Abstract
Here we report a structure-activity relationship (SAR) study of analogues of 5/ 7-{[2-(4-Aryl-piperazin-1-yl)-ethyl]-propyl-amino}-5,6,7,8-tetrahydro-naphthalen-2-ol. Our SAR is focused on introduction of various substitutions in the piperazine ring of the hybrid template. The goal behind this study is to delineate the nature of the binding pocket for N-aryl substitution in the piperazine ring by observing the effect of various hydrophobic and other heteroaromatic substitutions on binding affinity (Ki), as measured with tritiated spiperone and HEK-293 cells expressing either D2 or D3 receptors. Functional activity of selected compounds was assessed with the GTPγS binding assay. Compound 8d was the most selective for the D3 receptor in the spiperone binding assay. An interesting similarity in binding affinity was observed between isoquinoline derivative D-301 and the 2-substituted pyridine derivative 8d, suggesting the importance of relative spatial relationships between the N-atom of the ligand and the molecular determinants of the binding pocket in D2/D3 receptors. Functional activity assays demonstrated high potency and selectivity of (+)−8a and (−) −28b (D2/D3 (ratio of EC50): 105 and 202, respectively) for the D3 receptor and both compounds were more selective compared to the reference drug ropinirole (D2/D3 (ratio of EC50): 29.5).
Introduction
Dopamine receptors play important roles in diverse physiological functions in the Central Nervous System (CNS).1 Imbalances of dopamine level have been implicated in psychiatric disorders such as schizophrenia and depression, and movement disorders including Parkinson’s disease.2, 3 Dopamine D2 and D3 receptors have been targeted for drug development for many years.4 Although D2 and D3 receptors have similar pharmacological properties, recent studies indicate important differences between these two receptors.5 Some of the differences are due to divergent neuroanatomical locations of D2 and D3 receptors, others to different signaling cascades associated with the two receptor subtypes.5, 6 Interestingly, the D3 receptor which is found in highest density in the nucleus accumben7, has been implicated in upregulation in neurotrophic factors and in neurogenesis in the substantia nigra.8, 9
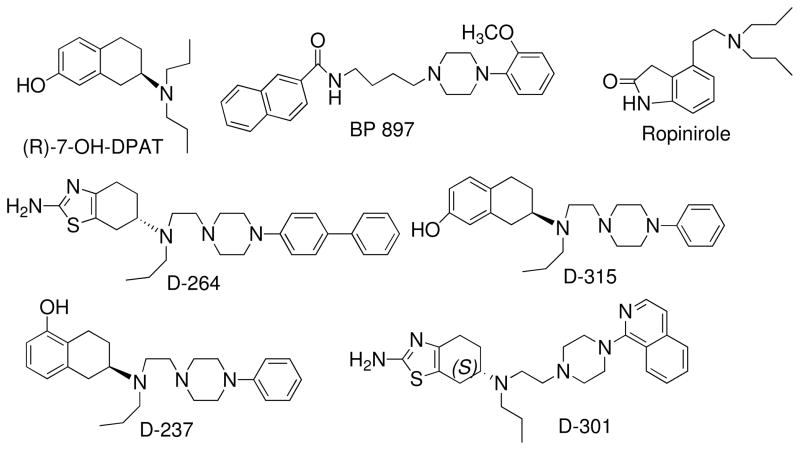
Considerable efforts have been expended to develop selective agonists and antagonists for the D3 receptor. These efforts resulted in the development of many ligands with varying selectivities for the D3 receptor.10 In general higher selectivity was achieved in newly developed antagonists than agonists.11, 12 This might be due to the fact that antagonist may not necessarily bind to the orthosteric binding site in the receptor as required by agonist,13 thereby, is able to exploit structural differences in D2 and D3 receptors to a greater degree than agonist. We have reported some time ago about our hybrid approach of drug development for D2/D3 receptors.14–16 This hybrid approach, which combined a known aminotetraline dopamine agonist with a substituted piperazine fragment via a suitable linker, produced potent preferential agonists for D3 receptors as shown by SAR studies. Some key findings from our recent SAR studies demonstrated that the linker length between the piperazine and aminotetralin fragments is important in potency and selectivity for the D3 receptor. In this regard, a two-methylene linker length was found to be optimal for such hybrid derivatives, in contrast to the 4-methylene length required for optimal affinity and selectivity for D3 antagonists derived from piperazine and benzamide fragments.17 Replacement of the phenolic moiety in D-237 by the bioisosteric amino thiazole moiety produced one of the highest selective D3 agonist. These hybrid derivatives were in general more potent than their parent 7-OH-DPAT or 5-OH-DPAT, thus, indicating contribution of the piperazine moiety in additional interaction.17 Some of our lead agonists are shown in Figure 1. Lead D3 preferring agonist developed from our studies exhibited potent in vivo activity in Parkinson’s disease animal models indicating their efficacy.18
Fig. 1.
A number of lead agonists.
In our current SAR studies, we anticipated to further extend our exploration of the influence of N-piperazine substitutions on affinity and selectivity for the D3 receptor. Compounds containing various aromatic heterocyclic rings and linearly fused biphenyl moieties were explored. The current SAR studies provide a more comprehensive picture of the nature of the binding pocket in D2/D3 receptors that accommodates N-piperazine substitutions in hybrid aminotetraline-piperazine derivatives.
Chemistry
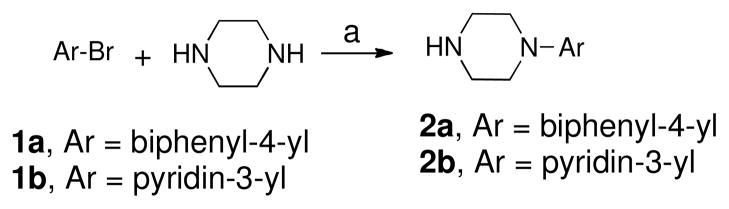
Scheme 1 outlines the syntheses of two aryl piperazines 2a and 2b used in the synthesis of target compounds. Compounds 2a and 2b were synthesized by palladium (II) catalyzed amination reaction with two corresponding aryl halides 4-bromobiphenyl and 3-bromopyridine, 1a and 1b, with excess piperazine.19
Scheme 1.
Reagents and Conditions : (a) 3–5 mol% PdCl2[P(o-tol)3]2, NaOt-Bu, Diglyme, reflux, 48h
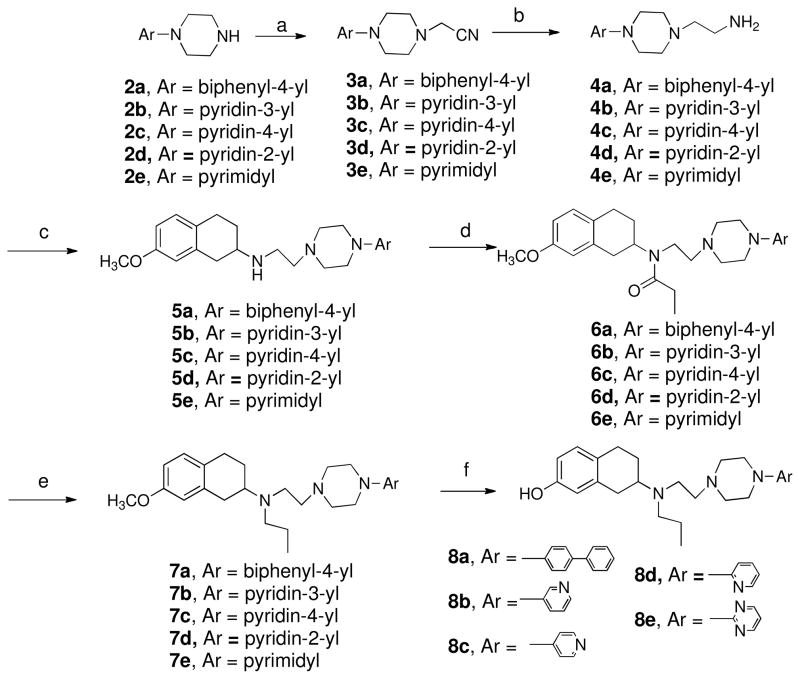
Scheme 2 describes the syntheses of five target compounds. Starting materials 2a and 2b and the rest commercially available starting materials 2c–2e were subjected to N-alkylation reaction with 2-chloroacetonitrile in presence of potassium carbonate in toluene to yield intermediates 3a–3e which were then reduced in presence of raney nickel using a Parr hydrogenetor resulted in amine intermediates 4a–4e. These amines were then treated with 7-methoxy-2-tetralone in standard reductive amination conditions to obtain intermediates 5a–5e which were subjected to N-alkylation reaction with propionyl chloride to afford amides 6a–6e. The amides were then reduced with LAH/THF followed by demethylation in presence of boron tribromide (1M) solution in dichloromethane at -40 °C temperature afforded final compounds 8a–8e.
