Introduction
The rapidly evolving progress in lymphangioleiomyomatosis (LAM) research is an ongoing story of exemplary patient advocacy, collaboration between private organizations and government, outstanding scientists, and good timing. LAM is a rare, slowly progressive disease characterized by the proliferation of smooth muscle-like cells and cystic lesions that gradually destroy the lungs. LAM also produces lesions in the lymphatic tissues and is often accompanied by renal angiomyolipomas. It may occur sporadically or it may be associated with tuberous sclerosis complex (TSC). LAM affects mostly young and middle-aged women and rarely affects men. In the mid 1990s, when the National Heart, Lung, and Blood Institute (NHLBI) first began supporting research on LAM, it was a disease of unknown origin with no effective treatment other than lung transplantation. Patients had no support network and were often being managed with high doses of progesterone or oophorectomy to abrogate the effect of estrogen. Since that time, establishment of LAM patient advocacy and support groups, increased support for research in LAM by the NHLBI, launching and completion of a LAM patient registry, and development of a LAM tissue bank have combined to result in substantial research accomplishments and progress in both basic and clinical LAM research.
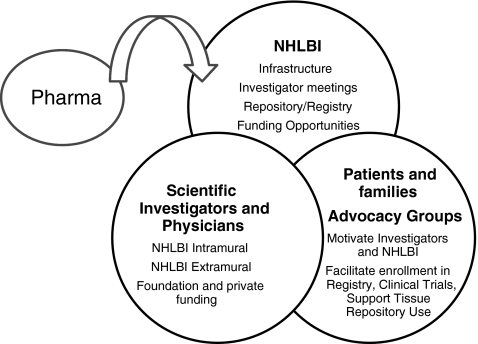
The role played by NHLBI in LAM research illustrates some of the unique features of the NIH in the American biomedical research enterprise. NHLBI's intramural program supported the first studies on this rare orphan disease, and established essential infrastructure to facilitate research. Patient registries and tissue repositories were created and made available to the scientific community. Patient advocates ensured that these resources were used and shared. Funding opportunities enabled investigators to link tissues and clinical data, rapidly advancing the cellular and molecular biologic understanding of the disease. Support of these activities is now more broadly supported, and the scientific opportunities explored by an increasing number of investigators. NHLBI looks forward to development of more effective therapies, bringing industry and other partners into this exciting field of science and medicine (Fig. 1).
FIG. 1.
Essential partners in biomedical research.
Patient Advocacy
LAM patients and patient interest groups have been and continue to be the vital core of the LAM research enterprise. They have participated directly in the research effort, giving, their time, blood, and tissues. LAM patients and advocates have also formed strong support networks, raised funds, organized patient and scientific meetings, lobbied, educated, served as advisors, and provided financial support for investigators, especially those at early stages of their careers. This effort was sparked by the parent of a LAM patient, and a tireless advocate who worked vigorously in the mid 1990s to garner support for developing a LAM patient registry and tissue bank. A “Labor of Love” campaign was launched that engaged patients, women's organizations, churches, schools, educators, and doctors in efforts to boost recognition and support for a registry. The timing was good for this type of patient involvement, as the scientific community was ripe for such a partnership following a decade of AIDS advocacy. The research establishment had come to appreciate patients working closely with investigators, having an active voice in the management of their disease, and lobbying vigorously for research and treatment.
The LAM Treatment Alliance was created in 2005 and is accelerating LAM research by many means, including fund raising, creating a seminar series for investigators, sponsoring scientific meetings, awarding grants, and raising public awareness. (LAM Treatment Alliance website http://lamtreatmentalliance.org/index.html). The Alliance has been instrumental in aiding in the continued collection of tissue and distribution of materials.
