Abstract
Introduction
Malignant pleural effusions (MPEs) complicate many advanced malignancies and the median prognosis for those who develop MPEs is 6 months. These effusions lead patients to suffer from significant dyspnea, which may consequently impair mobility and lead to reduced quality of life. There are several treatment options for those with MPE. Thoracentesis may be quick and relatively easy to perform, but has a high recurrence rate; chest tube placement with talc slurry is quite effective at achieving pleurodesis, but this procedure can be quite painful and requires hospitalization. An alternative option is outpatient placement of the Pleurx® catheter (Denver Biomedical Inc., Denver, CO) for home-based drainage of effusions.
Objective
To determine the incremental cost effectiveness of treating MPE with talc pleurodesis versus placement of Pleurx® catheter.
Methods
We used decision analysis to compare treatments for the management of MPE. Cost data for Pleurx® and talc treatments were obtained using Medicare reimbursement data for 2008, and outcome data (probability of treatment success and/or complication, and utility of health states) were obtained through literature review.
Results
Under our base-case analysis, treatment with talc was less costly than Pleurx® (talc, $8170.80; Pleurx®, $9011.60) with similar effectiveness (talc, 0.281 quality adjusted life years [QALYs]; Pleurx®, 0.276 QALYs). Pleurx® became more cost effective (<$100K/QALY) when life expectancy was 6 weeks or less.
Conclusion
The treatment choice (talc pleurodesis or Pleurx® catheter) for those with an MPE and a prognosis of 6 months should be based on the clinical situation and patient preferences, as well as local expertise and success rates of the procedures. A prospective study specific to the palliative care population might help to clarify which treatment is more cost effective in this population in which optimizing quality of life is essential.
Introduction
Malignant pleural effusions (MPE) complicate many advanced malignancies. The prognosis for those who develop MPEs is poor with a median 6-month survival.1–4 Patients with MPE suffer from significant dyspnea, which may impair mobility and lower quality of life.5,6 The prevalence of dyspnea may be as high as 50%, emphasizing the need for effective management of these patients who are already facing a limited life span.7
There are several treatment options for those with MPE, including thoracentesis, talc pleurodesis, or placement of a long-term indwelling catheter. Thoracentesis may be quick and relatively easy to perform, but has a 98% recurrence rate at 30 days.5 Repeat thoracentesis may be recommended for those patients with advanced disease.8
Chest tube placement with talc slurry is effective at achieving pleurodesis, but this procedure can be painful and requires hospitalization (mean stay was 7 days in two recent studies6,9). A newer treatment option involves the placement of a long-term indwelling catheter (Pleurx®, Denver Biomedical Inc., Denver, CO), which can be performed on an outpatient basis with a low complication rate.1–3,6,7,9,10 These catheters can be accessed for effusion drainage at home.
The ideal treatment approach for those with MPE should offer long-term relief of symptoms, avoid hospitalization, and have minimal side effects/complications.3 Some recent studies have reported positive results with placement of the Pleurx® catheter,1–3,5,6,7,9,10 yet there is no clear consensus regarding the optimal treatment approach. These catheters are being used more frequently for patients with MPE, especially in the field of palliative care. Although there has been some preliminary cost data published on the catheter,11 no data have yet been published that combine cost and quality of life estimates of these two competing approaches. The purpose of this analysis is to determine the incremental cost effectiveness of the “intervention,” i.e., Pleurx® catheter, compared with the “comparator,” i.e., talc pleurodesis, for management of MPE.
Methods
This is a decision analytic model comparing the costs and effects of the Pleurx® catheter to chest tube placement with talc pleurodesis for treatment of malignant pleural effusion. Table 1 describes base-case model inputs. Details of the model and data are described below.
Table 1.
