Abstract
Objective
To determine the relationship between disease severity and patient characteristics in endometriosis.
Design
Cross-sectional study of self-reported survey data.
Setting
Academic research setting.
Patient(s)
One thousand women in the Oxford Endometriosis Gene (OXEGENE) study.
Intervention(s)
None.
Main Outcome Measure(s)
Participants were assigned to one of two groups with predominantly revised AFS stage I–II (group I, n = 423) or III–IV disease (group II, n = 517). Their characteristics were compared by disease extent.
Result(s)
Most participants were white (96%) and of reproductive age (81%). Women in group I were significantly younger on entering the study (39.9 ± 0.5 vs. 44.5 ± 0.4 years). Overall time to diagnosis did not differ between groups. The most common symptoms leading to a diagnosis were dysmenorrhea (79%) and pelvic pain (69%). In group II, subfertility (21.5% vs. 30.0%) and an ovarian mass (7.3% vs. 29.4%) more commonly led to a diagnosis, whereas dyspareunia (51.1% vs. 39.5%) was significantly more common in group I. Subfertility (41.5% vs. 53.4%) remained more common in group II throughout reproductive life, although birth and miscarriage rates were similar.
Conclusion(s)
Pelvic pain is common to all with endometriosis and those with more extensive disease report higher rates of subfertility. Remarkably, the time to diagnosis was similar among women.
Keywords: Endometriosis, patient characteristics, extent of disease, pelvic pain, subfertility
Chronic pelvic pain, defined as persistent pain in the pelvis, is the most common symptom associated with endometriosis, yet there is no clear relationship between the severity of the pain experienced and the extent of disease, irrespective of which classification system is used. Researchers have found either no association between pain symptoms and disease stage (1–3) or observed a degree of association between pain and the presence of adnexal adhesions, rectal and vaginal infiltration, ovarian involvement, or endometriomas (2, 4, 5). The failure of many women with minimal endometriosis to respond to surgical treatment has led some investigators to question whether this is even a cause of pain (6), especially as it may be an incidental finding in asymptomatic women (7, 8).
The relationship between subfertility and disease stage is similarly uncertain. Some researchers have found no association between stage and subfertility (1), whereas others report that fertility decreases with increasing disease severity (9).
Although studies have reported an increased risk of other diseases among women with endometriosis (10–12), evidence is lacking as to how these other conditions are influenced by stage of disease. Women with endometriosis, regardless of stage, appear to have similar ages at menarche and menstrual patterns (13). Health and lifestyle factors, such as general health, depressive state, history of sexually transmitted diseases, or use of contraception have not been shown to differ by disease stage (1, 9), but those with milder forms of endometriosis have been reported to smoke more cigarettes per day (14). Assessing whether such factors are a cause or a consequence of the condition has been virtually impossible because of the retrospective study designs used (15), although such studies do provide a profile of the characteristics of women with different stages of disease.
The current study investigates survey responses from 1,000 women with surgically confirmed endometriosis who are participating in the Oxford Endometriosis Gene (OXEGENE) study (16). We analyzed their responses to determine whether women with extensive disease report more symptoms and more problems with their menses and reproductive health in general than women with milder disease. We also sought to investigate how family history, personal health, and lifestyle factors vary by extent of disease.
MATERIALS AND METHODS
Women were recruited into the OXEGENE study, part of the International Endogene Study (17) which aims to identify susceptibility genes in endometriosis. The study design and recruitment methods have been reported previously (16). In brief, women were identified from the clinic databases of collaborating clinicians in Britain, Ireland, and the United States, or through advertisements, by the OXEGENE website, or research centers throughout the world. The sample of women used in the present analyses comprised the first 1,000 that returned the survey questionnaire. Because of the genetic focus of the OXEGENE study, many women were related to each other, such that 570 women came from 257 families and had one or more affected family members participating in the study; 430 others did not have any relatives with endometriosis who participated. In addition to providing DNA for genetic linkage and association studies, women completed a survey at study entry that included questions relating to symptoms, the diagnosis of endometriosis, menstrual and reproductive history, personal health, and lifestyle factors.
The questionnaire was self-administered and gathered self-reported data in women with endometriosis confirmed through operative records. Women reported retrospectively on the age at symptom onset, the type of symptoms that led to a diagnosis (e.g., pelvic pain, painful periods, infertility, ovarian tumor or mass), the age at which medical attention was first sought, and age and type of first surgery confirming the diagnosis of endometriosis. Some questions related to the timing of diagnosis with endometriosis. For instance, the survey included questions specifically on the use of contraceptives before diagnosis rather than at any time. Several questions on reproductive and other medical history inquired about their presence before, after, or both before and after diagnosis with endometriosis. Women were instructed to answer questions on their menstrual patterns at the time the diagnosis was first made. Other questions related to lifetime prevalence (e.g., ever having a pregnancy and pregnancy outcomes, ever having had infertility lasting more than 12 consecutive months, ever having had allergies, a family member with endometriosis, smoked cigarettes, or used talc as body powder).
