Abstract
We have previously shown that a 'reversed chloroquine (RCQ)' molecule, composed of a chloroquine-like moiety and a resistance reversal-like moiety, can overcome chloroquine resistance in P. falciparum (Burgess, S. J.; Selzer, A.; Kelly, J. X.; Smilkstein, M. J.; Riscoe, M. K.; Peyton, D. H. J. Med. Chem. 2006, 49, 5623; Andrews, S.; Burgess, S. J.; Skaalrud, D.; Kelly, J. X.; Peyton, D. H. J. Med. Chem. 2010, 53, 916). Here, we present an investigation into the structure-activity relationship of the RCQ structures, resulting in an orally active molecule with good in vitro and in vivo antimalarial activity. We also present evidence of the mode of action, indicating that the RCQ molecules inhibit hemozoin formation in the parasite’s digestive vacuole in a manner similar to that of chloroquine.
Keywords: Chloroquine, Plasmodium falciparum, antimalarial, drug evaluation, accumulation, hemozoin, hematin, structure activity relationship, drug development, malaria, drug resistance
INTRODUCTION
Malaria remains among the most important diseases, with nearly half of the world’s population at risk of infection, and ∼250 million cases reported annually.1 Malaria kills nearly a million people each year, 90% of whom are African children. Of the different Plasmodium parasites known to give human malaria infections, P. falciparum is the most deadly, although the others still result in serious illness which can turn fatal. There are a number of drugs available to treat malaria, yet P. falciparum has developed resistance to almost all of them. Even the artemisinin class of drugs is now showing worrying signs of reduced efficacy,1–6 and so there is a continuing need for a pipeline of new antimalarial treatments to combat the disease.
Chloroquine (CQ) was first introduced in the 1940s and quickly became the drug of choice for the treatment of malaria. CQ has several advantages over other antimalarial drugs: its low cost made it available to everyone; its low toxicity meant it was safe for children and pregnant women, the most vulnerable victims of malaria; and its good efficacy meant the treatment regime was simple and easy to administer. However, resistance developed within about a decade and spread to such an extent that, today, the World Health Organization (WHO) recommends CQ not be used for the treatment of P. falciparum malaria, except in specific areas.1
CQ resistance in P. falciparum is strongly linked to mutations in the gene pfcrt that gives rise to the protein, PfCRT (P. falciparum chloroquine resistance transporter), located in the parasite’s digestive vacuole (DV) membrane.7–10 In chloroquine resistant (CQR) P. falciparum there is reduced accumulation of CQ in the DV, due to increased efflux.9, 11 PfCRT is a putative member of the drug/metabolite transporter superfamily, and recent evidence suggests that the mutated forms are, in fact, the transporters directly responsible for exporting CQ from the DV of P. falciparum.12
There are molecules such as verapamil and desipramine, which have been found to reverse the effects of CQ resistance.11, 13, 14 These are known as Reversal Agents (RAs), or chemosensitizers. Many of these compounds are existing drugs, such as antidepressants or antihistamines, and at the high doses often required to achieve optimal reversal activity there can be problems with unpleasant side effects.15 A pharmacophore, consisting of two aromatic rings and an aliphatic nitrogen a few angstroms away, has been derived from a set of such RAs.16
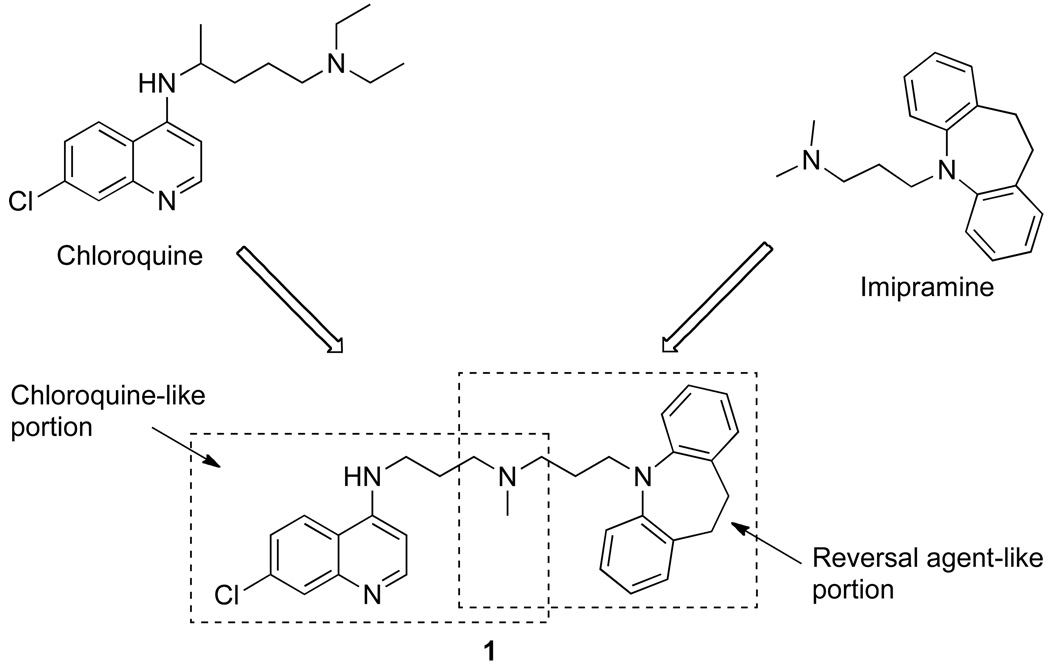
Our previous work demonstrated that the hybrid molecule 1 can be synthesized, containing elements from both CQ and the RA pharmacophore (Figure 1).17 This prototype “reversed chloroquine” (RCQ) molecule gave IC50 values lower than CQ for both CQR and chloroquine sensitive (CQS) P. falciparum strains, and demonstrated oral efficacy against P. chabaudi in mice. A subsequent structure-activity relationship (SAR) study demonstrated that the linkage between the 7-chloro-4-aminoquinoline moiety from CQ, and the aromatic rings of the RA head group could be varied in length without serious loss of activity, and that the RA portion itself could be substantially varied without serious loss of activity against CQR or CQS P. falciparum malaria strains.18 The work presented here is the result of a more extensive SAR study, with further variations to both the linkage and the aromatic head group of the RA moiety. The result is an orally efficacious molecule with good in vitro and in vivo antimalarial activity.
Figure 1.
The prototype Reversed Chloroquine molecule, 1. The dashed boxes show the chloroquine and reversal agent (imipramine) portions of the molecule.
CHEMISTRY
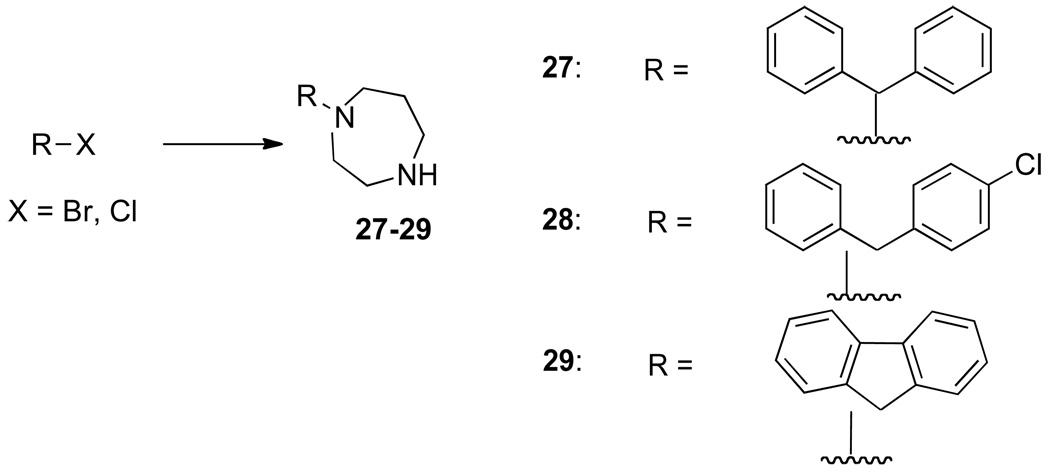
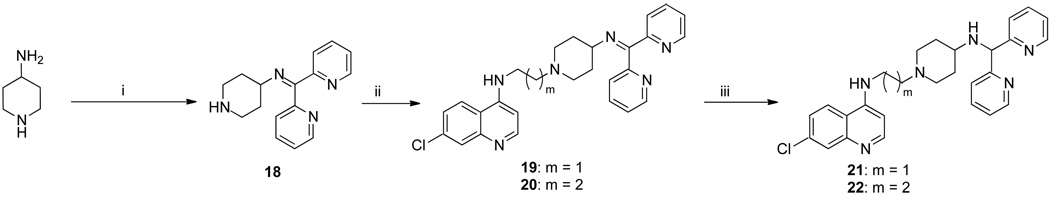
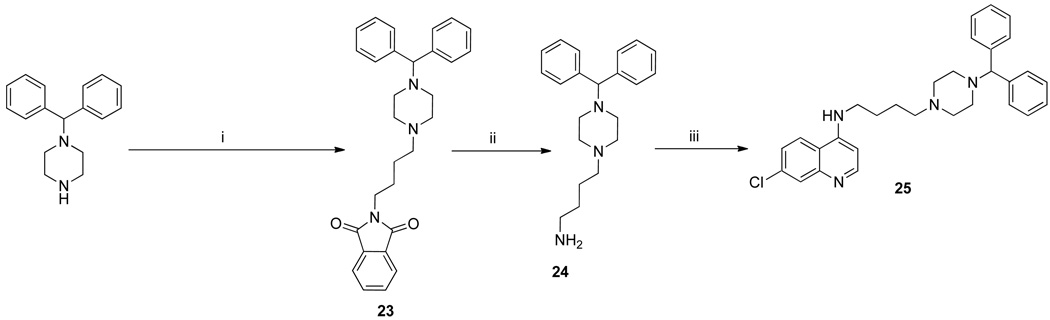
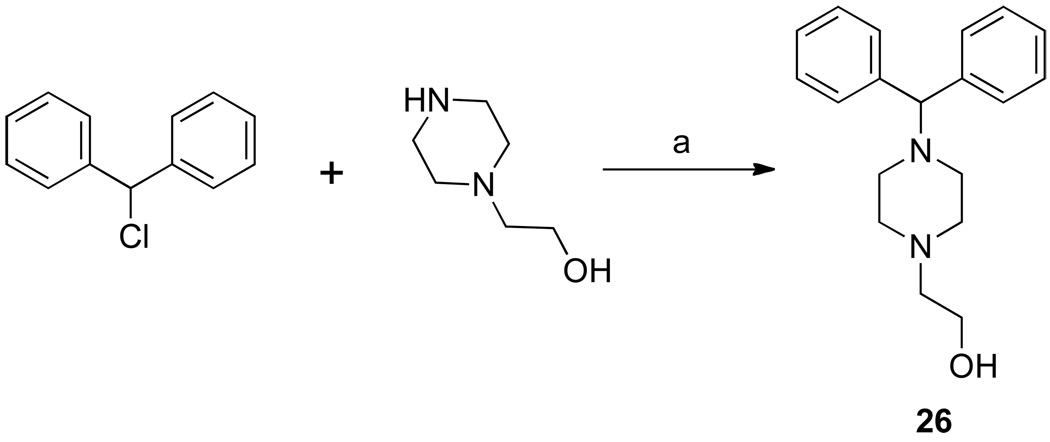
The synthesis of 3b has been previously described,17 and 3a was similarly synthesized (Scheme 1). These were treated with 1-(diphenylmethyl)piperazine or 27–29 (Scheme 2) to give the RCQ compounds 4–12. RCQ compound 13, the 2-carbon linker analogue of 1, was synthesized by treating 3a with desipramine hydrochloride. The intermediate compound 14 was made by first treating 2b with methane sulfonyl chloride, then adding an excess of piperazine (Scheme 1). This was subsequently treated with trityl chloride to give 15. 16 and 17 were synthesized from diphenylacetaldehyde and 2-adamantanone respectively, by reductive amination onto 14.19 The dipyridyl analogues were synthesized by first treating 4-aminopiperidine with 2,2’-dipyridyl ketone to give 18 (Scheme 3). This was then treated with methane sulfonate esters 3a and 3b to give 19 and 20 respectively, which were reduced in the presence of sodium borohydride to give 21 and 22. Initial attempts to make 25, the 4-carbon linker analogue of 4 and 5, by an analogous route, failed due to intramolecular cyclization of the activated alcohol to form a pyrrolidine ring at the 4-position of the quinoline. Therefore, a different route was employed (Scheme 4). 1-(diphenylmethyl)piperazine was treated with N-(4-bromobutyl)phthalamide to give 23, then deprotected with hydrazine to give 24. This was treated with 4,7-dichloroquinoline to give 25. To make the unattached RA head group 26, chlorodiphenylmethane was treated with 1-(2-hydroxyethyl)piperazine in the presence of potassium carbonate (Scheme 5).
Scheme 1.
Synthesis of the intermediate molecules 2a,b, 3a,b and 14, and the Reversed Chloroquine molecules 4–13, 15–17a
a Reagents and conditions: (i) 2-aminoethanol or 3-aminopropanol, 140°C; (ii) methanesulfonyl chloride, Et3N, dichloromethane, 5°C; (iii) appropriate piperazine or homopiperazine compound, Et3N, 3a or 3b, THF, 70°C; (iv) (1) desipramine HCl, NaHCO3, dichloromethane; (2) 3a, Et3N, THF, 60°C; (v) 2b, Et3N, methanesulfonyl chloride, THF, 0°C, piperazine, reflux; (vi) trityl chloride, K2CO3, acetonitrile, reflux; (vii) diphenylacetaldehyde, Na(AcO)3BH, acetic acid, THF, room temperature; (viii) 2-adamantanone, Na(AcO)3BH, acetic acid, THF, room temperature.
