Abstract
Fibromyalgia syndrome (FMS) is a prevalent and disabling chronic pain disorder. Past research suggests that obesity is a common comorbidity and may be related to the severity of FMS. The main objective of the present study was to evaluate the relationships between FMS and obesity in the multiple FMS-related domains: hyperalgesia, symptoms, physical abilities, and sleep. A total of 215 FMS patients completed a set of self-report inventories to assess FMS-related symptoms and underwent the tender point (TP) examination, physical performance testing, and 7 day home sleep assessment. Forty seven percent of our sample was obese and additional 30% was overweight. Obesity was related significantly to greater pain sensitivity to TP palpation particularly in the lower body areas, reduced physical strength and lower-body flexibility, shorter sleep duration, and greater restlessness during sleep. The results confirmed that obesity is a prevalent comorbidity of FMS that may contribute to the severity of the problem. Potential mechanisms underlying the relationship are discussed.
Perspectives
This report presents how obesity may be interrelated to fibromyalgia pain, disability, and sleep. We found that obesity is common in FMS. Approximately a half of our patients were obese and additional 30% were overweight. We also found that obesity in FMS was associated with greater pain sensitivity, poorer sleep quality, and reduced physical strength and flexibility. The results suggest that obesity may aggregate FMS and weight management may need to be incorporated into treatments.
Keywords: Fibromyalgia, Obesity, Pain Sensitivity, Sleep, Physical Ability
INTRODUCTION
Fibromyalgia syndrome (FMS) is a prevalent musculoskeletal pain disorder affecting 3–5% of the population in the US [57]. The cardinal features of FMS are widespread pain and hyperalgesia to palpation on at least 11 of the 18 specific tender points (TPs) [58]. A range of other functional problems typically co-occur with FMS such as chronic fatigue, non-restorative sleep, functional disability, and mood disturbance [2; 60]. Etiology of FMS is not known. A number of factors are thought to contribute to pain and associated disability of FMS. They include abnormal regulation of central pain modulation [1], dysregulated hypothalamic-pituitary-adrenal axis (HPA) [41], and immunological vulnerability [56].
The aforementioned dysregulations and vulnerability are not FMS specific but also found in obesity. Sensitivity to experimentally induced nociceptive stimuli is greater in obese humans [35; 44] as well as in obese rodents [48]. Pain complaints and chronic pain disorders are common in obese people [29; 43; 54]. Obese individuals typically exhibit abnormalities in the regulation of the HPA axis [7; 49]. Obesity is also known to be related to the level of pro-inflammatory indices, such as Interlukin-6 (IL-6) and C reactive protein (CRP) [9; 27].
Available evidence suggests that obesity is common in FMS. Studies report that 32%–50% of FMS patients are obese and additional 21–28% are overweight [39; 40; 61]. FMS patients show greater body mass index (BMI) relative to pain-free individuals [19]. The results from the recent internet survey with 2569 FMS patients also show the average BMI to be in the obese range [4]. Greater BMI seems to be related to greater pain/tender sensitivity as well as poorer quality of life and reduced physical functioning in FMS [39; 40; 61]. Our preliminary data [40] have suggested that BMI is linearly related to IL-6, CRP, cortisol, and epinephrine levels in FMS. However, because these results are based upon a small number of patients, they need to be considered preliminary.
Another potential link between obesity and FMS is sleep disturbance. Evidence supports the positive relationship between obesity and shorter sleep duration in the general population [8; 17; 52]. Numerous reports suggest that FMS is associated with disturbed sleep architecture [10; 14] and self-reported poor quality of sleep [13; 33; 53]. Sleep quality appears to be a significant contributor for fatigue and pain in FMS [53]. Our preliminary results suggest that obesity in FMS is related to shorter sleep durations, lower sleep efficiency, longer minutes awoken after sleep onset, and restless sleep [40].
The primary purpose of the present study was to evaluate the relationship between FMS and obesity in the multiple domains relevant to FMS, including pain, hyperalgesic response, sleep, physical abilities, and mood, with a larger sample of FMS patients than previously reported. We hypothesized that obesity significantly would add disease and disability burden to FMS patients. In this paper we reserve the noun “obesity” to describe the continuum ranging from normal to obese and use the appropriate adjective (i.e., “overweight” or “obese” patients) to refer to clinical categories within the continuum.
METHOD
The research protocol was approved by the Institutional Review Board at the University of Utah. All subjects provided written consent prior to entering the study.
Participants
A total of 215 FMS patients, who were recruited to be a part of a larger clinical study, were included in this study. The sample includes 38 subjects whose data were reported in the previous paper [40]; these people’s data were included in the present study because a greater range of factors, most of which had not been studied in the previous paper, were tested in this study. The participants were mostly females (n=205, 95%) and 45 years old on average (SD=11 years, range 21–64), with the average FMS duration of 12.77 years (SD=7.21). Mean height was 65.14 inches (SD 2.93) and mean weight was 184.00 lb. (SD=47.39), yielding the mean BMI of 30.53. General background information of the patients is shown in Table 1.
Table 1.
Background Information (n=215)
| Age | 45.30 (SD=11.04) |
| Pain Duration in Years | 12.77 (SD=10.80) |
| Sex (female) | 95% |
| Race (white) | 95% |
| Education (> HS) | 97% |
| Marital (married) | 64% |
| Employment | |
| Working full time | 30% |
| Working part time | 13% |
| Unemployed due to pain | 19% |
| Pain Onset (insidious) | 48% |
| Medications | |
| Non-opioid analgesics | 70% |
| Opioid analgesics | 35% |
| Tricyclics | 15% |
| SSRI/SNRI | 52% |
| Antieplectic | 23% |
| Muscle relaxant | 27% |
| Benzodiazepine | 23% |
| Nonbenzo sedatives | 37% |
Procedures
As a part of the larger clinical study, participants underwent a comprehensive FMS evaluation, including standardized TP examination, physical performance testing, and home sleep assessment. In addition, they were asked to complete self-report inventories.