Base-Case Inputs
| Variable | Estimate | Range | Source |
|---|---|---|---|
| Efficacy of treatment | |||
| Effusion resolved, talc | 0.80 | 0.62–0.80 | Shaw 2004, Stefani 2006 |
| Effusion resolved, pleurx® | 0.45 | 0.21–0.70 | Tremblay 2006, 2007 |
| Effusion resolved after infection, with talc | 0.90 | 0.80–0.95 | Estimate |
| Effusion resolved after infection, with Pleurx® | 0.65 | 0.60–0.85 | Estimate |
| Time to pleurodesis with Pleurx | 10 weeks | 1 mo–2.5 mo | Warren 2008, van den Toorn 2005 |
| Probabilities | |||
| Complication with talc | 0.015 | 0.015–0.02 | Dresler 2005 |
| Probability of infection, given complication with talc | 0.667 | Adjusted for conditional probability | |
| Probability of death, given complication with talc | 0.333 | Adjusted for conditional probability | |
| Complication with Pleurx® | 0.075 | 0.02–0.13 | Tremblay 2006, 2007 |
| Probability of infection, given complication with pleurx | 0.9999 | Adjusted for conditional probability | |
| Probability of death, given complication with pleurx | 0.0001 | Adjusted for conditional probability | |
| Utilities | |||
| Effusion resolved, still with cancer/fatigue | 0.599 | Nafees 2008 | |
| Effusion not resolved | 0.473 | Nafees 2008 | |
| Utility with pleurx catheter | 0.58 | Liem 2008 | |
| In hospital with chest tube or infection | 0.40 | Estimate | |
| Costs (2008 U.S. dollars) | |||
| Admission for talc | $5279 | Medicare DRG | |
| Admission for infection | $7877 | Medicare DRG | |
| Home visit (R.N. 1 hour) | $85 | Paul 2004 | |
| Visit with physician | $100 | Estimate | |
| Placement of Pleurx® | $1956 | Medicare CPT | |
| Case of Pleurx® supplies (10 boxes) | $750 | $500–$1500 | Denver Biomedical, Inc. (Denver, CO) |
Target population
This analysis includes patients over 50 years of age with recurrent MPE with a goal to achieve successful pleurodesis and relief of symptoms. Patients with any type of cancer and MPE are eligible for inclusion. This analysis pertains only to the treatment for a single effusion. We assume that the diagnosis of MPE has already been established at the time of the intervention.
Perspective and time horizon
Analysis is performed from the perspective of the third-party payer; only direct health care costs to patients and direct insurance-covered costs are considered. We did not include direct non-health care costs (such as costs of transportation and child care) and productivity costs in our analysis. The time horizon for this model is 6 months.
Model description
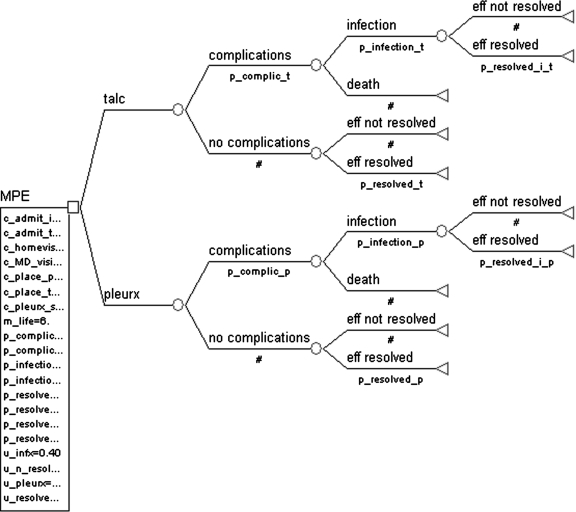
This analysis was performed using a static decision tree model. The decision tree is shown in Figure 1, and was constructed using Tree Age 4.0 software (Treeage Software Inc., Williamstown, MA, 2009). Analysis was performed as a cost-effective analysis, with costs valued in 2008 dollars and effects valued in utilities (which were transformed to quality-adjusted life years, QALYs). The decision node (square shape, MPE) in the tree represents the alternate treatment approaches of Pleurx® catheter placement versus talc pleurodesis for treatment of MPE. Only serious complications of infection and death are considered. These complications are represented as branch points from the chance node (circle shape) entitled “complications” and “no complications” after each treatment option.
FIG. 1.
Decision Tree: Pleurx Catheter versus Talc Pleurodesis.
Other less common complications, such as fever and pain, are not included; we assume these complications would be managed conservatively, do not incur differential additional costs, and effects on utilities are transient.
