Abstract
Background:
Protocol-driven early goal-directed therapy (EGDT) has been shown to reduce mortality in patients with severe sepsis and septic shock in the ED. EGDT appears to be underused, even in centers with formalized protocols. The aim of our study was to identify factors associated with not initiating EGDT in the ED.
Methods:
This was a cohort study of 340 EGDT-eligible patients presenting to a single center ED from 2005 to 2007. EGDT eligibility was defined as a serum lactate ≥ 4 mmol/L or systolic BP< 90 mm Hg after volume resuscitation. EGDT initiation was defined as the measurement of central venous oxygen saturation via central venous catheter. Multivariable logistic regression was used to adjust for potential confounding.
Results:
EGDT was not initiated in 142 eligible patients (42%). EGDT was not completed in 43% of patients in whom EGDT was initiated. Compliance with the protocol varied significantly at the physician level, ranging from 0% to 100%. Four risk factors were found to be associated independently with decreased odds of initiating EGDT: female sex of the patient (P = .001), female sex of the clinician (P = .041), serum lactate (rather than hemodynamic) criterion for EGDT (P = .018), and nonconsultation to the Severe Sepsis Service (P < .001).
Conclusions:
Despite a formalized protocol, we found that EGDT was underused. We identified potential barriers to the effective implementation of EGDT at the patient, clinician, and organizational level. The use of a consultation service to facilitate the implementation of EGDT may be an effective strategy to improve protocol adherence.
Severe sepsis and septic shock are associated with significant morbidity and mortality.1 Early goal-directed therapy (EGDT), a strategy of early hemodynamic optimization that targets central venous pressure (CVP), mean arterial pressure (MAP), and central venous oxygen saturation (Scvo2) goals, reduced mortality when implemented in the ED of a single center.2 Several observational studies have validated the effectiveness of protocol-directed resuscitation3-8 and its use has been advocated in practice-based guidelines for sepsis management.9
The failure to translate evidence into practice has been identified as one of the great challenges of modern medicine.10 Despite advances in the care of critically ill patients, evidence-based interventions remain underused.11-15 To date, few institutions have adopted a formal protocol for the delivery of EGDT.16-19 In institutions that have adopted protocol-based resuscitation, compliance ranges from 50% to 60%.20,21 Despite evidence that the introduction of an EGDT protocol at our institution was associated with lower mortality compared with historical controls,4 we recently reported that EGDT was underused in patients with severe sepsis and septic shock at our institution for unclear reasons.22 Because mortality exceeded 35% in these patients, it is important to understand why a potentially life-saving intervention like EGDT was not implemented.22
Why EGDT is underused in centers that have adopted an EGDT protocol has not been studied. The primary goal of this study was to identify potential barriers to the implementation of EGDT in the ED. We hypothesized that patient, clinician, and organizational factors would be associated with the noninitiation of EGDT.
Materials and Methods
The Institutional Review Board of the University of Pennsylvania approved the study with an informed consent waiver.This was a retrospective cohort study of EGDT-eligible patients admitted through the University of Pennsylvania ED between 2005 and 2007. In late 2004, our ED institutionalized a Severe Sepsis protocol to identify and treat all EGDT-eligible patients. EGDT eligibility was defined as a serum lactate ≥ 4 mmol/L in hemodynamically stable patients (occult shock) or systolic BP < 90 mm Hg after volume resuscitation (1,500 mL).2-5,8,20,23,24 A Severe Sepsis Consultation Service was available to facilitate the effective implementation of EGDT. The Sepsis Service was staffed by one of two attending ED physicians continuously.
EGDT-eligible patients were identified within our ED severe sepsis cohort.22 Admitted patients were screened for inclusion if serum lactate was measured in the ED or a physician documented any of the following in the ED electronic medical record (EMR): sepsis, severe sepsis, cryptic septic shock, septic shock, or EGDT. The EMR of these patients was then evaluated for evidence of severe sepsis in the ED. Sepsis, severe sepsis, and septic shock were defined according to the International Sepsis Definitions Conference criteria.23,24 Subjects were excluded if criteria for severe sepsis were not met, if serum lactate was not measured ( < 2% of exclusions), or if the placement of a central venous catheter (CVC) was refused by the patient or their proxy (n = 15). Subjects with a do-not-resuscitate order were not excluded. The details of our ED severe sepsis cohort have been previously published.22
Data Collection and Exposures
Candidate risk factors hypothesized to be associated with the noninitiation of EGDT were categorized into patient, clinician, and organizational factors (Table 1). Candidate risk factors were selected based on previous literature,11,13-15,25-28 biologic plausibility, and/or consensus opinion of the investigators based on their clinical experience. Candidate risk factors were not meant to be exhaustive; to minimize potential type 1 error, patient factors were limited to sociodemographic factors and factors that could alter the perceived risk-to-benefit ratio of initiating EGDT (eg, less severely ill patients may not have EGDT initiated). Acute Physiology and Chronic Health Evaluation (APACHE) II score was calculated based on initial variables obtained in the ED.27 Consistent with the APACHE methods,27 organ dysfunction was categorized as absent if unmeasured. Our assessment of clinician factors was limited to the attending physician.28
Table 1.
—Candidate Risk Factors For Noninitiation of Early Goal-Directed Therapy in Eligible Septic Patients
| Patient-Specific Factors | Organizational Factors |
| Age | Criteria for EGDTa |
| Sex | Admitting serviceb |
| Severity of illness | Time and day of presentation |
| Comorbiditiesc | ED census (occupancy)d |
| Organ dysfunctionc | Activation of Severe Sepsis Consultation Service |
| Do-not-attempt resuscitation | Attending of record |
| Cause of severe sepsis |
EGDT = early goal-directed therapy.