NHLBI and LAM Research
The LAM Registry
In 1995, the Division of Lung Diseases, NHLBI convened a Lymphangioleiomyomatosis Working Group at the National Jewish Center for Immunology and Respiratory Medicine in Denver, Colorado to discuss what was known about LAM and to make recommendations for future investigative work that might be undertaken to determine the cause and appropriate therapy of LAM. They concluded that more data were needed on the natural history, pathogenesis, and prognostic factors of LAM and that patient samples, both lung tissue and blood, were needed for studying pathogenetic mechanisms (National Heart, Lung, and Blood Institute Lymphangiomyomatosis Working Group Report, 1995.) The Working Group advised that a valuable registry should include multiple sites; have high quality data; protect participant privacy; and link patient data should be to biospecimens.
In 1997, the NHLBI, with co-funding from the Office of Research for Women's Health, awarded a research grant to the Cleveland Clinic Foundation with collaborating investigators at six clinical sites to establish a Lymphangiomyomatosis Registry. The NHLBI LAM Registry was modeled on the Alpha-1-Antitrypsin and the Primary Pulmonary Hypertension registries. Between 1998 and 2001 the program enrolled 243 subjects with LAM. Underscoring the key role of patient involvement in advancing the science of rare diseases, most patient referrals came through the LAM Foundation. The goal of the program was to learn more about the prevalence, distribution, and the clinical course of the disease, including radiographic features, changes in pulmonary function, course after lung transplantation and quality of life. Baseline characteristics and data on lung transplantation in LAM patients were published.1,2
The NHLBI Intramural Program
The Pulmonary Branch of NHLBI Division of Intramural Research, under the leadership of Dr. Joel Moss, developed the first research program focused on LAM.3,4 In September 1995, an observational natural history study, the Disease Process of LAM, was started. The intent of this study was to describe the clinical course of the disease with the expectation that better understanding would eventually elucidate the cellular and molecular pathogenesis, ultimately leading to the development of effective therapies. Data from this study and the large patient population followed at the NIH Clinical Center have impacted the management of LAM patients. A study of the decline of lung function in patients treated with progesterone found no significant difference in decline of lung function in progesterone-treated patients (n = 139) as compared to untreated patients (n = 136).5 The group described molecular similarities between LAM and TSC.6 The TSC2 gene had been cloned in 1993,7 the TSC1 gene was cloned in 1997,8 and basic understanding of the biology of TSC was expanding rapidly. The NHLBI Intramural group also delineated the nature of the lymphatic manifestations9 and described biomarkers and prognostic indicators.10,11 The NHLBI intramural laboratory has provided data on dissemination of neoplastic cells and gene expression in LAM, and in the relation of maximal oxygen uptake to the severity of disease.11–13
The NHLBI Intramural Program follows several hundred LAM patients, the largest patient population followed anywhere, and has information on 400 patients diagnosed with LAM between 1998 and 2003. In 1999, the group published a comprehensive evaluation of 35 patients with LAM.14 The group conducted a phase II study of octreotiede between 2000 and 2008 for reducing lymphangioleiomyomas and/or chylous effusions/ascites, peripheral lymphedema, and chyluria in 14 symptomatic LAM patients. Currently, the intramural pulmonary program is a participating site in the MILES trial to test the effectiveness of sirolimus as a treatment for LAM.
Expansion of LAM tissue collection efforts
Progress in LAM research has depended on the availability of tissue, especially lung tissue and tissue from renal angiomyolipomas (AML). Many LAM research protocols involve attempts to establish cell lines, which require a source of fresh LAM tissue; others require fresh frozen tissue. Lung explants at the time of lung transplantation have been the source of most lung tissue. Fresh tissue must be collected immediately after removal under sterile conditions and distributed the same day. The NHLBI intramural laboratory has worked in close collaboration with the LAM Foundation to identify patients scheduled for lung transplants to ensure that tissue reaches qualified investigators.
The NHLBI LAM Registry collected and stored serum, DNA, RNA, and LAM tissue in the NHLBI biorepository for future research. As the program evolved, the LAM Registry took over the collection and distribution of LAM tissue, which required addressing privacy and consent issues.
In 2007, NHLBI began supporting the National Disease Research Interchange (NDRI) to collect and distribute fresh LAM tissue to qualified investigators and to manage and fill requests for archived tissue. The LAM Treatment Alliance has played a role in the continued collection of tissue and distribution of materials when funding for the LAM Registry ended in 2003. As of October 2008, NDRI had greatly facilitated access to LAM tissue, procuring more than a thousand surgical and postmortem samples and distributing over 900 tissue samples to LAM investigators.