Scheme 2.
Reagents and Conditions : (a) Chloroacetonitrile, K2CO3, toluene, reflux, 3h; (b) Raney nickel, H2, 60 psi, 8 h; (c) 7-methoxy-2-tetralone, NaCNBH3, AcOH, dichloroethane, RT, overnight; (d) propionyl chloride, Et3N, CH2Cl2, 0 oC to RT, 4h; (e) LiAlH4, THF, reflux, 4 h; (f) BBr3, CH2Cl2, −40 °C to RT, overnight.
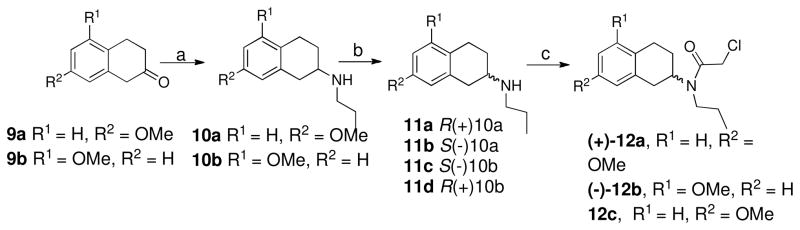
Scheme 3 describes the preparation of some intermediates that were used towards the synthesis of target compounds shown in Scheme 4. In this scheme either 7-methoxy or 5-methoxy-2-tetralone was subjected to reductive amination with n-propyl amine in standard reductive amination conditions to give amino-tetraline moieties 10a and 10b. These amines were resolved to their S(−) or R(+) enantiomers.16, 20 N-amidation of amines using chloroacetyl chloride in presence of triethyl amine produced the chloro-intermediates 12a–c.
Scheme 3.
Reagents and Conditions : (a) n-Propylamine, NaCNBH3, CH3COOH, dichloroethane, RT, overnight; (b) chlocyphos, EtOH; (c) chloroacetyl chloride, Et3N, dichloromethane, 0 °C, 30 min.
Scheme 4.
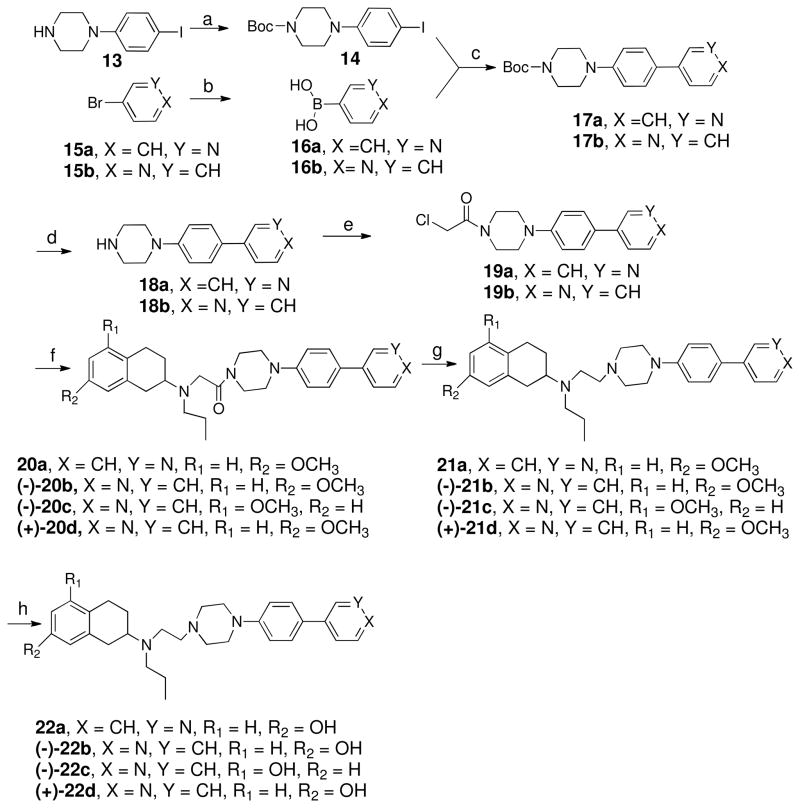
Reagents and Conditions: (a) (Boc)2O, CH2Cl2, 0°C, 2 h; (b) n-BuLi, (i-PrO)3B, toluene, THF, -78 °C to rt; ii. NaOH, 50%; (c) 1,2-dimethoxyethane, t-BuOK, 5 mole% Pd(PPh3)4, H2O, 90 °C; (d) TFA/DCM (1/1), RT, overnight; (e) chloroacetyl chloride, Et3N, dichloromethane, 0 °C, 30 min; (f) 10a/11a/11b/11c (tetralins) K2CO3, KI, Acetonitrile, 60 °C, 4 h; (g) LiAlH4, THF, reflux, 2 h; (h) BBr3, -78° C, CH2Cl2, overnight or 48% Aq. HBr, reflux, 2h.
Scheme-4 depicts the syntheses of four final compounds. Commercially available 1-(4-iodophenyl)piperazine was treated with Boc-anhydride to make mono Boc protected intermediate which was then exposed to Suzuki coupling reaction21, 22 with 3- or 4- pyridinyl boronic acid to give 17a and 17b. Pyridinyl boronic acid was made from pyridinyl bromides using the reported procedure.21 The amine protecting group, Boc was removed using trifluoroacetic acid and subjected to N-amidation reaction with Chloroacetylchloride to get intermediates 19a and 19b which was then treated with either racemic or enantiomerically pure 7-methoxy or 5-methoxy 2-amino tetralin in standard N-alkylation reaction condition to produce corresponding amides 20a–d which after LAH reduction gave the amines 21a–d. Demethylation in presence of boron tribromide in dichloromethane at −40 °C or in Aq. HBr at reflux yielded the final compounds 22a–d.
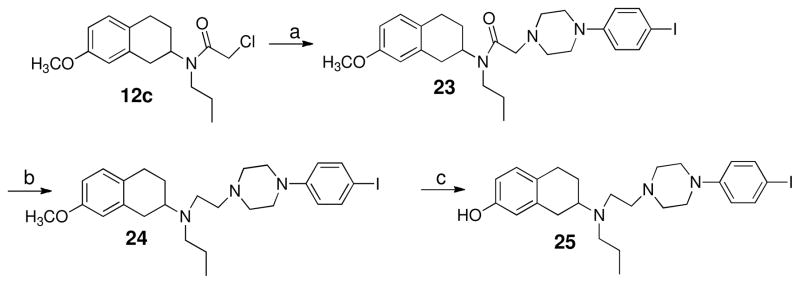
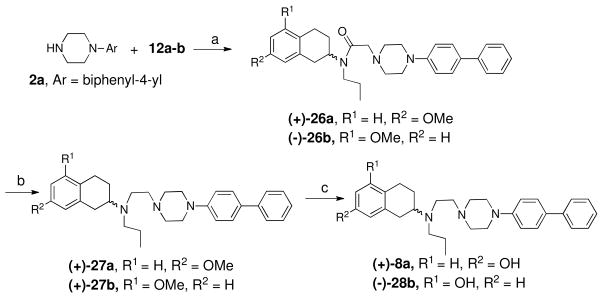
Scheme 5 shows the preparation of final compound 25. Racemic 7-methoxy-2-tetralin was treated with chloroacetylchloride to give intermediate 12c which was then subjected to N-alkylation reaction with 1-(4-iodophenyl)piperazine to produce amide 23. Next LAH reduction gave the amine 24 and the final compound 25 was afforded by demethylation of the amine using borontribromide solution (1M in dichloromethane). Scheme 6 represents the preparation of two enantiomerically pure compounds. One of the intermediates 2a described in the Scheme 1 was subjected to N-alkylation reaction with 12a and 12b to get the corresponding amides which were then reduced by LAH in THF to produce amine intermediates 27a–b. Demethylation yielded the final compounds (+)−8a and (−) −28b.