EGDT initiation would be less likely in patients fulfilling the serum lactate rather than the hemodynamic criterion; as such, eligibility criteria could affect protocol implementation. Serum lactate and hemodynamic data were analyzed in the patient factor model.
The initiation of EGDT may be influenced by the service admitting the septic patient, as nonmedical services can assume primary care for the patient in the ED. All patients admitted to nonmedical services (surgery, obstetrics and gynecology, neurology, and neurosurgery) fulfilled criteria for sepsis and EGDT in the ED.
EGDT initiation would be less likely in patients with certain comorbidities (congestive heart failure, end-stage renal disease, or chronic liver failure) and characteristics (ie, increased bleeding risk secondary to coagulopathy, thrombocytopenia).
Occupancy, the percentage of ED beds filled at the time of patient triage, was used to measure ED crowding.25
Using a predrafted case report form, three trained investigators recorded the relevant patient, clinician, and organizational data from the EMR. ED therapies, including EGDT interventions and resuscitation end points (eg, Scvo2), were also recorded, as was mortality. Each case was assessed by a second investigator for completeness and accuracy.
Outcomes
The primary outcome was the noninitiation of EGDT. EGDT initiation was defined as the measurement of Scvo2 via CVC in the ED. Scvo2 was the resuscitation end point that distinguished the EGDT group in the trial by Rivers et al2 and its measurement has been used to define EGDT initiation previously.20 EGDT was categorized as initiated in 13 patients in whom Scvo2 was measured via preexisting CVC (eg, peripherally inserted central catheter). The secondary outcome was EGDT completion. EGDT was completed if the following targets were achieved in the ED: CVP ≥ 8 mm Hg, MAP ≥ 65 mm Hg, and Scvo2 ≥ 70%.2,9,20
Statistical Analysis
Comparisons between patients in whom EGDT was and was not initiated were tested using the Student t test or Wilcoxon rank-sum test for continuous variables or the χ2 statistic or Fisher exact test for categorical variables. We used the nonparametric test for trends across ordered groups and the Mantel-Haenszel statistic for stratified analyses.
Multivariable logistic regression was used to adjust for potential confounding and the results are presented as adjusted odds ratios (OR) with 95% CI. Separate models were created for patient and organizational factors. Candidate risk factors for the noninitiation of EGDT were included in the models if the P value was < .20 in univariate analyses. Potential confounders, including attending information, were added one at a time to the model and maintained if the point estimate for the OR of any of the candidate risk factors was altered by > 10%.29 Nonnormally distributed continuous variables were categorized. Multicollinearity, assessed using variance inflation factors,30 was detected between APACHE II score and age, MAP, and hematocrit; these variables were not included in the model separately. Finally, patient, clinician, and organizational risk factors found to be significantly associated with the noninitiation of EGDT at the ≤ 0.05 level were included in a model simultaneously. Statistical significance was defined as a P value of ≤ 0.05. Analyses were conducted using Stata 10.1 software (Stata Datacorp; College Station, TX).
Results
Baseline Characteristics
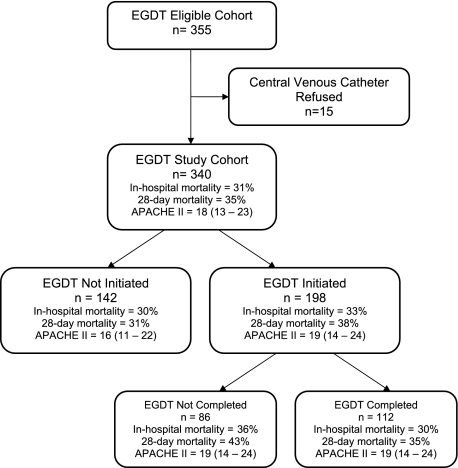
The EGDT-eligible cohort included 340 adults (Fig 1). The age range of the cohort was 18 to 101 years and 54% were men. Septic shock was the criterion for EGDT in 183 patients (54%); 157 (46%) fulfilled occult shock criteria.
Figure 1.
Patients meeting criteria for EGDT, EGDT compliance, and severity of illness measures and outcomes by subgroup. The primary comparison was between the EGDT-initiated and noninitiated subgroups. APACHE = Acute Physiology and Chronic Health Evaluation; EGDT = early goal-directed therapy.
EGDT was not initiated in 142 patients (42%). The EGDT group (n = 198) received more IV fluids (P < .001), vasoactive agents (P < .001), and central venous catheterizations (P < .001) (Table 2). The inhospital mortality rate was 33% in the EGDT-initiated group and 30% in the EGDT noninitiated group (P = .52). The odds of inhospital death, adjusted for each patient, clinician, and organizational factor found to be associated independently with the noninitiation of EGDT in separate multivariable models, was not significantly different in the EGDT-initiated group (adjusted OR = 0.95; 95% CI: 0.52, 1.75; P = .87).
Table 2.
—Interventions Received and Measurements Obtained in the ED by Whether EGDT Was Initiated
| ED Interventions | EGDT Not Initiated (n = 142) | EGDT Initiated (n = 198) | P Value |
| IV fluids (mL) | 2,675 (2,000-4,000) | 3,750 (2,605-4,975) | < .001 |
| Vasoactive agents | 13, 9.2% | 61, 30.8% | < .001 |
| Blood transfusion | 13, 9.2% | 25, 12.6% | .32 |
| Central venous catheterization | 31, 21.8%a | 185, 93.4%b | < .001 |
Continuous measures are presented as medians with interquartile ranges (25th, 75th percentile). Categorical variables are presented as counts and percents. CVC = central venous catheter. See Table 1 for expansion of other abbreviation.