Effects data: outcomes and assumptions
The studies that we reviewed refer to treatment success with talc or Pleurx® placement as resolution of the effusion and catheter removal. Thus, our treatment outcome (represented by terminal nodes [triangles]) is effusion resolution (Fig. 1, “eff resolved” and “eff not resolved”). If the effusion is resolved, symptoms related to the effusion are considered to have resolved. We assume that patients still live for the remainder of the 6 months with a baseline level of fatigue even if the effusion has resolved. We also assume that these treatments are palliative; they have no differential effects on mortality and all effects are expressed in terms of patient utilities.
Effects data: treatment success/time to success
Base-case and range estimates for treatment effects are reported in Table 1, with a more detailed explanation of these estimates shown in Tables 2 and 3. Estimates for success of treatment with talc ranged from 0.62 to 0.80. Based on a recent large meta-analysis, we chose 0.80 as the base-case estimate for success of talc pleurodesis.12 Several studies were identified for treatment with Pleurx® (Table 3), and from these studies, we defined the base-case probability of success with the catheter as 0.45. This value was chosen after careful review of the literature with more weight placed on those studies with larger numbers of patients and higher methodological quality.
Table 2.
Talc Data
| Author | Year | n | Type | Infection | Death | Success |
|---|---|---|---|---|---|---|
| Stefani | 2006 | 37 | Prospective | 5% | 0% | 62% |
| Dresler | 2005 | 196 | Prospective | 2% | 3% | 71% |
| Shaw | 2004 | 1499 | Meta Analysis | NR | NR | 80% |
NR, not reported.
Table 3.
Pleurx Data
| Author | Year | n | Type | Infection | Success/time to success |
|---|---|---|---|---|---|
| Sioris | 2008 | 51 | Prospective | 6% | 22% |
| Warren | 2007 | 231 | Retrospective | 2% | Mean 29 days |
| Tremblay | 2007 | 109 | Retrospective | 5% | 70% |
| Tremblay | 2006 | 250 | Retrospective | 3% | 43%, Mean 56 days |
| van den Toorn | 2005 | 17 | Retrospective | 12% | Mean 69 days |
| Musani | 2004 | 24 | Retrospective | 13% | 53%, Mean 39 days |
| Putnam | 2000 | 100 | Retrospective | 5% | 21% |
No studies reported on deaths related to the placement of Pleurx® catheter (Denver Biomedical Inc., Denver, Co).
Using data shown in Table 3, we chose the “time to pleurodesis” for patients receiving Pleurx® catheter to be 10 weeks. This time point represents the most clinically relevant estimate based on our consultation with a local thoracic surgeon. This variable was important in the costing of supplies for those with the catheter, and for calculating utilities for those with resolved or unresolved effusions.
Effects data: treatment failure
The clinical approach for those who fail initial treatment for MPE is not well defined in the literature. We assume that patients who do not respond to talc treatment rarely receive a second talc treatment, and would be managed conservatively with medications and/or oxygen. Thus, we do not include an option for further treatment for those for whom treatment with talc fails. With regard to those treated with the catheter, a small minority of patients who fail to obtain effusion resolution elect talc treatment for symptom control.
We therefore performed a separate analysis and included a branch for second-line talc treatment only in those with Pleurx® placement without effusion resolution. We assume that 10% of patients will elect this second-line treatment. We assume that those with complications after Pleurx® treatment will not elect to have an additional chest tube placement and talc procedure.
Effects data: treatment complications
Tables 2 and 3 document treatment complication rates. The most frequently reported complication for both procedures was infection. The most infrequently reported complication was death, but given the serious nature of this complication, it was included in the model. Other reported complications were fever and pain, but for model simplicity we assumed that these complications did not incur additional costs or change utility estimates.
The base-case probability of infection with talc was defined as 0.01 with a range of 0.01 to 0.05. The base-case probability of death with talc was 0.005, with a range of 0.005 to 0.03. To obtain an overall probability of complications for talc, we summed the base-case probabilities to obtain an overall probability of complications of 0.015. We then adjusted the base-case probabilities to reflect conditional probability, and calculated a probability of infection (given complications) as 0.667 and probability of death (given complications) as 0.333 (Table 1).