TP Examination
A nurse practitioner (NP) under the supervision of a physician specialized in pain medicine, conducted a standardized TP examination, Manual Tender Point Survey (MTPS) [42]. In this protocol, the NP digitally palpated 18 TPs that are described in the classification criteria for FMS by in the American College of Rheumatology[58] and three additional non-TP points (“control points”) that are located in the mid forehead, left thumbnail, and dorsum right forearm. After each palpation, the NP asked the patient to rate the level of pain, on a scale of 0 (no pain) to 10 (worst pain). The MTPS specifies the precise location of the palpation sites, positioning of a patient and examiner, order of palpations, and pressure application technique (digital pressure with a thumb pad, gradually increasing by 1 kg force per second over 4 seconds).
Physical performance testing
A licensed physical therapist evaluated each patient’s flexibility, strength, and walking. Prior to the actual testing, each patient verbally rated the pain level on the 10 point numerical scale.
Flexibility
Trunk Flexion
With a patient standing in a neutral position, the examiner measured 10 cm cephalad to the superior margin of the gluteal cleft, marking the spot. Then the patient was verbally cued to bend forward as far as he/she comfortably could. The increased distance of the 10 cm marking due to the flexion was recorded.
Cervical Range of Motion (ROM)
With a patient in a neutral sitting position, Goniometric motion was assessed for neck flexion, extension, sidebending (left and right), and rotation (left and right), with the verbal cue to move into position as far as he/she comfortably could.
Straight Leg Raise
With a patient in the supine position, the examiner asked the patient to relax while the examiner passively lifted each leg into straight leg hip flexion, with the verbal cue to allow the lift to be as far as he/she could manage comfortably.
Strength
There were three strength tests.
Standing push-ups
A patient stood facing a 110 cm high counter. The distance between the base of the counter and the patient’s feet was measured. The standard distance is 60 cm but this needed to be adjusted for each subject’s height and comfort. The majority (90%) of the patients stood 50–70 cm away from the cabinet, whereas 3% stood at <50 cm and 7% at >70 cm. Then he/she was asked to perform as many as push-ups he/she was able to do comfortably with the hands placed on the counter, shoulder width apart. The number of pushups was counted. The maximum was set at 30.
Phantom Chair
A patient was asked to stand against the wall with head, shoulder blades, and buttocks touching the wall. The patient’s toes were placed shoulder width apart and 70 cm away from the wall. The patient was visually and verbally cued to slide down wall until knees were bent either 60 or 90 degrees (as measured by the examiner). For the majority of the patients, 60 (81%) or 90 (10%) degrees were used, but three patients were able to do only at 30–46 degrees. The patient was asked to hold the position as long as he/she could comfortably. The amount of time in seconds that they were able to support themselves in this position was measured.
Pelvic Bridging
A patient was placed in the hooklying position (ie, lying supine on an examination table with feet on the surface and knees flexed), arms at sides. The patient was visually and verbally cued to lift buttocks off the surface until there was a “straight line from knees through hips to shoulders”. With no pause at the terminal motion, buttocks were returned to the surface. The patient was asked to perform this as many repetitions as he/she could comfortably, up to a maximum of 30.
Walking Test
A patient was asked to walk on a treadmill at his/her preferred speed, as long as he/she was able, up to 20 minutes. Speed and time on the treadmill were recorded and distance walked was then calculated. The patient’s heart rate was continuously monitored and the maximum heart rate achieved was also recorded.
Self-Report Inventories
In order to assess FMS-related symptoms and mood, each patient was asked to complete the Fibromyalgia Impact Questionnaire (FIQ) [11] and the Center for the Epidemiological Study of Depression Scale (CESD) [46]. The FIQ has 10 disability questions that patients rate on a Likert-type scale as well as a series of visual analog scales to assess pain, fatigue, not feeling refreshed in the morning, stiffness, anxiety, and depression. The psychometric values of the FIQ have been extensively reviewed [3]. The CESD is a commonly used measure of depression, consisting of 20 items. Respondents are asked to indicate how frequently they experienced each symptom in the past week, ranging from 0 (less than one day) to 3 (5–7 days). The total possible score ranging from 0 to 60, reflecting both the number of symptoms and the frequency of their occurrence. The internal consistency of the CES-D has been reported to be .84–.90 [46].
Sleep Assessment
Following the in-clinic evaluation, each patient was asked to continuously wear a Mcro Mini Motionlogger Actigraph (Ambulatory Monitoring, Ardsley, NY, USA), a wristwatch like device that measures three-dimensional movement, for 7 days. Sleep is defined if the probability of sleep exceeds the criterion in the epoch, based upon the algorithm defined in Sadeh et al.[50]. The software Action series (Ambulatory Monitoring Inc.) calculates common sleep parameters (total sleep time, sleep efficiency, sleep onset latency, # nocturnal awaking, and time awake after sleep onset), using the algorithm developed by Cole et al. [16]. Actigraph data has shown high agreement with polysomnographic data (91–93%) [50] as well as minute-by-minute agreement with EEG [16].
Statistical Analyses
Because there was a clear ordering among the categories of the obesity dimension (normal [X1] < overweight [X2] < obese [X3]), we conducted all group comparisons with the Jonckheere-Terpstra test[23; 25], which evaluates the null hypothesis that the cumulative distributions are equal in the population (F(X1) = F(X2) = F(X3)) versus the alternative hypothesis that either F(X1) < F(X2) < F(X3) or F(X1) > F(X2) > F(X3), two-sided. Thus the test detects the differences among groups that have an orderly relationship (in our case, weight ranges). When the groups represent ordered levels of a factor, the Jonckheere-Terpstra test has more statistical power than the general analysis of variance [36]. P-values for the Jonckheere-Terpstra tests were calculated using consensus estimates from StatXact 7, SPSS 17, and SAS 9.1 as Monte Carlo approximations to the exact permutational distributions with sufficient simulations (usually 250,000) to yield 99% confidence intervals of p +/− .001. Hypothesis tests yield the probability of the evidence under the null hypothesis of exact equality, but provide no information regarding the pragmatic magnitude of the effect in question. A natural index of effect size for these comparisons is provided by Spearman’s rho correlation between obesity levels and the dependent variables. In our sample, p-values of .05, .01, .001, and .0001 correspond approximately to Spearman’s rho coefficients of .14, .18, .23, and .27, respectively.