Of the 31 patients in whom EGDT was not initiated, 16 received femoral central venous catheterization, seven received EGDT upon arrival to the ICU, six received central venous catheterization for inadequate peripheral venous access, and three for central venous pressure measurement only.
Thirteen patients had EGDT initiated via preexisting CVC.
EGDT was not completed in 86 of 198 (43%) patients in whom EGDT was initiated. The inhospital mortality rate was 36% in the 86 patients in whom EGDT was initiated but not completed, compared with 30% in those in whom EGDT was completed (P = .40). The CVP target was not achieved in 43 patients, the MAP target was not achieved in 18 patients, and the ScvO2 target was not achieved in 44 patients. EGDT was completed in 33% of the overall cohort. The rate of EGDT completion significantly decreased over the 2-year observational period (P = .003); by 6-month interval, rate of completion decreased from 48% to 35% to 25% to 22%.
The patients were managed by 33 physicians (Table 3). Compliance with EGDT (initiation and completion) varied significantly by attending, ranging from 0% to 100%. EGDT was less likely to be initiated by physicians who were female (P = .017) and in practice for more years (P = .026). The use of the Sepsis Service also differed significantly by attending, ranging from 0% to 100%. The Sepsis Service was less likely to be activated by physicians who were women (P = .017), older (P = .028) and in practice for more years (P = .002).
Table 3.
—Characteristics of the ED Attending Physicians and Association With the Initiation of EGDT
| Attending Characteristics | Attending Physicians (n = 33) | EGDT Initiated, % | P Value |
| Age category, y | .078 | ||
| 31-40 | 13, 39.4% | 61.3 | |
| 41-50 | 13, 39.4% | 60.0 | |
| 51-70 | 7, 21.2% | 43.2 | |
| Sex | .017 | ||
| Female | 12, 36.4% | 48.5 | |
| Male | 21, 63.6% | 62.4 | |
| Years in practice since residency | .026 | ||
| 0-5 | 14, 42.4% | 63.6 | |
| 6-10 | 2, 6.1% | 66.7 | |
| 11-15 | 10, 30.3% | 55.7 | |
| > 15 | 7, 21.2% | 46.2 |
Categorical data presented as counts and percents. Nonparametric test for trend across ordered groups was used to test for association between categorized demographics and the initiation of EGDT. See Table 1 for expansion of abbreviation.
We identified nine patient-specific factors that were associated with the noninitiation of EGDT in unadjusted analyses (Table 4). EGDT was less likely to be initiated in women (P = .001). This association was independent of the sex of the physician; female subjects were less likely to have EGDT initiated whether the attending was a woman (OR = 0.29; 95% CI: 0.13, 0.65) or a man (OR = 0.54; 95% CI: 0.32, 0.92; P < .001). EGDT initiation was less likely in patients who were younger (P = .043) and less severely ill, as measured by higher initial MAP (P = .002), lower lactate levels (P = .014), lower APACHE II scores (P = .001), and less coagulation dysfunction. Finally, patients with a preexisting CVC (P = .013) and patients with bacteremia as the cause of sepsis (P = .02) were less likely to have EGDT initiated.
Table 4.
—Univariate Comparisons of Patient-Specific Factors and the Initiation of EGDT
| Patient-Specific Factors | EGDT Not Initiated (n = 142) | EGDT Initiated (n = 198) | P Value |
| Demographics | |||
| Age, y | 56 (44-71) | 59 (50-71) | .16 |
| Age, y, categorized | .043 | ||
| < 47 (lowest quartile) | 42, 29.6% | 38, 19.2% | |
| 47-70 | 61, 43.0% | 109, 55.0% | |
| > 70 (highest quartile) | 39, 27.4% | 51, 25.8% | |
| Sex, female | 81 (57.0%) | 76 (38.4%) | .001 |
| Racea | .56 | ||
| White | 70, 51.5% | 87, 45.3% | |
| Black | 62, 45.6% | 99, 51.6% | |
| Other | 4, 2.9% | 6, 3.1% | |
| Initial ED vital signs | |||
| Temperature | 37 (36.5-38.3) | 36.9 (36.2-38.3) | .43 |
| Heart rate | 116 (92-130) | 114 (98-132) | .40 |
| Respiratory rate | 20 (16-24) | 20 (18-25) | .94 |
| Mean arterial pressure | 81 (68-96) | 74 (60-89) | .002 |
| Baseline ED laboratory values | |||
| WBC count | 12.3 (7.0-19.3) | 12.9 (8.3-18.9) | .55 |
| Hematocrit | 36 (31-40) | 34 (27-40) | .12 |
| Platelet count | 216 (149-295) | 217 (139-304) | .85 |
| Thrombocytopenic (< 100) | 21, 14.8% | 34, 17.2% | .56 |
| Creatinine, mg/dL | 1.3 (1.0-2.5) | 1.6 (1.1-2.4) | .074 |
| Glucose, mg/dL | 122 (94-178) | 124 (96-189) | .64 |
| Total bilirubin,b mg/dL | 0.8 (0.4-1.6) | 0.9 (0.5-2.6) | .14 |
| Hepatic failureb | 13, 9.2% | 26, 13.1% | .26 |
| PT, sb | 14.1 (12.7-15.8) | 15.1 (13.4-18.9) | .004 |
| Coagulopathicb | 17, 12.0% | 48, 24.2% | .005 |
| Lactate, mmol/L | 4.4 (2.9-5.9) | 5.1 (3.9-6.8) | .014 |
| Hypoperfusion, lactate ≥ 4 mmol/L | 95, 66.9% | 145, 73.2% | .21 |
| Severity of illness | |||
| APACHE II (baseline) | 16 (11-22) | 19 (14-24) | .001 |
| Comorbiditiesa | |||
| Coronary artery disease | 10, 7.0% | 24, 12.1% | .12 |
