Abstract
Background
Lymphatic pump techniques (LPT) are used clinically by osteopathic practitioners for the treatment of edema and infection; however, the mechanisms by which LPT enhances lymphatic circulation and provides protection during infection are not understood. Rhythmic compressions on the abdomen during LPT compress the abdominal area, including the gut-associated lymphoid tissues (GALT), which may facilitate the release of leukocytes from these tissues into the lymphatic circulation. This study is the first to document LPT-induced mobilization of leukocytes from the GALT into the lymphatic circulation.
Methods and Results
Catheters were inserted into either the thoracic or mesenteric lymph ducts of dogs. To determine if LPT enhanced the release of leukocytes from the mesenteric lymph nodes (MLN) into lymph, the MLN were fluorescently labeled in situ. Lymph was collected during 4 min pre-LPT, 4 min LPT, and 10 min following cessation of LPT. LPT significantly increased lymph flow and leukocytes in both mesenteric and thoracic duct lymph. LPT had no preferential effect on any specific leukocyte population, since neutrophil, monocyte, CD4+ T cell, CD8+ T cell, IgG+B cell, and IgA+B cell numbers were similarly increased. In addition, LPT significantly increased the mobilization of leukocytes from the MLN into lymph. Lymph flow and leukocyte counts fell following LPT treatment, indicating that the effects of LPT are transient.
Conclusions
LPT mobilizes leukocytes from GALT, and these leukocytes are transported by the lymphatic circulation. This enhanced release of leukocytes from GALT may provide scientific rationale for the clinical use of LPT to improve immune function.
Introduction
The lymphatic system collects proteins and excess interstitial fluid into afferent lymphatic vessels. This lymph also carries antigens and antigen-bearing cells from infected tissues to lymph nodes, where antigen-specific immune responses are initiated.1 The resulting primed lymphocytes then exit the lymph nodes via efferent lymphatic vessels and re-enter the lymphatic circulation. The thoracic duct is the largest lymphatic vessel, and it transports lymph from most body tissues, excluding the right arm, the right side of the head, neck and chest, and the right lung and lower left lung lobe, which are drained by the right lymphatic duct.2 Lymph from the thoracic duct enters the blood circulation at the left subclavian vein, allowing primed lymphocytes to enter general blood circulation.3 This lymphocyte recirculation facilitates interactions of lymphocytes with foreign antigens in blood and tissue, and is an important component of the immune system.
Unlike circulating blood, the movement of lymph through lymphatic vessels is not maintained by the pumping of the heart. Instead, lymphatic circulation is maintained through the rhythmic, phasic contraction of lymph vessel walls and external compression of the lymph vessels. A series of one-way valves along the vessels ensures unidirectional lymph flow toward their junction with the blood circulation.1,2 Forces external to the lymph vessels such as respiration, intestinal peristalsis, and muscle contraction facilitate lymph flow.3 In addition, activities such as exercise,4,5 passive limb movement,5,6 and body-based manipulative medicine techniques4,7,8 have been shown to increase thoracic duct lymph flow.
Diseases that result in congestion of the lymphatic circulation, such as infection and lymphedema, can inhibit leukocyte recirculation and exacerbate the disease process.9–11 Therefore, interventions that relieve lymphedema and enhance lymph-tissue recirculation of immune cells, immune products, or pharmaceuticals should aid in the treatment of infectious disease. Specifically, limb elevation and compression garments reduce filtration of fluid from vascular capillaries and accelerate removal of excess interstitial fluid by the lymphatic drainage.12 This lymphatic drainage can be further stimulated by intermittent, external pneumatic compression.13 In addition, manual therapies used by osteopathic practitioners, physical therapists, and massage therapists have been reported to reduce lymphedema.14,15
Osteopathic physicians believe that removing obstructions to tissue blood and lymph flow is one of the most effective ways to promote and restore health.9,15,16 A group of osteopathic manipulations known collectively as the lymphatic pump techniques (LPT) are designed to enhance lymph return from specific areas of the body. These techniques include the thoracic, liver, splenic, and pancreatic pumps, the pedal pump, and the abdominal lymphatic pump.9,16 In addition to reducing edema, the increased lymph flow that results from these treatments is thought to accelerate the removal of cellular wastes, toxins, and bacteria from the interstitial fluid.17 These lymphatic pump techniques are also reported to enhance immune function.8,18–20 In clinical studies, LPT increased vaccine-specific antibodies,21,22 reduced antibiotic use during infection,23–25 and reduced the duration of hospital stay in elderly patients with pneumonia.25 Collectively, these studies suggest that LPT stimulates immune responses, which may accelerate the clearance of infection. However, the mechanisms by which LPT may enhance immunity and provide protection during infection are still poorly understood.