Scheme 5.
Reagents and Conditions: (a) 4-iodophenylpiperazine, K2CO3, KI, Acetonitrile, 60 °C, 4 h; (b) LiAlH4, THF, reflux, 2 h; (c) BBr3, −78° C, CH2Cl2, overnight
Scheme 6.
Reagents and Conditions: (a) K2CO3, KI, acetonitrile, 60 oC, 4 h; (b) LiAlH4, THF, reflux, 2 h; (c) BBr3, −78 °C, CH2Cl2, overnight
Results and Discussion
As mentioned before, linearly fused N-biphenyl and N-isoquinoline moieties in the piperazine ring of D-264 and D-301 were tolerated well as these compounds exhibited potent binding affinity and high selectivity for the D3 receptor. The results of D-264 and D-301 are consistent with the interpretation that compounds containing a thiazolidinium moiety generally exhibit high selectivity for the D3 receptor, although N-aromatic substitutions in the piperazine ring might also play a role in selectivity. Our next goal was to explore replacement of the thiazolidinium ring in D-264 by a hydroxy-phenolic moiety with an hydroxyl group located at either the 5- or 7-position, and to observe the effect of such replacement on the binding affinity compared to D-264 and D-301. Racemic 7-hydroxy derived 8a was potent at D3 and was moderately potent at D2 (Ki; D2 = 64 and D3 = 5.22 nM), Table 1. Next, compound (+)−8a was selectively synthesized as the (+)-isomeric form of the 7-hydroxy derived hybrid molecules consistently exhibited higher affinity than its (−)-enantiomeric counterpart.15, 16 Compound (+)−8a exhibited two fold higher affinity at D3 compared to racemic 8a whereas the affinity at D2 did not change appreciably (ki; D3 = 2.79 nM, D2/D3 = 20.77). However, the selectivity compared to D-264 was far less. Similarly, 5-hydroxy derived (−) −28b was synthesized selectively as it has been found almost in all the cases the (−)-isomer in this series is more active than the (+)-counterpart.15, 16 Compound (−) −28b was found to exhibit a profile similar to (+)−8a (Ki; D3 = 2.36 nM, D2/D3 = 22.69), Table 1. In comparison to D-264 and D-301, both compounds (+)−8a and (−) −28b were similar in binding affinity for the D3 receptor, but D-264 was more selective for the D3 receptor due to its lower affinity for D2 receptor. Effect of replacement of the phenyl ring by iodine in the biphenyl moiety as shown in 25 reduced the affinity for the D3 receptor but maintained the affinity for D2.
Table 1.
Ki values (nM) are for inhibition of [3H] spiroperidol binding to HEK-D2/D3 cells and are given as the mean ± SEM for 3 to 6 independent experiments carried out in triplicate.
| Compound | Ki, (nM), D2 [3H]Spiperone | Ki, (nM), D3 [3H]Spiperone | D2/D3 | CLogP |
|---|---|---|---|---|
| (±)-7-OH-DPAT | 311 ± 47 | 6.19 ± 1.4 | 4.00 | |
| (−)-5-OH-DPAT | 58.8 ± 11.0 | 1.36 ± 0.28 | 43.2 | 4.00 |
| Ropinirole | 2,674 ± 305 | 29.3 ± 4.2 | 91 | 2.79 |
| D-315 | 40.6 ± 3.6 | 1.77 ± 0.42 | 22.9 | 5.46 |
| D-237a | 26.0 ± 7.5 | 0.825 ± 0.136 | 31.5 | 5.46 |
| D-264b | 264 ± 40 | 0.92 ± 0.23 | 253 | 6.10 |
| D-301 | 269 ± 16 | 2.23 ± 0.60 | 121 | 4.44 |
| D-214 (8a) | 64.1 ± 14.8 | 5.22 ± 0.73 | 7.35 | |
| D-216 (8d) | 103 ± 28 | 1.96 ± 0.19 | 52.6 | 4.51 |
| D-222 (8e) | 165 ± 21 | 6.84 ± 1.83 | 24.1 | 3.75 |
| D-243 (8b) | 21.7 ± 4.8 | 2.89 ± 0.52 | 7.51 | 4.51 |
| D-288 (8c) | 68.3 ± 8.5 | 14.1 ± 2.11 | 4.84 | 4.51 |
| D-292 (22a) | 59.1 ± 0.9 | 3.01 ± 0.88 | 19.6 | 5.96 |
| D-293 (25) | 46.4 ± 3.2 | 7.66 ± 1.56 | 6.06 | 6.75 |
| (+)-D-335 (8a) | 58.0 ± 14.7 | 2.79 ± 0.73 | 20.8 | 7.35 |
| (−)-D-352 (28b) | 53.6 ± 12.3 | 2.36 ± 0.87 | 22.7 | 7.35 |
| (−)-D-304 (22c) | 13.2 ± 1.3 | 1.53 ± 0.15 | 8.63 | 5.96 |
| (−)-D-305 (22b) | 399 ± 16 | 16.2 ± 1.8 | 24.6 | 5.96 |
| (+)-D-414 (22d) | 24.7 (4) ± 5.8 | 0.780 ± 0.22 | 32 | 5.96 |
Ref # 15, 16
Next, we wanted to modify the biphenyl moiety in (+)−8a and (−) −28b to a phenyl-pyridine linearly fused moiety to observe the introduction of pyridine on affinity and selectivity. An N-containing pyridine ring can potentially provide additional interactions besides predominant hydrophobic interactions from the phenyl group. Thus, compounds 22a, (−) −22b, (−) −22c and (+)−22d were designed. 5-Hydroxy derived compound (−) −22c displayed very high affinity for both D2 and D3 receptors (Ki; D2 = 13.2 nM & D3 = 1.53 nM). Similarly, 7-hydroxy derived compound (+)−22d exhibited relatively higher affinity at D3 receptor with improved selectivity for D3 (Ki; D3 = 0.78 nM; D2/D3 = 32). As expected, 7-hydroxy derived (−) −22b exhibited much weaker potency (Ki; D2 = 399 nM & D3 = 16.2 nM). Racemic 22a, which is a 7-hydroxy derived 3-pyridine derivative, displayed high affinity for D2/D3 receptor (Ki; D2 = 59 nM & D3 = 3 nM), Table 1.
Next, we synthesized the three isomeric N-pyridine analogues 8d, 8b, 8c, and the 2-substituted pyrimidine derivative 8e. Among the three pyridine derivatives, 2- substituted derivative 8d exhibited highest affinity and selectivity for the D3 receptor (Ki; D3 = 1.96 nM; D2/D3 = 53). This compound bears a structural resemblance to the isoquinoline compound D-301 where the relative position of the N-atom in the N- isoquinoline substitution is similar to the 2-pyridine substitution in 8d. If isoquinoline is considered a phenyl ring with fused 2-substituted pyridine ring then for both the compounds, 8d and D-301, the N-atoms should have similar locations in the space with respect to the target receptors. It is apparent that such a position of the heterocyclic N-atom in both the molecules leads to production of an unfavorable interaction with the dopamine D2 receptor, thereby, increasing the selectivity for D3. Binding data for 8b bear a striking resemblance to 22a. 8b resembles structurally to 22a as they are both 3-substituted pyridine derivatives although in 22a pyridine ring is part of a biphenyl system. Current results from three isomeric pyridine substituted compounds indicate that the location of N-atom in substituted pyridine is important for interaction when the 2-substituted pyridine ring produced highest affinity and selectivity. On the other hand, 4-substituted pyridine compound, 8c, was least active and selective (Ki; D3 = 14.1 nM; D2/D3 = 4.5), indicating generation of unfavorable interaction. Finally, we have added ClogP values (calculated from ChemDraw program) of all the compounds in Table 1 which indicates a broad range of values depending on the structure of the target compounds.