Scheme 2.
Synthesis of RA head groups 27–29a
a Reagents and conditions: R-Br or R-Cl, homopiperazine, chloroform, reflux.
Scheme 3.
Synthesis of the dipyridyl compounds 21 and 22a
aReagents and conditions: (i) 2,2’-dipyridylketone, pTSA, toluene, reflux; (ii) 3a or 3b, K2CO3, acetonitrile, 70°C; (iii) NABH4, methanol, room temperature.
Scheme 4.
Synthesis of the 4-carbon analogue, 25a
a Reagents and conditions: bromobutylphthalimide, K2CO3, acetonitrile, reflux; (ii) hydrazine hydrate, ethanol, reflux; (iii) 4,7-dichloroquinoline, Et3N, ethanol, reflux.
Scheme 5.
Synthesis of 26, the RA head group without a quinoline ring attached.a
aReagents and conditions: K2CO3, KI, DMF, 70°C.
RESULTS AND DISCUSSION
After the success of the prototype molecule 1 in overcoming CQ resistance, compounds 4 and 5 were designed to modify the structure of the RA head group slightly; moving away from the initial, tricyclic antidepressant, imipramine structure. In keeping with the published pharmacophore,16 the 1-(diphenylmethyl)piperazine retained the two aromatic rings and the protonatable nitrogen. However, the connector was now a piperazinyl ring which, it was hoped, would make the compounds more stable to metabolic cleavage. A secondary advantage was that it was easy to design a SAR study around this structure from inexpensive and readily available starting materials. Thus compounds 6–12, 15–17 were also synthesized, either varying the length of the chain from the quinoline to the piperazine ring, changing the piperazine to a homopiperazine, or varying the RA aromatic head group. Compound 13 gave the 2-carbon linker analogue of 1. As can be seen in Table 1, the in vitro results from these first compounds against P. falciparum malaria strains were favorable. While some did not quite match CQ against CQS D6, they all were more active than CQ against the two tested CQR strains.
Table 1.
Table of IC50 values of RCQ compounds against three P. falciparum strains, CQS D6 and CQR Dd2 and 7G8. Also included are ClogP values, cytotoxicity data against mouse spleen lymphocytes and a therapeutic index value calculated from the cytotoxicity and Dd2 IC50 values.
| Compound | IC50 (nM) for P. falciparuma |
Cytotoxicity (nM)b |
Therapeutic Index (Dd2 strain)c |
ClogPd | ||
|---|---|---|---|---|---|---|
| D6 | Dd2 | 7G8 | ||||
| CQ | 6.9 | 102 | 106 | 12000 | 122 | 5.1 |
| 1 | 2.9 | 5.3 | 4.0 | 700 | 132 | 8.8 |
| 4 | 1.0 | 3.6 | 4.3 | 1900 | 528 | 7.2 |
| 5 | 1.9 | 2.6 | 11 | 1100 | 423 | 7.4 |
| 6 | 4.8 | 4.1 | 15 | 700 | 171 | 7.4 |
| 7 | 2.6 | 2.0 | 10 | 1400 | 700 | 7.8 |
| 8 | 3.2 | 3.5 | 12 | 800 | 229 | 8.0 |
| 9 | 6.6 | 5.7 | 22 | 900 | 158 | 8.0 |
| 10 | 2.0 | 2.4 | 34 | 4100 | 1710 | 7.6 |
| 11 | 9.2 | 9.6 | 41 | 2500 | 260 | 7.0 |
| 12 | 21 | 15 | 56 | 2500 | 167 | 7.3 |
| 13 | 3.6 | 5.2 | 21 | 1400 | 269 | 8.5 |
| 14 | 4.3 | 6.8 | nte | 23000 | 3380 | 3.4 |
| 15 | 2.0 | 4.2 | 10 | 57000 | 13600 | 8.9 |
| 16 | 0.5 | 3.3 | 3.4 | 23000 | 6970 | 6.4 |
| 17 | 0.8 | 1.8 | 10 | 130000 | 69400 | 6.5 |
| 21 | 1.1 | 3.9 | 7.4 | 29000 | 7310 | 3.3 |
| 22 | 0.9 | 1.6 | 1.8 | 6500 | 4060 | 3.6 |
| 25 | 1.3 | 1.3 | 7.2 | 1100 | 846 | 7.5 |
| 26 | >2500 | >2500 | >2500 | 130000 | >50 | 3.2 |
Averages of at least 3 runs (± 15%). The uncertainties are estimated based on weighing uncertainties for the various compounds (free-bases and often oils), and on variability between determinations that were performed on different weeks. In order to compare results run on different days, and with different batches of each stain; CQ was run as a positive control. All results obtained were ‘normalized’ to the CQ values of 6.9 nM for D6, 102 nM for Dd2 and 108 nM for 7G8. For example, the normalized IC50 for an RCQ compound tested against a D6 strain = [6.9 / IC50 CQ (D6)] × IC50 RCQ compound (D6).
Cytotoxicity is tested against mouse spleen lymphocytes.
Therapeutic index for the Dd2 strain is given by the ratio of the cyctoxicity divided by the IC50 value for Dd2.
ClogP values calculated for the unionized forms of the compounds, using ChemDrawUltra 11.0
nt – not tested
The general cytotoxic effect was assessed by functional assay using mitogen-stimulated murine spleenic lymphocytes. The therapeutic indices for the RCQ compounds were calculated as the ratio of cytotoxicity IC50 to antimalarial IC50 against Dd2 (Table 1).
Although the in vitro data for 1, 4, 5–13 and 15–17 was promising, all of these compounds suffer from high ClogP values. This suggested that they could have limited water solubility, and so oral availability might be impaired, although formulating as salts and/or co-crystals, or other strategies may mitigate this concern. A modification to the aromatic head group was designed, using pyridines in place of the phenyl groups. Compounds 21 and 22 had ClogP values of 3.3 and 3.6 respectively, and IC50 values comparable to the best of the previous compounds (Table 1). For comparison, CQ has a ClogP value of 5.1. The therapeutic index values for these compounds are substantially greater than 10-fold above that of CQ. The result of the SAR is a collection of compounds with high potencies against CQS and CQR malaria strains, low cytotoxicities, and with ClogP values bracketing that of CQ.
It has previously been shown that simply changing the chain length of CQ can circumvent the PfCRT-associated CQR mechanism.20–22 All of the RCQ molecules thus far synthesized had either a 2- or 3-carbon chain between the tertiary nitrogen and the aminoquinoline, so 25, which has the same length linker between the quinoline ring and the aliphatic nitrogen, was made and evaluated by the in vitro methods applied above. As can be seen in Table 1, the activity of 25 against both CQS and CQR P. falciparum is better than that of CQ, and comparable to both 4 and 5, the 2- and 3- carbon analogues. These results, combined with other work from our group,18 demonstrate that the ability to overcome CQR by the addition of a RA head group to the 4-aminoquinoline ring is, in fact, independent of the chain length between them, at least if the chain length is between 2 and 4 carbons.
In vivo efficacy against Plasmodium berghei
1, 4, 15 and 22 were tested in vivo in a P. berghei rodent model. Three sets of conditions were used: 1 dose of 30 mg/kg administered orally, 1 dose of 30 mg/kg administered subcutaneously, and 4 × 30 mg/kg dose delivered orally. The subcutaneous series provided a convenient alternative to the oral route, in case absorption across the intestine was problematic. The % activity was calculated as the difference between the mean percent parasitemia for the control and treated groups expressed as a percent relative to the control group:
%Activity = 100 × [(parasitemia control group – parasitemia treated group) / parasitemia control group].
The results for 1 × 30 mg/kg dose experiments were varied (Table 2). In terms of the activity of the drug, 1 did poorly when administered orally, but better when given subcutaneously. 4 and 15 were opposite to this, and 22 was fairly good both orally and subcutaneously. However, the average survival time of the mice in all cases was under 10 days, indicating that even if the drug dramatically reduced the parasitemia initially, there was recrudescence. In this protocol, CQ gave similar results. Improvements were seen in some cases of the 4 × 30 mg/kg dosage experiment: 1 showed little difference, 4 showed high activity and about a 10 day increase in survival days compared to control animals, and both 15 and 22 were encouraging, with 22 resulting in two of the three treated mice being parasite-free on day 30 post-infection. The change from phenyls in 4 to pyridyls in 22 seemed to result in a drug which was orally active and showed no obvious signs of toxicity when administered to mice. 15 was a surprise in that it showed good oral activity, but was poor when administered subcutaneously, even though it had a ClogP value of 8.9. It should be remembered that ClogP is a calculated value which gives only an indication of aqueous solubility, but should not be used on its own as an equivalent to bioavailability.23 Another possible reason for the good in vivo activity of 15 could be that the trityl group was being cleaved from the rest of the molecule after oral administration. This would result in the starting material, 14, which has a ClogP value of 3.4 (consistent with good oral activity) and low IC50 values against P. falciparum strains (Table 1).
Table 2.
In vivo data for mice infected with P. berghei, tested at the Swiss Tropical and Public Health Institute. Historical data for CQ is included for comparison. The no-drug controls were euthanized on day 4.
| Drug | Dosage (mg/kg) |
Route | % Activity | Mouse survival (days) |
|---|---|---|---|---|
| CQ | 1 × 30 | Oral | 99.7 | 9 |
| 1 × 30 | Subcutaneous | 99.5 | 10 | |
| 4 × 30 | Oral | 99.9 | 23 (3/10 mice cured) | |
| 1 | 1 × 30 | Oral | <40 | Mice euthanized day 3 |
| 1 × 30 | Subcutaneous | 92 | 7 | |
| 4 × 30 | Oral | 96 | 8 | |
| 4 | 1 × 30 | Oral | >99.9 | 8 |
| 1 × 30 | Subcutaneous | <40 | Mice euthanized day 3 | |
| 4 × 30 | Oral | >99.9 | 16 | |
| 15 | 1 × 30 | Oral | 99.7 | 7 |
| 1 × 30 | Subcutaneous | <40 | Mice euthanized day 3 | |
| 4 × 30 | Oral | >99.9 | 20 (1/3 mice cured) | |
| 22 | 1 × 30 | Oral | 94 | 7 |
| 1 × 30 | Subcutaneous | 99.3 | 9 | |
| 4 × 30 | Oral | >99.9 | 27 (2/3 mice cured) | |
Due to the difference in molecular weights, a dose of 30 mg/kg of CQ actually resulted in many more molecules being administered to the mouse, than in a dose of 30 mg/kg of any of the RCQ compounds. Accordingly, a second in vivo experiment was carried out for 21 and 22, wherein the dosage was adjusted such that each compound was administered in an equimolar amount to 30 mg/kg of CQ. In this test 22 was administered to 10 mice, to obtain a more reliable evaluation of oral activity in this mammalian model (Table 3).
Table 3.
Second in vivo trial against P. berghei, with dosage adjusted to be equimolar to 30 mg/kg CQ. Historical data for CQ is included for comparison.
| Drug | Dosage (mg/kg) |
Route | % Activity | Mouse survival (days) |
|---|---|---|---|---|
| CQ | 4 × 30 | Oral | 99.9 | 23 (3/10 mice cured) |
| 21 | 4 × 44 | Oral | >99.9 | >30 (3/3 mice cured) |
| 22 | 4 × 46 | Oral | >99.9 | 30 (9/10 mice cured) |
21 had 3 out of 3 mice cured, and 22 had 9 out of 10, with the 10th mouse reaching 29 days. Parasitemia determination on day 30 showed that the surviving mice were parasite free.
Accumulation of 1 in the DV of P. falciparum
When reversal agents such as verapamil or imipramine are co-administered with CQ, the enhanced CQ activity is limited to CQR parasites; no effect is seen against CQS P. falciparum.11–13 However, several of the RCQ compounds show IC50 values lower than CQ against even CQS parasites. One hypothesis to explain this enhanced potency against CQS parasites could be that the RA head group also had antimalarial potency, and that its intrinsic antimalarial effect was enhancing activity of the total RCQ molecule. To investigate this possibility, compound 26, which lacks the quinoline moiety, was tested against both CQS and CQR P. falciparum. As can be seen in Table 1, 26 showed no effect up to 2500 nM, and so the RA head group has no strong intrinsic antimalarial activity.