Previously, we demonstrated that abdominal LPT increased leukocyte counts in thoracic lymph;8 however, the tissue source of these mobilized leukocytes was unknown. Studies to identify the source of thoracic duct lymphocytes indicate that the majority of thoracic duct lymphocytes are derived from the gut-associated lymphoid tissue (GALT).26–29 Rhythmic compressions of the abdomen during LPT most likely compress regional lymphoid tissues, including the gastrointestinal mucosa, which may release pooled leukocytes into the lymph circulation. The purpose of this study was to determine if, in fact, abdominal LPT increases leukocytes in both thoracic and mesenteric duct lymph, and determine if the mesenteric lymph nodes (MLN) are a source of these leukocytes.
Materials and Methods
Animal procedures
This study was approved by the Institutional Animal Care and Use Committee and conducted in accordance with the Guide for the Care and Use of Laboratory Animals (NIH Publication no. 85-23, revised 1996). Seventeen adult mongrel dogs, free of clinically evident signs of disease were used for this study.
Surgical techniques
Dogs were anesthetized with sodium pentobarbital (30 mg/kg, i.v.). After endotracheal intubation, the dogs were ventilated with room air supplemented with O2 to maintain normal arterial blood gases. A femoral arterial catheter provided blood samples to measure arterial blood gases, and this catheter was also connected to a transducer to monitor arterial blood pressure, which remained normal throughout the experimental protocols. A femoral venous catheter was used to administer supplementary anesthetic.
Collection of thoracic duct lymph
In six dogs, the chest was opened by a thoracotomy in the left fourth intercostal space. The thoracic duct was isolated from connective tissue and ligated. Caudal to the ligation, a PE 60 catheter (i.d. 0.76 mm, o.d. 1.22 mm) was inserted into the duct and secured with a ligature. Approximately 60 min following cannulation of the thoracic duct, thoracic lymph was collected at 1 min intervals during 4 min pre-LPT (baseline), during 4 min of LPT, and at 2–5 min intervals for 10 min following cessation of LPT (post-treatment condition). Lymph flow rate was computed from the volume of lymph collected during these time intervals.
Collection of mesenteric duct lymph
For collection and analysis of mesenteric lymph, six dogs were surgically prepared for experimentation as described above, except that instead of opening the chest, a midline abdominal incision was made to expose a large mesenteric lymph duct. This duct was isolated, ligated, and a PE60 catheter was inserted into the duct and secured with a ligature. This catheter was exteriorized through the abdominal incision, which was then closed with 2-0 silk suture. Approximately 60 min following cannulation of the mesenteric lymph duct, mesenteric lymph samples were collected, and flow was measured as described above for thoracic duct lymph.
Fluorescent labeling of mesenteric lymph nodes in situ
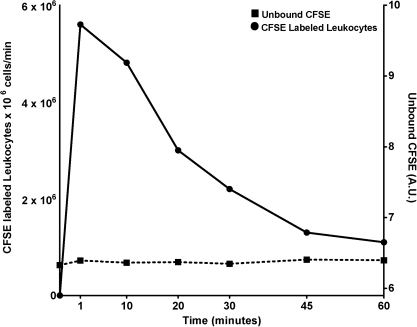
Five dogs were surgically prepared for thoracic duct lymph collection as described above, with incisions in both the left chest and in the midline abdomen. After the abdominal cavity was opened, readily visible mesenteric lymph nodes (MLN) were labeled as previously described.30 Briefly, 12 mg of lyophilized 5(6)-Carboxyfluorescein diacetate N-succinimidyl ester (CFSE) were dissolved in 5 ml dimethyl sulfoxide (Sigma, St Louis, MO). Then, each MLN was directly injected with 100–200 μl of CFSE, depending on the size of the node. The abdominal cavity was closed with 2-0 silk suture. The thoracic lymph duct was then catheterized for collection of lymph as described above. Thoracic duct lymph samples were collected at 1, 10, 20, 30, 45, and 60 min after labeling the MLN with CFSE, and these lymph samples were analyzed for CFSE-labeled leukocytes and free CFSE (see Figure 1). When the concentration of CFSE labeled leukocytes in thoracic duct lymph had reached a steady state, at 45–60 min post-labeling, lymph was collected during 4 min pre-LPT (min 61, 62, 63, and 64), during 4 min of LPT (min 65, 66, 67, and 68), and for 10 min post-LPT (78 min). The number of CFSE-labeled leukocytes was measured in these lymph samples.
FIG. 1.
Thoracic duct lymph was collected at 0 (pre-injection) and at 1, 10, 20, 30, 45, and 60 min following intranodal injection of the MLN with CFSE. Lymph was assayed for the numbers of CFSE-labeled leukocytes per minute and the amount of free (unbound) CFSE.
Lymphatic pump technique (LPT)
The anesthetized animal was placed in a supine position. To perform abdominal LPT, the operator contacted the animal with the hands placed bilaterally at the costo-diaphragmatic junction. Pressure was exerted medially and cranially sufficient to compress the lower ribs until significant resistance was encountered, and then the pressure was released. Rib compressions were administered at a rate of approximately 1/sec for a total of 4 min of LPT.