Disease stage was assessed by one gynecologist (S.K.) from the surgical records using the revised American Fertility Society (AFS) classification system (18). Of the 1,000 women who completed the survey, 940 (94.0%) had sufficient information in their operative records to classify disease severity into four categories: stage A (revised AFS stage I); stage A+(defined as superficial ovarian disease plus some adhesions, i.e., similar to revised AFS stage II); stage B (revised AFS stage III–IV disease); and stage C (isolated rectovaginal nodules) (17). This simplified system was used because it proved difficult to assign revised AFS stage II retrospectively using the clinical records gathered from hundreds of different gynecologists. If a woman had had more than one operation, disease stage was based on the most severe findings; however, if there was ever any uncertainty about the extent of disease present, a less severe stage was always assigned. Women were excluded from the study if their operative records were unavailable or the diagnosis was questionable. In the United Kingdom, the study received approval from the regional Multi-centre Research Ethics Committee and Local Research Ethics Committees; the appropriate approval was also obtained in collaborating centers. An exemption from review by the Investigational Review Board (IRB) was granted by the Office of Human Subjects Research at the National Institutes of Health, Bethesda, Maryland, for the evaluation of this anonymized and unlinked survey.
For the purpose of analysis, the women were divided into two groups: group I (stages A and A+) and group II (stages B and C). Demographic characteristics, symptoms, diagnosis, menstrual history, reproductive health and infertility, contraception, comorbid diseases, family history, and lifestyle factors were described using frequency distributions. Overall comparisons of categorical variables were made using χ2 tests, whereas specific proportions for contingency tables with more than two rows or columns were compared using Z-tests. Post-hoc ANOVA was used to compare the number of symptoms by times to seeking medical attention and diagnosis from onset of symptoms, and time from seeking medical attention to diagnosis. Continuous variables such as subject age, and ages at and times to seeking medical attention and diagnosis were compared by the Van Der Waerden nonparametric tests as data were not normally distributed.
To account for any recall differences based on time since diagnosis, the mean time between the age at diagnosis and completion of the questionnaire was calculated and compared using nonparametric tests. Logistic regression analyses were used to control for the potential confounding of subject age and time since diagnosis when assessing the association between extent of disease and subfertility, pregnancy outcomes, use of contraception and oral contraceptive (OC) pills, and lifestyle factors. Heavy menstrual flow was also controlled for when the associations between extent of disease and use of regular tampons or overnight tampon use were assessed.
Related cases within a family may influence the frequency of the assessed characteristics in group I and group II, because it may cause dependence between individuals and the factors assessed. Therefore, all analyses were repeated using only unrelated cases, which included a randomly selected case from each family and all women without any participating relatives affected with endometriosis (total number of cases: 687 [272 in group I, 361 in group II, 54 could not be classified]).
All statistical tests were two-sided, with statistical significance defined as a P value of less than or equal to .05. Analyses were performed using SAS system software, release 8.2 (1999–2001; SAS Institute, Inc., Cary, NC) and PEPI v 4.0 (PM Gahlinger & JH Abramson, 1993–2001, Sagebrush Press, Salt Lake City, UT).
RESULTS
The participants live predominantly in the United Kingdom (68%) and United States (15%), followed by representation from Ireland and other European countries (14%), Australia and New Zealand (2%), and Canada (1%). Most participants were white (96%), had received postsecondary education (64%), and been diagnosed at laparoscopy (75%) (Table 1). About one-fifth of women in the sample reported that their mother had a history of endometriosis, and this proportion did not differ by group. Half in each group reported at least one sister with endometriosis. Of the 940 participants with sufficient data to classify disease, 556 (59%) were related: 254 (60%) in group I and 302 (58%) in group II. Related women in the same families were also evenly distributed between the two groups. When the study sample was limited to only unrelated women (n = 633, see Materials and Methods section), the characteristics of women described did not change significantly.
TABLE 1.
Distribution of demographic and diagnostic characteristics of 940 women with surgically diagnosed endometriosis completing the OXEGENE questionnaire at study entry.