To ascertain the comparability of the three groups at baseline on background and demographic variables, we conducted exact chi-square tests of the equality of the three distributions.
RESULTS
OBESITY
Of 215 patients, four patients had their BMI ranges in the below normal range (M=17.07, SD=.58). Forty seven patients were in the normal range (M=22.46, SD=1.59). Given the small numbers in the underweight group, we combined these two groups to form Normal Weight group (n=51, BMI M=22.04, SD=2.11). Sixty four patients were categorized as Overweight (BMI M=27.71, SD=1.39) and one hundred patients were in the obese range (BMI M=36.67, SD=5.36). In our sample of FMS patients, 47% were in the obese range with an additional 30% falling into the overweight range.
The three groups were comparable in most of the background variables, except for marital status (χ2(6)=16.35, p<.01) and the use of non-benzodiazepine sedatives (χ2(2)=13.87, p<.001), as shown in Table 2. Greater proportions of single patients appeared to be in the normal weight range whereas more overweight patients were separated or divorced. For the use of sedatives, much larger proportions of the normal weight patients (57%) reported to use of the sedatives compared to the obese patients (26%).
Table 2.
Marital Status and Use of non-benzodiazepine sedatives by BMI Groups
| Normal | Overweight | Obese | |
|---|---|---|---|
| MARITAL | |||
| Never Married | 22% | 2% | 22% |
| Married | 57% | 63% | 68% |
| Separated/Divorced | 22% | 33% | 20% |
| Widowed | 0% | 3% | 1% |
| Using Non-Benzodiazepine Sedatives | |||
| Yes | 57% | 38% | 26% |
Table 3 lists subjects’ vital signs at the time of initial evaluation. The two-sided Jonckheere-Terpstra tests showed that all variables differed significantly with respect to obesity: systolic blood pressure (p<.001), diastolic blood pressure (p<.001), heart rate (p<.001), and oxygen saturation rate (p<.001).
Table 3.
Vital Signs at Baseline by BMI Groups
| Normal | Overweight | Obese | |
|---|---|---|---|
| Systolic Blood Pressure (mmHG)** | 117.02 (14.87) | 121.20 (13.25) | 127.02 (13.92) |
| Diastolic Blood Pressure (mmHG)** | 68.22 (12.64) | 71.53 (9.96) | 74.76 (10.45) |
| Heart Rate** | 81.14 (11.13) | 80.40 (11.27) | 87.62 (13.95) |
| Oxygen Saturation (%)** | 96.88 (3.63) | 96.05 (2.05) | 95.74 (1.80) |
p<.001
TENDER POINTS
The distributions of positive tender points were quite skewed (see Figure 1), with the majority (78%) of patients having all 18 points positive. The exact Jonckheere-Terpstra test is still valid under these conditions, and revealed a significant overall effect for Obesity (p=.048, two-tailed).
Figure 1.
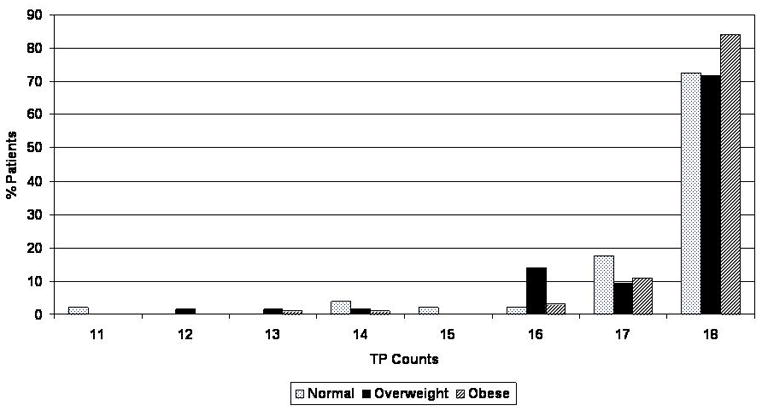
Distribution of positive tender point counts
The groups did not differ in the pain severity ratings of the three control points, but the groups were significantly different in the pain ratings for TPs (p<.001). Table 4 lists the mean TP pain scores for each group over the total 18 TPs as well as nine biolateral TP sites. The univariate analyses revealed that the groups were significantly different in the Gluteal(p<.001), 2nd Rib (p=.046), Lateral Epicondyle (p=.006), Greater Trochanter (p<.001), and Knee (p<.001).
Table 4.
TP Pain Ratings by BMI Groups
| Normal | Overweight | Obese | |
|---|---|---|---|
| All 18 TPs** | 5.02 (1.71) | 4.98 (1.66) | 5.92 (1.74) |
| Bilateral Sites | |||
| Occiput | 5.45 (2.18) | 4.70 (1.98) | 5.71 (2.09) |
| Trapezius | 5.57 (2.15) | 5.34 (2.38) | 5.95 (1.99) |
| Supraspinatus | 5.31 (2.47) | 4.31 (2.48) | 5.27 (2.26) |
| Gluteal ** | 4.65 (2.27) | 5.30 (2.54) | 6.11 (2.57) |
| Low Cervical | 5.38 (2.30) | 5.42 (2.07) | 5.63 (2.38) |
| 2nd Rib* | 4.78 (2.33) | 4.70 (2.50) | 5.11 (2.38) |
| Lateral Epicondyle* | 4.06 (2.62) | 4.09 (2.27) | 5.06 (2.42) |
| Greater Trochanter** | 5.71 (2.36) | 6.27 (2.47) | 7.41 (2.25) |
| Knee** | 4.09(2.24) | 4.85 (2.50) | 6.65 (2.34) |
| Upper Body TPs | 5.09 (1.86) | 4.76 (1.80) | 5.53 (1.71) |
| Lower Body TPs** | 4.81 (1.81) | 5.47 (2.11) | 6.72 (2.02) |
p<.05
p<.001
The mean pain values in each of the bilateral sites seem to suggest that the group differences are more pronounced in the lower body sites. Thus, we grouped Gluteal, Greater Trochanter, and Knee sites as the lower body TPs and the rest as the upper body TPs. The mean pain ratings for the upper and lower body TPs are listed at the bottom of Table 4. The lower body TPs, but not the upper body TPs, varied significantly with Obesity (p<.001 versus p=.078).