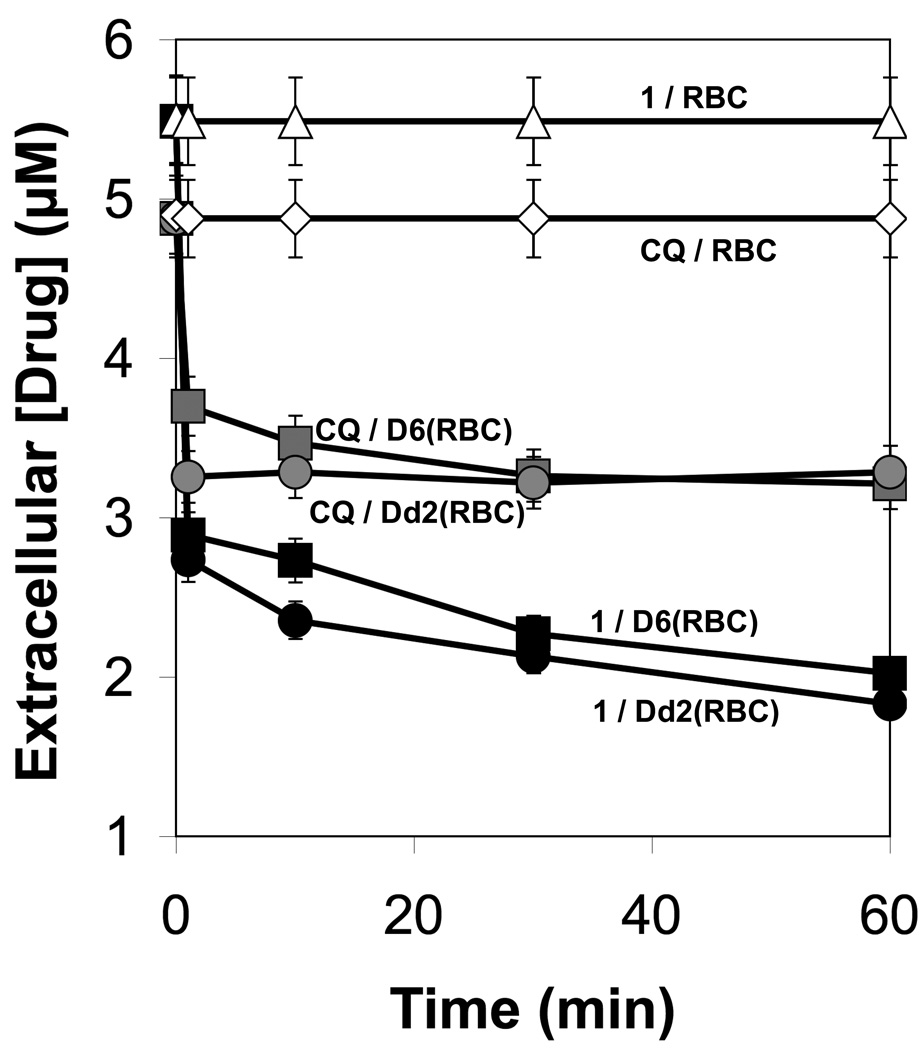
A second hypothesis to explain the enhanced activity of the RCQ molecules is that there was increased accumulation in the DV of the parasite. To test this, an accumulation experiment was devised, similar to one used by Kelly et al. to show accumulation of xanthones in the DV of Plasmodium parasites.24 The experimental design provides two results: first it allows the amount of drug accumulated in the DV to be measured; second, by demonstrating that the retention time is unchanged for the released drug, it shows that the vast majority of the drug is not modified by the parasite. As shown in Figure 2, 1 accumulated in the parasitized red blood cells (PRBCs) in a manner similar to that of CQ (Figure 2), in that there was a rapid initial uptake over the first minute, followed by a slow uptake until an equilibrium state was reached by about 60 minutes. After this time the accumulated amount remained nearly constant.
Figure 2.
Time dependent decline of drug concentration in culture medium for each drug/P. falciparum strain combination. The graph shows the first 60 minutes. The uncertainties are estimated to be ±5% based on variations measured in multiple trials of similar experiments performed under identical experimental conditions.
The initial concentration of 1 was slightly higher than CQ (5.5 µM to 4.9 µM), but after 1 hour the concentration of 1 in the medium was lower than CQ in both the D6 and Dd2 experiments. Addition of the ammonium chloride to the PRBCs resulted in a rapid return of each drug into the culture medium (data not shown). Both 1 and CQ returned to very close to their respective starting levels, and the HPLC retention times were not changed, indicating that the chemical composition of each of the drugs was, in fact, not altered while in the PRBCs over the course of the experiment.
By taking the difference between the amount of each drug in the medium after 60 minutes of incubation, and the amount after the addition of ammonium chloride to the medium, it is possible to deduce how much of each drug was taken up by the parasites. To estimate the concentration of the drug in the DV, it was first considered that each flask contained a 50 µL pellet of red blood cells (RBCs), and that each RBC has a volume of 80 fL.25 Therefore each flask contained 6 × 108 cells. Given the parasitemia level was about 10%, there were 6 × 107 PRBCs present. Assuming the DV has an average volume of 4 fL,25 the total DV volume for the population of parasites is 6 × 107 × 4 × 10−15 = 2.5 × 10−7 liters. The concentration of each drug in the DV can then be calculated (Table 4). This calculation is based on the assumption that the drug is completely released by the ammonium chloride. However, a small portion of the drug may still be associated with heme, and thus not be released to the medium.
Table 4.
Uptake, DV concentration and accumulation ratio for drug/P. falciparum strain combinations. The concentration in DV is calculated assuming 6 × 107 PRBCs present and an average DV volume of 4 fL. The uncertainties are estimated to be ±5%, based on variations measured in multiple trials of similar experiments performed under identical experimental conditions.
| Drug/Pf strain | Uptakea(moles) | Concentration in DV (mM) |
Accumulation ratiob |
|---|---|---|---|
| 1/D6 | 1.6 × 10−8 | 65 | 32,000 |
| 1/Dd2 | 1.8 × 10−8 | 70 | 38,000 |
| CQ/D6 | 0.7 × 10−8 | 29 | 9,000 |
| CQ/Dd2 | 0.5 × 10−8 | 20 | 6,000 |
Uptake is the number of moles of drug taken up by the 6 × 107 PRBCs after 1 hour of incubation, calculated by the ratio: [(concentration in medium after NH4Cl addition – concentration in medium after 1 hour of incubation) / 5 mL (the volume of medium used)].
Accumulation ratio is [concentration in DV / concentration in medium].
The accumulation ratio for each drug can be estimated from this data, with the assumption that the drugs accumulate within the parasite DV; we provide evidence for this, below. From an initial medium concentration of ∼ 5 µM, 1 accumulates in the DV of D6 P. falciparum to an equilibrium concentration of about 65 mM, a 13,000-fold increase, yielding an accumulation ratio ([drug in the DV] / [drug in medium at equilibrium]) of 32,000. Most significantly, the accumulation ratio of 1 is more than triple that of CQ. This is in agreement with the IC50 values, in that the values for CQ are well over twice those of 1, even for CQS D6 P. falciparum (Table 1). However, the DV is known to swell as it accumulates quinoline-based antimalarial drugs,26, 27 and so the accumulation ratio presented here may be too large as the calculation is based on a DV volume of 4 fL. Yet whether the drug accumulation causes, or is enabled by, the swelling of the DV, does not detract from the fact that the molar uptake of 1 is more than double that of CQ, irrespective of DV volume. In any event, these experiments were carried out at significantly higher drug concentrations than the IC50 determinations presented in Table 1, and may reflect mechanisms in addition to simple differences in accumulation and inhibition of hemozoin formation.28, 29 In fact, the higher concentrations may represent at least a significant portion of the CQ blood concentration time-course during malaria chemotherapy.30, 31
In vitro heme binding and β-hematin inhibition
It is known that CQ binds to heme dimers in vitro, and also can inhibit the formation of β-hematin, the in vitro analogue of hemozoin.32–35 A selection of RCQ compounds was tested as to whether addition of the RA head group affected the compounds’ abilities to bind to heme and to inhibit the formation of β-hematin. As can be seen from Table 5, there was no significant difference between CQ and the RCQ compounds’ ability to bind heme in solution (Table 5; Kd values). Although the numbers range from 1 to 8.3 µM, they are all in the micromolar range, with CQ about in the middle. A weak, positive correlation was noted between the RCQ in vitro potency (Table 1) and its Kd value (R2 ∼ 0.17). 26 was tested and showed no activity, suggesting that the quinoline portion of the molecules physically interacted with the heme.
Table 5.
Apparent dissociation equilibrium constants obtained from optical titration of heme with antimalarial agents, assuming a 2:1 heme to drug stoichiometry, and IC50 values for the inhibition of β-hematin by RCQ compounds.
| Drug | Kd [µM (app)] | β-hematin inhibition (µM) |
|---|---|---|
| CQ | 4.0 | 24 |
| 1 | 8.6 | 9.0 |
| 4 | 3.3 | 2.5 |
| 5 | 3.0 | 3.5 |
| 13 | 7.3 | 10 |
| 15 | 5.4 | insoluble |
| 17 | 2.5 | 14 |
| 21 | 1.0 | 2.0 |
| 22 | 1.7 | 1.6 |
Regarding β-hematin inhibition, the IC50 values ranged from 24 µM for CQ to about 2 µM for 4, 21, and 22 (Table 5). While these are all in the micromolar range, there is a trend toward (R2 ∼ 0.66) enhanced potency, coinciding with lower IC50 values for the RCQ compounds against P. falciparum strains (Table 1).
Hemozoin inhibition in vivo
The hemozoin inhibition properties of 1 and 22 in cell culture were examined in a series of experiments, monitoring in parallel with microscopy (to characterize morphological change of the parasites) and a colorimetric assay (to provide a semi-quantitative assessment of hemozoin suppression). The images obtained by microscopy show hemozoin in the control samples, and even in the 100 nM CQ samples, but compound 22, at as low as 10 nM, appeared to preclude hemozoin formation in both D6 and Dd2 strains. 100 nM 1 showed some hemozoin present in the D6 sample, so inhibition was not complete even at this concentration in a CQS strain. This was also the case with the CQR Dd2 stain (not shown).
The images (Figure 3) also show an enlarged DV in the parasites incubated in the presence of drugs. In the drug-free control, the DV is fairly small relative to the parasite, and is almost entirely filled by several large crystals of hemozoin. It is known that the DV swells in the presence of quinoline-based antimalarial drugs,26, 27 and this can clearly be seen with both 1 and 22 (and to a lesser extent with CQ), where the almost clear DV is easy to distinguish from the darker parasite.
Figure 3.
Microscope images from showing D6 and Dd2 parasites in RBCs. Top – D6 parasites clearly showing hemozoin in the control and CQ samples, and the enlarged DV with little or no hemozoin with 1 and 22. Bottom – Dd2 parasites with 22, showing little or no hemozoin formation.
For the colorimetric assay, synchronized D6 and Dd2 P. falciparum parasites were incubated with various concentrations of each drug for 24 hours, then lysed by treatment with a saponin-containing lysis buffer. The hemozoin was extracted by centrifugation, then washed with acetone and PBS buffer to remove residual protein. The pellet was then dissolved in 0.2 N sodium hydroxide solution, and the absorbance at 400 nm was measured. Using an extinction coefficient of 91,000 cm−1M−1, the amount of heme was calculated.36–38 The amount of heme per parasitized erythrocyte was calculated based on the number of erythrocytes in the culture and the percent parasitemia obtained after growing synchronized culture for 24 hours. Baseline hemozoin production was calculated by processing the cell culture at 0 hour in an identical way.
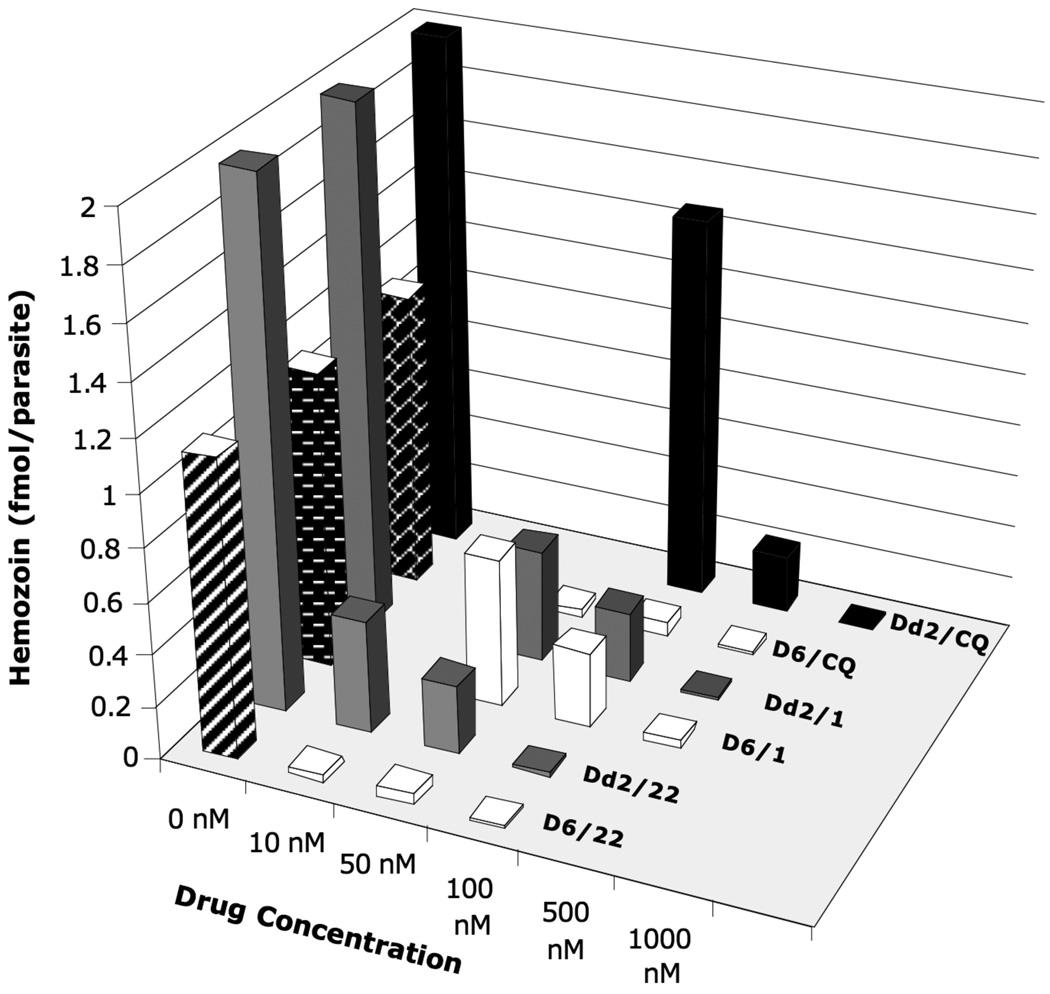
The results from the colorimetric assays indicate that the RCQ molecules did indeed decrease the hemozoin production of P. falciparum, in a manner analogous to, but more potently than CQ (Figure 4). At 10 nM against D6, 22 nearly completely inhibited hemozoin production. Dd2 required a 100 nM concentration of 22 for the same percentage decrease, but this was much lower than the 1000 nM required by CQ.