Analytical procedures
Leukocyte enumeration
Leukocytes were enumerated using the Hemavet 950 (Drew Scientific, Waterbury, CT). To compute the leukocyte flux, the total number of a specific leukocytes population was multiplied by the lymph flow per minute.
Determination of total fluorescence and fluorescing leukocytes in lymph samples
Two-color immunofluorescent staining and flow cytometry were performed to identify lymphocyte populations. Fluorescein isothiocyanate (FITC) labeled anti-canine CD3, phycoerythrin (PE)-anti-canine B cell, PE-anti-canine CD4, AlexaFluor 647-anti-canine CD8, FITC-anti-canine IgA, or FITC-anti-canine IgG monoclonal antibodies (mAb) (Serotech, Raleigh, NC) were used. A total of 106 cells were incubated with the mAb as described by the manufacturer. The cells were washed in staining buffer consisting of Mg2+-free, Ca2+-free phosphate buffered saline supplemented with 2% fetal bovine serum (HyClone Laboratories, Logan, UT) and fixed with 0.05% paraformaldehyde until analyzed.
Following intranodal labeling of the MLN, lymph was centrifuged and CFSE in supernatants of thoracic duct lymph was measured with a Cary Eclipse spectrofluorometer (Varian Inc., Palo Alto, CA). The excitation wavelength was 480 nm, and the emission signal was collected at wavelengths from 500 to 570 nm. Each sample was scanned three times and a mean value was computed.
Fluorescently labeled lymphoid cells were analyzed using a Cytomics FC 500 flow cytometer (Beckman Coulter, Fullerton, CA). Lymphocyte gates and detector voltages were set using isotype control stained cells, and stained cell populations were seen as distinct peaks or clusters of cells. The proportion of each cell population was expressed as the percentage of the number of stained cells. To determine the total number of a specific lymphocyte population in a milliliter of lymph, their percentage was multiplied by the total number of cells. To determine the flux of a leukocyte population (cells/min), the cell population number was multiplied by the thoracic duct lymph flow.
Statistical analyses
Data are presented as arithmetic means ± standard error (SE). Values at individual time points were analyzed and are plotted in figures. Mean values for pre-LPT, LPT, and post-LPT conditions were computed for each animal; these values were analyzed and are reported in tables. For statistical evaluation, data were subjected to repeated measures analysis of variance followed by a Tukey–Kramer multiple comparisons post test. Analyses were performed with Graphpad Prism version 5.0 for Windows, (GraphPad Software, San Diego, CA). Differences among mean values with P ≤ 0.05 were considered statistically significant.
Results
Abdominal LPT increases leukocytes in thoracic duct lymph
During the pre-LPT condition, thoracic duct lymph contained 12.5 ± 2.2 × 106 leukocytes/ml, a value consistent with prior measurements in dogs,8 rats,27,31 and humans.26 LPT quickly increased leukocyte count in thoracic duct lymph to a peak value 34.0 ± 15.0 × 106 cells/ml at 2 min of treatment (P < 0.05, vs pre-LPT). For 4 min of LPT, the lymph leukocyte count averaged 32.2 ± 9.4 × 106 cells/ml (P < 0.01, vs pre-LPT). By 10 min post-LPT, the lymph leukocyte count (11.8 ± 2.0 × 106 cells/ml) was not significantly greater than the pre-LPT value (P > 0.05).
The thoracic duct lymph flow pre-LPT was 0.62 ± 0.12 ml/min, and during the 4 min of LPT, the lymph flow averaged 4.2 ± 0.39 ml/min (P < 0.01, vs pre-LPT). By 10 min post-LPT, thoracic duct lymph flow was still elevated at 2.0 ± 0.77 ml/min (P < 0.05, vs pre-LPT).
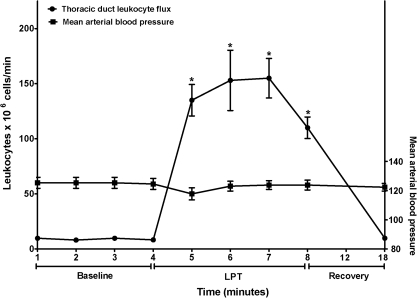
Leukocyte flux was computed from the product of thoracic duct lymph flow and cell count. Figure 2 illustrates dynamics of leukocyte flux in the thoracic duct. LPT markedly accelerated leukocyte flux in the thoracic duct from 8.88 ± 2.0 × 106 cells/min to a peak value of 153 ± 67 × 106 cells/min (P < 0.01, vs pre-LPT). For 4 min of LPT, the leukocyte flux averaged 138 ± 43 × 106 cells/ml (P < 0.01, vs pre-LPT). By 10 min post-LPT, the leukocyte flux was significantly greater than pre-LPT values (P < 0.05 vs pre-LPT). Fluxes of leukocyte subpopulations in the thoracic duct are summarized in Table 1. LPT had no preferential effect on any of these components, since neutrophils, monocytes, total lymphocytes, CD4+ T cells, CD8+ T cells, and IgA+ and IgG+ B cell numbers were similarly increased. By 10 min post-LPT, flux of each of these cell types had decreased, but tended to exceed pre-LPT values.