PHYSICAL PERFORMANCE TESTING
Table 5 lists the mean values from the initial physical performance testing. The flexibility data shows that the groups were comparable in their cervical and lumbar flexibility; however, they were significantly different in the hamstring flexibility (straight leg raise), (p=.014).
Table 5.
Mean physical ability indices by BMI groups
| Normal | Overweight | Obese | |
|---|---|---|---|
| Flexibility | |||
| Cervical Range of Motion | |||
| Side Bend (degrees) | 32.16 (8.97) | 28.80 (9.11) | 31.70 (13.82) |
| Rotate (degrees) | 61.93 (16.13) | 59.13 (15.09) | 59.20 (14.82) |
| Cervical Flexion | 58.36 (25.57) | 55.32 (23.68) | 59.49 (24.26) |
| Cervical Extension | 62.66 (23.80) | 54.72 (25.17) | 60.78 (24.68) |
| Lumber Flexion (cm) | 10.80 (2.42) | 10.08 (2.45) | 11.15 (2.61) |
| Straight Leg Raise (degree)* | 69.40 (18.45) | 70.71 (21.78) | 63.20 (18.35) |
| Strength | |||
| Standing Push-ups (reps, max 30)* | 15.06 (10.12) | 15.32 (10.62) | 11.63 (8.51) |
| Phantom chair (sec)* | 27.06 (22.41) | 25.56 (26.81) | 18.34 (13.39) |
| Bridging* | 16.33 (9.36) | 17.02 (11.07) | 13.79 (9.70) |
| Endurance | |||
| Treadmill Speed (mph) | 2.17 (.70) | 2.22 (.57) | 2.05 (.52) |
| Min walked | 13.06 (6.92) | 13.94 (5.75) | 12.09 (6.56) |
| Walking Distance (miles) | .53 (.36) | .53 (.28) | .44 (.29) |
| Max HR during walking** | 105.46 (18.36) | 107.38 (13.70) | 117.21 (18.94) |
| % patients walked 20 min | 41% | 35% | 32% |
p<.05
p<.001
The strength data also showed consistent Obesity differences. The groups were significantly different in the number of push-ups (p=.018), duration of phantom chair in seconds (p=.013), and the number of bridging reps (p=.020). The distributions of the phantom chair angles are comparable across groups (Normal Weight group: 74% at 60° and 25% at 90°, Overweight group: 1% at 45°, 83% at 60°, and 15% at 90 °, Obesity group: 1% at 30°, 1% at 45°, 84% at 60° and 14% at 90°). The majority of the patients did not reach the maximal repetitions of 30 set for the push-up and bridging tests (7% and 5%, respectively). However, the obese patients appeared to be less likely to reach the maximal repetitions. Two percent of the obese patients relative to 12% of normal weight and 11% of overweight patients completed 30 push-ups (χ2(2)=7.15, p<.03). For the bridging, 2% of the obese patients relative to 8% of the normal weight and 6% of overweight patients reached 30; however, this difference did not reach the statistical significance ((χ2(2)=3.15).
The results of the walking tests showed slightly declined speed, duration, and distance in the obese patients but the differences failed to reach the statistical significance. Obesity was associated with significantly higher heart rate during the test (p<.001). However, as shown in Table 3, the groups were also different in their baseline heart rates. Obesity was also associated with significantly greater changes from baseline heart rate levels (p=.032). The mean changes in their heart rates were 24.12 (SD=16.41) for the normal weight patients, 26.60 (SD=13.80) for the overweight patients, and 29.54 (SD=14.31) for the obese patients.
SLEEP AND SELF-REPORT FMS SYMPTOMS
Sleep data from the actigraph are listed in Table 6. All sleep variables except latency were significantly associated with Obesity (Duration, p<.001; Efficiency, p=.004; Minutes Woken after Sleep Onset, p=.033; and Sleep Activity, p=.007
Table 6.
Sleep Parameters by BMI Group
| Normal | Overweight | Obese | |
|---|---|---|---|
| Sleep Duration (min)** | 414.05 (65.64) | 387.37 (74.30) | 373.02 (77.39) |
| Sleep Efficiency (%)* | 93.69 (5.37) | 92.25 (7.22) | 90.76 (9.86) |
| Sleep Latency (min) | 10.37 (5.16) | 13.00 (15.95) | 13.46 (12.28) |
| Waking after Sleep Onset (min)* | 31.28 (27.61) | 35.36 (27.69) | 37.99 (26.65) |
| Activity Index during Sleep* | 54.71 (13.10) | 56.66 (15.41) | 61.29 (14.10) |
p<.05
p<.001
The mean scale scores of the FIQ, CESD, and numerical rating scale (NRS) of pain score for each BMI group are shown in Table 7. No self-report symptom variable was significantly associated with Obesity.
Table 7.
Self-Report FMS symptoms by BMI groups
| Normal | Overweight | Obese | |
|---|---|---|---|
| Pain Drawing | 24.26 (9.34) | 25.52 (9.19) | 25.52 (8.89) |
| FIQ | |||
| Disability | 1.46 (.63) | 1.69 (1.37) | 1.59 (.62) |
| Pain VAS | 70.71 (17.38) | 68.50 (15.84) | 68.80 (15.48) |
| Fatigue VAS | 76.46 (24.56) | 80.03 (16.79) | 80.86 (17.51) |
| Not Refreshed AM VAS | 76.46 (19.94) | 79.79 (15.23) | 77.73 (20.25) |
| Stiffness VAS | 65.46 (25.74) | 73.40 (18.73) | 74.88 (17.70) |
| Anxiety VAS | 57.61 (25.05) | 58.81 (25.05) | 57.93 (25.27) |
| Depression VAS | 48.31 (28.42) | 47.80 (26.39) | 51.65 (26.24) |
| CESD | 24.96 (11.03) | 24.81 (9.51) | 26.08 (11.88) |
| Pain NRS | 4.39 (2.01) | 4.82 (1.04) | 4.72 (1.77) |
Discussion
The present study confirmed the results from our earlier study [40] that increased BMI is common in FMS. Approximately the half of our sample was in the obese range, with an additional 30% in the overweight range. According to the recent Behavioral Risk Factor Surveillance Survey, the state of Utah has an obesity rate of 23.1% and overweight rate of 35.1% [37]. Our sample had over twice the rate of the obesity in the state. National rates are estimated at 32.2% for obesity and 34.1% for overweight [22].