| Chronic renal insufficiency | 18, 12.7% | 28, 14.1% | .70 |
| Congestive heart failure | 16, 11.3% | 26, 13.1% | .61 |
| COPD | 8, 6.2% | 16, 8.8% | .31 |
| Diabetes mellitus | 34, 23.9% | 56, 28.4% | .24 |
| End-stage renal disease | 11, 7.5% | 8, 4.1% | .16 |
| HIV | 7, 4.9% | 14, 7.1% | .41 |
| Hypertension | 54, 38.0% | 84, 42.4% | .42 |
| Chronic liver failure | 11, 7.8% | 24, 12.1% | .19 |
| Oncology | 52, 36.6% | 61, 31.0% | .28 |
| Transplant | 14, 9.9% | 17, 8.6% | .70 |
| Do-not-resuscitate | 12, 8.4% | 10, 5.0% | .21 |
| Preexisting central venous access | 37, 26.1% | 30, 15.2% | .013 |
| Etiology of sepsis | |||
| Bacteremia | 25, 17.6% | 18, 9.1% | .02 |
| Respiratory | 39, 27.5% | 49, 24.8% | .57 |
| Urosepsis | 24, 16.9% | 43, 21.7% | .27 |
| Gastrointestinal | 25, 17.6% | 32, 16.2% | .72 |
| Soft-tissue infection | 7, 4.9% | 14, 7.1% | .42 |
| Surgical-site related | 1, 0.7% | 4, 2.0% | .40 |
| Central nervous system | 5, 3.5% | 5, 2.5% | .75 |
Continuous data presented as medians with interquartile ranges. Categorical data presented as counts and percents. APACHE = Acute Physiology and Chronic Health Evaluation; INR = international normalized ratio; PT = protime; PTT = partial thromboplastin time. See Table 1 for expansion of other abbreviation.
Comorbidities and race not reported in all patients (< 5% missing).
Reported in those in whom a measurement was obtained. Coagulopathy defined as an INR > 1.5 or PTT (s) > 60. Hepatic failure defined as a total bilirubin > 4 mg/dL.
We identified three organizational factors that were associated with the noninitiation of EGDT in unadjusted analyses (Table 5). First, EGDT was less likely to be initiated when the Sepsis Service was not consulted (P < .001). Second, EGDT was less likely to be initiated in occult shock patients, compared with patients fulfilling the hemodynamic criterion (50% vs 65%, P = .006). Follow-up lactate levels were decreasing in 98% (60/61) of the occult shock patients who did not receive EGDT; levels remained ≥ 4 mmol/L in 25% (15/61) of cases. Finally, EGDT was less likely to be initiated in patients admitted to nonmedical services (P = .049). The rate of EGDT initiation did not significantly decline over the 2-year period of observation (P = .22); by 6-month interval, use was 61%, 60%, 59%, and 51%. The use of the Sepsis Service declined over the 2-year interval (P = .005); use decreased from 61% to 48% to 40% in the last two epochs. The association between consultation to the Sepsis Service and EGDT initiation was observed within each 6-month interval (P < .001 in each epoch). In patients in whom EGDT was initiated, EGDT was less likely to be completed when the Sepsis Service was not consulted (44% vs 62%, P = .02).
Table 5.
—Univariate Comparisons of Organizational Factors and the Initiation of EGDT
| Organizational Factors | EGDT Not Initiated (n = 142) | EGDT Initiated (n = 198) | P Value |
| Criterion for EGDT protocol eligibility | .006 | ||
| Serum lactate (occult shock) | 78, 54.9% | 79, 39.9% | |
| Hemodynamic (shock) | 64, 45.1% | 119, 60.1% | |
| Admitting service | .049 | ||
| Medical | 120, 84.5% | 181, 91.4% | |
| Nonmedicala | 22, 15.5% | 17, 8.6% | |
| Time of presentation | .43 | ||
| 7:00 am-11:59 am | 24, 16.9% | 45, 22.7% | |
| 12:00 pm-6:59 pm | 63, 44.4% | 89, 45.0% | |
| 7:00 pm-11:59 pm | 34, 23.9% | 36, 18.2% | |
| 12:00 am-6:59 am | 21, 14.8% | 28, 14.1% | |
| Day of presentation | .84 | ||
| Weekend (Saturday or Sunday) | 38, 26.8% | 51, 25.8% | |
| Weekday (Monday to Friday) | 104, 73.2% | 147, 74.2% | |
| Protocol duration | .22 | ||
| First 6 mo of protocol | 31, 23.3% | 49, 24.8% | |
| Second 6 mo of protocol | 43, 29.5% | 65, 32.8% | |
| Third 6 mo of protocol | 36, 24.7% | 51, 25.8% | |
| Fourth 6 mo of protocol | 32, 22.6% | 33, 16.7% | |
| ED occupancyb | 73 (60-83) | 70 (53-85) | .37 |
| Activation of the Severe Sepsis Consultation Service | 21, 14.8% | 141, 71.2% | < .001 |
Continuous data presented as medians with interquartile ranges. Categorical data presented as counts and percents. See Table 1 for expansion of definition.
Surgery, obstetrics and gynecology, neurology and neurosurgery.
Percentage of ED beds filled at time of ED triage at the patient level.
Independent patient-specific risk factors associated with decreased odds of initiating EGDT included: female sex (P = .002), lower severity of illness, as measured by the ED APACHE II score (P = .013), and the absence of coagulopathy (P = .007) (Table 6). Organizational factors associated with the noninitiation of EGDT included: serum lactate (rather than hemodynamic) criterion for EGDT eligibility (P = .024), admission to a nonmedical service (P = .021), and failure to consult the Sepsis Service (P < .001). The six risk factors identified remained statistically significant when attending demographics were included in each model.