FIG. 2.
Thoracic duct lymph was collected 1) pre-LPT, 2) during 4 min LPT, and 3) during 10 min post-LPT. Data are means × 106 total leukocytes/minute ± SE or mean arterial blood pressure ± SE from 6 animals. *Greater than Pre-LPT and Post-LPT (P < 0.001).
Table 1.
Abdominal LPT Increases Leukocyte Flux in Thoracic Duct Lymph
| Pre-LPT | LPT | Post-LPT | |
|---|---|---|---|
| Neutrophils | 0.27 ± 0.12 | 3.67 ± 0.96** | 0.29 ± 0.01 |
| Monocytes | 0.34 ± 0.14 | 4.24 ± 1.18* | 0.36 ± 0.10 |
| Lymphocytes | 10.32 ± 4.53 | 81.1 ± 22.2** | 7.30 ± 2.30 |
| CD4+ T cells | 3.25 ± 0.62 | 43.7 ± 5.57** | 5.10 ± 1.90 |
| CD8+ T cells | 1.24 ± 0.37 | 16.3 ± 4.12** | 2.23 ± 0.76 |
| IgA+ B cells | 0.65 ± 0.18 | 9.02 ± 0.86** | 0.60 ± 0.21 |
| IgG+ B cells | 1.06 ± 0.21 | 13.4 ± 4.81* | 0.78 ± 0.18 |
Data are means × 106 leukocytes/min ± SE from 6 experiments.
Greater than Pre-LPT and Post-LPT (P < 0.01), **Greater than Pre-LPT and Post-LPT (P < 0.001).
Abdominal LPT mobilizes leukocytes into mesenteric duct lymph
The average pre-LPT leukocyte count in mesenteric lymph was 6.3 ± 0.67 × 106 cells/ml, consistent with values previously reported for sheep32,33 and rats.27 Thus, basal mesenteric leukocyte count was approximately 50% of that of thoracic duct lymph. LPT quickly increased leukocyte count in mesenteric lymph to a peak value 18.0 ± 4.11 106 cells/ml (P < 0.01, vs pre-LPT). For 4 min of LPT, the lymph leukocyte count averaged 16.7 ± 3.8 106 cells/ml (P < 0.01, vs pre-LPT). During LPT, the mesenteric leukocyte count remained approximately 50% of that thoracic duct lymph, so proportional increases in leukocyte counts were observed in both the mesentery and thoracic ducts. By 10 min post-LPT, the lymph leukocyte count had fallen but returned to near the pre-LPT value (P > 0.05, vs pre-LPT).
The average pre-LPT, mesenteric lymph flow was 0.35 ± 0.07 ml/min. During the first 2 min of LPT, the duct lymph flow increased to an average of 1.4 ± 0.42 ml/min (P < 0.05, vs pre-LPT). This initial increase in mesenteric flow was proportionally similar to that observed for 4 min in the thoracic duct. However, during the last 2 min of LPT, the mesenteric duct lymph flow average fell to 0.87 ± 0.14 ml/min (P < 0.05 vs pre-LPT). This finding suggests that LPT mobilizes mesenteric lymph from a fluid pool that becomes partially depleted during 4 min of LPT treatment. By 10 min post-LPT, the mesenteric lymph flow was similar to pre-LPT.
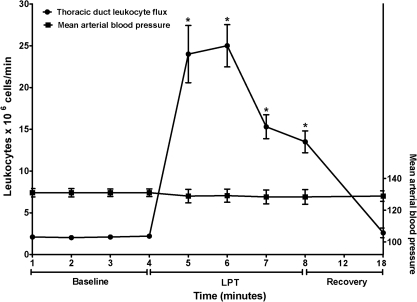
As illustrated in Figure 3, LPT markedly increased flux of leukocytes in mesenteric lymph. This flux increased from an average pre-LPT value of 2.1 ± 0.25 cells/min to a peak of 25.0 ± 6.2 cells/min at 2 min LPT (P < 0.01, vs baseline). During the first 2 min LPT, mesenteric leukocyte flux averaged 25 ± 7.3 cell/min (P < 0.01, vs baseline). During the later 2 min of LPT, mesenteric leukocyte flux subsided due to less of an increment in lymph flow. LPT also increased flux of CD4+ T cells, CD8+ T cells, and IgA+ and IgG+ B cells in mesenteric lymph (Table 2).