In accordance with the previous findings [39], obesity was related to increased TP pain sensitivity. The results are also consistent with the findings that obesity is a risk factor for chronic pain [6; 29; 30; 43], is associated with increased severity in visceral pain [5; 15], and is generally related to high prevalence of pain complaints [54]. The mechanisms underlying the obesity-pain sensitivity link are not clear at this time. Poor physical conditioning has been considered as one of the potential contributors of pain sensitivity; conversely, aerobic fitness programs reduce TP pain sensitivity in FMS patients [32; 34]. In out study, obesity was associated with reduced strength and flexibility but there was no group difference in the walking test. As the recent systematic review [12] indicates, accumulated evidence suggests the benefit of an aerobic program with some suggestive benefit of strengthening. Muscle strengthening of the neck has resulted in reduced pain thresholds of the area in chronic neck pain patients [59]. Although these findings are suggestive, whether exercise capacity mediates the obesity-TP pain sensitivity link warrants further investigations.
Another possible mechanism may be related to the endogenous opioid system. Animal studies suggest that obesity may affect sensitivity to noxious stimuli via obesity-related alteration in the endocrine and opioid systems [28; 48]. In our previous study [40], we have reported that obesity in FMS is related to the greater levels of proinflammatory indices. Results from animal research strongly suggests the involvement of proinflammatory cytokines in central sensitization [26] and the development of chronic latent hyperalgesia in muscles [18]. Whether and how these endogenous changes associated with obesity play a role in heightened pain sensitivity in obese FMS patients may be of interest in future research.
Interestingly, however, the heightened pain sensitivity to TPs in obese patients appeared to be more pronounced in the lower body areas. In addition to centrally modulated pain sensitivity, the pain sensitivity of obese patients may also be influenced by the mechanical loads of having to carry extra weight. While it is well known that obesity is a risk factor for pain disorders involving bones and joints [21; 31], recent evidence also suggests that obesity may also be a significant contributor for soft-tissue related pain [47; 55]. Furthermore, a study of people with spinal cord injury suggests that obesity may contribute to increased tissue loading, leading to deep tissue injury [20], Thus, it is likely that multiple factors appear to be involved in the relationship between obesity and pain sensitivity.
Our results suggest that the degree of hyperalgesia can vary across body sites in FMS, and the assessment of pain sensitivity should take different body sites into consideration. Some pain testing methods, such as the TP examination used in this study and thermal pain sensitivity, may be applied to various body sites, whereas others, such as cold presser and ischemic pain tests are not very practical. Using the multiple methods to determine the levels of pain sensitivity is likely desirable. There is one methodological concern related to this result. The TP examination protocol in our study was standardized so that the order of palpation was same for all patients, raising a question of the order effect. The differences were most pronounced in the lower body sites including gluteal, greater trochanter and knee. Without the random order palpation we cannot totally rule out the possibility of order effect. However, given that the patients in the normal weight range did not show the differential pain sensitivity to upper vs lower body TPs, and that there were palpations of upper body sites after the gluteal sites, the greater difference in pain sensitivity for those lower body sites are unlikely due to the order of the palpations.
As discussed earlier, our results indicate that obesity influences the physical fitness ability to some extent in FMS. Given this, failure to find the group differences in the walking ability was surprising; however, this may have been influenced by the fact that all patients were very sedentary and there was a 20 minute cap and self-determined pace in the walking test. The maximum heart rate during the walking test was significantly higher in the obese patients than normal weight patients. Although this seems to have been accounted for by the baseline heart rate levels, which were significantly elevated in the obese patients, we have shown significance with respect to change as well. The increases in heart rate during the walking test were significantly greater for the obese patients, potentially making the test more laboring for these patients. If so, this may have significant implications for rehabilitation of obese FMS patients. Inclusion of perceived exertion may be helpful in future research to clarify this point further.
The obese patients also showed reduced flexibility in the lower body areas (ie, straight leg raise), as well as reduced strengths in general. Anecdotally, the physical performance testing examiner (LLB) observed that many of the obese patients appeared to put less effort to due to increased pain. This observation is consistent with the report that obese women tend to stop their exercise testing due to musculoskeletal pain [24]. The results suggest that the heightened pain sensitivity in the lower body areas of the obese FMS patients may bring up an additional barrier for activating therapy.
The results also replicated our earlier findings that obesity in FMS is related to the poorer quality of sleep. Obese patients exhibited reduced sleep duration and increased activities during sleep. However, our results failed to show group difference in self-report symptoms of FMS except for stiffness. It is interesting to note that the factors that were associated with obesity were obtained based upon observed or provoked testing process (ie, TP exam, physical performance, actigraphic sleep data), whereas most of self-reported symptoms show little relationship to obesity. These results seem consistent with previous findings by others [39; 61]. Yunus et al [61] also found no difference in VAS measured pain, fatigue, and global severity of FMS between normal weight FMS patients and FMS patients with BMI greater than 25. FMS is a multifactorial condition and the results suggest that obesity does not necessarily affect all aspects of FMS. At this time, it seems reasonable to conclude that the adverse impact of obesity in FMS mostly affects hyperalgesia, disability, quality of life and as we replicated in this study with larger sample, sleep.
However, a recent study evaluating a behavioral weight loss program for FMS suggests that weight loss improves FMS symptoms [51]. Given the absence of significant association between FMS symptoms and obesity, we must speculate that the relationship between weight loss and symptom reduction is not direct but is mediated by other factors. One possibility is changes in lifestyle. Behavioral weight loss programs typically involve some significant changes in eating habits, physical activities and coping. Shifting towards a healthy lifestyle may positively influence how patients manage their symptoms, how they perceive their plight, and overall quality of life.
