, and 31 of the biomarkers are present in both models.
The 19 CAD event samples were examined utilizing these five models (three for DN, two for “general chronic renal damage”) to calculate an additive single score for renal disease, RDScore. The RDScore was evaluated for a relationship with other markers of renal dysfunction collected in the present study, including AER, albuminuria status (normal, microalbuminuria, or macroalbuminuria), cystatin C, and serum creatinine.
Statistical analysis
Study participants were matched for sex and diabetes status and were matched within 5 years by age and diabetes duration (for participants with T1D). Variables that were non-normally distributed were log transformed and included albumin excretion rate, cystatin C, visceral fat volume, and triglycerides. Characteristics of study participants were compared using a paired t test. Conditional logistic regression was used to model the relationship between the urinary proteome CAD score and CAD case status. A series of conditional logistic regression models were run to adjust for potential confounders and known CAD risk factors. Because of the sample size, potential confounders were adjusted for individually.
The analysis for the two biomarker panels for diabetes and the DNscore and RDscore were limited to participants with CAD, since the controls were selected to match the CAD cases and therefore were not representative of the underlying population. The relationship between T1D and the two biomarkers panels for diabetes was examined using a Wilcoxon rank sum test. The association between the diabetic nephropathy score (DNscore) and the renal disease score (RDscore) and other measures of renal function (AER, cystatin C, serum creatinine) were examined using generalized linear models. Correlations between DNscore, RDscore, and other renal function markers were examined using Pearson correlation coefficients. A value of P < 0.05 was considered statistically significant.
Results
Characteristics of the study participants at the baseline visit are shown in Table 1. CAD cases did not differ from controls on the matching characteristics of age, duration of diabetes (among participants with T1D, diabetes' duration was 31.6 ± 8.1 years among cases and 31.4 ± 8.0 years among controls), and gender. Cases experienced a first CAD event on average (± SD) 1.4 ± 1.3 years following the baseline visit (range, 0.2–4.5 years). Median time to event was similar for MI (183 days) and CABG (209 days) but longer in patients with angioplasty (721 days, P = 0.047), and the CAD score did not differ by type of event; the median (range) for each event was: MI, 15.5 (13.2–18.6); CABG, 15.3 (10.9–17.6); and angioplasty, 14.1 (11.5–16.0) (P = 0.31).
Table 1.
Characteristics of Study Participants
| Patients with CAD (n = 19) | Controls (n = 19) | |
|---|---|---|
| Age (years) | 47.3 ± 7.5 | 48.0 ± 7.2 |
| T1D (% yes) | 21% | 21% |
| Gender (% male) of n = 19 | 63% | 63% |
| Those with T1D (% male) | 53% | 53% |
| Those without diabetes (% male) | 100% | 100% |
| Blood pressure (mm Hg) | ||
| Systolic | 128.3 ± 12.8a | 117.1 ± 13.3 |
| Diastolic | 79.6 ± 7.0 | 77.0 ± 8.6 |
| Cholesterol (mg/dL) | ||
| Total | 195 ± 44 | 186 ± 33 |
| LDL | 122 ± 34 | 110 ± 29 |
| HDL | 51 ± 17 | 58 ± 18 |
| Triglycerides (mg/dL) | 102.5 (1.5) | 83.5 (1.5) |
| BMI (kg/m2) | 27.7 ± 3.5a | 25.0 ± 2.8 |
| Waist circumference (cm) | 94.5 ± 11.6a | 87.0 ± 11.0 |
| Intraabdominal fat (cm2) | 58.7 ± 30.1a | 43.3 ± 20.8 |
| AER (μg/min) | 13.8 ± 3.8 | 6.5 ± 3.2 |
| Cystatin C (mg/L) | 0.9 (1.4) | 0.8 (1.2) |
| Estimated GFR | ||
| GFR-MDRD | 55.9 ± 14.5a | 61.3 ± 11.5 |
| GFR-CG | 79.1 ± 23.5a | 77.4 ± 21.6 |
| GFR-MCQ | 72.6 ± 21.5a | 81.0 ± 17.5 |
| HbA1c (%) | ||
| Overall | 7.9 ± 1.6 | 7.4 ± 1.8 |
| T1D | 8.5 ± 1.3 | 7.9 ± 1.7 |
| Without diabetes | 5.9 ± 0.4 | 5.7 ± 0.3 |
| CAD score | ||
| Overall | 14.6 ± 2.0a | 12.7 ± 2.7 |
| T1D | 14.9 ± 2.1 | 13.3 ± 2.5 |
| Without diabetes | 13.4 ± 0.8 | 10.6 ± 2.3 |
| Coronary calcium score (Agatston units) | 125 (0–2,272) | 0 (0–52) |
Data are mean ± SD, geometric mean (SD), or median (minimum – maximum) values. BMI, body mass index; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
P < 0.05.