FIG. 3.
Mesenteric duct lymph was collected 1) pre-LPT, 2) during 4 min LPT, and 3) during 10 min post-LPT. Data are means × 106 total leukocytes/minute ± SE or mean arterial blood pressure ± SE from 6 animals. *Greater than Pre-LPT and Post-LPT (P < 0.01).
Table 2.
Abdominal LPT Increases Leukocyte Flux in Intestinal Duct Lymph
| Baseline | LPT | Recovery | |
|---|---|---|---|
| Neutrophils | 0.12 ± 0.03 | 1.79 ± 0.47** | 0.22 ± 0.07 |
| Monocytes | 0.10 ± 0.02 | 1.10 ± 0.30** | 0.29 ± 0.13 |
| Lymphocytes | 1.85 ± 0.23 | 13.1 ± 4.43** | 1.90 ± 0.98 |
| CD4+ T cells | 1.36 ± 0.36 | 9.00 ± 3.47** | 1.30 ± 0.50 |
| CD8+ T cells | 0.27 ± 0.08 | 1.84 ± 0.55** | 0.29 ± 0.10 |
| IgA+ B cells | 0.28 ± 0.13 | 4.10 ± 3.42* | 0.14 ± 0.05 |
| IgG+ B cells | 0.14 ± 0.06 | 2.00 ± 1.29* | 0.45 ± 0.04 |
Data are means × 106 leukocytes/min ± SE from 6 experiments.
Greater than baseline (P < 0.05). **Greater than baseline (P < 0.01) and recovery (P < 0.05).
Abdominal LPT mobilizes leukocytes from the mesenteric lymph nodes into thoracic duct lymph
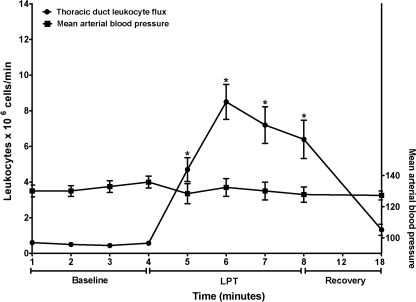
Immediately following intranodal labeling of the MLN with CFSE, 80%–90% of leukocytes in samples of thoracic duct lymph were labeled with CFSE. This percentage then gradually fell until, by 45–60 min postlabeling, the percentage of labeled leukocytes in thoracic duct lymph was nearly constant at approximately 16%. Thus, in this steady-state baseline condition, about 16% of the thoracic duct leukocytes had originated from labeled MLN. The initial greater percentage of labeled leukocytes in thoracic duct lymph was most likely due to extranodal labeling of leukocytes with CFSE that had escaped from the injection sites. This view is supported by detection of unbound CFSE in supernatant of samples of thoracic duct lymph collected soon after injection of CFSE. However, by 45–60 min following intranodal injections of CFSE, unbound CFSE was no longer detected in thoracic duct lymph. Thus, by 45–60 min postlabeling, the amount of CFSE in thoracic duct lymph provided a stable index of the contribution of MLN-derived leukocytes to total leukocytes in thoracic duct lymph. This percentage of CFSE-labeled leukocytes in the thoracic duct samples collected were 16 ± 3.1% pre-LPT, 19 ± 4.8% during LPT, and 21 ± 7.7% post-LPT. If the MLN were not a source of the leukocytes released into lymphatic circulation during LPT, the percentage of CFSE-labeled leukocytes would have decreased markedly during LPT, since thoracic duct flow increased. While LPT did not significantly increase the percentage of CFSE labeled leukocytes in the thoracic duct lymph, LPT did increase the lymphatic flux of CFSE labeled leukocytes (Fig. 4), since thoracic duct lymph flow was increased approximately 4-fold. Considering the flux of leukocytes in the thoracic duct, and the percentage of these leukocytes that were derived from MLN, these data demonstrate that 4 min of LPT produced an incremental mobilization of 27 × 106 leukocytes from MLN. Thus, the hypothesis that LPT increases mobilization of leukocytes from the MLN into the lymphatic circulation is supported.
FIG. 4.
Sixty minutes after labeling the MLN in situ with CFSE, thoracic duct lymph was collected 1) pre-LPT, 2) during 4 min LPT, and 3) during 10 min post-LPT. Data are means × 106 of CFSE labeled leukocytes/minute ± SE or mean arterial blood pressure ± SE from 5 animals. *Greater than Pre-LPT (P < 0.05).