Following binding evaluation, optically active compounds (+)−8a and (−) −28b were evaluated in the GTPγS binding functional assay for D2 and D3 receptors. The assays were carried out with the cloned human D2 and D3 receptors expressed in CHO-cells and ropinirole was used as a reference compound for comparison purpose. Both compounds (+)−8a and (−) −28b exhibited high potency for the D3 receptor whereas (−) −28b was more potent compared to (+)−8a (EC50; 1.03 and 0.25 nM for 28a and 28b, respectively), Table 2. In regards to selectivity for D3 receptor with respect to D2 receptor, both compounds exhibited high selectivity for D3 receptor while compound (−) −28b was more selective compared to (+)−8a (D2/D3 (Ratio of EC50): 105 vs. 202), Table 2. Compared to reference ropinirole, both compounds (+)−8a and (−) −28b exhibited higher potency and selectivity for D3 receptor.
Table 2.
EC50 values (nM) for stimulating [35S]GTPγS binding. Results are means + SEM for 3–5 experiments each performed in triplicate.
| Compound | CHO-D2 | CHO-D3 | |||
|---|---|---|---|---|---|
| EC50 (nM) [35S]GTPγS | %Emax | EC50 (nM) [35S]GTPγS | %Emax | D2/D3 | |
| Dopamine | 209 ± 29 | 100 | 4.76 ± 0.87 | 100 | 43.9 |
| Ropinirole | 304 ± 11 | 83.9 ± 0.3 | 10.3 ± 1.5 | 66.6 ± 8.1 | 29.5 |
| (+)-D-335 (8a) | 108 ± 39 | 42.1 ± 7.0 | 1.03 ± 0.47 | 69.1 ± 7.4 | 105 |
| (−)-D-352 (28b) | 52.6 ± 10.9 | 71.8 ± 5.3 | 0.26 ± 0.058 | 74.6 ± 4.7 | 202 |
Conclusion
In this report we have shown that various N-aromatic and bulky substitutions on the piperazine moiety were tolerated well by both D2 and D3 receptors. Compound 8d turned out to be the most selective for the D3 receptor in the binding assay. The similarity in binding affinity observed for the isoquinoline derivative D-301 and the 2-substituted pyridine derivative 8d, suggest the importance of relative spatial relationships between the N-atom of the ligand and the molecular determinants of the binding pocket in D2/D3 receptors. In the functional activity assay, compounds (+)−8a and (−) −28b were more potent and selective for the D3 receptor compared to the reference drug ropinirole.
Experimental
Analytical silica gel-coated TLC plates (Silica Gel 60 F254) were purchased from EM Science and were visualized with UV light or by treatment with phosphomolybdic acid (PMA). Flash chromatography was carried out on Baker Silica Gel 40 mM. 1H NMR spectra were routinely obtained on GE-300 MHz and Varian 400 MHz FT NMR. The NMR solvent used was either CDCl3 or CD3OD or DMSO-d6 as indicated. TMS was used as an internal standard. Elemental analyses were performed by Atlantic Microlab, Inc and were within ± 0.4% of the theoretical value.
Procedure A. Synthesis of 1-(4-Biphenylyl)piperazine (2a)
Into a solution of 4-bromobiphenyl 1a (3 g, 12.87 mmol) and piperazine (4.43 g, 51.48 mmol) in 100 ml of diglyme was added K-t-butoxide (4.33 g, 38.61 mmol). The reaction mixture was stirred for few minutes before the addition of palladium catalyst, dichlorobis(tri-o-tolylphosphine)palladium (0.386 g, 0.386 mmol) and refluxed at 170 °C for 48 h. The reaction mixture was cooled and the diglyme was evaporated under reduced pressure. The solid residue was partitioned between ethyl acetate and water. The aqueous layer was extracted with ethyl acetate (3×100 ml). The combined organic layer was dried (Na2SO4), evaporated under reduced pressure to obtain the crude product which was purified by column chromatography (ethylacetate :MeOH 9:1) to yield 1.99 g of pure compound 2a ( 65 %) as a yellow color solid. 1H NMR (400 MHz, CDCl3): δ ppm 3.05 (t, 4H, J = 4.4 Hz), 3.20 (t, 4H, J = 5.2 Hz), 6.99–7.01 (d, 2H, J = 8.8 Hz), 7.26–7.30 (m, 1H), 7.40 (t, 2H, J = 7.6 Hz), 7.51–7.57 (m, 4H).
Synthesis of 1-(pyridin-3-yl)piperazine (2b)
Compound 2b was prepared from 3-bromopyridine 1b (3.10 mL, 31.6 mmol) and piperazine (10.9 g, 12.66 mmol) according to the procedure A to afford 3.15 g of resinous compound 2b (61 %). 1H NMR (400 MHz, CDCl3): δ ppm 3.05 (t, 4H, J = 4.4 Hz), 3.20 (t, 4H, J = 5.2 Hz); 7.17–7.19 (m, 2H); 8.120–8.135 (dd, 1H, J1 = 2 Hz, J2 = 4 Hz); 8.31–8.31 (s, 1H).
Procedure B. Synthesis of 2-(4-(biphenyl-4-yl)piperazin-1-yl)acetonitrile (3a)
A suspension of 1-(biphenyl-4-yl)piperazine (2a) (2.5 g, 10.5 mmol), potassium carbonate (2.9 g, 21 mmol), and 2-chloroacetonitrile (1.3 mL, 21 mmol) in toluene was refluxed for 3 h. Toluene was removed under reduced pressure, and the residue was diluted with ethyl acetate, washed with water and brine, dried over sodium sulfate (Na2SO4), concentrated, and purified by column chromatography (ethyl acetate/hexane = 1:1) to afford the product 3a as a thick yellow solid (2.33 g, 80%): 1H NMR (400 MHz, CDCl3) δ ppm 2.76–2.79 (t, 4H, J = 6 Hz); 3.27–3.30 (t, 4H, J = 6 Hz); 3.58 (s, 2H); 6.98–7.00 (d, 2H, J = 8 Hz); 7.25–7.30 (m, 1H); 7.39–7.42 (t, 2H, J = 6 Hz); 7.50–7.57 (m, 4H).
Synthesis of 2-(4-(pyridin-3-yl)piperazin-1-yl)acetonitrile (3b)
Compound 3b was synthesized from 1-(pyridin-3-yl)piperazine, 2b (3.15 g, 19.28 mmol) and 2-chloroacetonitrile (3.66 mL, 57.9 mmol) according to the procedure B to afford product 3b as a yellow mass (3.08 g, 79%): 1H NMR (400 MHz, CDCl3) δ ppm 2.76–2.78 (t, 4H, J = 4 Hz); 3.26–3.27 (t, 4H, J = 6 Hz), 3.56 (s, 2H); 7.17–7.19 (m, 2H); 8.120– 8.135 (dd, 1H, J1 = 2 Hz, J2 = 4 Hz); 8.31–8.31 (s, 1H).