Figure 4.
Graph of hemozoin production in synchronized D6 and Dd2 P. falciparum, when incubated with various concentrations of CQ, 1 and 22. After the parasites were lysed, the hemozoin was collected by centrifugation, washed and dissolved in 0.2 N sodium hydroxide solution. The absorbance at 400 nm was measured, and the amount of heme was calculated using an extinction coefficient of 91,000 cm−1M−1 The amount of heme per parasitized erythrocyte was calculated based on the number of erythrocytes in the culture and the percent parasitemia obtained after growing synchronized culture for 24 hours. Blank regions represent concentration of drug not tested for a particular cell line. Uncertainties are estimated to be at least ±15%, based on estimated uncertainties due to the extraction of hemozoin from the cells.
The results for 1 against Dd2 are between those for 22 and CQ, a result consistent with the in vitro IC50 values (Table 1). The relatively low activity of 1 against D6 seems inconsistent with its low IC50 value against CQS P. falciparum. However, these results are for very high concentrations. While it is possible that hemozoin inhibition is not the only significant mode of action for the RCQ molecules, and that the low IC50 value is due, in part, to another antimalarial mechanism, further investigation would be needed before such a conclusion were reached.
These in vivo hemozoin inhibition experiments indicate that the RCQ molecules act in a manner similar to that of CQ against CQS P. falciparum. The enlarged DV caused by accumulation of the drugs, can be seen clearly, as can the reduction in hemozoin production. These effects are most pronounced for 22, requiring substantially lower concentrations than CQ to effect almost complete inhibition of hemozoin.
SUMMARY AND CONCLUSION
This work strongly supports the hypothesis that an improved drug can be made by combining elements of CQ and a reversal agent. The in vitro results clearly show that the RCQ compounds have great efficacy against P. falciparum, and the in vivo results demonstrate that the compounds are efficacious in a mouse model. In the in vivo test, 22 stands out as an excellent lead compound for full preclinical testing, with good activity via the oral route of administration, a low ClogP, and no obvious signs of toxicity. Further testing will also be carried out using a wider range of drug-resistant parasite strains to demonstrate that these compounds truly are promising lead compounds for all CQR Plasmodia.
While the mode of action of these compounds has not been fully elucidated, the experiments described above show that the RCQ compounds appear to act in a manner similar to that of CQ. Compound 22 showed the highest level of hemozoin inhibition, both in vitro and in vivo. Taken together, the results suggest that the addition of the RA moiety to the 4-aminoquinoline can enhance the mode of antimalarial activity, at least in part by acting to increase the accumulation in the parasite’s DV.
EXPERIMENTAL
Chemistry
All chemicals were obtained from Sigma-Aldrich Chemical Co. Purities of all final products were ≥95% as determined by HPLC, measuring by UV detection at 254 and 325 nm, using a Varian ProStar 325 UV/Vis dual wavelength detector. HPLC Method A was done with a Microsorb-MV 100 CN 5 µm 4.6 mm × 250 mm column, eluting with 100% methanol for 30 minutes unless otherwise stated. HPLC Method B was performed using a SUPELCO Ascentis RP-Amide 5 µm 4.6 mm × 150 mm column, eluting with 100% methanol for 30 minutes unless otherwise stated. HPLC Method C was performed using a SUPELCO Ascentis C18 5 µm 4.6 mm × 150 mm column, eluting with a 30 minute gradient, from 95:5 to 5:95 water with 0.1 % formic acid (v/v): acetonitrile. High resolution mass spectrometry was performed on a Bruker micrOTOF-Q instrument. Results were obtained using electospray ionization (ESI) in the positive mode, at a flow rate of 0.4 mL/min with 1:1 methanol water. 1H, 13C and 2D NMR experiments were run on a Bruker 400MHz AMX or AVANCE-II+ instrument, using the standard pulse sequences provided, including zg30, zgpg30 cosygpqf, hsqcetgpsi2, hmbcgplpndqf and noesyph, at 25°C.
The syntheses of 1, 2b and 3b have been previously described.17
2-(7-Chloroquinolin-4-ylamino)ethan-1-ol (2a)
A mixture of 4,7-dichloroquinoline (4.95 g, 0.025 mol) and ethanolamine (15.27 g, 15.0 mL, 0.25 mol) were heated with stirring at 130–140 °C for 24 hours. After cooling, the reaction was poured into water (150 mL) and filtered. After air drying the solid was boiled in methanol (100 mL), allowed to cool to room temperature then cooled in ice. The solid was filtered, then washed with a small amount of ice cold methanol to give 2a (3 g, 54%) as an off-white solid. HPLC (method A) tR = 6.99 min (99% pure). 1H NMR δ (ppm)(400 MHz, CH3OH-d4): 3.48–3.55 (2 H, m), 3.85 (2 H, q, J = 5.80 Hz), 6.60 (1 H, d, J = 5.67 Hz), 7.43 (1 H, dd, J = 9.02, 2.20 Hz), 7.80 (1 H, d, J = 2.19 Hz), 8.11 (1 H, t, J = 9.02 Hz), 8.38 (1 H, d, J = 5.64 Hz). 13C NMR δ (ppm)(100 MHz, CH3OH-d4): 46.2, 60.7, 99.7, 118.8, 124.3, 126.1, 127.6, 136.4, 149.7, 152.5, 152.9.
2-(7-Chloroquinolin-4-ylamino)ethyl methanesulfonate (3a)
To a suspension of 2a (1.5 g, 6.7 mmol) in anhydrous dichloromethane (25 mL) under a nitrogen atmosphere was added triethylamine (2 mL, 14.3 mmol). The mixture was cooled to below 0 °C. Methanesulfonyl chloride (0.57 mL, 7.41 mmol) was added slowly, keeping the temperature below 5 °C, and the reaction was stirred in an ice bath for 1 hour. The reaction was added to a saturated NaHCO3 solution (100 mL), and the organic layer was separated and washed with saturated NaHCO3 solution (25 mL). The combined aqueous layers were extracted with dichloromethane (2 × 20 mL). The combined organic extracts evaporated to leave 3a (1.19 g, 59%) as an off-white solid.
General procedure for the preparation of compounds 4–13
A mixture of the respective piperazine or homopiperazine compound (1.2 equiv), triethylamine (2.0 equiv) and appropriate methylsulfonate ester (1.0 equiv) was heated to 70°C in THF for 3 days with stirring. After cooling to room temperature, 50% K2CO3 solution was added. The mixture was shaken and the THF layer was separated. The aqueous layer was extracted with ethyl acetate. The extracts were combined with the THF layer, and washed with water. After drying and evaporating, the residue was purified.
N-(2-(4-Benzhydrylpiperazin-1-yl)ethyl)-7-chloroquinolin-4-amine (4)
The title compound was prepared from 1-(diphenylmethyl)piperazine (0.4 g, 0.0016 mol), triethylamine (0.27 g, 0.0027 mol) and 3a (0.4 g, 0.00133 mol) in THF (12 mL) according to the general procedure. The crude compound was purified by recrystallization in ethyl acetate to give an off white solid (0.13 g, 21%). HPLC (method B) tR = 2.74 min (97% pure). 1H NMR δ (ppm)(400 MHz, CHCl3-d) 2.51 (8 H, d, J = 40.09 Hz), 2.78 (2 H, t, J = 5.91 Hz), 3.29 (2 H, q, J = 5.32 Hz), 4.26 (1 H, s), 5.96 (1 H, s), 6.35 (1 H, d, J = 5.36 Hz), 7.21-7.14 (2 H, m), 7.31-7.25 (4 H, m), 7.39 (1 H, dd, J = 8.90, 2.20 Hz), 7.46-7.40 (4 H, m), 7.65 (1 H, d, J = 8.92 Hz), 7.95 (1 H, d, J = 2.16 Hz), 8.52 (1 H, d, J = 5.31 Hz). 13C NMR δ (ppm)(100 MHz, CHCl3-d) 38.9, 52.1, 52.9, 55.4, 76.2, 99.3, 117.4, 121.1, 125.3, 127.0, 127.9, 128.5, 128.8, 134.8, 142.6, 149.1, 149.8, 152.2. MS (ESI): m/z 457.2149 M + H (Calculated 457.2154).
N-(3-(4-Benzhydrylpiperazin-1-yl)propyl)-7-chloroquinolin-4-amine (5)
The title compound was prepared from 1-(diphenylmethyl)piperazine (0.56 g, 0.0022 mol), triethylamine (0.43 g, 0.0042 mol) and 3b (0.65 g, 0.0021 mol) in THF (15 mL) according to the general procedure. The crude compound was purified by recrystallization in ethyl acetate to give an off white solid (0.32 g, 31%). HPLC (method B) tR = 3.22 min (97% pure). 1H NMR δ (ppm)(400 MHz, CHCl3-d) 1.97-1.89 (2 H, m), 2.87-2.32 (10 H, m), 3.35 (2 H, q, J = 5.12 Hz), 4.42 (1 H, s), 6.29 (1 H, d, J = 5.40 Hz), 7.13 (1 H, dd, J = 8.92, 2.18 Hz), 7.28-7.20 (2 H, m), 7.36-7.29 (4 H, m), 7.46-7.41 (4 H, m), 7.61 (1 H, s), 7.78 (1 H, d, J = 8.95 Hz), 7.92 (1 H, d, J = 2.16 Hz), 8.48 (1 H, d, J = 5.35 Hz). 13C NMR δ (ppm)(100 MHz, CHCl3-d) 23.3, 44.5, 51.6, 54.0, 58.9, 75.9, 98.4, 117.5, 122.4, 124.6, 127.2, 128.2, 128.5, 128.6, 134.6, 141.9, 149.1, 150.6, 152.2. MS (ESI): m/z 471.2293 M + H (Calculated 471.2310).
N-(3-(4-Benzhydryl-1,4-diazepan-1-yl)propyl)-7-chloroquinolin-4-amine (6)
The title compound was prepared from 27 (0.22 g, 0.001 mol), triethylamine (0.18 g, 0.0018 mol) and 3b (0.20 g, 0.0006 mol) in THF (15 mL) according to the general procedure. The crude compound was purified by chromatography on alumina eluting with ethyl acetate/hexanes 40:60) to give a solid (0.07 g, 24%). HPLC (method C) tR = 10.76 min (95% pure). 1H NMR δ (ppm)(400 MHz, CHCl3-d): 1.85–1.86 (4 H, m), 2.72–2.73 (6 H, m), 2.78 (2 H, d, J = 6.40 Hz), 2.87 (2 H, t, J = 5.56 Hz), 3.34 (2 H, q, J = 5.22 Hz), 4.63 (1 H, s), 6.29 (1 H, d, J = 5.39 Hz), 7.18–7.20 (2 H, m), 7.27-7.27 (5 H, m), 7.43 (4 H, d, J = 7.75 Hz), 7.62 (1 H, s), 7.72 (1 H, d, J = 8.92 Hz), 7.92 (1 H, d, J = 2.18 Hz), 8.48 (1 H, d, J = 5.34 Hz). 13C NMR δ (ppm)(100 MHz, CHCl3-d): 24.5, 27.8, 44.5, 52.9, 53.0, 54.3, 57.0, 57.9, 75.7, 98.5, 117.6, 122.1, 124.8, 127.0, 128.0, 128.5, 128.6, 134.6, 143.2, 149.2, 150.6, 152.2. MS (ESI): m/z 485.2454 M + H (Calculated 485.2467).
7-Chloro-N-(2-(4-((4-chlorophenyl)(phenyl)methyl)piperazin-1-yl)ethyl)quinolin-4-amine (7)
The title compound was prepared from 1-[(4-chlorophenyl)(phenyl)methyl]piperazine (0.46 g, 0.0016 mol), triethylamine (0.27 g, 0.0027 mol) and 3a (0.4 g, 0.00133 mol) in THF (12 mL) according to the general procedure. The crude compound was purified by recrystallization in ethyl acetate to give an off white solid (0.32 g, 31%). The crude compound was purified by recrystallization in ethyl acetate/hexanes (70/30) to give an off white solid (0.20 g, 30%). HPLC (method A) tR = 7.30 min (96% pure). 1H NMR δ (ppm)(400 MHz, CHCl3-d) 2.50 (8 H, d, J = 44.37 Hz, 2.78 (2 H, t, J = 5.91 Hz), 3.29 (2 H, q, J = 5.32 Hz), 4.24 (1 H, s), 5.94 (1 H, s), 6.35 (1 H, d, J = 5.36 Hz), 7.31-7.19 (5 H, m), 7.41-7.35 (5 H, m), 7.64 (1 H, d, J = 8.93 Hz), 7.95 (1 H, d, J = 2.16 Hz), 8.52 (1 H, d, J = 5.31 Hz). 13C NMR δ (ppm)(100 MHz, CHCl3-d) 38.9, 52.0, 52.8, 55.4, 75.4, 99.3, 117.3, 121.1, 125.3, 127.2, 127.8, 128.6, 128.7, 128.8, 129.1, 132.6, 134.8, 141.2, 142.1, 149.1, 149.7, 152.1. MS (ESI): m/z 491.1745 M + H (Calculated 491.1764).