All cases without diabetes were male, while 53% of cases with T1D were male. Systolic blood pressure was significantly higher in CAD cases than in matched controls. Body mass index, waist circumference, and intraabdominal fat volume were all significantly increased among CAD cases compared to age-, gender-, and diabetes-matched controls. There was no significant difference in any of the lipid concentrations between CAD cases and controls.
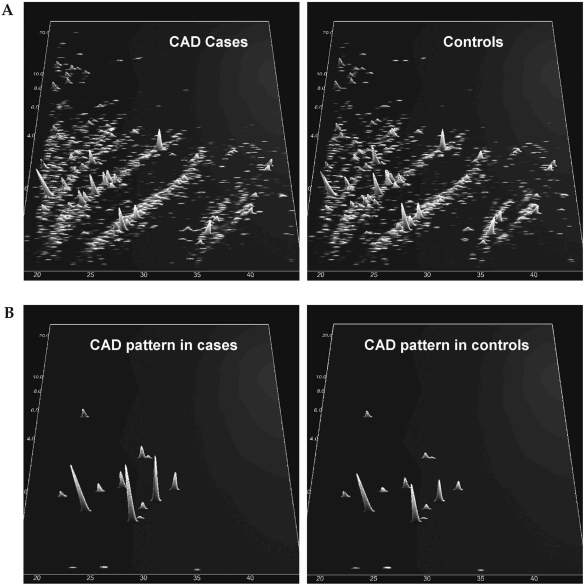
The polypeptide patterns compiled of the CAD cases and controls are shown in Figure 1A. The CAD score based on the proteome pattern developed by Zimmerli et al.20 was significantly increased among CAD cases compared to controls (14.6 ± 2.0 vs. 12.7 ± 2.7, P = 0.002), and the distribution of biomarkers in the proteome pattern among CAD cases and controls is shown in Figure 1B.
FIG. 1.
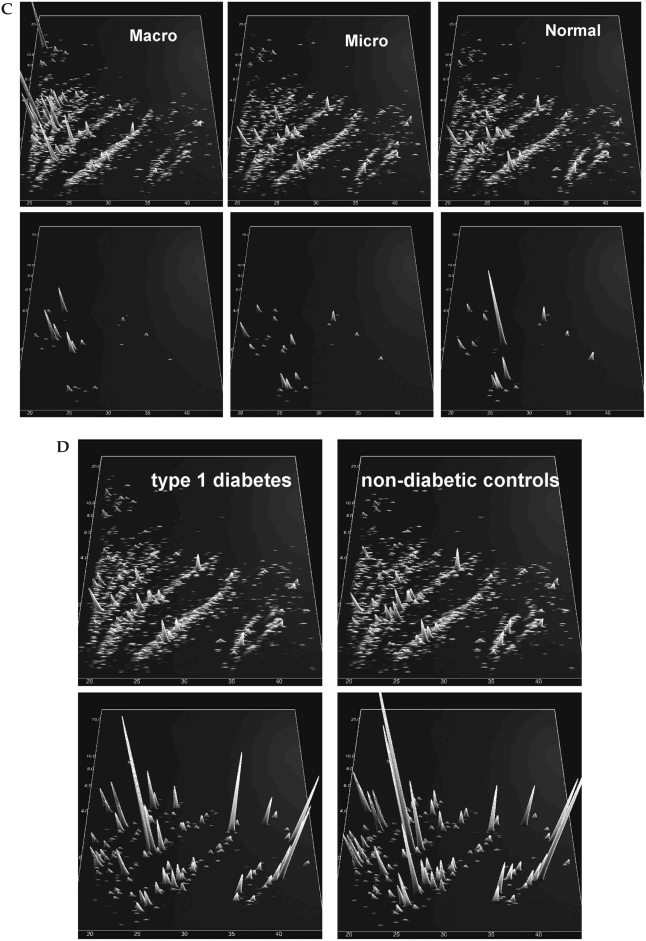
(A) Compiled polypeptide patterns of the patients and controls examined. Shown are compiled patterns consisting of all samples from patients with a subsequent CAD event and from controls with no CAD event during the observation period. The molecular mass (0.8–25 kDa, on a logarithmic scale) is plotted against normalized migration time (18–45 min). Signal intensity is encoded by peak height and color. (B) Distribution of the biomarkers for CAD in the same patients as shown in (A), with signal intensity amplified 10-fold in comparison to (A). (C) (Top panels) Compiled polypeptide patterns of all subjects in the study, according to albuminuria (29 normoalbuminuric, six microalbuminuric, and three macroalbuminuric patients). (Bottom panels) Distribution of the 33 biomarkers for chronic renal disease (RD1) in the three groups, with signal intensity amplified 10-fold in comparison to top panels. (D) (Top panels) Compiled polypeptide patterns of all subjects in the study, according to metabolic status (30 with diabetes, eight without diabetes). (Bottom panels) Distribution of the 261 biomarkers for diabetes defined previously in the two groups, with signal intensity amplified 10-fold in comparison to top panels.