Synthesis of 2-(4-(pyridin-4-yl)piperazin-1-yl)acetonitrile (3c)
Compound 3c was synthesized from commercially available 1-(pyridin-4-yl)piperazine (2c) (4.00 g, 24.5 mmol) and 2-chloroacetonitrile (4.6 mL, 73.52 mmol) according to the procedure B to afford product 3c (2.57 g, 52 %) as yellow oil: 1H NMR (400 MHz, CDCl3) δ ppm 2.76–2.78 (t, 4H, J = 4 Hz); 3.26–3.27 (t, 4H, J = 6 Hz), 3.56 (s, 2H); 6.99–7.03 (m, 2H); 8.20–8.22 (d, 2H, J = 8 Hz).
Synthesis of 2-(4-(pyridin-2-yl)piperazin-1-yl)acetonitrile (3d)
Compound 3d was also synthesized from commercially available 1-(pyridin-2- yl)piperazine (2d) (2.24 mL, 15.32 mmol) and 2-chloroacetonitrile (2.9 mL, 45.95 mmol) according to the procedure B to afford product 3d (3.02 g, 97.5 %) as yellow thick oil: 1H NMR (400 MHz, CDCl3) δ ppm 2.63–2.66 (t, 4H, J = 6 Hz); 3.52 (s, 2H); 3.54–3.57 (t, 4H, J = 6 Hz), 6.58–6.62 (m, 2H); 7.42–7.46 (t, 1H, J = 8 Hz); 8.14–8.15 (d, 1H, J = 4 Hz).
Synthesis of 2-(4-(pyrimidin-2-yl)piperazin-1-yl)acetonitrile (3e)
Compound 3e was synthesized from commercially available 2-(piperazin-1-yl)pyrimidine (2e) (2.16 mL, 15.22 mmol) and 2-chloroacetonitrile (2.89 mL, 45.67 mmol) according to the procedure B to afford product 3e as viscous oil (2.4 g, 77.6 %): 1H NMR (400 MHz, CDCl3) δ ppm 2.63–2.66 (t, 4H, J = 6 Hz); 3.58 (s, 2H); 3.88–3.91 (t, 4H, J = 6 Hz), 6.50–6.53 (t, 1H, J = 6 Hz); 8.31–8.32 (d, 2H, J = 4 Hz).
Procedure C. Synthesis of 2-(4-(biphenyl-4-yl)piperazin-1-yl)ethanamine (4a)
A solution of compound 3a in methanol (2.33 g, 8.41 mmol) was hydrogenated in a parr hydrogenator apparatus in the presence of raney nickel catalyst at a pressure of 60 psi for 12 h. The reaction mixture was passed through celite, dried over Na2SO4, evaporated, and purified over a silica gel column using the solvent system ethyl acetate/methanol/triethylamine (80:15:5) to afford compound 4a as thick oil (2.73 g, 93%): 1H NMR (400 MHz, CDCl3) δ ppm 2.47–2.50 (t, 2H, J = 6 Hz); 2.68–2.70 (t, 4H, J = 4 Hz); 2.87–2.90 (t, 2H, J = 6 Hz); 3.13–3.15 (t, 4H, J = 4 Hz); 6.97–6.99 (d, 2H, J = 8 Hz); 7.26–7.30 (m, 1H); 7.38–7.41 (t, 2H, J = 6 Hz); 7.49–7.55 (m, 4H).
Synthesis of 2-(4-(pyridin-3-yl)piperazin-1-yl)ethanamine (4b)
Compound 4b was synthesized from 2-(4-(pyridin-3-yl)piperazin-1-yl)acetonitrile, 3b (3.08 g, 15.13 mmol) according to the procedure C to afford product 4b as thick oil (3.01 g, 96 %): 1H NMR (400 MHz, CDCl3) δ ppm 2.47–2.50 (t, 2H, J = 6 Hz); 2.68–2.70 (t, 4H, J = 4 Hz); 2.87–2.90 (t, 2H, J = 6 Hz); 3.13–3.15 (t, 4H, J = 4 Hz); 7.17–7.19 (m, 2H); 8.120–8.135 (dd, 1H, J1 = 2 Hz, J2 = 4 Hz); 8.31–8.31 (s, 1H).
Synthesis of 2-(4-(pyridin-4-yl)piperazin-1-yl)ethanamine (4c)
Compound 4c was synthesized from 2-(4-(pyridin-4-yl)piperazin-1-yl)acetonitrile, 3c (2.57 g, 12.7 mmol) according to the procedure C to afford product 4c as thick oil (2.43 g, 93 %): 1H NMR (400 MHz, CDCl3) δ ppm 2.46–2.49 (t, 2H, J = 6 Hz); 2.67–2.71 (t, 4H, J = 8 Hz); 2.86–2.89 (t, 2H, J = 6 Hz); 3.14–3.16 (t, 4H, J = 4 Hz); 6.96–7.04 (m, 2H); 8.18–8.20 (d, 2H, J = 8 Hz).
Synthesis of 2-(4-(pyridin-2-yl)piperazin-1-yl)ethanamine (4d)
Compound 4d was synthesized from 2-(4-(pyridin-2-yl)piperazin-1-yl)acetonitrile, 3d (3.02 g, 14.9 mmol) according to the procedure C to afford product 4d as thick oil (2.91 g, 94.5 %): 1H NMR (400 MHz, CDCl3) δ ppm 2.33–2.36 (t, 2H, J = 6 Hz); 2.63–2.66 (t, 4H, J = 6 Hz); 2.71–2.86 (m, 2H); 3.64–3.67 (t, 4H, J = 6 Hz), 6.58–6.62 (m, 2H); 7.42–7.46 (t, 1H, J = 8 Hz); 8.14–8.15 (d, 1H, J = 4 Hz).
Synthesis of 2-(4-(pyrimidin-2-yl)piperazin-1-yl)ethanamine (4e)
Compound 4e was synthesized from 2-(4-(pyrimidin-2-yl)piperazin-1-yl)acetonitrile, 3e (2.4 g, 11.81 mmol) according to the procedure C to afford product 4e as thick oil (2.35 g, 97 %): 1H NMR (400 MHz, CDCl3) δ ppm 2.33–2.36 (t, 2H, J = 6 Hz); 2.63–2.66 (t, 4H, J = 6 Hz); 3.58 (m, 2H); 3.88–3.91 (t, 4H, J = 6 Hz); 6.50–6.53 (t, 1H, J = 6 Hz); 8.31–8.32 (d, 2H, J = 4 Hz).
Procedure D. Synthesis of N-(2-(4-(biphenyl-4-yl)piperazin-1-yl)ethyl)-7-methoxy-1,2,3,4-tetrahydronaphthalen-2-amine (5a)
A mixture of compound 4a (1.2 g, 4.3 mmol), 7-methoxy-2-tetralone (0.82 g, 4.7 mmol), and glacial acetic acid (HOAc) (0.25 mL) in 1,2-dichloroethane (50 mL) was stirred at room temperature under N2 atmosphere for 20 min. Sodium cyanoborohydride (NaCNBH3) (1.08 g, 17.2 mmol) dissolved in a minimum volume of methanol was added to the reaction mixture. The reaction mixture was stirred at room temperature under nitrogen atmosphere for 12 h. The solvent was evaporated, and saturated NaHCO3/H2O (50 mL) was added to the mixture, which was then extracted with ethyl acetate (3 × 100 mL). The combined organic phase was dried over Na2SO4 and evaporated to afford the crude product, which was purified by flash chromatography (EtOAc/MeOH/Et3N = 95:4:1) to give the product 5a as brown solid (0.55 g, 30%): 1H NMR (400 MHz, CDCl3) δ ppm 1.66–1.69 (m, 2H); 2.09 (bs, 1H); 2.59–2.72 (m, 6H); 2.81–3.02 (m, 6H); 3.23–3.25 (t, 4H, J = 4 Hz); 3.77 (s, 3H); 6.62 (s, 1H); 6.67–6.70 (d, 1H, J = 12 Hz); 6.98–7.01 (m, 3H); 7.26–7.30 (t, 1H J = 8 Hz); 7.38–7.42 (t, 2H, J = 8 Hz); 7.50–7.57 (m, 4H).