| Characteristics | Group Ia (N = 423) | Group IIb (N = 517) | Total (N = 940) | P valuec |
|---|---|---|---|---|
| Age when questionnaire completed | 39.9 ± 0.5 | 44.5 ± 0.4 | 42.2 ± 0.3 | <.001 |
| Ethnicity | .37 | |||
| White | 406 (96.0) | 498 (96.3) | 904 (96.2) | |
| Mixed race | 10 (2.3) | 8 (1.6) | 18 (1.9) | |
| Asian | 0 | 2 (0.4) | 3 (0.3) | |
| Hispanic/Latino | 2 (0.5) | 1 (0.2) | 3 (0.3) | |
| All others | 5 (1.2) | 8 (1.5) | 12 (1.3) | |
| Education | .15 | |||
| Primary grade | 8 (1.9) | 7 (1.4) | 15 (1.6) | |
| Secondary/high school | 142 (33.6) | 177 (34.2) | 319 (33.9) | |
| College | 138 (32.6) | 154 (29.8) | 292 (31.1) | |
| Graduate | 50 (11.8) | 90 (17.4) | 140 (14.9) | |
| All others | 85 (20.1) | 89 (17.2) | 174 (18.5) | |
| Diagnosis | ||||
| Laparoscopy | 372 (87.9) | 330 (63.8) | 702 (74.7) | <.001 |
| Laparotomy | 23 (5.4) | 145 (28.1) | 168 (17.9) | <.001 |
| Age at: | ||||
| Symptom onset | 21.7 ± 0.4 | 22.9 ± 0.4 | 22.4 ± 0.3 | <.03 |
| Seeking medical attention | 24.2 ± 0.4 | 25.6 ± 0.4 | 25.0 ± 0.3 | .004 |
| Diagnosis | 29.2 ± 0.4 | 31.1 ± 0.3 | 30.3 ± 0.2 | <.001 |
| Time to: | ||||
| Seeking medical attention from symptom onset | 2.5 ± 0.2 | 2.6 ± 0.2 | 2.5 ± 0.1 | .40 |
| To diagnosis from symptom onset | 7.4 ± 0.3 | 8.1 ± 0.3 | 7.8 ± 0.2 | .47 |
| Seeking medical attention to diagnosis | 4.9 ± 0.3 | 5.5 ± 0.3 | 5.2 ± 0.2 | .35 |
Note: Data are presented as mean (± standard error) in years, or n (%).
Women with milder disease, defined as stage A (revised AFS stage I) and stage A+(defined as some ovarian disease plus some adhesions).
Women with more severe disease, defined as stage B (revised AFS stage III–IV disease) and stage C (isolated rectovaginal nodules).
Determined by Van Der Waerden nonparametric test, χ2 test, or Z-test for proportions of women in group I versus group II depending on type of variable being compared.
The majority (81%) were still of reproductive age (≤50 years) at the time of completing the questionnaire. Those in group I (n = 423) were younger (Table 1). Compared to those in group II (n = 517), they also reported a significantly younger age at: [1] symptom onset, [2] the time of first seeking medical help, and [3] diagnosis; however, these differences were only about 1 year (Table 1). In addition, the time from diagnosis to completing the questionnaire was shorter for those women in group I (10.6 ± 0.3 vs. 12.8 ± 0.4 years, P<.001).
The times from the onset of symptoms to first seeking medical help and eventual diagnosis were not different between groups (Table 1). The most commonly reported symptoms leading to a diagnosis were dysmenorrhea (79%) and pelvic pain (69%) in all women. Among the other symptoms leading to a diagnosis, subfertility (21.5% vs. 30.0%) and an ovarian mass (7.3% vs. 29.4%) were more commonly reported in group II, whereas dyspareunia was more common in group I (51.1% vs. 39.5%) (Table 2). Comparing subfertility and all pain symptoms grouped together (pelvic pain, dysmenorrhea, dyspareunia, dysuria, and painful bowel movements) as presenting problems, women in group II were more likely to have reported both pain and subfertility (16.3% vs. 26.8%, P<.001), whereas those in group I were more likely to have reported pain alone (77.2% vs. 67.1%, P<.001). It was uncommon for women to have been asymptomatic (2%). Most women (88%) reported having up to five symptoms before diagnosis, but the number of symptoms was not statistically different between groups. Interestingly, however, the more symptoms women reported, the longer the times from symptom onset to first seeking medical help and eventual diagnosis (P=.03 and P<.001, respectively).
TABLE 2.
Presenting symptoms for endometriosis diagnosis based on self-reported data from 940 women with surgically diagnosed endometriosis completing the OXEGENE study questionnaire.
| Symptoms that led to diagnosis | Group Ia (N = 423) | Group IIb (N = 517) | Total (N = 940) | P valuec |
|---|---|---|---|---|
| Dysmenorrhea | 332 (78.5) | 408 (78.9) | 740 (78.7) | .95 |
| Pelvic pain | 302 (71.4) | 350 (67.7) | 652 (69.4) | .25 |
| Dyspareunia | 218 (51.5) | 204 (39.5) | 422 (44.9) | <.001 |
| Bowel upset (e.g., constipation, diarrhea) | 143 (33.8) | 199 (38.5) | 342 (36.4) | .29 |
| Bowel pain | 114 (27.0) | 159 (30.8) | 273 (29.0) | .23 |
| Infertility | 91 (21.5) | 155 (30.0) | 246 (26.2) | .004 |
| Ovarian mass/tumor | 31 (7.3) | 152 (29.4) | 183 (19.5) | <.001 |
| Dysuria | 48 (11.4) | 45 (8.7) | 93 (9.9) | .21 |
| Other urinary problems | 24 (5.7) | 34 (96.6) | 58 (6.2) | .67 |
Note: Data are presented as n (%).
Women with milder disease, defined as stage A (revised AFS stage I) and stage A+(defined as some ovarian disease plus some adhesions).