The CAD score was associated with CAD events in univariate analysis (odds ratio 2.2, 95% confidence interval 1.3–5.2, P = 0.0016). When adjustment was made for other known CAD risk factors and potential confounders, the polypeptide CAD score remained significantly associated with CAD, as shown in Table 2.
Table 2.
Odds of CAD Predicted by Urinary Peptide Biomarker Panel
| Adjusted for | OR (95% CI) for CAD biomarker |
|---|---|
| Univariate | 2.2 (1.3–5.2)a |
| Age (years) | 2.0 (1.1–5.2)a |
| AER (μg/min) | 2.0 (1.2–4.6)a |
| Cystatin C (mg/L) | 1.94 (1.03–5.94)b |
| Blood pressure (mm Hg) | |
| Systolic | 2.2 (1.2–6.1)b |
| Diastolic | 2.1 (1.2–5.0)a |
| BMI (kg/m2) | 1.9 (1.1–5.3)b |
| Waist circumference (cm) | 3.0 (1.3–20.2)a |
| Intraabdominal fat (cm2) | 2.0 (1.2–4.6)a |
| HbA1c (%) | 2.7 (1.3–12.5)a |
| Cholesterol (mg/dL) | |
| Total | 2.1 (1.3–5.0)a |
| LDL | 2.5 (1.3–7.0)a |
| HDL | 2.1 (1.2–5.1)a |
| Triglycerides (mg/dL) | 2.2 (1.2–6.1)a |
BMI, body mass index; 95% CI, 95% profile likelihood confidence interval; HDL, high-density lipoprotein; LDL, low-density lipoprotein; OR, odds ratio.
Likelihood ratio: aP < 0.01; bP < 0.05.
Biomarkers of diabetes and nephropathy
The DNScore and RDScore biomarkers were correlated with log-transformed AER, cystatin C, and serum creatinine, as shown in Table 3. GFR was estimated using the Modification of Diet in Renal Disease (MDRD), Cockcroft-Gault, and Mayo Clinic Quadratic (MCQ) equations,29 and there were significant correlations between calculated GFR and the biomarker scores with the exception of DNScore and the GFR estimated by the MDRD equation, as shown in Table 3. The DNScore and RDScore also differed significantly by albuminuria status when adjusted for age, diabetes, and gender (DNScore Least Square Mean ± SE, 3.3 ± 1.6, −0.8 ± 1.3, and −2.7 ± 0.7 for normal, micro-, and macroalbuminuria [P = 0.009]; RDScore Least Square Mean ± SE, 9.5 ± 2.5, −0.4 ± 2.0, and −4.4 ± 1.0 for normal, micro-, and macroalbuminuria [P = 0.0004]). The distribution of 33 biomarkers for renal disease (RD1) are shown in Figure 1C, by albuminuria status.
Table 3.