There are some limitations of the study we must discuss. First, the study design was cross-sectional and thus the results are all correlational. No causality can be ascertained. Second, the definition of obesity we used in this study was solely based upon BMI. BMI is the vital measure for classifying obesity today and perhaps because of the simplicity, it is most used as a proxy measure of body composition. Although BMI is generally correlated with total body fat [38], BMI fails to take various individual factors into consideration, such as muscle mass, tallness, age, and ethnic factors [45]. The use of the direct measures of body fat, such as X-ray absorptiometry or densitometry clearly should be ideal but may not be feasible. A large, worldwide INTERHEART study suggests that waist-to-hip ratio may provide the best anthropometric measure to estimate abdominal fat that predicts cardiovascular morbidity [62]. The waist-to-hip ratio is easily measured and future studies should benefit from including this measure as a surrogate measure of abdominal fat.
In summary, obesity is a common co-morbidity that may complicate the clinical picture of FMS. Obesity in FMS adversely affects both the quality and quantity of sleep, physical strength and flexibility, and pain sensitivity to pressure particularly in the lower body. Future research needs to clarify the mechanisms of how the obesity and its associated specific and relevant factors influence FMS as well as how successful weight management changes the expression of FMS.
Acknowledgments
The authors would like to thank Reiko Mitsunaga RN, Diana Mayer, Sarah Featherstone, and Mark Herrera JD, for their technical and administrative assistance for the project. The preparation of this manuscript was supported by the NIAMS grant (R01AR4888) to the first author. There are no conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Arendt-Nielsen L, Graven-Nielsen T. Central sensitization in fibromyalgia and other musculoskeletal disorders. Curr Pain Headache Rep. 2003;7(5):355–361. doi: 10.1007/s11916-003-0034-0. [DOI] [PubMed] [Google Scholar]
- 2.Baumstark K, Buckelew S. Fibromyalgia: clinical signs; research findings; treatment implications; and future directions. Ann Beh Med. 1992;14:282–291. [Google Scholar]
- 3.Bennett R. The Fibromyalgia Impact Questionnaire (FIQ): a review of its development, current version, operating characteristics and uses. Clin Exp Rheumatol. 2005;23(5 Suppl 39):S154–162. [PubMed] [Google Scholar]
- 4.Bennett RM, Jones J, Turk DC, Russell IJ, Matallana L. An internet survey of 2,596 people with fibromyalgia. BMC Musculoskelet Disord. 2007;8:27. doi: 10.1186/1471-2474-8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernstein MS, Costanza MC, Morabia A. Association of physical activity intensity levels with overweight and obesity in a population-based sample of adults. Prev Med. 2004;38(1):94–104. doi: 10.1016/j.ypmed.2003.09.032. [DOI] [PubMed] [Google Scholar]
- 6.Bigal ME, Liberman JN, Lipton RB. Obesity and migraine: a population study. Neurology. 2006;66(4):545–550. doi: 10.1212/01.wnl.0000197218.05284.82. [DOI] [PubMed] [Google Scholar]
- 7.Bjorntorp P, Holm G, Rosmond R. Hypothalamic arousal, insulin resistance and Type 2 diabetes mellitus. Diabet Med. 1999;16(5):373–383. doi: 10.1046/j.1464-5491.1999.00067.x. [DOI] [PubMed] [Google Scholar]
- 8.Bjorvatn B, Sagen IM, Oyane N, Waage S, Fetveit A, Pallesen S, Ursin R. The association between sleep duration, body mass index and metabolic measures in the Hordaland Health Study. J Sleep Res. 2007;16(1):66–76. doi: 10.1111/j.1365-2869.2007.00569.x. [DOI] [PubMed] [Google Scholar]
- 9.Bluher M, Fasshauer M, Tonjes A, Kratzsch J, Schon MR, Paschke R. Association of interleukin-6, C-reactive protein, interleukin-10 and adiponectin plasma concentrations with measures of obesity, insulin sensitivity and glucose metabolism. Exp Clin Endocrinol Diabetes. 2005;113(9):534–537. doi: 10.1055/s-2005-872851. [DOI] [PubMed] [Google Scholar]
- 10.Branco J, Atalaia A, Paiva T. Sleep cycles and alpha-delta sleep in fibromyalgia syndrome. J Rheumatol. 1994;21(6):1113–1117. [PubMed] [Google Scholar]
- 11.Burckhardt CS, Clark SR, Bennett RM. The fibromyalgia impact questionnaire: development and validation. J Rheumatol. 1991;18(5):728–733. [PubMed] [Google Scholar]
- 12.Busch AJ, Barber KA, Overend TJ, Peloso PM, Schachter CL. Exercise for treating fibromyalgia syndrome. Cochrane Database Syst Rev. 2007;(4):CD003786. doi: 10.1002/14651858.CD003786.pub2. [DOI] [PubMed] [Google Scholar]
- 13.Cappelleri JC, Bushmakin AG, McDermott AM, Dukes E, Sadosky A, Petrie CD, Martin S. Measurement properties of the Medical Outcomes Study Sleep Scale in patients with fibromyalgia. Sleep Med. 2009;10(7):766–770. doi: 10.1016/j.sleep.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Carette S, Oakson G, Guimont C, Steriade M. Sleep electroencephalography and the clinical response to amitriptyline in patients with fibromyalgia. Arthritis Rheum. 1995;38(9):1211–1217. doi: 10.1002/art.1780380906. [DOI] [PubMed] [Google Scholar]
- 15.Clements RH, Gonzalez QH, Foster A, Richards WO, McDowell J, Bondora A, Laws HL. Gastrointestinal symptoms are more intense in morbidly obese patients and are improved with laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2003;13(4):610–614. doi: 10.1381/096089203322190835. [DOI] [PubMed] [Google Scholar]