Women with more severe disease, defined as stage B (revised AFS stage III–IV disease) and stage C (isolated rectovaginal nodules).
Determined by Z-test for proportions of women in group I versus group II.
Considering the number of gynecologic pain symptoms leading to a diagnosis that were reported by individual women, a third (34%) reported having pelvic pain, dysmenorrhea, and dyspareunia, and a quarter reported pelvic pain and dysmenorrhea (Fig. 1). About 70% of women had at least two of the three types of gynecologic pain. Those in group I were more likely to have reported all three pain symptoms than those in group II (39.5% vs. 30.2%, P=.003).
FIGURE 1.
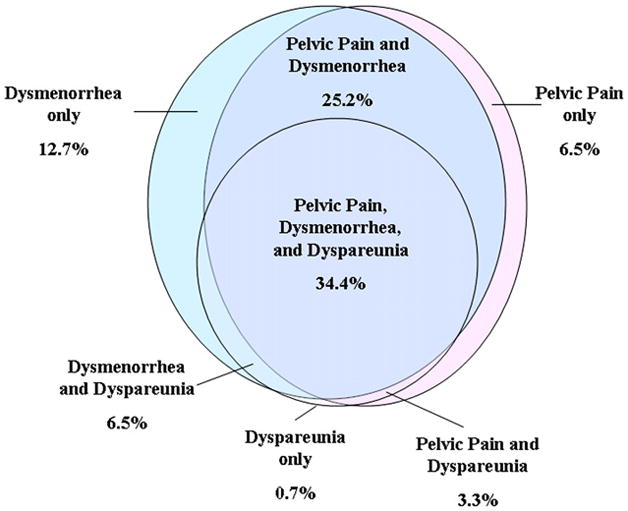
Prevalence and overlap of gynecologic pain symptoms that led to the surgical diagnosis of 940 women with endometriosis who participated in the OXEGENE study. Footnote: 10.7% of women did not report any gynecologic pain symptoms.
As for other symptoms (Table 2), bowel pain and bowel upset (e.g., constipation, diarrhea) were reported by 29% and 36%, respectively, with 46% reporting either or both; urinary pain or symptoms were reported less commonly. Approximately 3% of women reported both urinary and bowel pain/symptoms, without any statistical differences between groups.
Menstrual characteristics did not differ between groups at the time of diagnosis. Menses usually lasted 5–7 days (63%), with almost one-quarter of women reporting that menses lasted 8 days or more. Nearly all, regardless of extent of disease, reported moderate to heavy menstrual flow (92%) and a striking two-thirds (66%) had heavy flow. A third (32%) reported a 28-day cycle length and 16% reported cycle lengths of 24 days or less. Most women (85%) experienced menarche between the ages of 11 and 14, and 77% reported having regular cycles at the time of diagnosis.
When adjusted for heavy menstrual flow, subject age, and time since diagnosis, use of regular tampons was more likely in group I (odds ratio [OR] = 1.94, 95% confidence interval [CI]: 1.27, 2.96). Not surprisingly, pads and tampons were more commonly used together among those with heavy menstrual flow (35%), and overnight tampon use at the time of diagnosis was reported by 46% of women, with no observed differences by extent of disease. Women who frequently slept with tampons in place were not more likely to be in group II, even after adjusting for heavy menstrual flow, age, and time since diagnosis.
During their reproductive life, almost half (48%) of all women met the definition of having been subfertile at some point, as they reported having tried to get pregnant for 12 or more consecutive months without success (Table 3). After adjusting for subject age and time since diagnosis, those in group II continued to have a higher rate of subfertility after diagnosis. Therapeutic abortions (9%) and ectopic pregnancies (2%) were infrequently reported. Miscarriages, on the other hand, were relatively common (33%) among those who had ever been pregnant. Most women who had been pregnant reported carrying to delivery (85%) with cesarean sections performed in 21%. With regard to these outcomes, only therapeutic abortions differed by extent of disease and occurred more frequently among those in group I with adjustments for age and time since diagnosis (Table 3). Some women (37%) reported no change in their menstrual cycles after a live birth, although a substantial number reported less regular periods (20%) and decreased duration of flow (18%).
TABLE 3.
Self-reported reproductive characteristics in 940 women with surgically diagnosed endometriosis who participated in the OXEGENE study.
| Reproductive characteristics | Group Ib (N = 423) | Group IIc (N = 517) | Adjusted ORd (95% CI) |
|---|---|---|---|
| Infertility | 159 (41.5) | 253 (53.4) | 1.57 (1.19, 2.08) |
| Ever been pregnant | 258 (63.1) | 280 (56.8) | 1.62 (1.2, 2.2) |
| Carried to terma | 213 (83.2) | 235 (86.1) | 0.82 (0.47, 1.45) |
| Miscarriagea | 83 (32.4) | 91 (33.3) | 1.05 (0.71, 1.56) |
| Ectopic | 10 (2.4) | 6 (1.2) | 2.88 (0.94, 8.81) |
| Therapeutic abortion | 49 (11.6) | 33 (6.4) | 1.76 (1.05, 2.96) |
Note: Data are presented as n (%).