Pearson Correlations of DNScore and RDScore with Renal Function Measurements, Adjusted for Age and Diabetes Status
| |
|
|
|
GFR |
||
|---|---|---|---|---|---|---|
| AERa | Cystatin Ca | Serum creatinine | GFR-MDRD | GFR-CG | GFR-MCQ | |
| DNScore | 0.80b | 0.68c | 0.58c | −0.43 | −0.56c | −0.50c |
| RDScore | 0.87b | 0.83b | 0.76b | −0.62c | −0.66c | −0.64c |
| AER | 0.72c | 0.62c | −0.62c | −0.73c | −0.59c | |
| Cystatin C | 0.98b | −0.83b | −0.77b | −0.85b | ||
| Serum creatinine | −0.85b | −0.72b | −0.87b | |||
Log transformed.
P < 0.001,
P < 0.05.
Study participants with T1D had significantly higher diabetes biomarker scores compared to individuals without diabetes for both model diabetes 4c (10.5 ± 9.7 vs. −12.9 ± 10.1, P = 0.0005) and model diabetes 7 (9.7 ± 5.2 vs. −3.4 ± 8.9, P = 0.001). When adjusted for age, gender, AER, and glycosylated hemoglobin (HbA1c), the model diabetes 4c marker remained significantly different by diabetes status (Least Square Mean ± SE, 14.7 ± 1.3 for patients with T1D, −8.2 ± 3.3 for controls [P < 0.001]) as did the model diabetes 7 marker (Least Square Mean ± SE, 9.6 ± 1.8 for patients with T1D, −2.3 ± 4.4 for controls [P = 0.04]). The distribution of the 261 biomarkers in model diabetes 7 is shown in Figure 1D.
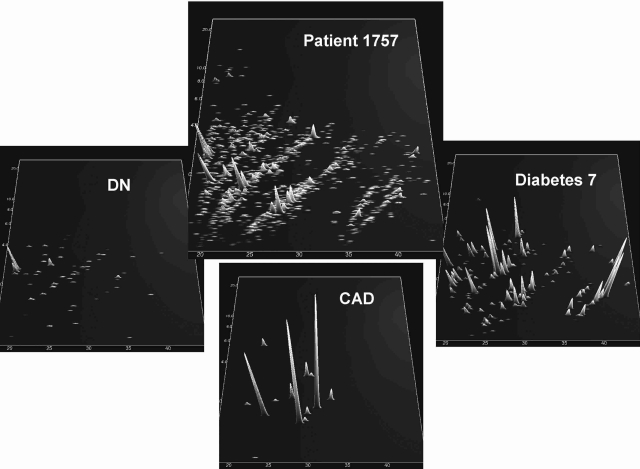
The proteomic profile of an individual study participant is shown in Figure 2, demonstrating the blinded analysis and its correspondence with clinical data.
FIG. 2.
Evaluation of a blinded sample for one patient. (Center panel) Data on all peptides and proteins identified. (Surrounding panels) Distribution of only biomarkers for diabetes (model 7), CAD, and DN (RD1). According to our assessment, this sample scored as being from a patient with diabetes, early DN, and a high probability of CAD. Upon unblinding, this patient, 1757, had diabetes, was microalbuminuric, and experienced a cardiovascular event 270 days after the sample was taken.
Discussion
In the present study we demonstrate that urinary proteome analysis based on the online combination of CE and ESI MS may contribute to the identification of patients at risk of undergoing a CAD event. The urinary protein patterns examined were associated with the development of clinical CAD, even when adjusted for known CAD risk factors and potential confounders.
In addition, there were distinct differences in the urinary polypeptide patterns among CAD cases who were individuals without diabetes compared to patients with T1D, as well as significant differences between CAD cases with normal AER and those with albuminuria.
Further investigation is needed to identify and characterize the urinary proteins in the panels that distinguished diabetes, diabetic nephropathy, and CAD events from controls. Such biomarker identification could elucidate pathophysiologic mechanisms operative in these conditions and be targets for therapeutic intervention. One identified class of biomarkers used in this study is fragments of collagen α-1(I), which are decreased in diabetes and even more so in DN. Decreased levels of urinary collagen fragments may be a result of both decreased degradation as well as increased resistance of collagen fibers towards proteolysis. The latter may be a result of increased advanced glycation end products, protecting against proteolytic processing,30 while the former may be related to decreased level or activity of elastase.11,12,31 This decrease in elastase leads to the accumulation of elastin in the macula densa, collecting ducts, and pelvicalyceal epithelia of the kidney. These changes are consistent with the theory that thickening and expansion of the extracellular matrix plays a major role in diabetes-associated complications.