Synthesis of 7-methoxy-N-(2-(4-(pyridin-3-yl)piperazin-1-yl)ethyl)-1,2,3,4-tetrahydronaphthalen-2-amine (5b)
Compound 4b (0.96 g, 4.5 mmol) was reacted with 7-methoxy-2-tetralone (1.16 g, 6.6 mmol), NaCNBH3 (1.17 g, 18.75 mmol), and HOAc (0.28 mL) in 1,2-dichloroethane (50 mL) to yield 5b as brown mass (1.11 g, 65%) (procedure D): 1H NMR (400 MHz, CDCl3) δ ppm 1.73–1.82 (m, 1H); 1.99–2.03 (m, 1H); 2.15 (s, 1H); 2.28 (bs, 1H); 2.53–2.92 (m, 6H); 3.11–3.26 (m, 3H); 3.65–3.77 (m, 9H); 6.58 (s, 1H); 6.65–6.68 (m, 2H); 6.94–6.98 (t, 2H, J = 8 Hz); 7.11–7.16 (m, 1H); 8.01–8.22 (m, 1H).
Synthesis of 7-methoxy-N-(2-(4-(pyridin-4-yl)piperazin-1-yl)ethyl)-1,2,3,4-tetrahydronaphthalen-2-amine (5c)
Compound 4c (1.2 g, 5.82 mmol) was reacted with 7-methoxy-2-tetralone (1.53 g, 8.73 mmol), NaCNBH3 (1.09 g, 17.45 mmol), and HOAc (0.7 mL) in 1,2-dichloroethane (50 mL) to yield 5c as brown color solid (1.3 g, 61%) (Procedure D): 1H NMR (400 MHz, CDCl3) δ ppm 1.69–1.78 (m, 1H); 1.96–2.01 (m, 1H); 2.13 (s, 1H); 2.31 (bs, 1H); 2.51–2.91 (m, 6H); 3.09–3.21 (m, 3H); 3.65–3.77 (m, 9H); 6.63–6.65 (m, 2H); 6.81–6.82 (d, 2H, J = 4 Hz); 6.98–7.00 (d, 1H, J = 8 Hz), 8.27–8.29 (d, 2H, J = 8 Hz).
Synthesis of 7-methoxy-N-(2-(4-(pyridin-2-yl)piperazin-1-yl)ethyl)-1,2,3,4-tetrahydronaphthalen-2-amine (5d)
Compound 4d (1.3 g, 6.3 mmol) was reacted with 7-methoxy-2-tetralone (1.33 g, 7.56 mmol), NaCNBH3 (1.58 g, 25.21 mmol), and HOAc (0.4 mL) in 1,2-dichloroethane (50 mL) to yield 5d (1.23 g, 69.2 %) (procedure D): 1H NMR (400 MHz, CDCl3) δ ppm 1.94–1.99 (m, 2H); 2.37–2.46 (m, 2H); 2.56–2.85 (m, 8H); 3.42–3.59 (m, 6H); 3.73 3.76 (t, 4H, J = 6 Hz); 6.58–6.73 (m, 4H), 6.96–7.02 (dd, 1H, J1 = 8.4 Hz, J2 = 14.8 Hz); 7.43–7.45 (t, 1H, J = 4 Hz); 8.16–8.17 (d, 1H, J = 4 Hz).
Synthesis of 7-methoxy-N-(2-(4-(pyrimidin-2-yl)piperazin-1-yl)ethyl)-1,2,3,4-tetrahydronaphthalen-2-amine (5e)
Compound 4e (1.59 g, 7.7 mmol) was reacted with 7-methoxy-2-tetralone (1.62 g, 9.2 mmol), NaCNBH3 (1.93 g, 30.68 mmol), and HOAc (0.45 mL) in 1,2-dichloroethane (50 mL) to yield 5e as semisolid (1.42 g, 50 %) (Procedure D): 1H NMR (400 MHz, CDCl3) δ ppm 1.55–1.59 (m, 1H); 1.79 (bs, 1H); 1.98–2.02 (m, 2H); 2.44–2.52 (m, 7H), 2.71–2.92 (m, 5H); 3.7 (s, 3H); 3.76–3.78 (t, 4H, J = 4 Hz); 6.39 (t, 1H, J = 4 Hz); 6.57 (s, 1H); 6.62–6.64 (d, 1H, J = 8 Hz); 6.92–6.95 (d, 1H, J = 12 Hz); 8.23–8.24 (d, 2H, J = 4 Hz).
Procedure E. Synthesis of N-(2-(4-(biphenyl-4-yl)piperazin-1-yl)ethyl)-N-(7-methoxy-1,2,3,4-tetrahydronaphthalen-2-yl)propionamide (6a)
Propionyl chloride (0.33 mL, 3.75 mmol) was added into a solution of compound 5a (0.55 g, 1.25 mmol) and Et3N (1.0 mL) in anhydrous methylene chloride at 0 °C under N2 atmosphere and then stirred at room temperature for 4 h. The reaction was diluted with CH2Cl2 and washed with water and brine, and the organic layer was dried over Na2SO4, evaporated, and purified by flash chromatography (EtOAc/MeOH/Et3N = 95:4:1) to yield 6a as solid (0.78 g, 90 %): 1H NMR (400 MHz, CDCl3) δ ppm 1.12–1.14 (m, 3H); 1.66–1.69 (m, 2H); 2.09 (bs, 1H); 2.59–2.72 (m, 6H); 2.81–3.02 (m, 6H); 3.19–3.22 (bs, 2H); 3.23–3.25 (t, 4H, J = 4 Hz); 3.77 (s, 3H); 6.62 (s, 1H); 6.67–6.70 (d, 1H, J = 12 Hz); 6.98–7.01 (m, 3H); 7.26–7.30 (t, 1H J = 8 Hz); 7.38–7.42 (t, 2H, J = 8 Hz); 7.50–7.57 (m, 4H).
Synthesis of N-(7-methoxy-1,2,3,4-tetrahydronaphthalen-2-yl)-N-(2-(4-(pyridin-3-yl)piperazin-1-yl)ethyl)propionamide (6b)
Compound 5b (0.80 g, 2.2 mmol) was reacted with propionyl chloride (0.57 mL, 6.55 mmol) and Et3N (2.0 mL) in CH2Cl2 (20 mL) (procedure E). The crude product was purified by flash chromatography using solvent system EtOAc/MeOH = 90:10 to yield pure compound 6b as semisolid (0.48 g, 52 %): 1H NMR (400 MHz, CDCl3) δ ppm 1.05–1.08 (m, 3H); 1.72–1.82 (m, 1H); 1.98–2.03 (m, 1H); 2.14 (s, 1H); 2.28–2.30 (bs, 1H); 2.54–2.94 (m, 6H); 3.21–3.26 (m, 3H); 3.67–3.74 (m, 11H); 6.56 (s, 1H); 6.64–6.67 (m, 2H); 6.93–6.98 (t, 2H, J = 8 Hz); 7.12–7.15 (m, 1H); 8.11–8.22 (m, 1H).
Synthesis of N-(7-methoxy-1,2,3,4-tetrahydronaphthalen-2-yl)-N-(2-(4-(pyridin-4-yl)piperazin-1-yl)ethyl)propionamide (6c)
Compound 5c (1.3 g, 3.55 mmol) was reacted with propionyl chloride (0.93 mL, 10.64 mmol) and Et3N (3.0 mL) in CH2Cl2 (20 mL) (Procedure E). The crude product was purified by flash chromatography using solvent system EtOAc/MeOH = 90:10 to yield pure compound 6c as solid (0.70 g, 61 %): 1H NMR (400 MHz, CDCl3) δ ppm 1.05–1.08 (m, 3H); 1.71–1.82 (m, 1H); 1.91–2.03 (m, 1H); 2.13 (s, 1H); 2.32 (bs, 1H); 2.74–2.91 (m, 6H); 3.19–3.26 (m, 3H); 3.67–3.74 (m, 11H); 6.63–6.65 (m, 2H); 6.81–6.82 (d, 2H, J = 4 Hz); 6.98–7.00 (d, 1H, J = 8 Hz), 8.27–8.29 (d, 2H, J = 8 Hz).