There were 538 women who had been pregnant, and n (%) is based on 529 with nonmissing responses to term delivery and miscarriage.
Women with milder disease, defined as stage A (revised AFS stage I) and stage A+(defined as some ovarian disease plus some adhesions).
Women with more severe disease, defined as stage B (revised AFS stage III–IV disease) and stage C (isolated rectovaginal nodules).
OR = odds ratio; CI = confidence interval; data adjusted for subject age and time since diagnosis using logistic regression modeling the group (I vs. II) with the higher prevalence.
Use of contraception before diagnosis was reported frequently (90%). The OC pills were the most popular type used by nearly all women (90%). Many reported OC pill use for contraception only (42%), or for both menstrual symptoms and contraception (41%) before a confirmed diagnosis of endometriosis. Those women in group II were more likely to have reported OC pill use for contraception only (35% vs. 48%, P<.001) before definite diagnosis, even after adjusting for age and time since diagnosis (OR = 1.55, 95% CI: 1.13, 2.11). However, women in group I used OC pills for the first time at a younger age than those in group II (18.2 ± 0.2 vs. 19.2 ± 0.2 years, P<.001).
Fibroids were common in the study sample (16%), with a higher prevalence in group II (13.4% vs. 18.8%, P=.02). Those women with fibroids were also older than those without (48.4 ± 0.8 years vs. 41.0 ± 0.3 years, P<.001). Cancers of the breast, ovary, and skin were reported in similar proportions in both groups, and were rare. Allergies were frequently reported (51.6%), with no differences by extent of disease. Depression requiring medication or medical consultation occurred in a third of the sample (33%), more commonly among those in group I (36.6% vs. 30.0%, P=.03).
Having ever smoked was common (41%) and two-thirds of women reported growing up in a household where at least one parent smoked. Women, on average, reported having smoked cigarettes for 14 years, with no differences noted by group when adjusted for age and time since diagnosis. Regardless of disease extent, equal proportions of women (45%) engaged in regular, vigorous exercise. Regular use of talc as body powder was common (23%), with many (62%) having used it for more than 10 years, and no differences noted between groups.
DISCUSSION
In this analysis of the first 1,000 questionnaires returned from women recruited into the OXEGENE study, those in group II (with revised AFS stage III–IV disease or isolated rectovaginal nodules) were about 1 year older at symptom onset and at diagnosis than those in group I (with less severe forms of the disease), although this age difference may not be clinically relevant. The time to diagnosis was similar between the groups. Dysmenorrhea and pelvic pain were common symptoms leading to the diagnosis in both groups; however, sub-fertility or an ovarian mass were more likely to have led to a diagnosis in group II and dyspareunia in group I. Women in group II more commonly had fibroids (most likely due to older age of this group), but were less likely to have had a therapeutic abortion or used OC pills at a younger age than those in group I. There were no differences in smoking habits between the groups. Surprisingly, women in group I reported depression more frequently.
Previous research has not found any consistent associations between the presenting gynecologic pain symptoms of dysmenorrhea, pelvic pain, or dyspareunia and stage of disease. For example, consistent with the current study, some researchers have found no association between disease stage and the presence of dysmenorrhea (2, 5, 13, 19), whereas others have reported such an association (1, 4, 14, 20, 21). Whereas most researchers have found that dyspareunia is not related to disease stage (2, 5, 19, 22), in the current study dyspareunia was a more common symptom leading to diagnosis in women with group I disease. Because the cul de sac and uterosacral ligaments are the most common sites of endometriosis (23) and these areas are stretched or moved during intercourse, it is understandable why dyspareunia might be a common complaint.
The relationship between pelvic pain and endometriosis, however, is more complex than a comparison of extent of disease and gynecologic pain. To illustrate, animal experiments have suggested that it is the location and innervation patterns of the endometrial deposits that probably are more important for nociception (pain perception) than visual extent of disease (24), which could explain our observation. Research in twins showed that part of the heritability of chronic pelvic pain related to endometriosis was explained by somatic distress—indicative of increased nociception (25), which could possibly result from complex feedback mechanisms in the central nervous system resulting from innervation of endometrial deposits. Another possible explanation, however, is that many women with seemingly small amounts of disease in the cul de sac may actually have undiagnosed rectovaginal nodules. Interestingly, women in this study commonly reported bowel symptoms suggesting that irritable bowel syndrome might have been a comorbidity. In contrast, bladder pain, which might indicate interstitial cystitis, was infrequently reported.
Subfertility has previously been both related (9) and unrelated (1) to disease stage. In this study subfertility appears to have been related to the extent of disease in that women in group II were more likely to have presented with subfertility or an ovarian mass at the time of diagnosis, and have experienced subfertility at some time during their reproductive lives. Approximately a quarter of the women with subfertility had had IVF treatment, and more women in group II reported treatment to aid conception. Interestingly, the live birth and miscarriage rate were not different between the groups. Although one research team has reported no differences in induced abortions between stages (22), in this study, women in group I were more likely to have had therapeutic abortions. The clinical significance of these findings is uncertain, although it is possible that these women were simply more fertile.