Table 6.
—Multivariable Logistic Regression Models Demonstrating Adjusted Odds Ratio for Initiating EGDT
| Model (N = 340) | Adjusted OR (95% CI) | P Value |
| Patient factor model | ||
| Sex, female | 0.48 (0.30-0.75) | .002 |
| Coagulopathic | 2.57 (1.30-5.06) | .007 |
| APACHE II (baseline)a | 1.05 (1.01-1.09) | .013 |
| Serum lactatea | 0.98 (0.91-1.06) | .60 |
| Serum creatininea | 1.04 (0.90-1.21) | .56 |
| Chronic liver failure | 1.03 (0.44-2.40) | .95 |
| End-stage renal disease | 0.44 (0.14-1.38) | .16 |
| Preexisting central catheter | 0.71 (0.38-1.33) | .28 |
| Bacteremia as cause of sepsis | 0.57 (0.27-1.22) | .15 |
| Organizational factor model | ||
| Eligibility criterion (serum lactate inclusion criterion) | 0.54 (0.32-0.92) | .024 |
| Admitting service (nonmedicalb) | 0.37 (0.16-0.86) | .021 |
| Activation of the Severe Sepsis Consultation Service | 14.85 (8.37-26.35) | < .001 |
| Complete model | ||
| Sex, female | 0.38 (0.22-0.68) | .001 |
| Coagulopathic | 1.94 (0.90-4.18) | .089 |
| APACHE II (baseline)a | 1.04 (1.00-1.08) | .064 |
| Eligibility criterion (serum lactate inclusion criterion) | 0.50 (0.28-0.89) | .018 |
| Admitting service (nonmedicalb) | 0.42 (0.17-1.05) | .065 |
| Activation of the Severe Sepsis Consultation Service | 13.38 (7.36-24.36) | < .001 |
| Attending sex, female | 0.50 (0.26-0.97) | .041 |
| Attending experience, years in practice | ||
| 0-5 | Referent | Referent |
| 6-10 | 3.74 (0.78-18.04) | .10 |
| 11-15 | 0.86 (0.46-1.60) | .63 |
| > 15 | 0.73 (0.31-1.70) | .47 |
An adjusted OR < 1 indicates that the factor is associated with lower odds of EGDT being initiated. OR = odds ratio. See Tables 1 and 4 for expansion of other abbreviations.
OR for each 1-unit increase in baseline APACHE II score, serum lactate, or serum creatinine.
Surgery, obstetrics and gynecology, neurology, and neurosurgery.
When patient, clinician, and organizational risk factors were included simultaneously in a separate multivariable logistic regression model, four risk factors were found to be associated independently with decreased odds of initiating EGDT: female sex of the patient (P = .001), female sex of the clinician (P = .041), lactate (rather than hemodynamic) criterion for EGDT (P = .018), and failure to consult the Sepsis Service (P < .001). Severity of illness, as measured by higher APACHE II score and the presence of coagulation dysfunction, was not associated independently with EGDT initiation in the complete model, nor was admission to a nonmedical service (Table 6).
Discussion
In this single-center cohort study, we found that EGDT was not initiated in 42% of eligible patients and was incomplete in 43% of patients in whom EGDT was initiated. We identified potential barriers to the initiation of EGDT at the patient, clinician, and organizational level. At the patient level, sex and severity of illness appeared to influence the decision to initiate EGDT. We found that the rate of EGDT use varied widely at the physician level and the sex of the physician appeared to influence whether EGDT was initiated; at the organizational level, we found that when the Severe Sepsis Service was not activated, EGDT was significantly less likely to be initiated and less likely to be completed in those in whom EGDT was initiated.
Our findings demonstrate the challenges that exist in translating evidence into clinical practice effectively. Despite evidence that adoption of EGDT led to improved outcomes at our institution,4 EGDT was underused. Even when EGDT was initiated, CVP and ScvO2 targets were not achieved in > 20% of cases. Prior studies2-8,31-35 suggest that the observed mortality in the cohort would have been lower had EGDT been implemented effectively and these benefits appear to last beyond the initial hospitalization.36 Despite significantly higher APACHE II scores in the EGDT-initiated group, their observed mortality was comparable to the EGDT non-initiated group, suggesting both a benefit for initiating and completing EGDT in patients who are more severely ill and potential harm when EGDT is not initiated or not completed. Given recent evidence that failure to achieve target Scvo2 within the first 6 h is associated with increased mortality,37 it would be reasonable to focus future efforts on achieving goal Scvo2 to improve outcomes.
Our observed compliance rates are consistent with those reported previously.19-21 Nguyen and colleagues20 reported 51% compliance with EGDT initiation at the inception of their EGDT protocol, which increased to 83% after 2 years; similarly, the rate of EGDT completion increased from 8% to 54%. More recently, Ferrer et al35 reported that despite a nationwide education effort to improve compliance with processes of care for patients with severe sepsis, EGDT was initiated in < 40% of eligible patients and < 10% had EGDT completed.
We did not observe an increase in the rate of EGDT initiation at our institution; however, we did observe a significant decline in the rate of EGDT completion. Importantly, we did not use a formal feedback mechanism to providers as others have implemented successfully.20,38 In lieu of a formal feedback mechanism, we developed a consultation service to facilitate the implementation of EGDT. The use of the consultation service was strongly associated with EGDT initiation and EGDT completion; however, the service was underused at the inception of the protocol and its use decreased over time, a finding that may explain the decline in EGDT completion rate. Potential reasons that the service was used less over time include physician fatigue with the service, physician dissatisfaction with the services provided, and the perception that consultation, over time, was no longer necessary to implement the protocol effectively. Furthermore, the use of the service, and EGDT itself, varied significantly at the practitioner level, which serves to highlight the fact that some physicians will adopt protocols more readily, whereas the inertia of previous practice may prove to be a barrier to protocol adherence for others.11 We are directing our current efforts to understand provider-specific reasons for not using the consultation service, as the service appears to be an effective strategy to improve protocol adherence.