The main complication for catheter treatment was infection, and reports of infection probabilities in the literature (Table 3) ranged from 0.02 to 0.13. When considering data from studies with the most patients, and using more recently published studies, we chose the base case probability of infection with Pleurx® as 0.075. No studies reported on deaths from the catheter, but since this outcome was viewed as clinically possible and relevant, we estimated this probability to be 0.00001. We defined the probability of complications as 0.07501, and calculated a probability of infection given complications as 0.9999 and a probability of death given complications as 0.0001 (Table 1).
An important assumption of our model is that “infection” from either talc treatment or Pleurx® catheter is equivalent to an infection of the pleural fluid (empyema). We also assume that an infection requires 1 week of hospitalization and placement of a chest tube.
Utility data
The utilities for patients with pleural effusion were obtained from a recent study documenting health state utilities for lung cancer.13 In this study, the authors document societal utility values (with a utility of 1.0 defined as perfect health, and utility of 0 defined as dead) for patients with cancer. We used the utilities reported for the following states in our analysis: “progressive cancer” (0.473) corresponds to those with an unresolved effusion and continued dyspnea, and “cancer, responding and fatigue” (0.599) corresponds to those with a resolved effusion. No utility was given for hospitalization, so we estimated this utility as 0.40 (Table 1).
To assign a utility for patients with the catheter, we used the results of a recent meta-analysis of patients on peritoneal dialysis (PD).14 We chose the peritoneal dialysis population because both patient groups (those with Pleurx® and those on PD) live with an indwelling catheter that is accessed regularly for drainage. From this analysis, we inferred a base-case utility for patients with Pleurx® catheter as 0.58.
Cost data
Cost data was estimated using Medicare Diagnosis-Related Group (DRG) reimbursement data from 2008. Cost data is summarized in Table 1: the DRG for “pleural effusion without complications” (DRG: 188) was used to cost admission for talc and the DRG for “pleural effusion with complications” (DRG: 186) was used to cost admission for infection. The Medicare Current Procedural Terminology (CPT) code for placement of the pleural catheter was used for this cost variable, and the cost estimate for Pleurx® supplies was obtained from several Internet vendors and verified with a local apothecary.
The cost of a nursing visit was obtained from a cost-effectiveness study of pediatric home visits15 and was adjusted for inflation to reflect 2008 dollars (Table 1). This cost was also verified with a local home nursing agency. The cost of a physician office visit was estimated from billing data at our institution. In both treatment arms, patients require one monthly visit with a physician. We also assume that patients with Pleurx® require three visits from a nurse per week. In all patients for whom the effusion does not resolve, we assume that home nursing visits occur once per week for the remaining month(s) of life.
Analysis
We determined the incremental cost-effectiveness ratio (ICER) of Pleurx® catheter placement compared with talc pleurodesis for the base-case assumptions. In circumstances where the catheter was less effective and more costly than talc pleurodesis, we considered the catheter to be dominated. When the catheter is both more effective and more costly compared with the talc pleurodesis we calculate and report an incremental cost-effectiveness (C/E) ratio. For incremental ratios up to $100,000/QALY, we consider such ranges as likely to be cost effective.
To examine the stability of our results, univariate sensitivity analysis was performed. For each analysis, one parameter of the model was varied over a range as defined in Table 1, while keeping other parameters at their baseline. Parameters considered for sensitivity analysis were those that appeared most variable in the literature review.
Results
Base-case analysis
Table 4 provides the results of the incremental cost-effectiveness analysis under base-case conditions. Under the base-case, the catheter and the talc pleurodesis therapies cost $9011.60 and $8170.80, respectively. The aggregate numbers of QALYs for the catheter and talc treatment are 0.276 and 0.281, respectively. Therefore, treatment with talc provides 0.006 additional QALYs compared to the Pleurx® catheter at a lower cost by $840.80. These small differences in cost and effects suggest that the choice of treatment should be based on patient preferences and the clinical situation.
Table 4.