7-Chloro-N-(3-(4-((4-chlorophenyl)(phenyl)methyl)piperazin-1-yl)propyl)quinolin-4-amine (8)
The title compound was prepared from 1-[(4-chlorophenyl)(phenyl)methyl]piperazine (0.63 g, 0.0022 mol), triethylamine (0.43 g, 0.0042 mol) and 3b (0.65 g, 0.0021 mol) in THF (15 mL) according to the general procedure. The crude compound was purified by recrystallization from ethyl acetate to give an off white solid (0.38 g, 34%). HPLC (method A – 40 min) tR = 9.67 min (98% pure). 1H NMR δ (ppm)(400 MHz, CHCl3-d) 1.92 (2 H, p, J = 5.38 Hz), 2.70-2.52 (10 H, m), 3.34 (2 H, q, J = 5.11 Hz), 4.37 (1 H, s), 6.29 (1 H, d, J = 5.40 Hz), 7.15 (1 H, dd, J = 8.90, 2.17 Hz), 7.32-7.22 (3 H, m), 7.38-7.30 (2 H, m), 7.42-7.35 (4 H, m), 7.52 (1 H, s), 7.76 (1 H, d, J = 8.95 Hz), 7.92 (1 H, d, J = 2.15 Hz), 8.48 (1 H, d, J = 5.35 Hz). 13C NMR δ (ppm)(100 MHz, CHCl3-d) 23.3, 44.4, 51.6, 53.9, 58.8, 75.3, 98.4, 117.4, 122.3, 124.6, 127.5, 128.1, 128.6, 128.7, 129.3, 132.7, 134.6, 140.8, 141.2, 149.1, 150.5, 152.2. MS (ESI): m/z 505.1911 M + H (Calculated 505.1920).
7-Chloro-N-(3-(4-((4-chlorophenyl)(phenyl)methyl)-1,4-diazepan-1-yl)propyl)quinolin-4-amine (9)
The title compound was prepared from 28 (0.17 g, 0.00057 mol), triethylamine (0.18 g, 0.0018 mol) and 3b (0.20 g, 0.00064 mol) in THF (15 mL) according to the general procedure. The crude compound was purified by chromatography on alumina eluting with ethyl acetate/hexanes (40:60) to give a solid (0.21 g, 71%). HPLC (method B) tR = 4.66 min (97% pure). 1H NMR δ (ppm)(400 MHz, CHCl3-d) 1.81–1.92 (4 H, m), 2.74–2.75 (8 H, m), 2.88 (2 H, t, J = 5.53 Hz), 3.37 (2 H, q, J = 5.13 Hz), 4.61 (1 H, s), 6.31 (1 H, d, J = 5.41 Hz), 7.26–7.28 (5 H, m), 7.35–7.40 (4 H, m), 7.53 (1 H, s), 7.72 (1 H, d, J = 8.93 Hz), 7.93 (1 H, d, J = 2.16 Hz), 8.50 (1 H, d, J = 5.36 Hz). MS (ESI): m/z 519.2079 M + H (Calculated 519.2077).
N-(3-(4-(Bis(4-fluorophenyl)methyl)piperazin-1-yl)propyl)-7-chloroquinolin-4-amine (10)
The title compound was prepared from 1-(bis(4-fluorophenyl)methyl)piperazine (0.52 g, 0.0018 mol), triethylamine (0.20 g, 0.0019 mol) and 3b (0.41 g, 0.0013 mol) in THF (15 mL) according to the general procedure. The crude compound was purified by chromatography on alumina eluting with ethyl acetate/hexanes (40:60) to give a solid (0.12 g, 18%). HPLC (method B) tR = 3.3 min (98% pure). 1H NMR δ (ppm)(400 MHz, CHCl3-d) 1.97 (3 H, m), 2.57 (8 H, bs), 2.68 (3 H, t, J = 5.60 Hz), 3.40 (3 H, m), 4.38 (1 H, s), 6.34 (1 H, d, J = 5.47 Hz), 7.04 (4 H, t, J = 8.55 Hz), 7.22 (1 H, dd, J = 8.90, 2.05 Hz), 7.40 (4 H, dd, J = 8.47, 5.45 Hz), 7.67 (1 H, bs), 7.84 (1 H, d, J = 8.89 Hz), 8.00 (1 H, s), 8.51 (1 H, d, J = 5.44 Hz).
N-(2-(4-(9H-Fluoren-9-yl)-1,4-diazepan-1-yl)ethyl)-7-chloroquinolin-4-amine (11)
The title compound was prepared from 29 (0.24 g, 0.0010 mol), triethylamine (0.69 g, 0.0068 mol) and 3a (0.30 g, 0.0010 mol) in THF (15 mL) according to the general procedure. The crude compound was purified by chromatography on alumina eluting with ethyl acetate/hexanes (40:60) to give a solid (0.05 g, 11%). HPLC (method C) tR = 10.88 min (95% pure). 1H NMR δ (ppm)(400 MHz, CHCl3-d): 1.79–1.80 (2 H, m), 2.67–2.70 (2 H, m), 2.74–2.78 (2 H, m), 2.84 (2 H, t, J = 5.93 Hz), 2.91 (4 H, dt, J = 12.45, 5.98 Hz), 3.23 (2 H, q, J = 5.25 Hz), 4.90 (1 H, s), 6.17 (1 H, s), 6.34 (1 H, d, J = 5.34 Hz), 7.26 (2 H, s), 7.37 (3 H, d, J = 7.82 Hz), 7.63 (1 H, s), 7.69 (3 H, d, J = 7.26 Hz), 7.96 (1 H, d, J = 2.18 Hz), 8.52 (1 H, d, J = 5.28 Hz). 13C NMR δ (ppm)(100 MHz, CHCl3-d): 29.4, 39.7, 53.4, 54.9, 56.5, 71.6, 99.3, 119.8, 121.2, 125.3, 125.5, 127.1, 128.1, 128.8, 134.8, 140.8, 144.9, 149.1, 149.9, 152.1. MS (ESI): m/z 469.2143 M + H (Calculated 469.2154).
N-(3-(4-(9H-Fluoren-9-yl)-1,4-diazepan-1-yl)propyl)-7-chloroquinolin-4-amine (12)
The title compound was prepared from 29 (0.31 g, 0.0012 mol), triethylamine (0.25 g, 0.0025 mol) and 3b (0.37 g, 0.0012 mol) in THF (15 mL) according to the general procedure. The crude compound was purified by chromatography on alumina eluting with ethyl acetate/hexanes (40:60) to give a solid (0.29 g, 50%). HPLC (method C) tR = 11.24 min (97% pure). 1H NMR δ (ppm)(400 MHz, CHCl3-d): 1.88–1.93 (4 H, m), 2.08 (1 H, s), 2.77 (3 H, s), 2.83 (2 H, t, J = 5.89 Hz), 2.94 (2 H, t, J = 5.29 Hz), 3.00 (1 H, s), 3.34 (2 H, t, J = 5.75 Hz), 4.92 (1 H, s), 6.27 (1 H, d, J = 5.53 Hz), 7.38 (2 H, t, J = 7.47 Hz), 7.64 (2 H, d, J = 7.49 Hz), 7.69 (2 H, d, J = 7.56 Hz), 7.96-7.96 (2 H, m),8.48 (1 H, s). 13C NMR δ (ppm)(100 MHz, CHCl3-d): 23.3, 24.1, 27.6, 43.5, 50.2, 51.7, 53.5, 56.8, 57.4, 71.3, 98.3, 117.5, 119.9, 122.7, 125.1, 125.5, 127.2, 127.7, 128.3, 135.0, 140.9, 144.5, 148.3, 151.0, 151.3, 177.3. MS (ESI): m/z 483.2297 M + H (Calculated 483.2310).
N1-(7-chloroquinolin-4-yl)-N2-(3-(10,11-dihydro-5H-dibenzo[b,f]azepin-5-yl)propyl)-N2-methylethane-1,2-diamine (13)
Desipramine hydrochloride (0.44 g, 0.00145 mol) was dissolved in water (7 mL), and solid NaHCO3 (0.24 g, 0.0029 mol) was added with stirring. After addition of dichloromethane (8 mL), two clear layers resulted. The aqueous layer was removed and extracted with dichloromethane (2× 7 mL). The combined organic layers were evaporated to leave desipramine free base as a yellow oil. To this oil were added anhydrous THF (12 mL) and 3a (0.35 g, 0.00116 mol) followed by triethylamine (0.32 mL, 0.00232 mol). After being stirred at 50–60°C for 72 h, the reaction was allowed to cool to room temperature. The reaction was diluted with 50% K2CO3 solution (30 mL), and the THF layer was separated. The aqueous was extracted with ethyl acetate (2 × 10 mL). The extracts were combined with the THF layer, and washed with water (10 mL). After drying and evaporation, the residue was chromatographed on alumina (MCB type F20, 80–200 mesh), eluting with ethyl acetate:hexanes (70:30) to give a yellow oil (0.42 g, 77%). HPLC (method A) tR = 9.12 min (96% pure). 1H NMR δ (ppm)(400 MHz, CHCl3-d) 1.78 (2 H, p, J = 6.79 Hz), 2.23 (3 H, s), 2.46 (2 H, t, J = 7.00 Hz), 2.68 (2 H, t, J = 5.81 Hz), 3.03 (4 H, s), 3.26-3.16 (2 H, m), 3.79 (2 H, t, J = 6.54 Hz), 5.82 (1 H, s), 6.32 (1 H, d, J = 5.35 Hz), 6.84 (2 H, td, J = 7.14, 1.61 Hz), 7.09-6.95 (6 H, m), 7.23 (1 H, dd, J = 8.90, 2.19 Hz), 7.44 (1 H, d, J = 8.93 Hz), 7.95 (1 H, d, J = 2.16 Hz), 8.52 (1 H, d, J = 5.31 Hz). 13C NMR δ (ppm)(100 MHz, CHCl3-d) 24.7, 31.0, 38.5, 40.9, 47.0, 53.3, 54.3, 98.2, 116.3, 118.6, 120.0, 121.5, 124.3, 125.3, 127.7, 128.8, 133.1, 133.7, 147.0, 148.1, 148.6, 151.0. MS (ESI): m/z 471.2316 M + H (Calculated 471.2310).
7-Chloro-N-(3-(piperazin-1-yl)propyl)quinolin-4-amine (14)
2b (2.7g, 0.0116 mol) was finely ground and suspended on dry THF (50 mL). Triethylamine (2.9g, 0.29 mol) was added and the mixture was cooled to below 0°C. Methane sulfonyl chloride(1.46g, 0.0128 mol) was added slowly, keeping the temperature below 5°C. After stirring in an ice bath for 1 hour, TLC (alumina plate, run in ethyl acetate) indicated no 2b left in the reaction. Piperazine (10g, 0.116 mol) was added, and the reaction was heated to reflux. After 2 hours TLC indicated the reaction was complete, and it was allowed to cool to room temperature. 150 mL of saturated NaHCO3 solution was added and the solution was extracted with ethyl acteate (3 × 50 mL). The combined organic extracts were washed with water (5 × 25 mL), dried and evaporated give a cream solid (1.2 g, 34%). HPLC (method C) tR = 2.81 min (93% pure). 1H NMR δ (ppm)(400 MHz, CH3OH-d4): 1.79–1.89 (2 H, m), 2.38–2.49 (6 H, m), 2.76–2.83 (4 H, m), 3.33 (2 H, t, J = 6.79 Hz), 6.44 (1 H, d, J = 5.69 Hz), 7.27–7.33 (1 H, m), 7.68 (1 H, d, J = 2.18 Hz), 7.98 (1 H, d, J = 9.01 Hz), 8.25 (1 H, d, J = 5.65 Hz). 13C NMR δ (ppm)(100 MHz, CH3OH-d4): 25.8, 42.6, 46.1, 54.6, 58.0, 99.6, 118.7, 124.3, 126.0, 127.6, 136.4, 149.6, 152.4, 152.8. MS (ESI): m/z 305.1538 M + H (Calculated 305.1528).