- 16.Cole RJ, Kripke DF, Gruen W, Mullaney DJ, Gillin JC. Automatic sleep/wake identification from wrist activity. Sleep. 1992;15(5):461–469. doi: 10.1093/sleep/15.5.461. [DOI] [PubMed] [Google Scholar]
- 17.Di Milia L, Mummery K. The association between job related factors, short sleep and obesity. Ind Health. 2009;47(4):363–368. doi: 10.2486/indhealth.47.363. [DOI] [PubMed] [Google Scholar]
- 18.Dina OA, Green PG, Levine JD. Role of interleukin-6 in chronic muscle hyperalgesic priming. Neuroscience. 2008;152(2):521–525. doi: 10.1016/j.neuroscience.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elert J, Kendall SA, Larsson B, Mansson B, Gerdle B. Chronic pain and difficulty in relaxing postural muscles in patients with fibromyalgia and chronic whiplash associated disorders. J Rheumatol. 2001;28(6):1361–1368. [PubMed] [Google Scholar]
- 20.Elsner JJ, Gefen A. Is obesity a risk factor for deep tissue injury in patients with spinal cord injury? J Biomech. 2008;41(16):3322–3331. doi: 10.1016/j.jbiomech.2008.09.036. [DOI] [PubMed] [Google Scholar]
- 21.Gelber AC, Hochberg MC, Mead LA, Wang NY, Wigley FM, Klag MJ. Body mass index in young men and the risk of subsequent knee and hip osteoarthritis. Am J Med. 1999;107(6):542–548. doi: 10.1016/s0002-9343(99)00292-2. [DOI] [PubMed] [Google Scholar]
- 22.Healton CG, Vallone D, McCausland KL, Xiao H, Green MP. Smoking, obesity, and their co-occurrence in the United States: cross sectional analysis. Bmj. 2006;333(7557):25–26. doi: 10.1136/bmj.38840.608704.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hollander BM, Wolfe D. Nonparametric statistical methods. New York, NY: John Wiley & Sons; 1999. [Google Scholar]
- 24.Hulens M, Vansant G, Lysens R, Claessens AL, Muls E. Exercise capacity in lean versus obese women. Scand J Med Sci Sports. 2001;11(5):305–309. doi: 10.1034/j.1600-0838.2001.110509.x. [DOI] [PubMed] [Google Scholar]
- 25.Jonckheere AR. A distribution-free. k-sample test against. ordered alternatives. Biometrika. 1954;41:133–145. [Google Scholar]
- 26.Kawasaki Y, Zhang L, Cheng JK, Ji RR. Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. J Neurosci. 2008;28(20):5189–5194. doi: 10.1523/JNEUROSCI.3338-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khaodhiar L, Ling PR, Blackburn GL, Bistrian BR. Serum levels of interleukin-6 and C-reactive protein correlate with body mass index across the broad range of obesity. JPEN J Parenter Enteral Nutr. 2004;28(6):410–415. doi: 10.1177/0148607104028006410. [DOI] [PubMed] [Google Scholar]
- 28.Kutlu S, Canpolat S, Sandal S, Ozcan M, Sarsilmaz M, Kelestimur H. Effects of central and peripheral administration of leptin on pain threshold in rats and mice. Neuro Endocrinol Lett. 2003;24(3–4):193–196. [PubMed] [Google Scholar]
- 29.Lake JK, Power C, Cole TJ. Back pain and obesity in the 1958 British birth cohort. cause or effect? J Clin Epidemiol. 2000;53(3):245–250. doi: 10.1016/s0895-4356(99)00155-9. [DOI] [PubMed] [Google Scholar]
- 30.Liira JP, Shannon HS, Chambers LW, Haines TA. Long-term back problems and physical work exposures in the 1990 Ontario Health Survey. Am J Public Health. 1996;86(3):382–387. doi: 10.2105/ajph.86.3.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manek NJ, Hart D, Spector TD, MacGregor AJ. The association of body mass index and osteoarthritis of the knee joint: an examination of genetic and environmental influences. Arthritis Rheum. 2003;48(4):1024–1029. doi: 10.1002/art.10884. [DOI] [PubMed] [Google Scholar]
- 32.Martin L, Nutting A, MacIntosh BR, Edworthy SM, Butterwick D, Cook J. An exercise program in the treatment of fibromyalgia. J Rheumatol. 1996;23(6):1050–1053. [PubMed] [Google Scholar]
- 33.Martin S, Chandran A, Zografos L, Zlateva G. Evaluation of the impact of fibromyalgia on patients’ sleep and the content validity of two sleep scales. Health Qual Life Outcomes. 2009;7:64. doi: 10.1186/1477-7525-7-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCain GA, Bell DA, Mai FM, Halliday PD. A controlled study of the effects of a supervised cardiovascular fitness training program on the manifestations of primary fibromyalgia. Arthritis Rheum. 1988;31(9):1135–1141. doi: 10.1002/art.1780310908. [DOI] [PubMed] [Google Scholar]
- 35.McKendall MJ, Haier RJ. Pain sensitivity and obesity. Psychiatry Res. 1983;8(2):119–125. doi: 10.1016/0165-1781(83)90099-9. [DOI] [PubMed] [Google Scholar]
- 36.Mehta C, Patel N. In: Exact permutational inference for categorical and nonparametric data. Holyle R, editor. Book Title|, Vol. Volume|. City|: Publisher|, Year|. p.^pp. Pages|. [Google Scholar]
- 37.National Center for Chronic Disease Prevention & Health Promotion. Prevalence and Trends Data: Utah - 2008: Overweight and Obesity (BMI) [Google Scholar]
- 38.National Institute of Health. Clinical guidelines on the identificaiton, evaluation, and treatment of overweight and obesity in adults. [PubMed] [Google Scholar]
- 39.Neumann L, Lerner E, Glazer Y, Bolotin A, Shefer A, Buskila D. A cross-sectional study of the relationship between body mass index and clinical characteristics, tenderness measures, quality of life, and physical functioning in fibromyalgia patients. Clin Rheumatol. 2008;27(12):1543–1547. doi: 10.1007/s10067-008-0966-1. [DOI] [PubMed] [Google Scholar]