Base-Case Cost-Effectiveness Analysis
| Strategy | Cost | Inc. Cost | Effect (mos) | Eff. (yrs) | Inc. Effect (mos) | Inc. Effect (yrs) | Inc. C/E (mos) | Inc. C/E (yrs) |
|---|---|---|---|---|---|---|---|---|
| Talc | $8170.80 | 3.3826 | 0.281 | |||||
| Pleurx | $9011.60 | $840.80 | 3.3071 | 0.276 | −0.0756 | −0.006 | Dominated |
In our additional analysis allowing for the option of talc treatment for those patients who received Pleurx® catheter, did not have a complication, and did not have resolution of effusion, the catheter and the talc pleurodesis therapies cost $9288.00 and $8170.80, respectively (Table 5). The aggregate number of QALYs for the catheter and talc treatment are 0.277 and 0.281, respectively. Therefore, treatment with talc provides 0.004 additional QALYs compared to the catheter, at a lower cost by $1117.20.
Table 5.
Base-Case Cost-Effectiveness Analysis (Additional Scenario)
| Strategy | Cost | Inc. Cost | Effect (mos) | Eff. (yrs) | Inc. Eff. (mos) | Inc. Eff. (yrs) | Inc. C/E (yrs) |
|---|---|---|---|---|---|---|---|
| Talc | $8170.80 | 3.3826 | 0.281 | ||||
| Pleurx | $9288.00 | 1117.20 | 3.3219 | 0.277 | −0.06 | −0.005 | Dominated |
Although this additional analysis shows the catheter treatment arm to be slightly more effective than it was in the base-case, it is still less effective than talc with an even higher cost.
Sensitivity analyses
We performed a sensitivity analysis for those parameters in the base case that appeared most variable in the literature review (Table 6). For those patients with a prognosis of less than 6 weeks, treatment with the catheter costs less than $100,000/QALY and may be a cost-effective option. This is likely due to a lower cost of Pleurx® supplies (and not to a higher rate of treatment success) in these patients who have a very limited prognosis. Treatment with Pleurx® also enters the less than $100,000/QALY range when the probability of effusion resolution is greater than 0.87 for Pleurx® or less than 0.55 for talc. However, based on the current published studies that we identified (Tables 2 and 3), these values have not been reported and are unlikely in clinical practice.
Table 6.
Results of Sensitivity Analysis
| Variable | Pleurx® Reaches <$100,000/QALYa |
|---|---|
| Months of life | <6 weeks |
| Probability of effusion resolved with Pleurx | >0.87 |
| Probability of effusion resolved with talc | <0.55 |
Pleurx®, Denver Biomedical Inc., Denver, CO.
Discussion
We chose to evaluate the cost effectiveness of the Pleurx® catheter compared to talc pleurodesis for patients with MPE. Although our analysis was from a third-party insurance perspective, this analysis is clinically relevant for physicians and other providers wishing to maximize effects (quality of life) for this population of patients with such a limited prognosis. This analysis may also be of interest to hospice organizations although some costs such as pain medications and home aide services have not been incorporated in our model. One important goal of those providing hospice treatment for patients with MPE is to avoid less effective, more costly treatments, while maximizing patient quality of life.
In our base-case scenario, treatment with the catheter was only slightly more costly and less effective than treatment with talc, suggesting that treatment choice should be based on the clinical situation and patient preferences. When adding a possible talc treatment arm for those patients with MPE who received catheter treatment, had no complications, and did not have effusion resolution, this treatment was only minimally more effective than it was with the base-case model but was also more costly than in the base-case model.
Overall, the model demonstrates that the two treatments are comparable in terms of effects and minimally different in terms of costs. In fact, the difference in total costs for the two treatment branches is less than $1000 in our base-case model. The two treatments are nearly equally effective (in terms of QALYs), with a difference of only 0.07 months (2 quality-adjusted days) between talc and Pleurx® treatment in the base case. This information itself may be clinically useful for palliative care providers who advise patients on the appropriate treatment for MPE; when considering effects measured as QALYs, the treatments are quite similar and treatment choice may need to be individualized for each patient. There was a significant disutility associated with hospitalization for talc placement in our model, but the tradeoff in these patients is a higher rate of pleurodesis. Patients who opt for Pleurx® placement may avoid the disutility associated with hospitalization but have a lower rate of successful pleurodesis. Based on our sensitivity analysis, Pleurx® may be the preferred treatment for those with a limited prognosis of less than 6 weeks and in those patients hoping to avoid the hospitalization and disutility associated with talc treatment.