7-Chloro-N-(3-(4-tritylpiperazin-1-yl)propyl)quinolin-4-amine (15)
14 (0.3 g, 0.00098 mol) was dissolved in acetonitrile (15 mL) and potassium carbonate (0.2 g, 0.0015 mol) was added, followed by trityl chloride (0.25 g, 0.00089 mol). The mixture was heated to reflux with stirring for 3 hours, after which TLC indicated completion. After cooling to room temperature, the reaction was poured into water (50 mL) and ethyl acetate (20 mL) was added. The insoluble precipitate was filtered, washed with water and ethyl acetate, and dried. The ethyl acetate layer was separated and the aqueous layer extracted with ethyl acetate (2 × 20 mL). The combined organic phases were dried, filtered through an alumina plug, and evaporated. The residue was combined with the insoluble solid from above to give a white solid (0.15 g, 31%). HPLC (method A) tR = 8.63 min (97% pure). 1H NMR δ (ppm)(400 MHz, CHCl3-d): 1.90–1.97 (4 H, m), 2.53 (2 H, bs), 2.67–2.72 (2 H, m), 3.06 (2 H, bs), 3.16 (2 H, bs), 3.31 (2 H, q, J = 4.99 Hz), 6.22 (1 H, d, J = 5.41 Hz), 6.62 (1 H, dd, J = 8.95, 2.19 Hz), 7.26–7.34 (10 H, m), 7.52 (6 H, bs), 7.67 (1 H, s), 7.84 (1 H, d, J = 2.16 Hz), 8.43 (1 H, d, J = 5.35 Hz). 13C NMR δ (ppm)(100 MHz, CH3OH-d4): 22.8, 44.7, 47.8, 54.6, 59.6, 98.1, 117.2, 122.1, 125.0, 126.4, 127.2, 127.7, 127.8, 127.9, 129.4, 134.8, 146.9, 150.8, 151.5. MS (ESI): m/z 547.2637 M + H (Calculated 547.2623).
General method for the preparation of compounds 16 and 17
14 (0.5 g, 0.00164 mol) and the respective carbonyl compound were mixed together in dry THF (5 mL) then treated with sodium triacetoxyborohydride (0.52 g, 0.0025 mol) followed by acetic acid (0.1 g, 0.00175 mol).19 The mixture was stirred under nitrogen, and at room temperature, for 7 days. The reaction was quenched with saturated NaHCO3 solution (50 mL), and extracted into dichloromethane (3 × 10 mL). The extracts were washed with brine (10 mL), then dried and evaporated. The residue was chromatographed to give pure product.
7-Chloro-N-(3-(4-(2,2-diphenylethyl)piperazin-1-yl)propyl)quinolin-4-amine (16)
The title compound was prepared from diphenylacetaldehyde (0.32 g, 0.00164 mol) according to the general procedure. The crude compound was purified by chromatography on silica, eluting with ethyl acetate/ammonium hydroxide (99:1) to give an off white solid (0.43 g, 54%). HPLC (method A) tR = 7.87 min (98% pure). 1H NMR δ (ppm)(400 MHz, CHCl3-d): 1.87–1.94 (2 H, m), 2.48–2.62 (6 H, m), 2.64 (4 H, s), 3.07 (2 H, d, J = 7.54 Hz), 3.34 (2 H, q, J = 5.12 Hz), 4.24 (1 H, t, J = 7.52 Hz), 6.30 (1 H, d, J = 5.41 Hz), 7.17–7.23 (2 H, m), 7.24–7.33 (9 H, m), 7.58 (1 H, s), 7.87 (1 H, d, J = 8.94 Hz), 7.93 (1 H, d, J = 2.15 Hz), 8.49 (1 H, d, J = 5.36 Hz). 13C NMR δ (ppm)(100 MHz, CHCl3-d): 23.3, 44.5, 48.9, 53.5, 53.6, 58.8, 63.8, 98.5, 117.5, 122.5, 124.6, 126.3, 128.2, 128.4, 128.7, 134.6, 143.7, 149.1, 150.6, 152.2. MS (ESI): m/z 485.2479 M + H (Calculated 485.2467).
N-(3-(4-(Adamant-2-yl)piperazin-1-yl)propyl)-7-chloroquinolin-4-amine (17)
The title compound was prepared from 2-adamantanone (0.5 g, 0.00328 mol) according to the general procedure. The crude compound was purified by chromatography on silica, eluting with ethyl acetate/ammonium hydroxide (99.3:0.7) to give an off white solid (0.33 g, 46). HPLC (method A) tR = 11.75 min (96% pure). 1H NMR δ (ppm)(400 MHz, CHCl3-d): 1.42 (2 H, d, J = 11.72 Hz), 1.79 (5 H, m), 1.86–2.02 (5 H, m), 2.10 (5 H, m,), 2.60–2.66 (10 H, m), 3.38 (2 H, q, J = 5.12 Hz), 6.31 (1 H, d, J = 5.41 Hz), 7.32 (1 H, dd, J = 8.95, 2.14 Hz), 7.81 (1 H, s), 7.91–7.95 (2 H, m), 8.50 (1 H, d, J = 5.37 Hz). 13C NMR δ (ppm)(100 MHz, CHCl3-d): 23.2, 27.3, 27.6, 29.0, 31.4, 37.3, 37.8, 44.7, 49.7, 54.3, 59.0, 68.3, 98.4, 117.5, 122.7, 124.7, 128.6, 134.6, 149.2, 150.7, 152.2. MS (ESI): m/z 439.2631 M + H (Calculated 439.2623).
N-(Dipyridin-2-ylmethylene)piperidin-4-amine (18)
2,2’-Dipyridylketone (25.95 g, 0.141 mol) was dissolved in toluene (500 mL) and 4-aminopiperidine (16.2 g, 0.162 mol) was added followed by p-toluene sulfonic acid (∼0.5 g). The mixture was heated to reflux for 3 days, with a Dean and Stark trap to remove water. After cooling to room temperature, the toluene was removed to leave a crude oil (37.5 g, 99%), which was used without further purification.
7-Chloro-N-(2-(4-(dipyridin-2-ylmethyleneamino)piperidin-1-yl)ethyl)quinolin-4-amine (19)
18 (1.74 g, 0.0065 mol crude) was dissolved in acetonitrile (20 mL) and 3a (1.31 g, 0.00436 mol) and K2CO3 (1.2 g, 0.00871 mol) were added. The reaction was stirred at 70°C for 2 days. After cooling to room temperature, water (100 mL) was added and the mixture was stirred for 30 minutes. The solid was filtered, washed with water and recrystallized from toluene/hexanes twice to give a solid (0.68 g, 33%). HPLC (method A) tR = 7.80 min (99% pure). MS (ESI): m/z 471.2054 M + H (Calculated 471.2058).
7-Chloro-N-(3-(4-(dipyridin-2-ylmethyleneamino)piperidin-1-yl)propyl)quinolin-4-amine (20)
18 (1.02 g, 0.00382 mol crude) was dissolved in acetonitrile (12 mL) and 3b (1 g, 0.00318 mol) and K2CO3 (0.88 g, 0.00636 mol) were added. The reaction was stirred at 70°C overnight. TLC (alumina plate, run in ethyl acetate/methanol 9:1) indicated some 3b was still present, so a further solution of 18 (0.25 g, 0.000954 mol) in acetonitrile (5 mL) was added, and heating continued overnight. After cooling to room temperature, the solvent was evaporated and the residue was slurried in water (30 mL). The solid was filtered, washed with water and recrystallized from toluene/hexanes. The solid was dissolved in ethyl acetate/methanol 50:50 and stirred with alumina and charcoal for 30 minutes. After filtering through celite, the solvents were removed to give a solid (0.78 g, 50%). HPLC (method B) tR = 2.41 min (96% pure). MS (ESI): m/z 485.2230 M + H (Calculated 485.2215).
7-Chloro-N-(2-(4-(dipyridin-2-ylmethylamino)piperidin-1-yl)ethyl)quinolin-4-amine (21)
19 (0.53 g, 0.00113 mol) was dissolved in methanol (40 mL) and cooled in an ice/water bath. Sodium borohydride (0.13 g, 0.00338 mol) was added in portions and the reaction was stirred overnight at room temperature. After evaporating the methanol, water (40 mL) was added to the residue, and the resulting suspension was stirred for 30 minutes. The mixture was extracted with dichloromethane (3 × 20 mL) and the combined extracts were washed with water (10 mL), then dried and evaporated. Chromatography on alumina, eluting with dichloromethane/methanol (95:5) gave an oil (0.17 g, 34%). HPLC (method B) tR = 2.30 min (84% pure). 1H NMR δ (ppm)(CHCl3-d): 1.50–1.62 (2 H, m), 1.83 (1 H, s), 1.95 (2 H, d, J = 13.24 Hz), 2.04 (2 H, t, J = 11.64 Hz), 2.44–2.53 (1 H, m), 2.71 (2 H, t, J = 5.88 Hz), 2.89 (2 H, d, J = 11.30 Hz), 3.26 (2 H, q, J = 5.27 Hz), 5.24 (1 H, s), 6.09 (1 H, s), 6.34 (1 H, d, J = 5.37 Hz), 7.14 (2 H, ddd, J = 7.48, 4.87, 1.18 Hz), 7.36 (1 H, dd, J = 8.89, 2.19 Hz), 7.42 (2 H, dt, J = 7.88, 1.05 Hz), 7.62 (2 H, td, J = 7.67, 1.83 Hz), 7.64 (1 H, d, J = 8.94 Hz), 7.94 (1 H, d, J = 2.17 Hz), 8.51 (1 H, d, J = 5.32 Hz), 8.56 (2 H, ddd, J = 4.89, 1.81, 0.92 Hz). 13C NMR δ (ppm)(CHCl3-d): 33.0, 39.0, 51.9, 52.7, 55.3, 66.2, 99.2, 117.3, 121.2, 122.2, 122.4, 125.4, 128.7, 134.8, 136.7, 149.1, 149.2, 149.8, 152.1, 161.6. MS (ESI): m/z 473.2227 M + H (Calculated 473.2215).
7-Chloro-N-(3-(4-(dipyridin-2-ylmethylamino)piperidin-1-yl)propyl)quinolin-4-amine (22)
20 (0.60 g, 0.00124 mol) was dissolved in methanol (40 mL) and cooled in ice. Sodium borohydride (0.14 g, 0.0037 mol) was added in portions, and the reaction was stirred at room temperature overnight. After evaporating the methanol, the residue was stirred with water (50 mL) for 30 minutes then extracted with dichloromethane (3 × 20 mL). The extracts were washed with water (20 mL), dried and evaporated to give a solid (0.59 g, 99%). HPLC (method B) tR = 2.49 min (97% pure). 1H NMR δ (ppm)(400 MHz, CHCl3-d): 1.57–1.70 (2 H, m), 1.87–1.95 (2 H, m), 1.99 (5 H, d, J = 11.77 Hz), 2.56 (2 H, t, J = 5.17 Hz), 2.59–2.68 (1 H, m), 3.00 (2 H, d, J = 11.29 Hz), 3.35 (2 H, q, J = 5.10 Hz), 5.25 (1 H, s), 6.29 (1 H, d, J = 5.42 Hz), 7.16 (2 H, ddd, J = 7.47, 4.88, 1.19 Hz), 7.44 (1 H, dd, J = 8.91, 2.18 Hz), 7.47 (2 H, d, J = 7.89 Hz), 7.65 (2 H, td, J = 7.67, 1.83 Hz), 7.74 (1 H, s), 7.84 (1 H, d, J = 8.96 Hz), 7.91–7.94 (1 H, m), 8.49 (1 H, d, J = 5.37 Hz), 8.59 (2 H, ddd, J = 4.89, 1.80, 0.92 Hz). 13C NMR δ (ppm)(100 MHz, CHCl3-d): 23.7, 33.1, 44.5, 52.8, 53.1, 58.7, 66.5, 98.3, 117.6, 122.2, 122.4, 122.4, 125.0, 128.5, 134.6, 136.7, 149.1, 149.1, 150.7, 152.1, 162.1. MS (ESI): m/z 487.2355 M + H (Calculated 487.2371).
2-(4-(4-Benzhydrylpiperazin-1-yl)butyl)isoindoline-1,3-dione (23)
A mixture of N-(4-bromobutyl)phthalimide (0.5 g, 0.00177 mol), 1-(diphenylmethyl)piperazine (0.47 g, 0.00186 mol) and K2CO3 (0.61 g, 0.00443 mol) was stirred and heated in acetonitrile (25 mL) to reflux for 3 hours.39 After cooling, the acetonitrile was evaporated, and the residue partitioned between water (20 mL) and ethyl acetate (20 mL). The aqueous layer was extracted with ethyl acetate (2 × 10 mL) and the combined organic layers were dried and evaporated to give a solid (0.74 g, 92%), which was used without further purification.
4-(4-Benzhydrylpiperazin-1-yl)butan-1-amine (24)
23 (0.74 g, 0.00163 mol crude) was dissolved in ethanol (5 mL), and hydrazine hydrate (0.25 g, 0.0049 mol) was added.39 The mixture was stirred and heated to reflux for 3 hours, then allowed to cool to room temperature. The solid was filtered off, and the filter cake washed with cold ethanol. The filtrate was evaporated to give an oil (0.53 g, ∼100%), which solidified on contact with air. This was used without further purification.
N-(4-(4-Benzhydrylpiperazin-1-yl)butyl)-7-chloroquinolin-4-amine (25)
4,7-dichloroquinoline (0.24 g, 0.0012 mol) was dissolved in ethanol (10 mL) and 24 (0.53 g, 0.00163 mol crude) was added, followed by triethylamine (0.33 g, 0.00326 mol). The mixture was stirred and refluxed for 10 days, then allowed to cool to room temperature. The ethanol was evaporated, and the residue partitioned between saturated NaHCO3 solution (20 mL) and dichloromethane (20 mL). The organic layer was separated and the aqueous layer was extracted with dichloromethane (2 × 20 mL). The combined dichloromethane layers were washed with saturated NaHCO3 solution (20 mL), then dried and evaporated. The resulting oil was chromatographed on silica, eluting with ethyl acetate/triethylamine (99:1), to give an off-white solid (0.10 g, 17%). HPLC (method B) tR = 2.97 min (95% pure). 1H NMR δ (ppm)(400 MHz, CHCl3-d): 1.67 (2 H, t, J = 6.86 Hz), 1.80 (2 H, p, J = 6.95 Hz), 2.37–2.54 (10 H, m), 3.24–3.33 (2 H, m), 4.19–4.24 (1 H, m), 5.38 (1 H, s), 6.38 (1 H, t, J = 6.53 Hz), 7.14–7.22 (2 H, m), 7.25–7.31 (4 H, m), 7.32 (1 H, dd, J = 8.92, 2.20 Hz), 7.41 (4 H, d, J = 7.61 Hz), 7.66 (1 H, d, J = 8.94 Hz), 7.95 (1 H, d, J = 2.18 Hz), 8.52 (1 H, d, J = 5.36 Hz). 13C NMR δ (ppm)(100 MHz, CHCl3-d): 24.7, 26.4, 43.2, 51.8, 53.5, 57.7, 76.2, 99.0, 117.2, 121.1, 125.1, 127.0, 127.9, 128.5, 128.9, 134.8, 142.6, 149.2, 149.7, 152.1. MS (ESI): m/z 485.2456 M + H (Calculated 485.2467).