Discussion
This report provides the first data on the effects of LPT on mesenteric lymph flow and leukocytes in mesenteric lymph. Consistent with prior studies of this laboratory on the effects of LPT on thoracic duct flow and leukocyte counts,4,7,8 abdominal LPT produced significant elevations of mesenteric lymph flow and leukocyte counts and flux. While lymphatic leukocyte numbers remained statistically elevated during the entire 4 minutes of LPT, a decline in leukocytes was observed during the last 3-04 minutes of LPT, particularly in the mesenteric lymph. This was due primarily to reduced lymph flow, and suggests that LPT mobilizes mesenteric lymph from a fluid pool that can deplete within a few minutes of treatment. Mesenteric lymph dynamics are of particular importance since leukocytes from the GALT are transported by the mesenteric lymph circulation. LPT increased the numbers of IgA and IgG surface-bearing B cells in mesenteric and thoracic duct lymph, demonstrating that LPT is indeed able to mobilize mature, antigen-specific lymphocytes from GALT into the lymphatic circulation.
Of the leukocytes in thoracic duct lymph, approximately 16% came from labeled mesenteric lymph nodes. LPT markedly increased lymph flow with no decrement in the percentage of labeled cells, thus demonstrating the ability of LPT to significantly enhance mobilization of leukocytes from MLN. It is important to note that only 25%–50% of the MLN were labeled; therefore, the actual percentage of thoracic duct leukocytes that originate from the MLN would be much greater than our data indicate. The greatest increase in leukocyte concentrations was observed during the last two minutes of LPT (Fig. 4). These finding data indicate that it takes approximately 2 minutes for leukocytes released from the MLN to be transported into the thoracic duct.
LPT rapidly increased mesenteric leukocyte numbers in lymphatic circulation, suggesting the GALT is a tissue source that is sensitive to LPT. Leukocytes from the spleen exit via the splenic vein and take hours to enter peripheral blood circulation and lymphoid organs.30 Therefore, it is unlikely that LPT mobilized leukocytes from the spleen into lymphatic circulation within the 4 minutes of treatment. Furthermore, splenectomy does not significantly alter thoracic duct lymphocyte numbers,29 suggesting that leukocytes from the spleen do not readily migrate into thoracic duct lymph. Collectively, these findings suggest that spleen is not a source of the leukocytes mobilized into lymph during LPT.
While the release of leukocytes into lymph during LPT was transient, 4 minutes of LPT produced a net increase of 6 × 108 leukocytes into thoracic duct lymphatic circulation. Although this increase of leukocytes transported into circulation via lymph during LPT may seem relatively minor compared to the total leukocytes found in blood, increased mobilization of leukocytes could enhance immune surveillance and promote earlier responses to pathogens. Mature lymphocytes bearing antigen-specific receptors recirculate continually from the bloodstream through the peripheral or secondary lymphoid organs, and then return to the bloodstream via the lymphatic vessels.1,2 Most adaptive immune responses are initiated when these recirculating lymphocytes recognize specific antigens on the surface of an activated professional antigen presenting cell.2 If this circulation of lymphocytes is restricted in any way, there could be a delay in the immune response to a pathogen, which could compromise the health of an individual.9,11,16,19 Therefore, LPT may be an approach to not only to improve lymph flow but, more importantly may also increase the release of lymphocytes into circulation, resulting in earlier and more frequent encounters and responses to a pathogen.
The mucous membranes, which line the respiratory, digestive, and urogenital tracts, are a major site of antigen entry.1,2 Studies have shown that antigen-specific lymphocytes primed in the gastrointestinal tract can migrate into the lungs and provide protection during pulmonary infection.34–38 Furthermore, leukocytes from the GALT comprise a different array of immune specificities and responses than other tissues due to the antigens that they encounter.39 Thus, LPT may not only enhance circulation of leukocytes, but it may also facilitate the trafficking of local immune responses to other tissues, such as the lungs, that may benefit from their presence during infection.
In conclusion, clinical reports suggest that LPT, can stimulate immune responses,20–22 which may accelerate the clearance of infection.23–25 In support of this notion, we have demonstrated that LPT increases the release of leukocytes from the GALT into lymphatic circulation. The information gained from this study provides a rationale for the use of LPT to enhance immunity.
Footnotes
This work was supported by grants from the NIH: U19 AT002023 (H. Downey) and R01 AT004361 (L. Hodge).