The data we report are consistent with previous reports on type 1 and type 2 diabetes.10,18 Investigators at the Steno Diabetes Center identified a proteome pattern with 113 polypeptides that were able to distinguish between patients with T1D and albuminuria versus normoalbuminuric patients with T1D who were matched for age, gender, and duration of diabetes.10
The data in the present study as well as previously reported data18,28,32 indicate that peptides present in urine may reflect the turnover and dynamic changes in the extracellular matrix. Changes in these peptides may be an indicator of pathophysiologic alterations in the activity of proteases involved in the maintenance of the extracellular matrix. This dynamic balance of synthesis and degradation could not easily be accessed previously, but urinary proteome analysis may be an excellent tool to investigate this process in greater detail.
Strengths of this study include the use of previously validated biomarker panels: a well-described epidemiologic cohort; prospective data on development of CAD; and the use of well-established proteome analysis methodology performed in a highly reliable laboratory.
However, limitations include the small sample size included in this pilot study, which precludes regression analysis with adjustment for multiple variables simultaneously, and the lack of CAD events in women without diabetes. In addition, since most of the CAD events were revascularizations, it is possible that some of these “soft” events were related to other factors, including CAC score and physician characteristics. Urine samples were stored at −80°C for 6 years on average, and so it is possible that some degradation of samples occurred. Furthermore, while these urinary proteome patterns have identified novel biomarkers, it remains to be demonstrated whether this technique can add to current clinical prognostication.
The cardiovascular pattern that has been analyzed in the present study was developed based on the differences between patients who had CAD by angiography and healthy controls.20 The prognostic value of these biomarkers consequently was unknown but has been independently validated in this study. It is to be expected that evaluation of this and additional prospective studies will result in the definition of additional urinary biomarkers that may further increase the prognostic value of the biomarker model.
In conclusion, urinary proteome patterns are associated with CAD events with statistical significance. In addition, urinary proteome patterns associated with T1D and DN have been validated in this study.
Future work will include expanding this technology to larger samples to determine whether urinary proteomics can identify diabetes, DN, and CAD earlier in the pathologic course and/or add to or improve on current diagnostic techniques. Furthermore, identification of these biomarkers could provide targets for therapeutic intervention.
Acknowledgments
Support for this study was provided by National Heart, Lung, and Blood Institute grants R01 HL61753 and R01 HL079611 and by DERC Clinical Investigation Core grant P30 DK57516 from the National Institutes of Health. H.M. was supported in part by grant ETB-2006-016 from EURO-TRANS-BIO and European Union funding through InGenious HyperCare (grant LSHM-C7-2006-037093) and PREDICTIONS (grant LSHM-CT-2005-018733). The study was performed at the Adult General Clinical Research Center at the University of Colorado Health Sciences Center, Aurora, CO, supported by grant M01 RR00051 from the National Institutes of Health, at the Barbara Davis Center for Childhood Diabetes in Denver, CO, at Mosaiques Diagnostics and Therapeutics AG, Hannover, Germany, and at Colorado Heart Imaging Center in Denver, CO.
Author Disclosure Statement
J.K.S.-B., D.M.M., L.G.O., G.L.K., J.E.H., and M.R. have no competing financial interests to disclose. E.S. is employed by Mosaiques Diagnostics, and H.M. is co-founder and co-owner of Mosaiques Diagnostics, the company that developed and established the CE-MS proteome analysis of human urine.