We identified several potential barriers to the effective implementation of EGDT, in addition to nonconsultation to the Sepsis Service. First, sex appears to influence the decision of whether to initiate EGDT. EGDT was less likely to be initiated in women. Sex-based differences in care have been observed in cardiovascular medicine26 and, more recently, in critical care medicine.39,40 Females are less likely to be admitted to an ICU and to receive invasive procedures and these findings may adversely affect outcomes.39,40 Sex-based disparities may reflect differences in patient preferences or they may reflect preferences perceived by the patient’s proxy or physician.40 Alternatively, as seen in the critically ill elderly patient,41 physicians may underestimate female patients’ desire for aggressive care and, as a result, may withhold EGDT. Interestingly, we found that the sex of the physician may also influence EGDT use, as female physicians were less likely to initiate EGDT. Our finding that sex-based differences exist in the delivery of protocol-based resuscitation warrants further investigation.
Finally, patients experiencing occult shock were less likely to have EGDT initiated than patients fulfilling the hemodynamic EGDT criterion. Occult shock accounted for 46% of eligible patients; EGDT was not initiated in 50% of these patients. Although follow-up lactate measurements were decreasing in 98% of these patients, 25% remained eligible for EGDT and yet EGDT was still not initiated. Previous research suggests that patients with occult shock may derive an even greater mortality benefit from EGDT,42 yet serum lactate measurements remain underused.19 Within our observational study, it is impossible to determine how outcomes were affected by the failure to initiate and/or complete EGDT and it is unclear why EGDT was less likely to be initiated in occult shock patients. Admittedly, this observation may reflect how patient-specific disease severity influences the use of EGDT. Regardless, the potential impact of underusing EGDT in patients with occult shock warrants further investigation.
It is unclear how the factors we identified influenced the decision of whether to initiate EGDT. EGDT may not have been initiated because of barriers related to physician knowledge or attitudes regarding EGDT.11 For example, it is possible that physicians failed to recognize that patients were eligible for EGDT (eg, less ill, occult shock). Alternatively, physicians may have decided to implement EGDT in the most severely ill patients only, or to modify or not use EGDT in others based on objective data or inherent biases. As is the case for translating evidence into practice in general,14-17,26 disease severity likely affects the timely recognition of eligible patients as well as the decision to apply EGDT to a given patient.
There are several important limitations to our study. First, we acknowledge that relevant information pertaining to the complex decision to use EGDT may not have been identified, and a cause-and-effect relationship cannot be determined, through our observational study design. Furthermore, we are unable to comment on other important processes of care (eg, early and appropriate antibiotics) that affect the outcomes of septic patients. We are also unable to determine how outcomes were affected by underusing EGDT; confounding by indication impairs our ability to compare the observed mortality between the EGDT-initiated and EGDT non-initiated groups. Second, our study is subject to type 1 error given the multiple comparisons used. Although we adjusted for potential confounding and limited our hypotheses, our findings are hypothesis-generating and warrant confirmation. Third, we acknowledge the potential for misclassification bias based on our definition of EGDT initiation. Because EGDT resuscitation end points (eg, Scvo2) can be measured and fluids administered via preexisting CVCs, we categorized such patients as having EGDT initiated. Importantly, only 13 patients had EGDT initiated through a pre-existing CVC. A fourth limitation is the generalizability of our study; other centers may experience significantly different rates of EGDT use. Nevertheless, the barriers that we identified are likely to be experienced at other institutions. Finally, our use of the APACHE II score to assess severity of illness was not used as originally described;27 nevertheless, its use in the ED has construct and content validity and, given its association with mortality, criterion validity (data not shown).
In conclusion, our study revealed that EGDT was underused and we identified potential barriers to the effective implementation of EGDT. We found that a consultation service to facilitate the implementation of EGDT may be an effective strategy to improve protocol adherence. Whether EGDT underuse is due to underrecognition, disagreement with its use for specific patients, organizational barriers, or a combination thereof, is unknown and requires further investigation.
Acknowledgments
Author contributions: Dr Mikkelsen: contributed to study conception and design, data collection, analysis and interpretation of the data, drafting of the manuscript, and critical revision of the article for important intellectual content.
Dr Gaieski: contributed to study conception and design, data collection, analysis and interpretation of the data, drafting of the manuscript, and critical revision of the article for important intellectual content.
Dr Goyal: contributed to study conception and design, data collection, analysis and interpretation of the data, and drafting of the manuscript.
Ms Miltiades: contributed to study conception and design and data collection.
Dr Munson: contributed to study conception and design, analysis and interpretation of the data, drafting of the manuscript, and critical revision of the article for important intellectual content.
Dr Pines: contributed to data collection, analysis and interpretation of the data, drafting of the manuscript, and critical revision of the article for important intellectual content.
Dr Fuchs: contributed to study conception and design, analysis and interpretation of the data, and drafting of the manuscript.
Dr Shah: contributed to study conception and design, analysis and interpretation of the data, and drafting of the manuscript.
Dr Bellamy: contributed to analysis and interpretation of the data and drafting of the manuscript.