There are some limitations to our model, one of which is that we did not include costs such as medications or oxygen therapy for patients in the “effusion not resolved” groups. These costs are difficult to estimate, and depend highly on the clinical situation (for example, for patients who cannot tolerate oral medications, more expensive intravenous or transdermal medications might be required). Although we did not include these costs, it is likely that the Pleurx® treatment would continue to be dominated since the success of this treatment is lower than that of talc (and those with treatment failure are the ones who might require pain medications and/or oxygen). Our assumption that patients with the catheter require three nursing visits per week was also difficult to estimate; however, when we performed an analysis using two visits per week, Pleurx® continued to be the more costly treatment. Patients who are able to perform the drainage themselves, or have family to perform the drainage, may incur less nursing costs and shift the model toward a preference for Pleurx®. Another limitation of our model is that we did not include physician service fees for inpatients; these costs are highly dependent on the clinical situation. We were also limited by the heterogeneity of the data obtained from our review of the published literature and the assumptions we made regarding patient utilities, but we attempted to choose data for our model that best represents our clinical experience with such patients, and performed sensitivity analysis over the published ranges.
We believe that our model is highly representative of the clinical decision pathway for patients with MPE, and is an accurate depiction of the possible outcomes for those treated with Pleurx® catheter or talc pleurodesis. Further analysis of this model could include one from a societal perspective to account for the differences in indirect costs that might arise from the two treatment scenarios and the complications/outcomes of these treatments. Also, in the case that treatment success (as represented by resolution of the effusion) with Pleurx® catheter should improve in the future beyond the values reported in the literature, this option may become a more cost-effective option for MPE treatment.
Conclusion
For patients with MPE and a prognosis of 6 months, treatment with Pleurx® catheter is comparable to treatment with talc. Pleurx® may be more cost effective when patient prognosis is 6 weeks or less or if the effectiveness of Pleurx® increases to nearly 0.90. Measured in QALYs, both options are nearly equivalent in terms of treatment effects. The choice of treatment may need to be individualized based on provider and patient preferences and the specific clinical situation. A prospective study of these treatments in the palliative care population, including measurement of patient utilities for each health state, might help to further define the cost-effectiveness of these two treatment options. As the comparative effectiveness research agenda unfolds, decision modeling has the potential to play a key role in defining the best treatment options in the palliative care population.
Acknowledgments
The authors are grateful to Katia Noyes, Ph.D, M.P.H. and Alec O'Connor, M.D., M.P.H. for their assistance with the development of this model.
Author Disclosure Statement
No competing financial interests exist.
This project was partially supported by Grant Number 1 UL1 RR024160–01 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and the NIH Roadmap for Medical Research, and its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at www.ncrr.nih.gov/. Information on Re-engineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp.