Synthesis of N-(7-methoxy-1,2,3,4-tetrahydronaphthalen-2-yl)-N-(2-(4-(pyridin-2-yl)piperazin-1-yl)ethyl)propionamide (6d)
Compound 5d (2.74 g, 7.5 mmol) was reacted with propionyl chloride (0.98 mL, 11.2 mmol) and Et3N (3.0 mL) in CH2Cl2 (20 mL) (Procedure E). The crude product was purified by flash chromatography using solvent system EtOAc/MeOH = 90:10 to yield pure compound 6d as solid (1.81 g, 57.2 %): 1H NMR (400 MHz, CDCl3) δ ppm 1.12–1.16 (t, 3H, J = 8 Hz); 1.94–1.99 (m, 2H); 2.37–2.46 (m, 2H); 2.56–2.85 (m, 10H); 3.42–3.59 (m, 6H); 3.73 3.76 (t, 4H, J = 6 Hz); 6.58–6.73 (m, 4H), 6.96–7.02 (dd, 1H, J1 = 8.4 Hz, J2 = 14.8 Hz); 7.43–7.45 (t, 1H, J = 4 Hz); 8.16–8.17 (d, 1H, J = 4 Hz).
Synthesis of N-(7-methoxy-1,2,3,4-tetrahydronaphthalen-2-yl)-N-(2-(4-(pyrimidin-2-yl)piperazin-1-yl)ethyl)propionamide (6e)
Compound 5e (1.4 g, 3.81 mmol) was reacted with propionyl chloride (0.99 mL, 11.43 mmol) and Et3N (3.0 mL) in CH2Cl2 (20 mL) (Procedure E). The crude product was purified by flash chromatography using solvent system EtOAc/MeOH = 90:10 to yield pure compound 6e as solid (1.08 g, 67.5 %): 1H NMR (400 MHz, CDCl3) δ ppm 0.99–1.02 (t, 3H, J = 6 Hz); 1.55–1.59 (m, 1H); 1.79 (bs, 1H); 1.98–2.02 (m, 2H); 2.44–2.52 (m, 7H), 2.71–2.92 (m, 7H); 3.7 (s, 3H); 3.76–3.78 (t, 4H, J = 4 Hz); 6.39 (t, 1H, J = 4 Hz); 6.57 (s, 1H); 6.62–6.64 (d, 1H, J = 8 Hz); 6.92–6.95 (d, 1H, J = 12 Hz); 8.23–8.24 (d, 2H, J = 4 Hz).
Procedure F. Synthesis of N-(2-(4-(biphenyl-4-yl)piperazin-1-yl)ethyl)-7-methoxy-N-propyl-1,2,3,4-tetrahydronaphthalen-2-amine (7a)
Compound 6a (0.78 g, 1.6 mmol) in anhydrous THF (30 mL) was added dropwise into a suspension of lithium aluminum hydride (LiAlH4) (0.36 g, 9.44 mmol) in anhydrous THF (15 mL) at 0 °C under N2 atmosphere. The reaction mixture was re luxed for 8 h, cooled to room temperature, and then cooled further to 0 °C. Saturated NaOH/H2O (3 mL) was added drop wise to quench excess LiAlH4. The mixture was filtered, and the reaction mixture was dried over Na2SO4. The solvent was removed under vacuum to afford compound 7a as transparent viscous liquid (0.52 g, 68.3 %): 1H NMR (400 MHz, CDCl3) δ ppm 0.90–0.93 (t, 3H, J = 6 Hz); 1.26 (s, 1H); 1.48–1.53 (m, 2H); 2.02–2.2.05 (bs, 1H); 2.18 (s, 1H); 2.53–2.99 (m, 14H); 3.255–3.279 (t, 4H, J = 4.8 Hz); 3.78 (s, 3H); 6.64 (s, 1H); 6.68–6.71 (d, 1H, J = 12 Hz); 6.99–7.01 (m, 3H); 7.27–7.30 (t, 1H J = 6 Hz); 7.39–7.43 (t, 2H, J = 8 Hz); 7.51–7.57 (m, 4H).
Synthesis of 7-methoxy-N-propyl-N-(2-(4-(pyridin-3-yl)piperazin-1-yl)ethyl)-1,2,3,4-tetrahydronaphthalen-2-amine (7b)
Compound 6b (0.48 g, 1.14 mmol) was reacted with LiAlH4 (0.25 g, 6.8 mmol) in THF (20 mL) by following the Procedure F. The crude product was purified by flash chromatography using solvent system EtOAc/MeOH/Et3N = 95:4:1 to yield compound 7b as an oil (0.46 g, 90.5 %): 1H NMR (400 MHz, CDCl3) δ ppm 0.98–1.02 (t, 3H, J = 8 Hz ); 1.72–1.79 (m, 1H); 1.98–2.03 (m, 1H); 2.14 (s, 1H); 2.30–2.35 (bs, 1H); 2.62–2.92 (m, 8H); 3.12–3.25 (m, 3H); 3.63–3.72 (m, 11H); 6.57 (s, 1H); 6.64–6.67 (m, 2H); 6.93–6.97 (t, 2H, J = 8 Hz); 7.12–7.15 (m, 1H); 8.11–8.16 (m, 1H).
Synthesis of 7-methoxy-N-propyl-N-(2-(4-(pyridin-4-yl)piperazin-1-yl)ethyl)-1,2,3,4-tetrahydronaphthalen-2-amine (7c)
Compound 6c (0.50 g, 1.18 mmol) was reacted with LiAlH4 (0.26 g, 7.09 mmol) in THF (20 mL) by following the Procedure F. The crude product was purified by flash chromatography using solvent system EtOAc/MeOH/Et3N = 95:4:1 to yield compound 7c as thick liquid (0.37 g, 77.8 %): 1H NMR (400 MHz, CDCl3) δ ppm 0.88–0.90 (t, 3H, J = 4 Hz); 1.48 (bs, 2H); 1.59–1.62 (m, 1H); 1.996–2.034 (d, 1H, J = 15.2 Hz); 2.51–2.97 (m, 15H); 3.32–3.35 (t, 4H, J = 6 Hz); 3.77 (s, 3H); 6.63–6.65 (m, 4H), 6.98 (bs, 1H); 8.266 (bs, 2H).
Synthesis of 7-methoxy-N-propyl-N-(2-(4-(pyridin-2-yl)piperazin-1-yl)ethyl)-1,2,3,4-tetrahydronaphthalen-2-amine (7d)
Compound 6d (0.67 g, 1.58 mmol) was reacted with LiAlH4 (0.36 g, 9.5 mmol) in THF (20 mL) by following the Procedure F. The crude product was purified by flash chromatography using solvent system EtOAc/MeOH/Et3N = 95:4:1 to yield compound 7d as thick liquid (0.64 g, 97 %): 1H NMR (400 MHz, CDCl3) δ ppm 0.85–0.87 (t, 3H, J = 4 Hz); 1.18–1.22 (m, 1H); 1.41–1.49 (m, 2H); 1.99–2.00 (t, 2H, J = 2 Hz); 2.45–2.97 (m, 14H); 3.49–3.51 (t, 4H, J = 4 Hz); 3.7 (s, 3H).