- 40.Okifuji A, Bradshaw DH, Olson C. Evaluating obesity in fibromyalgia: neuroendocrine biomarkers, symptoms, and functions. Clin Rheumatol. 2009;28(4):475–478. doi: 10.1007/s10067-009-1094-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Okifuji A, Turk DC. Stress and psychophysiological dysregulation in patients with fibromyalgia syndrome. Appl Psychophysiol Biofeedback. 2002;27(2):129–141. doi: 10.1023/a:1016243710507. [DOI] [PubMed] [Google Scholar]
- 42.Okifuji A, Turk DC, Sinclair JD, Starz TW, Marcus DA. A standardized manual tender point survey. I. Development and determination of a threshold point for the identification of positive tender points in fibromyalgia syndrome. J Rheumatol. 1997;24(2):377–383. [PubMed] [Google Scholar]
- 43.Peres MF, Lerario DD, Garrido AB, Zukerman E. Primary headaches in obese patients. Arq Neuropsiquiatr. 2005;63(4):931–933. doi: 10.1590/s0004-282x2005000600005. [DOI] [PubMed] [Google Scholar]
- 44.Pradalier A, Willer JC, Boureau F, Dry J. Relationship between pain and obesity: an electrophysiological study. Physiol Behav. 1981;27(6):961–964. doi: 10.1016/0031-9384(81)90354-1. [DOI] [PubMed] [Google Scholar]
- 45.Prentice AM, Jebb SA. Beyond body mass index. Obes Rev. 2001;2(3):141–147. doi: 10.1046/j.1467-789x.2001.00031.x. [DOI] [PubMed] [Google Scholar]
- 46.Radloff L. A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–392. [Google Scholar]
- 47.Rano JA, Fallat LM, Savoy-Moore RT. Correlation of heel pain with body mass index and other characteristics of heel pain. J Foot Ankle Surg. 2001;40(6):351–356. doi: 10.1016/s1067-2516(01)80002-8. [DOI] [PubMed] [Google Scholar]
- 48.Roane DS, Porter JR. Nociception and opioid-induced analgesia in lean (Fa/-) and obese (fa/fa) Zucker rats. Physiol Behav. 1986;38(2):215–218. doi: 10.1016/0031-9384(86)90156-3. [DOI] [PubMed] [Google Scholar]
- 49.Rosmond R, Dallman MF, Bjorntorp P. Stress-related cortisol secretion in men: relationships with abdominal obesity and endocrine, metabolic and hemodynamic abnormalities. J Clin Endocrinol Metab. 1998;83(6):1853–1859. doi: 10.1210/jcem.83.6.4843. [DOI] [PubMed] [Google Scholar]
- 50.Sadeh A, Aster J, Urbach D, Lavie P. Actigraphcally based automatic bedtime sleep-wake scoring: validity and clinical application. J Ambulat Monit. 1989;2:209–216. [Google Scholar]
- 51.Shapiro JR, Anderson DA, Danoff-Burg S. A pilot study of the effects of behavioral weight loss treatment on fibromyalgia symptoms. J Psychosom Res. 2005;59(5):275–282. doi: 10.1016/j.jpsychores.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 52.Stamatakis KA, Brownson RC. Sleep duration and obesity-related risk factors in the rural Midwest. Prev Med. 2008;46(5):439–444. doi: 10.1016/j.ypmed.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Theadom A, Cropley M, Humphrey KL. Exploring the role of sleep and coping in quality of life in fibromyalgia. J Psychosom Res. 2007;62(2):145–151. doi: 10.1016/j.jpsychores.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 54.Wachholtz A, Binks M, Suzuki A, Eisenson H. Sleep disturbance and pain in an obese residential treatment-seeking population. Clin J Pain. 2009;25(7):584–589. doi: 10.1097/AJP.0b013e3181a0ff17. [DOI] [PubMed] [Google Scholar]
- 55.Walker-Bone KE, Palmer KT, Reading I, Cooper C. Soft-tissue rheumatic disorders of the neck and upper limb: prevalence and risk factors. Semin Arthritis Rheum. 2003;33(3):185–203. doi: 10.1016/s0049-0172(03)00128-8. [DOI] [PubMed] [Google Scholar]
- 56.Wallace DJ. Is there a role for cytokine based therapies in fibromyalgia. Curr Pharm Des. 2006;12(1):17–22. [PubMed] [Google Scholar]
- 57.White KP, Speechley M, Harth M, Ostbye T. The London Fibromyalgia Epidemiology Study: the prevalence of fibromyalgia syndrome in London, Ontario. J Rheumatol. 1999;26(7):1570–1576. [PubMed] [Google Scholar]
- 58.Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, Tugwell P, Campbell SM, Abeles M, Clark P, Campbell SM, Clark P, Fam AG, Farber SJ, Fiechtner JJ, Franklin CM, Gatter RA, Hamaty D, Lessard J, Lichtbroun AS, Masi AT, McCain GA, Reynolds WJ, Romano TJ, Russell IJ, Sheon RP. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee [see comments] Arthritis Rheum. 1990;33(2):160–172. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 59.Ylinen J, Takala EP, Nykanen M, Hakkinen A, Malkia E, Pohjolainen T, Karppi SL, Kautiainen H, Airaksinen O. Active neck muscle training in the treatment of chronic neck pain in women: a randomized controlled trial. Jama. 2003;289(19):2509–2516. doi: 10.1001/jama.289.19.2509. [DOI] [PubMed] [Google Scholar]
- 60.Yunus M, Masi AT, Calabro JJ, Miller KA, Feigenbaum SL. Primary fibromyalgia (fibrositis): clinical study of 50 patients with matched normal controls. Semin Arthritis Rheum. 1981;11(1):151–171. doi: 10.1016/0049-0172(81)90096-2. [DOI] [PubMed] [Google Scholar]
- 61.Yunus MB, Arslan S, Aldag JC. Relationship between body mass index and fibromyalgia features. Scand J Rheumatol. 2002;31(1):27–31. doi: 10.1080/030097402317255336. [DOI] [PubMed] [Google Scholar]
- 62.Yusuf S, Hawken S, Ounpuu S, Bautista L, Franzosi MG, Commerford P, Lang CC, Rumboldt Z, Onen CL, Lisheng L, Tanomsup S, Wangai P, Jr, Razak F, Sharma AM, Anand SS. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case-control study. Lancet. 2005;366(9497):1640–1649. doi: 10.1016/S0140-6736(05)67663-5. [DOI] [PubMed] [Google Scholar]