References
- 1.Libby P. Nathan DM. Abraham K. Brunzell JD. Fradkin JE. Haffner SM. Hsueh W. Rewers M. Roberts BT. Savage PJ. Skarlatos S. Wassef M. Rabadan-Diehl C. Report of the National Heart, Lung, and Blood Institute-National Institute of Diabetes and Digestive and Kidney Diseases Working Group on Cardiovascular Complications of Type 1 Diabetes Mellitus. Circulation. 2005;111:3489–3493. doi: 10.1161/CIRCULATIONAHA.104.529651. [DOI] [PubMed] [Google Scholar]
- 2.Kim WY. Astrup AS. Stuber M. Tarnow L. Falk E. Botnar RM. Simonsen C. Pietraszek L. Hansen PR. Manning WJ. Andersen NT. Parving HH. Subclinical coronary and aortic atherosclerosis detected by magnetic resonance imaging in type 1 diabetes with and without diabetic nephropathy. Circulation. 2007;115:228–235. doi: 10.1161/CIRCULATIONAHA.106.633339. [DOI] [PubMed] [Google Scholar]
- 3.Schiffrin EL. Lipman ML. Mann JF. Chronic kidney disease: effects on the cardiovascular system. Circulation. 2007;116:85–89. doi: 10.1161/CIRCULATIONAHA.106.678342. [DOI] [PubMed] [Google Scholar]
- 4.Yokoyama H. Aoki T. Imahori M. Kuramitsu M. Subclinical atherosclerosis is increased in type 2 diabetic patients with microalbuminuria evaluated by intima-media thickness and pulse wave velocity. Kidney Int. 2004;66:448–454. doi: 10.1111/j.1523-1755.2004.00752.x. [DOI] [PubMed] [Google Scholar]
- 5.Ishimura E. Shoji T. Emoto M. Motoyama K. Shinohara K. Matsumoto N. Taniwaki H. Inaba M. Nishizawa Y. Renal insufficiency accelerates atherosclerosis in patients with type 2 diabetes mellitus. Am J Kidney Dis. 2001;38(Suppl):S186–S190. doi: 10.1053/ajkd.2001.27440. [DOI] [PubMed] [Google Scholar]
- 6.Mischak H. Kaiser T. Walden M. Hillmann M. Wittke S. Herrmann A. Knueppel S. Haller H. Fliser D. Proteomic analysis for the assessment of diabetic renal damage in humans. Clin Sci (Lond) 2004;107:485–495. doi: 10.1042/CS20040103. [DOI] [PubMed] [Google Scholar]
- 7.Weissinger EM. Wittke S. Kaiser T. Haller H. Bartel S. Krebs R. Golovko I. Rupprecht HD. Haubitz M. Hecker H. Mischak H. Fliser D. Proteomic patterns established with capillary electrophoresis and mass spectrometry for diagnostic purposes. Kidney Int. 2004;65:2426–2434. doi: 10.1111/j.1523-1755.2004.00659.x. [DOI] [PubMed] [Google Scholar]
- 8.Julian BA. Wittke S. Novak J. Good DM. Coon JJ. Kellmann M. Zurbig P. Schiffer E. Haubitz M. Moldoveanu Z. Calcatera SM. Wyatt RJ. Sykora J. Sladkova E. Hes O. Mischak H. McGuire BM. Electrophoretic methods for analysis of urinary polypeptides in IgA-associated renal diseases. Electrophoresis. 2007;28:4469–4483. doi: 10.1002/elps.200700237. [DOI] [PubMed] [Google Scholar]
- 9.Decramer S. Wittke S. Mischak H. Zurbig P. Walden M. Bouissou F. Bascands JL. Schanstra JP. Predicting the clinical outcome of congenital unilateral ureteropelvic junction obstruction in newborn by urinary proteome analysis. Nat Med. 2006;12:398–400. doi: 10.1038/nm1384. [DOI] [PubMed] [Google Scholar]
- 10.Rossing K. Mischak H. Parving HH. Christensen PK. Walden M. Hillmann M. Kaiser T. Impact of diabetic nephropathy and angiotensin II receptor blockade on urinary polypeptide patterns. Kidney Int. 2005;68:193–205. doi: 10.1111/j.1523-1755.2005.00394.x. [DOI] [PubMed] [Google Scholar]
- 11.Fliser D. Novak J. Thongboonkerd V. Argiles A. Jankowski V. Girolami MA. Jankowski J. Mischak H. Advances in urinary proteome analysis and biomarker discovery. J Am Soc Nephrol. 2007;18:1057–1071. doi: 10.1681/ASN.2006090956. [DOI] [PubMed] [Google Scholar]