Dr Christie: contributed to study conception and design, analysis and interpretation of the data, drafting of the manuscript, and critical revision of the article for important intellectual content.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Abbreviations
- APACHE
Acute Physiology and Chronic Health Evaluation
- CVC
central venous catheter
- CVP
central venous pressure
- EGDT
early goal-directed therapy
- EMR
electronic medical record
- MAP
mean arterial pressure
- OR
odds ratio
- Scvo2
central venous oxygen saturation
Footnotes
Funding/Support: This study was supported in part by the National Institutes of Health, National Heart, Lung and Blood Institute, Bethesda, MD [Training Grant T32 HL07891].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (http://www.chestpubs.org/site/misc/reprints.xhtml).
References
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Rivers E, Nguyen B, Havstad S, et al. Early Goal-Directed Therapy Collaborative Group Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 3.Trzeciak S, Dellinger RP, Abate NL, et al. Translating research to clinical practice: a 1-year experience with implementing early goal-directed therapy for septic shock in the emergency department. Chest. 2006;129(2):225–232. doi: 10.1378/chest.129.2.225. [DOI] [PubMed] [Google Scholar]
- 4.Gaieski DF, McCoy J, Zeserson E, et al. Mortality benefit after implementation of early goal directed therapy protocol for the treatment of severe sepsis and septic shock. Ann Emerg Med. 2005;46(3):4. [Google Scholar]
- 5.Shapiro NI, Howell MD, Talmor D, et al. Implementation and outcomes of the Multiple Urgent Sepsis Therapies (MUST) protocol. Crit Care Med. 2006;34(4):1025–1032. doi: 10.1097/01.CCM.0000206104.18647.A8. [DOI] [PubMed] [Google Scholar]
- 6.Kortgen A, Niederprüm P, Bauer M. Implementation of an evidence-based “standard operating procedure” and outcome in septic shock. Crit Care Med. 2006;34(4):943–949. doi: 10.1097/01.CCM.0000206112.32673.D4. [DOI] [PubMed] [Google Scholar]
- 7.Micek ST, Roubinian N, Heuring T, et al. Before-after study of a standardized hospital order set for the management of septic shock. Crit Care Med. 2006;34(11):2707–2713. doi: 10.1097/01.CCM.0000241151.25426.D7. [DOI] [PubMed] [Google Scholar]
- 8.Jones AE, Focht A, Horton JM, Kline JA. Prospective external validation of the clinical effectiveness of an emergency department-based early goal-directed therapy protocol for severe sepsis and septic shock. Chest. 2007;132(2):425–432. doi: 10.1378/chest.07-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dellinger RP, Carlet JM, Masur H, et al. Surviving Sepsis Campaign Management Guidelines Committee Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit Care Med. 2004;32(3):858–873. doi: 10.1097/01.ccm.0000117317.18092.e4. [DOI] [PubMed] [Google Scholar]
- 10.Institute of Medicine . Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy Press; 2001. [PubMed] [Google Scholar]
- 11.Cabana MD, Rand CS, Powe NR, et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282(15):1458–1465. doi: 10.1001/jama.282.15.1458. [DOI] [PubMed] [Google Scholar]
- 12.Rubenfeld GD, Cooper C, Carter G, Thompson BT, Hudson LD. Barriers to providing lung-protective ventilation to patients with acute lung injury. Crit Care Med. 2004;32(6):1289–1293. doi: 10.1097/01.ccm.0000127266.39560.96. [DOI] [PubMed] [Google Scholar]
- 13.Kalhan R, Mikkelsen ME, Dedhiya P, et al. Underuse of lung protective ventilation: analysis of potential factors to explain physician behavior. Crit Care Med. 2006;34(2):300–306. doi: 10.1097/01.ccm.0000198328.83571.4a. [DOI] [PubMed] [Google Scholar]
- 14.Mikkelsen ME, Dedhiya PM, Kalhan R, Gallop RJ, Lanken PN, Fuchs BD. Potential reasons why physicians underuse lung-protective ventilation: a retrospective cohort study using physician documentation. Respir Care. 2008;53(4):455–461. [PubMed] [Google Scholar]
- 15.Umoh NJ, Fan E, Mendez-Tellez PA, et al. Patient and intensive care unit organizational factors associated with low tidal volume ventilation in acute lung injury. Crit Care Med. 2008;36(5):1463–1468. doi: 10.1097/CCM.0b013e31816fc3d0. [DOI] [PubMed] [Google Scholar]
- 16.Jones AE, Kline JA. Use of goal-directed therapy for severe sepsis and septic shock in academic emergency departments. Crit Care Med. 2005;33(8):1888–1889. doi: 10.1097/01.ccm.0000166872.78449.b1. [DOI] [PubMed] [Google Scholar]
- 17.Carlbom DJ, Rubenfeld GD. Barriers to implementing protocol-based sepsis resuscitation in the emergency department—results of a national survey. Crit Care Med. 2007;35(11):2525–2532. doi: 10.1097/01.ccm.0000298122.49245.d7. [DOI] [PubMed] [Google Scholar]
- 18.Varpula M, Karlsson S, Parviainen I, Ruokonen E, Pettilä V. Finnsepsis Study Group Community-acquired septic shock: early management and outcome in a nationwide study in Finland. Acta Anaesthesiol Scand. 2007;51(10):1320–1326. doi: 10.1111/j.1399-6576.2007.01439.x. [DOI] [PubMed] [Google Scholar]
- 19.Durthaler JM, Ernst FR, Johnston JA. Managing severe sepsis: a national survey of current practices. Am J Health Syst Pharm. 2009;66(1):45–53. doi: 10.2146/ajhp080067. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen HB, Corbett SW, Steele R, et al. Implementation of a bundle of quality indicators for the early management of severe sepsis and septic shock is associated with decreased mortality. Crit Care Med. 2007;35(4):1105–1112. doi: 10.1097/01.CCM.0000259463.33848.3D. [DOI] [PubMed] [Google Scholar]