No unaffected control group was included in this study and comparisons with population norms are difficult because the study sample is heterogeneous. Nevertheless, it is interesting that women in both groups reported seemingly shorter menstrual cycles and longer, heavier menstruation than is the norm in population studies (26, 27). Those in group I were more likely to have begun using OC pills (to relieve menstrual symptoms with or without need for contraception) at a younger age, albeit by 1 year, which might suggest that earlier continuous OC pill use may be protective against developing severe disease. However, this could indicate a cohort effect explained by a change in cultural norms. Perhaps it has become more common to begin OC pill use at younger ages, possibly explaining why the younger women in group I (on average by almost 5 years) began use earlier than those in group II. These findings together with the relationship between OC pill use and endometriosis deserve further study, as other reports have suggested that the risk of developing disease may increase with OC pill use (8).
We found no differences between groups in the times from symptom onset to first seeking medical help and eventual diagnosis. The average of almost 8 years from symptom onset to surgical diagnosis is shorter than previously reported (10). It can be divided into a 5- to 6-year delay in seeking medical help and a 2-year delay from that point until diagnosis. Taking 2 years after seeking medical attention to reach a diagnosis may be expected as empirical treatments and other investigations are often undertaken before surgery. However, waiting 5–6 years before seeking medical help suggests that these women may have been conditioned to believe such symptoms are normal or that access to healthcare may have been problematic. In this sample of women with pain, the times to medical attention and diagnosis correlated with the number of presenting symptoms, indicating perhaps that they were managed initially as if they had other conditions, such as irritable bowel syndrome and interstitial cystitis, before a diagnosis of endometriosis was made. This may have, in turn, complicated the diagnostic process and lengthened the time to diagnosis.
Research has previously linked breast and ovarian cancers to endometriosis (11, 28) and melanoma has been found to be associated with subfertility (29). In our study, however, these cancers were rarely reported, perhaps because the study sample was younger (mean age = 42 years) than those reported in other studies.
Fibroids were common in our study, but no more common than in the general population (30). Those women with fibroids were older and fibroids were more common in women with severe disease. Because the women in group II themselves were older, the higher prevalence of fibroids in women with severe disease is likely explained by age alone.
The women in our study were relatively healthy, as most did not smoke and almost half engaged in regular, vigorous exercise. We were surprised to find a lack of differences in smoking, which has been shown previously to be protective (31). The use of talc as body powder in those in group II may be explained by their older age rather than as a risk factor for more severe endometriosis.
The large sample size used in this study is one of its strengths compared to studies published previously. In addition, the survey collected detailed information regarding symptoms and diagnosis, reproductive health and menstruation, medical history, and lifestyle factors. Although the data were collected retrospectively, it was possible to control to some extent for recall differences between the groups based on time since diagnosis. Importantly, the extent of disease was obtained from surgical records and staging of disease was standardized.
There are, however, a few limitations to consider. Despite controlling for recall differences, such statistical adjustments cannot remedy all selection biases that may have arisen because of the recruitment methods of the study. An inherent assumption of the present analyses is that—although women choosing to participate in a genetic study such as the OXEGENE study may differ in their characteristics from other women with endometriosis who prefer not to do so—this bias would be similar for women with groups I and II stages of disease and therefore comparisons between different stages are valid. However, this may not be the case. Apart from age and time since diagnosis, there may be other factors that differ between women diagnosed with different stages of endometriosis, which may lead to differential interest in participating in a scientific study. For instance, women with milder stages of disease (group I) were more likely to seek medical attention at an earlier age—1 year earlier on average than those in group II. This may not be clinically relevant but might indicate that they experienced symptoms at an earlier age, or differed in some personality characteristics related to health care seeking behavior.
Next, reporting errors cannot be excluded as the data were collected retrospectively through self-administered questionnaires. Self-reported data are also limited because questions can be misinterpreted. Women were categorized according to the surgery at which the most severe disease was diagnosed rather than the most recent surgery. In addition, there is an inherent selection bias in studying predominantly white women, 53% of whom had a participating relative with endometriosis. However, adjusting for related families did not materially affect the results. In addition, comparisons of symptoms and other characteristics to the presence or absence of endometriosis could not be done as this was a study of women with mild and severe endometriosis without a control group, and findings can only be extrapolated to such a population.
In conclusion, our study describes the self-reported health characteristics of women with endometriosis as they relate to disease extent. As expected, even after adjusting for age, women with severe disease were more likely to be affected by subfertility, both before the diagnosis was made and throughout their reproductive lives. In contrast, women with milder disease were more likely to have unintended pregnancies resulting in therapeutic abortions (probably indicating comparatively increased fertility). Despite this difference in fertility, surprisingly, the rate of term births and miscarriage were similar between groups. Women in group I were more likely to report use of OC pills at a younger age to treat menstrual symptoms, regardless of whether they were also used for contraception. Because of the retrospective nature of our study, its results can only provide an indication of characteristics with which women present around their time of diagnosis. Only a prospective cohort study could determine to what extent any of these characteristics represent “risk-factors” for onset of (different stages of) disease. Our findings concerning the potentially complex relationship between disease severity and subfertility and pain symptoms may help focus future epidemiologic research regarding treatment and prevention strategies for this disease.