NHLBI Extramural Program Support for LAM Research
To stimulate basic research using cellular and molecular approaches for studying the development and subsequent progression of LAM, NHLBI issued a program announcement in December, 1995, Cellular and Molecular Mechanisms of lymphangioleiomyomatosis (PA96-011). One of the grants funded in response to this PA resulted in the discovery that mutations in the tuberous sclerosis complex gene TSC2 are a cause of sporadic pulmonary lymphangioleiomyomatosis.15,16 Subsequent studies demonstrated that metastatic LAM cells are the cause of recurrent lymphangiomyomatosis post lung transplantation.17,18 The fact that LAM cells are capable of metastasizing has become a key concept for understanding the pathogenesis of LAM as a cancer-like process.
A little more than 5 years after NHLBI first issued a Funding Opportunity Announcement for LAM research, an abundance of extramural research findings supported by the Institute were clarifying the biology of the underlying disorder. Studies of the smooth muscle-like cells in the lesions of LAM patients showed that tuberin, the protein produced by theTSC2 gene, negatively regulates the activity of ribosomal protein S6. In LAM cells, tuberin is defective and S6 is hyperphosphorylated. The LAM cells exhibit constitutive activation of p70 S6 kinase and dysregulated growth. Studies reported that the growth of the cells could be regulated by the introduction of wild-type tuberin or by addition of rapamycin (sirolimus), an immunosuppressant drug that inhibits hyperphosphorylation of S6, p70S6K activation, and DNA synthesis in LAM smooth muscle cells.19 This finding, which was rapidly translated into animal and then human studies, forms the basis of the use of sirolimus as an experimental treatment for LAM and TSC.20–22
The work that led to the identification of sirolimus as a possible treatment for LAM was partially supported by several NHLBI asthma grants to investigate mechanisms regulating airway smooth muscle. It also encompassed studies supported by one of the LAM program announcement grants. Importantly, many of the investigators involved in this work had been encouraged to interact and were receiving seed money from the LAM Foundation that enabled them to obtain preliminary data.
LAM Investigator Meetings
NHLBI co-funded the first LAM scientific meeting organized by the LAM Foundation held in New York in 1999. Over the years 2001–2008, NHLBI has provided funds for the annual Lymphangioleiomyomatosis Research Conference, held in Cincinnati. Co-funding from the NIH Office of Rare Diseases Research has been available for some meetings. This international annual meeting, sponsored by the LAM Foundation, brings together basic and clinical scientists and patients and their families. The meetings have been instrumental in bringing in scientists from a variety of disciplines and advancing LAM research rapidly from bench to bedside. A key part of the LAM meetings is that they provide a forum for patient participation and patient interaction with LAM investigators. (LAM foundation website http://lam.uc.edu/html/common.html)
Information on LAM for the public
The NHLBI developed a Fact Sheet on LAM for the public in the mid 1990s. Since then, the Fact Sheet has been updated and converted to the web-based Diseases Conditions Index (DCI) located on the NHLBI Website. The most recent version was revised in 2008.23
Future Directions and Perspective
Progress has been substantial, but much remains to be done. During the last 15 years, much of the molecular pathobiology of LAM and key findings such as the association of LAM with TSC has been elucidated, LAM tissue is available to qualified investigators, and clinical management has improved. A treatment target was identified and this work was rapidly translated into clinical studies. Research on the role of estrogen in LAM and the pathways regulated by the TSC genes has made it clear that multidrug regimens will be needed. This research is also yielding data on promising new therapeutic targets. One barrier that has hampered the progress of LAM research is the lack of good animal models. A new grant that has just been awarded by NHLBI will attempt to a develop a mouse model of LAM to study the role of estrogen and other hormones and the loss of TSC2 in the genesis and survival of smooth muscle-like LAM cells in the lung. LAM patients frequently have low-grade pulmonary hypertension which increases on exercise and contributes to their morbidity.24 Another new grant will use murine models of pulmonary hypertension to investigate the mechanisms of pulmonary hypertension and pulmonary vascular remodeling, which remain undefined.