- 21.Gao F, Melody T, Daniels DF, Giles S, Fox S. The impact of compliance with 6-hour and 24-hour sepsis bundles on hospital mortality in patients with severe sepsis: a prospective observational study. Crit Care. 2005;9(6):R764–R770. doi: 10.1186/cc3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mikkelsen ME, Miltiades AN, Gaieski DF, et al. Serum lactate is associated with mortality in severe sepsis independent of organ failure and shock. Crit Care Med. 2009;37(5):1670–1677. doi: 10.1097/CCM.0b013e31819fcf68. [DOI] [PubMed] [Google Scholar]
- 23.Bone RC, Balk RA, Cerra FB, et al. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest. 1992;101(6):1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 24.Levy MM, Fink MP, Marshall JC, et al. SCCM/ESICM/ACCP/ATS/SIS 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31(4):1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 25.Pines JM, Hollander JE. Emergency department crowding is associated with poor care for patients with severe pain. Ann Emerg Med. 2008;51(1):1–5. doi: 10.1016/j.annemergmed.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 26.Ayanian JZ, Epstein AM. Differences in the use of procedures between women and men hospitalized for coronary heart disease. N Engl J Med. 1991;325(4):221–225. doi: 10.1056/NEJM199107253250401. [DOI] [PubMed] [Google Scholar]
- 27.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–829. [PubMed] [Google Scholar]
- 28.Pines JM, Hollander JE, Isserman JA, et al. The association between physician risk tolerance and imaging use in abdominal pain. Am J Emerg Med. 2009;27(5):552–557. doi: 10.1016/j.ajem.2008.04.031. [DOI] [PubMed] [Google Scholar]
- 29.Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol. 1993;138(11):923–936. doi: 10.1093/oxfordjournals.aje.a116813. [DOI] [PubMed] [Google Scholar]
- 30.Stata Statistical Software . Release 9.0. College Station, TX: Stata Corporation; 2005. [Google Scholar]
- 31.Sebat F, Johnson D, Musthafa AA, et al. A multidisciplinary community hospital program for early and rapid resuscitation of shock in nontrauma patients. Chest. 2005;127(5):1729–1743. doi: 10.1378/chest.127.5.1729. [DOI] [PubMed] [Google Scholar]
- 32.Lin SM, Huang CD, Lin HC, Liu CY, Wang CH, Kuo HP. A modified goal-directed protocol improves clinical outcomes in intensive care unit patients with septic shock: a randomized controlled trial. Shock. 2006;26(6):551–557. doi: 10.1097/01.shk.0000232271.09440.8f. [DOI] [PubMed] [Google Scholar]
- 33.Sebat F, Musthafa AA, Johnson D, et al. Effect of a rapid response system for patients in shock on time to treatment and mortality during 5 years. Crit Care Med. 2007;35(11):2568–2575. doi: 10.1097/01.CCM.0000287593.54658.89. [DOI] [PubMed] [Google Scholar]
- 34.El Solh AA, Akinnusi ME, Alsawalha LN, Pineda LA. Outcome of septic shock in older adults after implementation of the sepsis “bundle”. J Am Geriatr Soc. 2008;56(2):272–278. doi: 10.1111/j.1532-5415.2007.01529.x. [DOI] [PubMed] [Google Scholar]
- 35.Ferrer R, Artigas A, Levy MM, et al. Edusepsis Study Group Improvement in process of care and outcome after a multicenter severe sepsis educational program in Spain. JAMA. 2008;299(19):2294–2303. doi: 10.1001/jama.299.19.2294. [DOI] [PubMed] [Google Scholar]
- 36.Puskarich MA, Marchick MR, Kline JA, Steuerwald MT, Jones AE. One year mortality of patients treated with an emergency department based early goal directed therapy protocol for severe sepsis and septic shock: a before and after study. Crit Care. 2009;13(5):R167. doi: 10.1186/cc8138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pope JV, Jones AE, Gaieski DF, Arnold RC, Trzeciak S, Shapiro NI. Emergency Medicine Shock Research Network (EMShockNet) Investigators Multicenter study of central venous oxygen saturation (ScvO(2)) as a predictor of mortality in patients with sepsis. Ann Emerg Med. 2010;55(1):40–46. doi: 10.1016/j.annemergmed.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jones AE, Shapiro NI, Roshon M. Implementing early goal-directed therapy in the emergency setting: the challenges and experiences of translating research innovations into clinical reality in academic and community settings. Acad Emerg Med. 2007;14(11):1072–1078. doi: 10.1197/j.aem.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Valentin A, Jordan B, Lang T, Hiesmayr M, Metnitz PG. Gender-related differences in intensive care: a multiple-center cohort study of therapeutic interventions and outcome in critically ill patients. Crit Care Med. 2003;31(7):1901–1907. doi: 10.1097/01.CCM.0000069347.78151.50. [DOI] [PubMed] [Google Scholar]
- 40.Fowler RA, Sabur N, Li P, et al. Sex-and age-based differences in the delivery and outcomes of critical care. CMAJ. 2007;177(12):1513–1519. doi: 10.1503/cmaj.071112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hamel MB, Teno JM, Goldman L, et al. SUPPORT Investigators. Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatment Patient age and decisions to withhold life-sustaining treatments from seriously ill, hospitalized adults. Ann Intern Med. 1999;130(2):116–125. doi: 10.7326/0003-4819-130-2-199901190-00005. [DOI] [PubMed] [Google Scholar]
- 42.Donnino MW, Nguyen B, Jacobsen G, et al. Cryptic septic shock: a sub-analysis of early goal directed therapy. Chest. 2003;124(4):90S. [Google Scholar]