2-(4-Benzhydrylpiperazin-1-yl)ethanol (26)
1-(2-Hydroxyethyl)piperazine (2 g, 0.0154 mol) was dissolved in DMF (20 mL), and potassium carbonate (4.27 g, 0.0308 mol) was added, followed by a catalytic amount of potassium iodide. The mixture was stirred at room temperature and chlorodiphenylmethane (3.12 g, 0.0154 mol) was added dropwise. After the addition, the reaction was stirred for a further 2 hours at room temperature, then warmed to 70°C, and held there overnight. After cooling to room temperature, water (100 mL) was added, and the mixture was extracted with diethyl ether (3 × 20 mL). The combined organic extracts were washed with brine solution (30 mL), then dried and evaporated to give an oil (1.7 g, 37%). HPLC (method B) tR = 2.25 min (95% pure). 1H NMR δ (ppm)(400 MHz, CHCl3-d): 2.43 (5 H, bs, J = 8.59 Hz), 2.54 (5 H, m, J = 5.45 Hz), 3.58 (2 H, t, J = 5.41 Hz), 4.22 (1 H, s), 7.14–7.19 (2 H, m), 7.24–7.30 (4 H, m), 7.38–7.43 (4 H, m). 13C NMR δ (ppm)(100 MHz, CHCl3-d): 52.0, 53.1, 57.7, 59.1, 76.2, 126.9, 127.9, 128.5, 142.7.
General method for the preparation of compounds 27–29
The halo compound was placed in chloroform and homopiperazine was added. The reaction was stirred and heated at reflux for 3 days. After cooling to room temperature, saturated NaHCO3 solution was added and the mixture was shaken. The organic layer was separated and washed with water. The aqueous layers were combined with the NaHCO3 layer and extracted with dichloromethane. The combined organic layers were dried and evaporated.
1-Benzhydryl-1,4-diazepane (27)
The title compound was prepared from Chlorodiphenylmethane (0.61 g, 0.003 mol) and homopiperazine (1.5 g, 0.015 mol) according to the general procedure. The crude compound was purified by chromatography on alumina, eluting with chloroform/methanol (95:5) to give an oil (0.35 g, 44%).
1-((4-Chlorophenyl)(phenyl)methyl)-1,4-diazepane (28)
The title compound was prepared from Chloro(4-chlorophenyl)phenylmethane (0.71 g, 0.003 mol) and homopiperazine (1.5 g, 0.015 mol) according to the general procedure. The crude compound was purified by chromatography on alumina, eluting with chloroform/methanol (95:5) to give an oil (0.39 g, 43%).
1-(9 H-Fluoren-9-yl)-1,4-diazepane (29)
The title compound was prepared from 9-bromofluorene (1.47 g, 0.006 mol) and homopiperazine (3 g, 0.03 mol) according to the general procedure. The crude compound was purified by chromatography on alumina, eluting with chloroform/methanol (95:5) to give an oil (0.55 g, 37%).
Inhibition of P. falciparum growth
CQS (D6) and CQR (Dd2 and 7G8) P. falciparum maintained continuously in culture were used.40 Asynchronous cultures were diluted with uninfected RBCs and complete medium (RPMI-1640 with 0.5% Albumax II) to achieve 0.2% parasitemia and 2% hematocrit. In 96-well microplates, CQ (positive control) or RCQ diluted in complete medium from 10 mM stock in DMSO were added to the cell mixture to yield triplicate wells with drug concentrations ranging from 0 to 10−4 M in a final well volume of 100 µL. After 72 hours of incubation under standard culture conditions, plates were harvested and read by the SYBR Green I fluorescence-based method40 using a 96-well fluorescence plate reader (Gemini-EM, Molecular Devices), with excitation and emission wavelengths at 497 and 520 nm, respectively. The fluorescence readings were plotted against log[drug], and the IC50 values were obtained from curve fitting performed by nonlinear regression using either Prism (GraphPad) or Excel (Microsoft) software. In order to better compare results run on different day, and with different batches of each stain, CQ was run as a positive control. All results obtained were ‘normalized’ to the CQ values of 6.9 nM for D6, 102 nM for Dd2 and 108 nM for 7G8. The normalization for D6 strains, using the CQ value of 6.9 nM as the control value is shown below:
‘normalized’ IC50 RCQ compound (D6) = [6.9 / IC50 CQ (D6)] × IC50 RCQ compound (D6)
Mouse efficacy against P. berghei
Compounds were formulated in a solution consisting of 70% Tween-80 (d = 1.08 g/mL) and 30% ethanol (d = 0.81 g/mL), followed by a 10-fold dilution in water. On day 0, heparinized blood (containing 100 µL of 200 u/mL Heparin) was taken from a donor NMRI mouse with approximately 30% parasitemia. The blood was diluted in physiological saline to 108 parasitized erythrocytes per mL. From this suspension 0.2 mL was injected intravenously (i.v.) into experimental groups of 3 female NMRI mice, and a control group of 5 mice. Compounds were administered in a volume of 10ml/kg either as single dose 24 hours after infection (day 1) either by oral gavage (p.o.) or subcutaneous injection, or as 4 consecutive daily p.o. doses 4, 24, 48 and 72 hours after infection (days 0–3).
On day 3 (with the single-dose regimen ) or on day 4 (with the quadruple-dose regimen), 1 µL tail blood was taken and dissolved in 1 mL PBS buffer. Parasitemia was determined with a FACScan (Becton Dickinson) by counting 100,000 RBCs. The difference between the mean value of the control group and those of the experimental groups was calculated and expressed as a percent relative to the control group (= activity). Animals receiving no compound would die typically 5–6 days post-infection and were therefore euthanized right after determination of parasitemia. The survival of the animals was monitored up to 30 days. Mice surviving for 30 days were checked for parasitemia and subsequently euthanized. A compound was considered curative if the animal survived to 30 days post-infection with no detectable parasites by microscopy, with a detection limit of 1 parasite in 10,000 erythrocytes (that is, 0.01%).
Accumulation experiment
Synchronized PRBCs were obtained following two cycles of sorbitol-induced lysis of an asynchronous stock culture. Incubation for an additional 20–24 hours provided a population of mature trophozoites that were added to the culture medium at 2% v/v (about/10% parasitemia).
An aliquot of a 10 mM solution of 1 was added to a culture flask containing 5 mL of PRBCs suspended in complete medium (10% parasitemia), such that the initial medium concentration of 1 was ∼ 5 µM. Samples were removed from the flask at various intervals and centrifuged; the supernatant fluid was then removed and refrigerated, before analysis. 1 was added to flasks containing both CQS D6- and CQR Dd2-infected RBCs, and as a control, to a flask containing uninfected RBCs. For the purpose of estimating the amount of 1 accumulated within the DV, NH4Cl (10 mM) was added 10 min prior to sampling in order to basify the acidic subcellular compartments and cause the release of accumulated 1. The samples were analyzed by reverse-phase HPLC, using a C18 column, eluting with an isocratic mixture of 75% acetonitrile: 25% 5 mM phosphate buffer (pH 11). A parallel experiment was preformed with CQ, also at ∼ 5 µM concentration, for comparative purposes. With these conditions 1 had a retention time of 14 minutes, and CQ 5 minutes. Each drug sample was monitored at 325 nm, and was compared to a standard curve for quantification.
In vitro heme binding and β-hematin inhibition
For heme-drug binding studies, a 1 mM stock solution of chloroquine or test compound was prepared in distilled water, methanol or dimethyl sulfoxide (DMSO), depending on solubility and sonicated to ensure complete dissolution. A 5 mM stock solution of heme was prepared by dissolving heme chloride in 0.1 mM NaOH by incubating at 37°C for 30 min. The solution was stored at 4°C for up to one month. At the beginning of each experiment, the stock heme solution was diluted to 5 µM in phosphate buffer (100 mM, pH 5.7) and allowed to equilibrate for four hours. The four hour equilibration allowed for the initial heme absorbance to stabilize prior to beginning the titration. Optical titrations with each compound were performed by successive addition of aliquots of its stock solution to the 5 µM heme solution. The pH was monitored throughout the procedure with only negligible (±0.05 pH units) changes. Equilibrium binding constants were determined by nonlinear least-squares analysis.41
Hemin chloride (16.3 mg) was dissolved in 1 ml of DMSO. The solution was passed through a 0.2 µm-pore membrane filter to remove insoluble particles and kept at 4°C for up to one month as a stock solution.42 In order to determine heme concentration of the stock solution, a sample was diluted in 2.5% sodium dodecyl sulfate in 0.1 M NaOH and an absorbance reading taken at 400 nm. The heme concentration was calculated using Beer’s law with a molar absorptivity ε = 105 mol L−1 cm−1. The optimal heme and Tween20 concentrations for promoting heme crystallization were calculated by the procedure described by Huy, 2002.43 The RCQ compounds were screened for their inhibitory capacity, and IC50 values were determined. Assays were run in duplicate twice. Incubations were conducted in the dark to ensure that light did not interfere. A series of solutions were made consisting of 300 µl of varying concentrations of the compound under study in distilled, 700 µl of 1 M acetate buffer, 300 µl of a 200 µM heme solution freshly buffered by 1 M sodium acetate (pH 4.8) and 200 µl of 0.0375 g/L Tween20 solution. This provided a final 40 µM heme solution buffered by 0.67 M sodium acetate at pH 4.8 and 0.0005 g/L Tween20, with the test compound ranging in concentration from 0–1000 µM. The mixtures were incubated for 24 hours at 37°C,35 then mixed and transferred cuvette for a 415/630 nm absorbance reading. IC50 values were calculated by (Dmax – Dinitial ) / 2 where Dmax represents the lowest concentration of compound under study to provide maximal absorbance readings indicating maximal free heme, and Dinitial represents the lowest concentration of drug to provide any increase in absorbance over a solution with no drug.
In vivo hemozoin inhibition experiment
P. falciparum strains D6 and Dd2 were synchronized to the ring stage (early trophozoites) with 5% sobitol solution.24 After the synchronization of the parasites, the erythrocytes were suspended in culture medium at 1% hematocrit and aliquots of 1 mM stock solution of drug were added to the culture flasks. Drug treated cultures, along with the no-drug control culture, were incubated for 24 hours at 37 °C under a gas mixture of 5% O2, 5% CO2, and 90% N2 then transferred to 15 ml centrifuge tubes. One 2 µL aliquot was used from each tube was used to obtain Giemsa-stained smear for determination of the parasitemia and morphology examination by microscopy.
Parasites were isolated by one freeze-thaw cycle at −20 °C and treated with a saponin-containing lysis buffer (Tris (20 mM; pH 7.5), EDTA (5 mM), saponin (0.008%; wt/vol), and Triton X-100 (0.08%; vol/vol)40 at 37 °C for 30 min. The hemozoin was pelleted by centrifugation at 215,000 g for 30 min at 25 °C. The supernatant was removed and the pellet consisting primarily of hemozoin was washed two times with acetone to remove residual proteins. Insoluble material was then washed three times with PBS (pH 7.4) buffer and collected by centrifugation at 215,000 g for 30 min at 25 °C. The pellet was dissolved with 0.2 N sodium hydroxide for 2 hours at 37 °C and periodic mixing of the sample. The absorbance at 400 nm was measured, and the amount of heme was calculated using an extinction coefficient of 91,000 cm−1M−1.36–38 The amount of heme per parasitized erythrocyte was calculated based on the number of erythrocytes in the culture and the percent parasitemia obtained after growing synchronized culture for 24 hours. Cultures at 0 hour processed with the identical method were used for determination of the baseline hemozoin production.
Acknowledgment
The authors thank the following for supporting this research: The Medical Research Foundation of Oregon (Grant 0530), and the National Institutes of Health (Grants AI067837 & AI072923) to DHP, as well as a grant from the Murdock Charitable Trust for the NMR instruments. The authors also thank Westin Morrill for his help in preparing this manuscript.