The story of LAM research provides a striking illustration of the power that strong, effective patient advocacy, prudent investment of federal research dollars, and active collaborations between private organizations, the NIH, and the pulmonary community can have to catalyze important progress towards understanding rare and complex diseases such as LAM. Effective strategies used to promote the role of patients and patient interest groups in LAM research have been shared with other patient organizations through the Patient Interest Organization meetings fostered by the NHLBI and through the Patient Advisory Roundtable of the American Thoracic Society. We look forward to continuing these efforts and partnerships as we work toward the common goal of discovering effective and safe treatments and new approaches to prevent LAM.
Author Disclosure Statement
Drs. Peavy, Gail, Kiley, and Shurin have no conflicts of interest or financial ties to report.
References
- 1.Maurer JR. Ryu J. Beck G, et al. Lung transplantation in the management of patients with lymphangioleiomyomatosis: Baseline data from the NHLBI LAM registry. J Heart Lung Transplant. 2007;26:1293–1299. doi: 10.1016/j.healun.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ryu JH. Moss J. Beck GJ, et al. The NHLBI Lymphangioleiomyomatosis Registry: Characteristics of 230 patients at enrollment. Am J Respir Crit Care Med. 2006;173:105–111. doi: 10.1164/rccm.200409-1298OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kawanami O. Ferrans VJ. Crystal RG. Subplasmalemmal linear densities in cells of the mononuclear phagocyte system in lung. Am J Pathol. 1980;100:131–149. [PMC free article] [PubMed] [Google Scholar]
- 4.Basset F. Ferrans VJ. Soler P. Takemura T. Fukuda Y. Crystal RG. Intraluminal fibrosis in interstitial lung disorders. Am J Pathol. 1986;122:443–461. [PMC free article] [PubMed] [Google Scholar]
- 5.Taveira–DaSilva AM. Stylianou MP. Hedin CJ. Hathaway A. Moss J. Decline in lung function in patients with lymphangioleiomyomatosis treated with or without progesterone. Chest. 2004;126:1867–1874. doi: 10.1378/chest.126.6.1867. [DOI] [PubMed] [Google Scholar]
- 6.Avila NA. Dwyer AJ. Rabel A. Moss J. Sporadic lymphangioleiomyomatosis and tuberous sclerosis complex with lymphangioleiomyomatosis: Comparison of CT features. Radiology. 2007;242:277–285. doi: 10.1148/radiol.2421051767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nellist M. Janssen B. Brookcarter PT, et al. Identification and characterization of the tuberous sclerosis gene on chromosome-16. cell. 1993;75:1305–1315. doi: 10.1016/0092-8674(93)90618-z. [DOI] [PubMed] [Google Scholar]
- 8.vanSlegtenhorst M. deHoogt R. Hermans C, et al. Identification of the tuberous sclerosis gene TSC1 on chromosome 9q34. Science. 1997;277:805–808. doi: 10.1126/science.277.5327.805. [DOI] [PubMed] [Google Scholar]
- 9.Avila NA. Dwyer AJ. Murphy–Johnson DV. Brooks P. Moss J. Sonography of lymphangioleiomyoma in lymphangioleiomyomatosis: Demonstration of diurnal variation in lesion size. Am J Roentgenol. 2005;184:459–464. doi: 10.2214/ajr.184.2.01840459. [DOI] [PubMed] [Google Scholar]