Acknowledgments
We thank Ignacy Gryczynski, Ph.D., and Pabak Sarkar, for assistance with the Eclipse spectrofluorometer, and Linda Howard for assistance with animal surgery. In addition, we thank Jerry Simecka, Ph.D., and Scott Stoll, D.O., Ph.D., for insightful discussions during the investigation.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Janeway C. Travers P. Walport M. Shlomchik M. Immunobiology: the immune system in health and disease. New York and London: Garland Publishing Inc; 2004. [Google Scholar]
- 2.Kindt G. Kuby . Immunology. New York: W.H Freeman and Company; 2007. [Google Scholar]
- 3.Galanzha EI. Tuchin VV. Zharov VP. Advances in small animal mesentery models for in vivo flow cytometry, dynamic microscopy, and drug screening. World J Gastroenterol. 2007;13:192–218. doi: 10.3748/wjg.v13.i2.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knott EM. Tune JD. Stoll ST. Downey HF. Increased lymphatic flow in the thoracic duct during manipulative intervention. J Am Osteopath Assoc. 2005;105:447–456. [PubMed] [Google Scholar]
- 5.Schad H. Brechtelsbauer H. Thoracic duct lymph flow and composition in conscious dogs and the influence of anaesthesia and passive limb movement. Pflugers Arch. 1977;371:25–31. doi: 10.1007/BF00580768. [DOI] [PubMed] [Google Scholar]
- 6.Lindena J. Küpper W. Friedel R. Trautschold I. Lymphatic transport of cellular enzymes from muscle into the intravascular compartment. Enzyme. 1979;24:120–131. doi: 10.1159/000458640. [DOI] [PubMed] [Google Scholar]
- 7.Downey HF. Durgam P. Williams AG., Jr Rajmane A. King HH. Stoll ST. Lymph flow in the thoracic duct of conscious dogs during lymphatic pump treatment, exercise, and expansion of extracellular fluid volume. Lymphat Res Biol. 2008;6:3–13. doi: 10.1089/lrb.2007.1017. [DOI] [PubMed] [Google Scholar]
- 8.Hodge LM. King HH. Williams AG, Jr, et al. Abdominal lymphatic pump treatment increases leukocyte count and flux in thoracic duct lymph. Lymphat Res Biol. 2007;5:127–133. doi: 10.1089/lrb.2007.1001. [DOI] [PubMed] [Google Scholar]
- 9.Degenhardt BF. Kuchera ML. Update on osteopathic medical concepts and the lymphatic system. J Am Osteopath Assoc. 1996;96:97–100. doi: 10.7556/jaoa.1996.96.2.97. [DOI] [PubMed] [Google Scholar]
- 10.Noll DR. Degenhardt BF. Stuart M. McGovern R. Matteson M. Effectiveness of a sham protocol and adverse effects in a clinical trial of osteopathic manipulative treatment in nursing home patients. J Am Osteopath Assoc. 2004;104:107–113. [PubMed] [Google Scholar]
- 11.Olszewski WL. The lymphatic system in body homeostasis: Physiological conditions. Lymphat Res Biol. 2003;1:11–21. doi: 10.1089/15396850360495655. [DOI] [PubMed] [Google Scholar]
- 12.Felty CL. Rooke TW. Compression therapy for chronic venous insufficiency. Semin Vasc Surg. 2005;18:36–40. doi: 10.1053/j.semvascsurg.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 13.Partsch H. Intermittent pneumatic compression in immobile patients. Int Wound J. 2008;5:389–397. doi: 10.1111/j.1742-481X.2008.00477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Badger C. Preston N. Seers K. Mortimer P. Physical therapies for reducing and controlling lymphoedema of the limbs. Cochrane Database Syst Rev. 2004;CD003141:1–39. doi: 10.1002/14651858.CD003141.pub2. [DOI] [PubMed] [Google Scholar]
- 15.Chikly BJ. Manual techniques addressing the lymphatic system: origins and development. J Am Osteopath Assoc. 2005;105:457–464. [PubMed] [Google Scholar]
- 16.Wallace E. McPartland JM. Jones JM., III Kuchera WA. Buser BR. Lymphatic system: Lymphatic manipulative techniques. In: Ward RC, editor. Foundations for Osteopathic Medicine. East Langsing, MI: Lippincott Williams & Wilkins; 2003. pp. 1056–1077. [Google Scholar]
- 17.Lesho EP. An overview of osteopathic medicine. Arch Fam Med. 1999;8:477–484. doi: 10.1001/archfami.8.6.477. [DOI] [PubMed] [Google Scholar]
- 18.Castlio Y. Ferris–Swift L. Effects of splenic stimulation in normal individuals on the active and differential blood cell counts and the opsonotic index. Kansas City College of Osteopathy and Surgery. 1932;16:10–16. [Google Scholar]
- 19.Measel JW., Jr Introduction: Thoughts on osteopathic practice and infectious diseases. Osteopath Ann. 1982;10:92–94. [Google Scholar]
- 20.Mesina J. Hampton D. Evans R, et al. Transient basophilia following the application of lymphatic pump techniques: A pilot study. J Am Osteopath Assoc. 1998;98:91–94. [PubMed] [Google Scholar]