Abbreviations
- CQ
chloroquine
- WHO
World Health Organization
- PfCRT
Plasmodium falciparum chloroquine resistance transporter
- DV
digestive vacuole
- CQR
chloroquine resistant
- CQS
chloroquine sensitive
- RA
reversal agent
- RCQ
reversed chloroquine compound
- SAR
structure-activity relationship
- RBC
red blood cell
- PRBC
parasitized red blood cell
REFERENCES
- 1.World malaria report 2009. Geneva, Switzerland: World Health Organization; 2009. [Google Scholar]
- 2.Jambou R, Legrand E, Niang M, Khim N, Lim P, Volney B, Ekala MT, Bouchier C, Esterre P, Fandeur T, Mercereau-Puijalon O. Resistance of Plasmodium falciparum field isolates to in-vitro artemether and point mutations of the SERCA-type PfATPase6. Lancet. 2005;366:1960–1963. doi: 10.1016/S0140-6736(05)67787-2. [DOI] [PubMed] [Google Scholar]
- 3.Andriantsoanirina V, Ratsimbasoa A, Bouchier C, Jahevitra M, Rabearimanana S, Radrianjafy R, Andrianaranjaka V, Randriantsoa T, Rason MA, Tichit M, Rabarijaona LP, Mercereau-Puijalon O, Durand R, Menard D. Plasmodium falciparum drug resistance in Madagascar: facing the spread of unusual pfdhfr and pfmdr-1 haplotypes and the decrease of dihydroartemisinin susceptibility. Antimicrob. Agents Chemother. 2009;53:4588–4597. doi: 10.1128/AAC.00610-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wongsrichanalai C, Meshnick SR. Declining artesunate-mefloquine efficacy against falciparum malaria on the Cambodia-Thailand border. Emerg. Infect. Dis. 2008;14:716–719. doi: 10.3201/eid1405.071601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, Lwin KM, Ariey F, Hanpithakpong W, Lee SJ, Ringwald P, Silamut K, Imwong M, Chotivanich K, Lim P, Herdman T, An SS, Yeung S, Singhasivanon P, Day NP, Lindegardh N, Socheat D, White NJ. Artemisinin resistance in Plasmodium falciparum malaria. N. Engl. J. Med. 2009;361:455–467. doi: 10.1056/NEJMoa0808859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giha HA. Artemisinin derivatives for treatment of uncomplicated Plasmodium falciparum malaria in Sudan: too early for too much hope. Parasitol Res. 2010;106:549–552. doi: 10.1007/s00436-009-1700-x. [DOI] [PubMed] [Google Scholar]
- 7.Zhang H, Paguio M, Roepe PD. The antimalarial drug resistance protein Plasmodium falciparum chloroquine resistance transporter binds chloroquine. Biochemistry. 2004;43:8290–8296. doi: 10.1021/bi049137i. [DOI] [PubMed] [Google Scholar]
- 8.Martin RE, Kirk K. The malaria parasite’s chloroquine resistance transporter is a member of the drug/metabolite transporter superfamily. Mol. Biol. Evol. 2004;21:1938–1949. doi: 10.1093/molbev/msh205. [DOI] [PubMed] [Google Scholar]
- 9.Bray PG, Martin RE, Tilley L, Ward SA, Kirk K, Fidock DA. Defining the role of PfCRT in Plasmodium falciparum chloroquine resistance. Mol. Microbiol. 2005;56:323–333. doi: 10.1111/j.1365-2958.2005.04556.x. [DOI] [PubMed] [Google Scholar]
- 10.Fidock DA, Nomura T, Talley AK, Cooper RA, Dzekunov SM, Ferdig MT, Ursos LM, Sidhu AB, Naude B, Deitsch KW, Su XZ, Wootton JC, Roepe PD, Wellems TE. Mutations in the P. falciparum digestive vacuole transmembrane protein PfCRT and evidence for their role in chloroquine resistance. Mol. Cell. 2000;6:861–871. doi: 10.1016/s1097-2765(05)00077-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krogstad DJ, Gluzman IY, Kyle DE, Oduola AM, Martin SK, Milhous WK, Schlesinger PH. Efflux of chloroquine from Plasmodium falciparum: mechanism of chloroquine resistance. Science. 1987;238:1283–1285. doi: 10.1126/science.3317830. [DOI] [PubMed] [Google Scholar]
- 12.Martin RE, Marchetti RV, Cowan AI, Howitt SM, Broer S, Kirk K. Chloroquine transport via the malaria parasite's chloroquine resistance transporter. Science. 2009;325:1680–1682. doi: 10.1126/science.1175667. [DOI] [PubMed] [Google Scholar]
- 13.Martin SK, Oduola AM, Milhous WK. Reversal of chloroquine resistance in Plasmodium falciparum by verapamil. Science. 1987;235:899–901. doi: 10.1126/science.3544220. [DOI] [PubMed] [Google Scholar]
- 14.Bitonti AJ, Sjoerdsma A, McCann PP, Kyle DE, Oduola AMJ, Rossan RN, Milhous WK, Davidson DE. Reversal of chloroquine resistance in malaria parasite Plasmodium falciparum by desipramine. Science. 1988;242:1301–1303. doi: 10.1126/science.3057629. [DOI] [PubMed] [Google Scholar]
- 15.Kelly JX, Smilkstein MJ, Cooper RA, Lane KD, Johnson RA, Janowsky A, Dodean RA, Hinrichs DJ, Winter R, Riscoe M. Design, synthesis, and evaluation of 10-N-substituted acridones as novel chemosensitizers in Plasmodium falciparum. Antimicrob. Agents Chemother. 2007;51:4133–4140. doi: 10.1128/AAC.00669-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhattacharjee AK, Kyle DE, Vennerstrom JL, Milhous WK. A 3D QSAR pharmacophore model and quantum chemical structure-activity analysis of chloroquine(CQ)-resistance reversal. J. Chem. Inf. Model. 2002;42:1212–1220. doi: 10.1021/ci0200265. [DOI] [PubMed] [Google Scholar]
- 17.Burgess SJ, Selzer A, Kelly JX, Smilkstein MJ, Riscoe MK, Peyton DH. A chloroquine-like molecule designed to reverse resistance in Plasmodium falciparum. J. Med. Chem. 2006;49:5623–5625. doi: 10.1021/jm060399n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andrews S, Burgess SJ, Skaalrud D, Kelly JX, Peyton DH. Reversal Agent and Linker Variants of Reversed Chloroquines: Activities against Plasmodium falciparum. J. Med. Chem. 2010;53:916–919. doi: 10.1021/jm900972u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abdel-Magid AF, Carson KG, Harris BD, Maryanoff CA, Shah RD. Reductive amination of aldehydes and ketones with sodium triacetoxyborohydride. Studies on direct and indirect reductive amination procedures. J. Org. Chem. 1996;61:3849–3862. doi: 10.1021/jo960057x. [DOI] [PubMed] [Google Scholar]
- 20.De D, Krogstad FM, Cogswell FB, Krogstad DJ. Aminoquinolines that circumvent resistance in Plasmodium falciparum in vitro. Am. J. Trop. Med. Hyg. 1996;55:579–583. doi: 10.4269/ajtmh.1996.55.579. [DOI] [PubMed] [Google Scholar]
- 21.Hofheinz W, Jaquet C, Jolidon S. Aminochinoline derivates useful in the treatment of malaria. 1995 EP0656353. [Google Scholar]
- 22.Ridley RG, Hofheinz W, Matile H, Jaquet C, Dorn A, Masciadri R, Jolidon S, Richter WF, Guenzi A, Girometta MA, Urwyler H, Huber W, Thaithong S, Peters W. 4-aminoquinoline analogs of chloroquine with shortened side chains retain activity against chloroquine-resistant Plasmodium falciparum. Antimicrob Agents Chemother. 1996;40:1846–1854. doi: 10.1128/aac.40.8.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin YC. A bioavailability score. J. Med. Chem. 2005;48:3164–3170. doi: 10.1021/jm0492002. [DOI] [PubMed] [Google Scholar]
- 24.Kelly JX, Winter RW, Cornea A, Peyton DH, Hinrichs DJ, Riscoe M. The kinetics of uptake and accumulation of 3,6-bis-ω-diethylamino-amyloxyxanthone by the human malaria parasite Plasmodium falciparum. Mol. Biochem. Parasit. 2002;123:47–54. doi: 10.1016/s0166-6851(02)00118-4. [DOI] [PubMed] [Google Scholar]
- 25.Sullivan DJ, Jr, Gluzman IY, Russell DG, Goldberg DE. On the molecular mechanism of chloroquine's antimalarial action. Proc. Natl. Acad. Sci. USA. 1996;93:11865–11870. doi: 10.1073/pnas.93.21.11865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacobs GH, Aikawa M, Milhous WK, Rabbege JR. An ultrastructural study of the effects of mefloquine on malaria parasites. Am. J. Trop. Med. Hyg. 1987;36:9–14. doi: 10.4269/ajtmh.1987.36.9. [DOI] [PubMed] [Google Scholar]
- 27.Jacobs GH, Oduola AM, Kyle DE, Milhous WK, Martin SK, Aikawa M. Ultrastructural study of the effects of chloroquine and verapamil on Plasmodium falciparum. Am. J. Trop. Med. Hyg. 1988;39:15–20. doi: 10.4269/ajtmh.1988.39.15. [DOI] [PubMed] [Google Scholar]
- 28.Cabrera M, Paguio MF, Xie C, Roepe PD. Reduced digestive vacuolar accumulation of chloroquine is not linked to resistance to chloroquine toxicity. Biochemistry. 2009;48:11152–11154. doi: 10.1021/bi901765v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gligorijevic B, Purdy K, Elliott DA, Cooper RA, Roepe PD. Stage independent chloroquine resistance and chloroquine toxicity revealed via spinning disk confocal microscopy. Mol Biochem Parasitol. 2008;159:7–23. doi: 10.1016/j.molbiopara.2007.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mzayek F, Deng H, Mather FJ, Wasilevich EC, Liu H, Hadi CM, Chansolme DH, Murphy HA, Melek BH, Tenaglia AN, Mushatt DM, Dreisbach AW, Lertora JJ, Krogstad DJ. Randomized dose-ranging controlled trial of AQ-13, a candidate antimalarial, and chloroquine in healthy volunteers. PLoS Clin Trials. 2007;2:e6. doi: 10.1371/journal.pctr.0020006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stepniewska K, White NJ. Pharmacokinetic determinants of the window of selection for antimalarial drug resistance. Antimicrob Agents Chemother. 2008;52:1589–1596. doi: 10.1128/AAC.00903-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chou AC, Chevli R, Fitch CD. Ferriprotoporphyrin IX fulfills the criteria for identification as the chloroquine receptor of malaria parasites. Biochemistry. 1980;19:1543–1549. doi: 10.1021/bi00549a600. [DOI] [PubMed] [Google Scholar]
- 33.Dorn A, Vippagunta SR, Matile H, Jaquet C, Vennerstrom JL, Ridley RG. An assessment of drug-haematin binding as a mechanism for inhibition of haematin polymerisation by quinoline antimalarials. Biochem. Pharmacol. 1998;55:727–736. doi: 10.1016/s0006-2952(97)00510-8. [DOI] [PubMed] [Google Scholar]
- 34.Egan TJ. Interactions of quinoline antimalarials with hematin in solution. J. Inorg. Biochem. 2006;100:916–926. doi: 10.1016/j.jinorgbio.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 35.Egan TJ, Ncokazi KK. Quinoline antimalarials decrease the rate of beta-hematin formation. J. Inorg. Biochem. 2005;99:1532–1539. doi: 10.1016/j.jinorgbio.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 36.Bonilla JA, Moura PA, Bonilla TD, Yowell CA, Fidock DA, Dame JB. Effects on growth, hemoglobin metabolism and paralogous gene expression resulting from disruption of genes encoding the digestive vacuole plasmepsins of Plasmodium falciparum. Int. J. Parasitol. 2007;37:317–327. doi: 10.1016/j.ijpara.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 37.Pandey AV, Singh N, Tekwani BL, Puri SK, Chauhan VS. Assay of β -hematin formation by malaria parasite. J. Pharm. Biomed. Anal. 1999;20:203–207. doi: 10.1016/s0731-7085(99)00021-7. [DOI] [PubMed] [Google Scholar]
- 38.Pandey AV, Joshi SK, Tekwani BL, Chauhan VS. A colorimetric assay for heme in biological samples using 96-well plates. Anal. Biochem. 1999;268:159–161. doi: 10.1006/abio.1998.2997. [DOI] [PubMed] [Google Scholar]
- 39.Leonardi A, Barlocco D, Montesano F, Cignarella G, Motta G, Testa R, Poggesi E, Seeber M, DeBenedetti PG, Fanelli F. Synthesis, screening, and molecular modeling of new potent and selective antagonists at the α1d adrenergic receptor. J. Med. Chem. 2004;47:1900–1918. doi: 10.1021/jm030944+. [DOI] [PubMed] [Google Scholar]
- 40.Smilkstein M, Sriwilaijaroen N, Kelly JX, Wilairat P, Riscoe M. Simple and inexpensive fluorescence-based technique for high-throughput antimalarial drug screening. Antimicrob. Agents Chemother. 2004;48:1803–1806. doi: 10.1128/AAC.48.5.1803-1806.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Connors KA. Binding constants : the measurement of molecular complex stability. New York: Wiley; 1987. [Google Scholar]
- 42.Trang DT, Huy NT, Uyen DT, Sasai M, Shiono T, Harada S, Kamei K. Inhibition assay of beta-hematin formation initiated by lecithin for screening new antimalarial drugs. Anal. Biochem. 2006;349:292–296. doi: 10.1016/j.ab.2005.11.022. [DOI] [PubMed] [Google Scholar]
- 43.Huy NT, Kamei K, Yamamoto T, Kondo Y, Kanaori K, Takano R, Tajima K, Hara S. Clotrimazole binds to heme and enhances heme-dependent hemolysis: proposed antimalarial mechanism of clotrimazole. J. Biol. Chem. 2002;277:4152–4158. doi: 10.1074/jbc.M107285200. [DOI] [PubMed] [Google Scholar]