- 10.Pacheco–Rodriguez G. Steagall WK. Crooks DM, et al. TSC2 loss in lymphangioleiomyomatosis cells correlated with expression of CD44v6, a molecular determinant of metastasis. Cancer Res. 2007;67:10573–10581. doi: 10.1158/0008-5472.CAN-07-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crooks DM. Pacheco–Rodriguez G. DeCastro RM, et al. Molecular and genetic analysis of disseminated neoplastic cells in lymphangioleiomyomatosis. Proc Natl Acad Sci USA. 2004;101:17462–17467. doi: 10.1073/pnas.0407971101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pacheco–Rodriguez G. Kristof AS. Stevens LA. Zhang Y. Crooks D. Moss J. Genetics and gene expression in lymphangioleiomyomatosis. Chest. 2002;121:56s–60s. doi: 10.1378/chest.121.3_suppl.56s. [DOI] [PubMed] [Google Scholar]
- 13.Taveira–DaSilva AM. Stylianou MP. Hedin CJ, et al. Maximal oxygen uptake and severity of disease in lymphangioleiomyomatosis. Am J Resp Crit Care Med. 2003;168:1427–1431. doi: 10.1164/rccm.200206-593OC. [DOI] [PubMed] [Google Scholar]
- 14.Chu SC. Horiba K. Usuki J, et al. Comprehensive evaluation of 35 patients with lymphangioleiomyomatosis. Chest. 1999;115:1041–52. doi: 10.1378/chest.115.4.1041. [DOI] [PubMed] [Google Scholar]
- 15.Carsillo T. Astrinidis A. Henske EP. Mutations in the tuberous sclerosis complex gene TSC2 are a cause of sporadic pulmonary lymphangioleiomyomatosis. Proc Natl Acad Sci USA. 2000;97:6085–6090. doi: 10.1073/pnas.97.11.6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smolarek TA. Wessner LL. McCormack FX. Mylet JC. Menon AG. Henske EP. Evidence that lymphangiomyomatosis is caused by TSC2 mutations: Chromosome 16p13 loss of heterozygosity in angiomyolipomas and lymph nodes from women with lymphangiomyomatosis. Am J Human Genet. 1998;62:810–815. doi: 10.1086/301804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karbowniczek M. Astrinidis A. Balsara BR, et al. Recurrent lymphangiomyomatosis after transplantation: genetic analyses reveal a metastatic mechanism. Am J Respir Crit Care Med. 2003;167:976–982. doi: 10.1164/rccm.200208-969OC. [DOI] [PubMed] [Google Scholar]
- 18.Karbowniczek M. Astrinidis A. Balsara BR, et al. Recurrent lymphangiomyomatosis after transplantation: Genetic analyses reveal a metastatic mechanism. Am J Respir Crit Care Med. 2003;167:976–982. doi: 10.1164/rccm.200208-969OC. [DOI] [PubMed] [Google Scholar]
- 19.Goncharova EA. Goncharov DA. Eszterhas A, et al. Tuberin regulates p70 S6 kinase activation and ribosomal protein S6 phosphorylation. A role for the TSC2 tumor suppressor gene in pulmonary lymphangioleiomyomatosis (LAM) J Biol Chem. 2002;277:30958–30967. doi: 10.1074/jbc.M202678200. [DOI] [PubMed] [Google Scholar]
- 20.Bissler JJ. McCormack FX. Young LR, et al. Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N Engl J Med. 2008;358:140–151. doi: 10.1056/NEJMoa063564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davies DM. Johnson SR. Tattersfield AE, et al. Sirolimus therapy in tuberous sclerosis or sporadic lymphangioleiomyomatosis. N Engl J Med. 2008;358:200–203. doi: 10.1056/NEJMc072500. [DOI] [PubMed] [Google Scholar]
- 22.Paul E. Thiele E. Efficacy of sirolimus in treating tuberous sclerosis and lymphangioleiomyomatosis. N Engl J Med. 2008;358:190–192. doi: 10.1056/NEJMe0707153. [DOI] [PubMed] [Google Scholar]
- 23.Fact Sheet on LAM. 2008. [Aug;2009 ]. http://www.nhlbi.nih.gov/health/dci/Diseases/lam/lam_whatis.html http://www.nhlbi.nih.gov/health/dci/Diseases/lam/lam_whatis.html
- 24.Taveira–DaSilva AM. Hathaway OM. Sachdev V. Shizukuda Y. Birdsall CW. Moss J. Pulmonary artery pressure in lymphangioleiomyomatosis. An echocardiographic study. Chest. 2007;132:1573–1578. doi: 10.1378/chest.07-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]