- 12.Mischak H. Julian BA. Novak J. High-resolution proteome/peptidome analysis of peptides and low-molecular weight proteins in urine. Proteomics Clin Appl. 2007;1:792–804. doi: 10.1002/prca.200700043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Good DM. Thongboonkerd V. Novak J. Bascands JL. Schanstra JP. Coon JJ. Dominiczak A. Mischak H. Body fluid proteomics for biomarker discovery: lessons from the past hold the key to success in the future. J Proteome Res. 2007;6:4549–4555. doi: 10.1021/pr070529w. [DOI] [PubMed] [Google Scholar]
- 14.Goodsaid F. Bandow JE. Mischak H. Grand rounds in proteomics at the FDA. Proteomics Clin Appl. 2007;1:1526–1531. doi: 10.1002/prca.200700575. [DOI] [PubMed] [Google Scholar]
- 15.Lescuyer P. Hochstrasser D. Rabilloud T. How shall we use the proteomics toolbox for biomarker discovery? J Proteome Res. 2007;6:3371–3376. doi: 10.1021/pr0702060. [DOI] [PubMed] [Google Scholar]
- 16.Schaub S. Wilkins J. Weiler T. Sangster K. Rush D. Nickerson P. Urine protein profiling with surface-enhanced laser-desorption/ionization time-of-flight mass spectrometry. Kidney Int. 2004;65:323–332. doi: 10.1111/j.1523-1755.2004.00352.x. [DOI] [PubMed] [Google Scholar]
- 17.Theodorescu D. Wittke S. Ross MM. Walden M. Conaway M. Just I. Mischak H. Frierson HF. Discovery and validation of new protein biomarkers for urothelial cancer: a prospective analysis. Lancet Oncol. 2006;7:230–240. doi: 10.1016/S1470-2045(06)70584-8. [DOI] [PubMed] [Google Scholar]
- 18.Rossing K. Mischak H. Dakna M. Zürbig P. Novak J. Julian BA. Good DM. Coon JJ. Tarnow L. Rossing P PREDICTIONS Network. Urinary proteomics in diabetes and CKD. J Am Soc Nephrol. 2008;19:1283–1290. doi: 10.1681/ASN.2007091025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meier M. Kaiser T. Herrmann A. Knueppel S. Hillmann M. Koester P. Danne T. Haller H. Fliser D. Mischak H. Identification of urinary protein pattern in type 1 diabetic adolescents with early diabetic nephropathy by a novel combined proteome analysis. J Diabetes Complications. 2005;19:223–232. doi: 10.1016/j.jdiacomp.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Zimmerli LU. Schiffer E. Zürbig P. Good DM. Kellmann M. Mouls L. Pitt AR. Coon JJ. Schmieder RE. Peter K. Mischak H. Kolch W. Delles C. Dominiczak AF. Urinary proteomic biomarkers in coronary artery disease. Mol Cell Proteomics. 2007;7:290–298. doi: 10.1074/mcp.M700394-MCP200. [DOI] [PubMed] [Google Scholar]
- 21.Mischak H. Apweiler R. Banks RE. Conaway M. Coon JJ. Dominiczak A. Ehrich JH. Fliser D. Girolami M. Hermjakob H. Hochstrasser DF. Jankowski V. Julian BA. Kolch W. Massy Z. Neususs C. Novak J. Peter K. Rossing K. Shanstra JP. Semmes OJ. Theodorescu D. Thongboonkerd V. Weissinger EM. Van Eyk JE. Yamamoto T. Clinical proteomics: a need to define the field and to begin to set adequate standards. Proteomics Clin Appl. 2007;1:148–156. doi: 10.1002/prca.200600771. [DOI] [PubMed] [Google Scholar]
- 22.Dabelea D. Kinney G. Snell-Bergeon JK. Hokanson JE. Eckel RH. Ehrlich J. Garg S. Hamman RF. Rewers M. Effect of type 1 diabetes on the gender difference in coronary artery calcification: a role for insulin resistance? The Coronary Artery Calcification in Type 1 Diabetes (CACTI) Study. Diabetes. 2003;52:2833–2839. doi: 10.2337/diabetes.52.11.2833. [DOI] [PubMed] [Google Scholar]