References
- 1.Musani AI. Haas AR. Seijo L. Wilby M. Sterman DH. Outpatient management of malignant pleural effusions with small-bore, tunneled pleural catheters. Respiration. 2004;71:559–66. doi: 10.1159/000081755. [DOI] [PubMed] [Google Scholar]
- 2.Sioris T. Sihvo E. Salo J. Räsänen J. Knuuttila A. Long-term indwelling pleural catheter (Pleurx®) for malignant pleural effusion unsuitable for talc pleurodesis. Eur J Surg Oncol. 2008 doi: 10.1016/j.ejso.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 3.Tremblay A. Michaud G. Single-center experience with 250 tunnelled pleural catheter insertions for malignant pleural effusion. Chest. 2006;129:362–8. doi: 10.1378/chest.129.2.362. [DOI] [PubMed] [Google Scholar]
- 4.Khaleeq G. Musani A. Emerging paradigms in the management of malignant pleural effusions. Respiratory Medicine. 2008;102:939–48. doi: 10.1016/j.rmed.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 5.Neragi-Miandoab S. Malignant pleural effusion, current and evolving approaches for its diagnosis and management. Lung Cancer. 2006;54:1–9. doi: 10.1016/j.lungcan.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 6.ATS Board of Directors: Management of Malignant Pleural Effusions. Am J Respir Crit Care Med. 2000;162:1987–2001. doi: 10.1164/ajrccm.162.5.ats8-00. [DOI] [PubMed] [Google Scholar]
- 7.van den Toorn LM. Schaap E. Surmont VFM. Pouw EM. van der Rijt KC. van Klaveren RJ. Management of recurrent malignant pleural effusions with a chronic indwelling pleural catheter. Lung Cancer. 2005;50:123–127. doi: 10.1016/j.lungcan.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 8.Tremblay A. Mason C. Michaud G. Use of tunnelled catheters for malignant pleural effusions in patients fit for pleurodesis. Eur Respir J. 2007;30:759–762. doi: 10.1183/09031936.00164706. [DOI] [PubMed] [Google Scholar]
- 9.Putnam JB. Walsh GL. Swisher SG. Roth JA. Suell DM. Vaporciyan AA. Smythe WR. Merriman KW. DeFord LL. Outpatient management of malignant pleural effusion by a chronic indwelling pleural catheter. Ann Thorac Surg. 2000;69:369–375. doi: 10.1016/s0003-4975(99)01482-4. [DOI] [PubMed] [Google Scholar]
- 10.Warren WH. Kalimi R. Khodadadian LM. Kim AW. Management of malignant pleural effusions using the Pleur(x) catheter. Ann Thorac Surg. 2008;85:1049–1055. doi: 10.1016/j.athoracsur.2007.11.039. [DOI] [PubMed] [Google Scholar]
- 11.Haas AR. Sterman DH. Musani AI. Malignant pleural effusions: Management options with consideration of coding, billing, and a decision approach. Chest. 2007;132:1036–1041. doi: 10.1378/chest.06-1757. [DOI] [PubMed] [Google Scholar]
- 12.Shaw P. Agarwal R. Pleurodesis for malignant pleural effusions. Cochrane Database Syst Rev. 2004. p. CD002916. [DOI] [PubMed]
- 13.Nafees B. Stafford M. Gavriel S. Bhalla S. Watkins J. Health state utilities for non small cell lung cancer. Health Qual Life Outcomes. 2008;6:84. doi: 10.1186/1477-7525-6-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liem YS. Bosch JL. Hunink MGM. Preference-based quality of life of patients on renal replacement therapy: A systematic review and meta-analysis. Value Health. 2008;11:733–741. doi: 10.1111/j.1524-4733.2007.00308.x. [DOI] [PubMed] [Google Scholar]
- 15.Paul IM. Phillips TA. Widome MD. Hollenbeak CS. Cost-effectiveness of postnatal home nursing visits for prevention of hospital care for jaundice and dehydration. Pediatrics. 2004;114:1015–1022. doi: 10.1542/peds.2003-0766-L. [DOI] [PubMed] [Google Scholar]
- 16.Dresler CM. Olak J. Herndon JE. Richards WG. Scalzetti E. Fleishman SB. Kernstine KH. Demmy T. Jablons DM. Kohman L. Daniel TM. Haasler GB. Sugarbaker DJ. Cooperative Groups Cancer and Leukemia Group B; Eastern Cooperative Oncology Group; North Central Cooperative Oncology Group; Radiation Therapy Oncology Group: Phase III intergroup study of talc poudrage vs talc slurry sclerosis for malignant pleural effusion. Chest. 2005;127:909–915. doi: 10.1378/chest.127.3.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stefani A. Natali P. Casali C. Morandi U. Talc poudrage versus talc slurry in the treatment of malignant pleural effusion. A prospective comparative study. Eur J Cardiothorac Surg. 2006;30:827–832. doi: 10.1016/j.ejcts.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 18.Pleurx® Catheter system: Cardinal Health 2008 Reimbursement Guide. www.denverbio.com/physician_Pleurx®_catheter.htmln. [Sep 4;2009 ]. www.denverbio.com/physician_Pleurx®_catheter.htmln