Acknowledgments
The authors would like to thank Dr. Susan Treloar for her help with the study design. This research was supported in part by the Intramural Program of the National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD.
References
- 1.Hassa H, Tanir HM, Uray M. Symptom distribution among infertile and fertile endometriosis cases with different stages and localisations. Eur J Obstet Gynecol Reprod Biol. 2005;119:82–6. doi: 10.1016/j.ejogrb.2004.07.025. [DOI] [PubMed] [Google Scholar]
- 2.Endometriosis GIplSd. Relationship between stage, site and morphological characteristics of pelvic endometriosis and pain. Hum Reprod. 2001;16:2668–71. doi: 10.1093/humrep/16.12.2668. [DOI] [PubMed] [Google Scholar]
- 3.Hurd WW. Criteria that indicate endometriosis is the cause of chronic pelvic pain. Obstet Gynecol. 1998;92:1029–32. doi: 10.1016/s0029-7844(98)00283-x. [DOI] [PubMed] [Google Scholar]
- 4.Chapron C, Fauconnier A, Dubuisson JB, Barakat H, Vieira M, Breart G. Deep infiltrating endometriosis: relation between severity of dysmenorrhoea and extent of disease. Hum Reprod. 2003;18:760–6. doi: 10.1093/humrep/deg152. [DOI] [PubMed] [Google Scholar]
- 5.Porpora MG, Koninckx PR, Piazze J, Natili M, Colagrande S, Cosmi EV. Correlation between endometriosis and pelvic pain. J Am Assoc Gynecol Laparosc. 1999;6:429–34. doi: 10.1016/s1074-3804(99)80006-1. [DOI] [PubMed] [Google Scholar]
- 6.Koninckx PR, Oosterlynck D, D’Hooghe T, Meuleman C. Deeply infiltrating endometriosis is a disease whereas mild endometriosis could be considered a non-disease. Ann N Y Acad Sci. 1994;734:333–41. doi: 10.1111/j.1749-6632.1994.tb21763.x. [DOI] [PubMed] [Google Scholar]
- 7.Moen MH, Stokstad T. A long-term follow-up study of women with asymptomatic endometriosis diagnosed incidentally at sterilization. Fertil Steril. 2002;78:773–6. doi: 10.1016/s0015-0282(02)03336-8. [DOI] [PubMed] [Google Scholar]
- 8.Balasch J, Creus M, Fabregues F, Carmona F, Ordi J, Martinez-Roman S, et al. Visible and non-visible endometriosis at laparoscopy in fertile and infertile women and in patients with chronic pelvic pain: a prospective study. Hum Reprod. 1996;11:387–91. doi: 10.1093/humrep/11.2.387. [DOI] [PubMed] [Google Scholar]
- 9.Ventolini G, Horowitz GM, Long R. Endometriosis in adolescence: a long-term follow-up fecundability assessment. Reprod Biol Endocrinol. 2005;3:14. doi: 10.1186/1477-7827-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sinaii N, Cleary SD, Ballweg ML, Nieman LK, Stratton P. High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic diseases among women with endometriosis: a survey analysis. Hum Reprod. 2002;17:2715–24. doi: 10.1093/humrep/17.10.2715. [DOI] [PubMed] [Google Scholar]
- 11.Brinton LA, Gridley G, Persson I, Baron J, Bergqvist A. Cancer risk after a hospital discharge diagnosis of endometriosis. Am J Obstet Gynecol. 1997;176:572–9. doi: 10.1016/s0002-9378(97)70550-7. [DOI] [PubMed] [Google Scholar]
- 12.Modugno F, Ness RB, Allen GO, Schildkraut JM, Davis FG, Goodman MT. Oral contraceptive use, reproductive history, and risk of epithelial ovarian cancer in women with and without endometriosis. Am J Obstet Gynecol. 2004;191:733–40. doi: 10.1016/j.ajog.2004.03.035. [DOI] [PubMed] [Google Scholar]
- 13.Parazzini F, Bertulessi C, Pasini A, Rosati M, Di Stefano F, Shonauer S, et al. Determinants of short term recurrence rate of endometriosis. Eur J Obstet Gynecol Reprod Biol. 2005;121:216–9. doi: 10.1016/j.ejogrb.2004.11.033. [DOI] [PubMed] [Google Scholar]
- 14.Montanino G, Montanino Oliva M, Gulemi L, Boninfante M, Cosmi EV. Menstrual patterns, pain symptoms, body mass index and smoking habits in women with endometriosis. Clin Exp Obstet Gynecol. 1995;22:132–6. [PubMed] [Google Scholar]
- 15.Zondervan KT, Cardon LR, Kennedy SH. What makes a good case-control study? Design issues for complex traits such as endometriosis. Hum Reprod. 2002;17:1415–23. doi: 10.1093/humrep/17.6.1415. [DOI] [PubMed] [Google Scholar]