- 23.Hokanson JE. MacKenzie T. Kinney G. Snell-Bergeon JK. Dabelea D. Ehrlich J. Eckel RH. Rewers M. Evaluating changes in coronary artery calcium: an analytic method that accounts for interscan variability. AJR Am J Roentgenol. 2004;182:1327–1332. doi: 10.2214/ajr.182.5.1821327. [DOI] [PubMed] [Google Scholar]
- 24.Taylor CF. Paton NW. Lilley KS. Binz PA. Julian RK., Jr Jones AR. Zhu W. Apweiler R. Aebersold R. Deutsch EW. Dunn MJ. Heck AJ. Leitner A. Macht M. Mann M. Martens L. Neubert TA. Patterson SD. Ping P. Seymour SL. Souda P. Tsugita A. Vandekerckhove J. Vondriska TM. Whitelegge JP. Wilkins MR. Xenarios I. Yates JR., 3rd Hermjakob H. The minimum information about a proteomics experiment (MIAPE) Nat Biotechnol. 2007;25:887–893. doi: 10.1038/nbt1329. [DOI] [PubMed] [Google Scholar]
- 25.Wittke S. Mischak H. Walden M. Kolch W. Radler T. Wiedemann K. Discovery of biomarkers in human urine and cerebrospinal fluid by capillary electrophoresis coupled to mass spectrometry: towards new diagnostic and therapeutic approaches. Electrophoresis. 2005;26:1476–1487. doi: 10.1002/elps.200410140. [DOI] [PubMed] [Google Scholar]
- 26.Neuhoff N. Kaiser T. Wittke S. Krebs R. Pitt A. Burchard A. Sundmacher A. Schlegelberger B. Kolch W. Mischak H. Mass spectrometry for the detection of differentially expressed proteins: a comparison of surface-enhanced laser desorption/ionization and capillary electrophoresis/mass spectrometry. Rapid Commun Mass Spectrom. 2004;18:149–156. doi: 10.1002/rcm.1294. [DOI] [PubMed] [Google Scholar]
- 27.Theodorescu D. Fliser D. Wittke S. Mischak H. Krebs R. Walden M. Ross M. Eltze E. Bettendorf O. Wulfing C. Semjonow A. Pilot study of capillary electrophoresis coupled to mass spectrometry as a tool to define potential prostate cancer biomarkers in urine. Electrophoresis. 2005;26:2797–2808. doi: 10.1002/elps.200400208. [DOI] [PubMed] [Google Scholar]
- 28.Coon JJ. Zürbig P. Dakna M. Dominczak AF. Decramer S. Fliser D. Frommberger M. Golovko I. Good DM. Herget-Rosenthal S. Jankowski J. Julian BA. Kellmann M. Kolch W. Massy Z. Novak J. Rossing K. Schanstra JP. Schiffer E. Theodorescu D. Vanholder R. Weissinger EM. Mischak H. Schmitt-Kopplin P. CE-MS analysis of the human urinary proteome for biomarker discovery and disease diagnostics. Proteomics Clin Appl. 2008;2:964–973. doi: 10.1002/prca.200800024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rule AD. Larson TS. Bergstralh EJ. Slezak JM. Jacobsen SJ. Cosio FG. Using serum creatinine to estimate glomerular filtration rate: accuracy in good health and in chronic kidney disease. Ann Intern Med. 2004;141:929–937. doi: 10.7326/0003-4819-141-12-200412210-00009. [DOI] [PubMed] [Google Scholar]
- 30.Ahmed N. Thornalley PJ. Advanced glycation endproducts: what is their relevance to diabetic complications? Diabetes Obes Metab. 2007;9:233–245. doi: 10.1111/j.1463-1326.2006.00595.x. [DOI] [PubMed] [Google Scholar]
- 31.Kafienah W. Buttle DJ. Burnett D. Hollander AP. Cleavage of native type I collagen by human neutrophil elastase. Biochem J. 1998;330:897–902. doi: 10.1042/bj3300897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rossing K. Mischak H. Rossing P. Schanstra JP. Wiseman A. Maahs DM. The urinary proteome in diabetes and diabetes-associated complications: new ways to assess disease progression and evaluate therapy. Proteomics Clin Appl. 2008;2:997–1007. doi: 10.1002/prca.200780166. [DOI] [PubMed] [Google Scholar]