- 21.Jackson KM. Steele TF. Dugan EP. Kukulka G. Blue W. Roberts A. Effect of lymphatic and splenic pump techniques on the antibody response to Hepatitis B vaccine: A pilot study. J Am Osteopath Assoc. 1998;98:155–160. [PubMed] [Google Scholar]
- 22.Measel JW., Jr The effect of lymphatic pump on the immune response: I. Preliminary studies on the antibody response to pneumococcal polysaccharide assayed by bacterial agglutination and passive hemagglutination. J Am Osteopath Assoc. 1982;82:28–31. [PubMed] [Google Scholar]
- 23.Allen TW. Pence TK. The use of the thoracic pump in treatment of lower respiratory tract disease. J Am Osteopath Assoc. 1967;67:408–411. [PubMed] [Google Scholar]
- 24.Kline CA. Osteopathic manipulative therapy, antibiotics, and supportive therapy in respiratory infections in children: Comparative study. J Am Osteopath Assoc. 1965;65:278–281. [PubMed] [Google Scholar]
- 25.Noll DR. Shores JH. Gamber RG. Herron KM. Swift J., Jr Benefits of osteopathic manipulative treatment for hospitalized elderly patients with pneumonia. J Am Osteopath Assoc. 2000;100:776–782. [PubMed] [Google Scholar]
- 26.Lemaire LC. van Deventer SJ. van Lanschot JJ. Meenan J. Gouma DJ. Phenotypical characterization of cells in the thoracic duct of patients with and without systemic inflammatory response syndrome and multiple organ failure. Scand J Immunol. 1998;47:69–75. doi: 10.1046/j.1365-3083.1998.00265.x. [DOI] [PubMed] [Google Scholar]
- 27.Mann JD. Higgins GM. Lymphocytes in thoracic duct, intestinal and hepatic lymph. Blood. 1950;5:177–190. [PubMed] [Google Scholar]
- 28.Morris B. The hepatic and intestinal contributions to the thoracic duct lymph. Q J Exp Physiol Cogn Med Sci. 1956;41:318–325. doi: 10.1113/expphysiol.1956.sp001195. [DOI] [PubMed] [Google Scholar]
- 29.Reinhardt WO. Yoffey JM. Thoracic duct lymph and lymphocytes in the guinea pig; effects of hypoxia, fasting, evisceration and treatment with adrenaline. Am J Physiol. 1956;187:493–500. doi: 10.1152/ajplegacy.1956.187.3.493. [DOI] [PubMed] [Google Scholar]
- 30.Pabst R. Binns RM. In vivo labeling of the spleen and mesenteric lymph nodes with fluorescein isithiocyanate for lymphocyte migration studies. Immunology. 1981;44:321–329. [PMC free article] [PubMed] [Google Scholar]
- 31.Williams AF. Gowans JL. The presence of IgA on the surface of rat thoracic duct lymphocytes which contain internal IgA. J Exp Med. 1975;141:335–345. doi: 10.1084/jem.141.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chin GW. Cahill NP. The appearance of fluorescien-labeled lymphocytes in lymph following in vitro or in vivo labeling: the route of lymphocyte recirculation through mesenteric lymph nodes. Immunology. 1984;52:341–347. [PMC free article] [PubMed] [Google Scholar]
- 33.Reynolds J. Heron I. Dudler L. Trnka Z. T-cell recirculation in the sheep: Migratory patterns of cells from lymph nodes. Immunology. 1982;47:415–421. [PMC free article] [PubMed] [Google Scholar]
- 34.Aldwell FE. Tucker IG. de Lisle GW. Buddle BM. Oral delivery of Mycobacterium bovis BCG in a lipid formulation induces resistance to pulmonary tuberculosis in mice. Infect Immun. 2003;71:101–108. doi: 10.1128/IAI.71.1.101-108.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DiGiandomenico A. Rao J. Goldberg JB. Oral vaccination of BALB/c mice with Salmonella enterica serovar Typhimurium expressing Pseudomonas aeruginosa O antigen promotes increased survival in an acute fatal pneumonia model. Infect Immun. 2004;72:7012–7021. doi: 10.1128/IAI.72.12.7012-7021.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sato J. Chida K. Suda T. Sato A. Nakamura H. Migratory patterns of thoracic duct lymphocytes into bronchus-associated lymphoid tissue of immunized rats. Lung. 2000;78:295–308. doi: 10.1007/s004080000033. [DOI] [PubMed] [Google Scholar]
- 37.Wallace FJ. Cripps AW. Clancy RL. Husband AJ. Witt CS. A role for intestinal T lymphocytes in bronchus mucosal immunity. Immunol. 1991;74:68–73. [PMC free article] [PubMed] [Google Scholar]
- 38.Zuercher AW. Jiang HQ. Thurnheer MC. Cuff CF. Cebra JJ. Distinct mechanisms for cross-protection of the upper versus lower respiratory tract through intestinal priming. J Immunol. 2002;169:3920–3925. doi: 10.4049/jimmunol.169.7.3920. [DOI] [PubMed] [Google Scholar]
- 39.Simecka JW. Mucosal immunity of the gastrointestinal tract and oral tolerance. Adv Drug Deliv Rev. 1998;34:235–259. doi: 10.1016/s0169-409x(98)00042-8. [DOI] [PubMed] [Google Scholar]