- 16.Kennedy S, Bennett S, Weeks DE. Affected sib-pair analysis in endometriosis. Hum Reprod Update. 2001;7:411–8. doi: 10.1093/humupd/7.4.411. [DOI] [PubMed] [Google Scholar]
- 17.Treloar S, Hadfield R, Montgomery G, Lambert A, Wicks J, Barlow DH, et al. The International Endogene Study: a collection of families for genetic research in endometriosis. Fertil Steril. 2002;78:679–85. doi: 10.1016/s0015-0282(02)03341-1. [DOI] [PubMed] [Google Scholar]
- 18.Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril. 1997;67:817–21. doi: 10.1016/s0015-0282(97)81391-x. [DOI] [PubMed] [Google Scholar]
- 19.Vercellini P, Trespidi L, De Giorgi O, Cortesi I, Parazzini F, Crosignani PG. Endometriosis and pelvic pain: relation to disease stage and localization. Fertil Steril. 1996;65:299–304. [PubMed] [Google Scholar]
- 20.Muzii L, Marana R, Pedulla S, Catalano GF, Mancuso S. Correlation between endometriosis-associated dysmenorrhea and the presence of typical or atypical lesions. Fertil Steril. 1997;68:19–22. doi: 10.1016/s0015-0282(97)81469-0. [DOI] [PubMed] [Google Scholar]
- 21.Dmowski WP, Lesniewicz R, Rana N, Pepping P, Noursalehi M. Changing trends in the diagnosis of endometriosis: a comparative study of women with pelvic endometriosis presenting with chronic pelvic pain or infertility. Fertil Steril. 1997;67:238–43. doi: 10.1016/S0015-0282(97)81904-8. [DOI] [PubMed] [Google Scholar]
- 22.Parazzini F, Ferraroni M, Fedele L, Bocciolone L, Rubessa S, Riccardi A. Pelvic endometriosis: reproductive and menstrual risk factors at different stages in Lombardy, northern Italy. J Epidemiol Community Health. 1995;49:61–4. doi: 10.1136/jech.49.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stratton P, Winkel CA, Sinaii N, Merino MJ, Zimmer C, Nieman LK. Location, color, size, depth, and volume may predict endometriosis in lesions resected at surgery. Fertil Steril. 2002;78:743–9. doi: 10.1016/s0015-0282(02)03337-x. [DOI] [PubMed] [Google Scholar]
- 24.Berkley KJ, Dmitrieva N, Curtis KS, Papka RE. Innervation of ectopic endometrium in a rat model of endometriosis. Proc Natl Acad Sci USA. 2004;101:11094–8. doi: 10.1073/pnas.0403663101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zondervan KT, Cardon LR, Kennedy SH, Martin NG, Treloar SA. Multivariate genetic analysis of chronic pelvic pain and associated phenotypes. Behav Genet. 2005;35:177–88. doi: 10.1007/s10519-004-1017-6. [DOI] [PubMed] [Google Scholar]
- 26.Vercellini P, De Giorgi O, Aimi G, Panazza S, Uglietti A, Crosignani PG. Menstrual characteristics in women with and without endometriosis. Obstet Gynecol. 1997;90:264–8. doi: 10.1016/S0029-7844(97)00235-4. [DOI] [PubMed] [Google Scholar]
- 27.Arumugam K, Lim JM. Menstrual characteristics associated with endometriosis. Br J Obstet Gynaecol. 1997;104:948–50. doi: 10.1111/j.1471-0528.1997.tb14357.x. [DOI] [PubMed] [Google Scholar]
- 28.Brinton LA, Lamb EJ, Moghissi KS, Scoccia B, Althuis MD, Mabie JE, et al. Ovarian cancer risk associated with varying causes of infertility. Fertil Steril. 2004;82:405–14. doi: 10.1016/j.fertnstert.2004.02.109. [DOI] [PubMed] [Google Scholar]
- 29.Brinton LA, Westhoff CL, Scoccia B, Lamb EJ, Althuis MD, Mabie JE, et al. Causes of infertility as predictors of subsequent cancer risk. Epidemiology. 2005;16:500–7. doi: 10.1097/01.ede.0000164812.02181.d5. [DOI] [PubMed] [Google Scholar]
- 30.Matchar DB, Myers ER, Barber MW, Couchman GM, Datta S, Gray RN, et al. Management of uterine fibroids. Evid Rep Technol Assess (Summ) 2001;(34):1–6. [PMC free article] [PubMed] [Google Scholar]
- 31.Cramer DW, Wilson E, Stillman RJ, Berger MJ, Belisle S, Schiff I, et al. The relation of endometriosis to menstrual characteristics, smoking, and exercise. JAMA. 1986;255:1904–8. [PubMed] [Google Scholar]