Synthesis of 7-methoxy-N-propyl-N-(2-(4-(pyrimidin-2-yl)piperazin-1-yl)ethyl)-1,2,3,4-tetrahydronaphthalen-2-amine (7e)
Compound 6e (1.00 g, 2.57 mmol) was reacted with LiAlH4 (0.58 g, 15.4 mmol) in THF (20 mL) by following the Procedure F. The crude product was purified by flash chromatography using solvent system EtOAc/MeOH/Et3N = 95:4:1 to yield compound 7e as thick oil (0.55 g, 53.2 %): 1H NMR (400 MHz, CDCl3) δ ppm 0.87–0.91 (t, 3H, J = 8 Hz); 1.57–1.66 (m, 1H); 1.99–2.03 (bs, 1H); 2.48–2.65 (m, 9H); 2.71–2.99 (m, 8H); 3.77 (s, 3H); 3.81–3.84 (t, 4H, J = 6 Hz); 6.46–6.48 (t, 1H, J = 4 Hz); 6.62 (s, 1H); 6.660–6.688 (dd, 1H, J1 = 2.4 Hz, J2 = 8.4 Hz); 6.97–6.99 (d, 1H, J = 8 Hz); 8.29–8.30 (d, 2H, J = 4 Hz).
Procedure G. Synthesis of 7-((2-(4-(biphenyl-4-yl)piperazin-1-yl)ethyl)(propyl)amino)-5,6,7,8-tetrahydronaphthalen-2-ol (8a)
Boron tribromide (1 M solution in dichloromethane) (3.22 mL, 3.22 mmol) was added into a solution of 7a (0.52 g, 1.07 mmol) in anhydrous methylene chloride (CH2Cl2) (30 mL) at −40 °C under N2 atmosphere. The reaction mixture was stirred at −40 °C for 2 h and was continued overnight at room temperature. The reaction was quenched by the addition of saturated NaHCO3 solution, and the mixture was extracted with CH2Cl2. The combined organic layer was dried over Na2SO4 and evaporated under vacuum, and the crude product was purified by flash chromatography (EtOAc/MeOH = 95:5) to afford compound 8a as white solid (0.27 g, 55 %): 1H NMR (400 MHz, CDCl3) δ ppm 0.90–0.93 (t, 3H, J = 6 Hz); 1.26 (s, 1H); 1.48–1.53 (m, 2H); 2.02–2.2.05 (bs, 1H); 2.18 (s, 1H); 2.53–2.99 (m, 14H); 3.25–3.27 (t, 4H, J = 4.8 Hz); 6.57 (s, 1H); 6.61–6.64 (d, 1H, J = 12 Hz); 6.94–6.96 (d, 1H, J = 8Hz); 6.99–7.01 (d, 2H, J = 8 Hz); 7.31–7.32 (d, 1H, J = 4 Hz); 7.39–7.43 (t, 2H, J = 8 Hz); 7.51–7.57 (m, 4H).
The product was converted into the corresponding trihydrochloride salt as white solid; mp: 180–182 °C, Anal. Calcd for (C31H42N3Cl3O, 0.7H2O) C, H, N.
Synthesis of 7-(propyl(2-(4-(pyridin-3-yl)piperazin-1-yl)ethyl)amino)-5,6,7,8-tetrahydronaphthalen-2-ol (8b)
Compound 7b (0.42 g, 1.3 mmol) was reacted with 1 M BBr3/CH2Cl2 (3.08 mL, 3.08 mmol) in CH2Cl2 (15 mL) by following the Procedure G to furnish 8b as white semi solid (0.245 g, 49.2 %): 1H NMR (400 MHz, CDCl3) δ ppm 0.90–0.95 (t, 3H, J = 10 Hz); 1.69 (bs, 3H); 2.165 (bs, 1H); 2.71–2.95 (m, 15H); 3.22–3.24 (t, 4H, J = 4 Hz); 6.95 (s, 1H); 6.44–6.67 (d, 2H, J = 12 Hz); 6.87–6.89 (d, 1H, J = 8 Hz); 7.19 (s, 1H); 8.08–8.09 (t, 1H, J = 2 Hz); 8.26 (s, 1H).
The product was converted into the corresponding tetrahydrochloride salt as white solid; mp: 184–186 °C, Anal. Calcd for (C24H38N4Cl4O, 0.5H2O) C, H, N.
Synthesis of 7-(propyl(2-(4-(pyridin-4-yl)piperazin-1-yl)ethyl)amino)-5,6,7,8-tetrahydronaphthalen-2-ol (8c)
Compound 7c (0.18 g, 0.44 mmol) was reacted with 1 M BBr3/CH2Cl2 (1.76 mL, 1.76 mmol) in CH2Cl2 (15 mL) by following the Procedure G to furnish 8c as white semi solid (0.10 g, 57.5 %): 1H NMR (400 MHz, CDCl3) δ ppm 1.06–1.09 (t, 3H, J = 6 Hz); 1.88–1.96 (m, 1H); 2.39 (bs, 1H), 2.90–2.94 (m, 2H); 3.10–3.31 (m, 11H); 3.46–3.54 (m, 4H), 3.73–3.84 (m, 4H); 6.60–6.62 (m, 2H); 6.93–6.96 (d, 1H, J = 12 Hz); 7.30–7.32 (d, 2H, J = 8 Hz); 8.255–8.274 (d, 2H, J = 7.6 Hz)
The product was converted into the corresponding oxalate salt as yellow solid, mp: 149– 152 °C . Anal. Calcd for (C30H40N4O13, 1.3H2O) C, H, N.
Synthesis of 7-(propyl(2-(4-(pyridin-2-yl)piperazin-1-yl)ethyl)amino)-5,6,7,8-tetrahydronaphthalen-2-ol (8d)
Compound 7d (0.63 g, 1.54 mmol) was reacted with 1 M BBr3/CH2Cl2 (4.63 mL, 4.63 mmol) in CH2Cl2 (25 mL) by following the procedure G to furnish 8d as white semi solid (0.298 g, 49 %): 1H NMR (400 MHz, CDCl3) δ ppm 0.86–0.88 (t, 3H, J = 4 Hz); 1.41–1.55 (m, 3H); 1.92–1.99 (bs, 1H); 2.49–2.72 (m, 14H); 2.91 (bs, 1H); 3.57–3.59 (t, 4H, J = 4 Hz); 6.47 (s, 1H), 6.55–6.57 (d, 1H, J = 8 Hz); 6.62–6.65 (m, 2H); 7.455–7.499 (t, 1H, 8.8 Hz); 8.17–8.18 (d, 1H, J = 4 Hz).
The product was converted into the corresponding trihydrochloride salt yellowish solid; mp: 190–192 °C, Anal. Calcd for (C24H37N4Cl3O, 1.4H2O) C, H, N.
Synthesis of 7-(propyl(2-(4-(pyrimidin-2-yl)piperazin-1-yl)ethyl)amino)-5,6,7,8-tetrahydronaphthalen-2-ol (8e)
Compound 7e (0.55 g, 1.37 mmol) was reacted with 1 M BBr3/CH2Cl2 (4.9 mL, 4.9 mmol) in CH2Cl2 (25 mL) by following the Procedure G to furnish 8e as semi solid (0.38 g, 71.3 %): 1H NMR (400 MHz, CDCl3) δ ppm 0.86–0.88 (t, 3H, J = 4 Hz); 1.41–1.54 (m, 1H); 1.92–1.98 (bs, 1H); 2.472.89 (m, 17H); 3.86–3.87 (t, 4H, J = 2 Hz); 6.48–6.58 (m, 3H); 6.86–6.88 (d, 1H, J = 8 Hz); 8.29–8.31 (d, 2H, J = 8 Hz).
The product was converted into the corresponding oxalate salt yellow solid; mp: 90–92 °C, Anal. Calcd for (C27H37N5O9) C, H, N.
Procedure H. Synthesis of (7-Methoxy-1,2,3,4-tetrahydro-naphthalen-2-yl)-propyl-amine (10a)
7-Methoxy-2-tetralone (10 g, 56.75 mmol) and acetic acid (13.5 ml, 226.9 mmol) were dissolved in dichloroethane (150 ml) and cooled to 0°C. n-Propylamine (11.7 ml, 141.87 mmol) was added and the mixture stirred under a N2 atmosphere for 30 min. NaCNBH3 (8.91 g, 141.87 mmol) in anhydrous MeOH (15 ml) was then added to the mixture and allowed to stir overnight at ambient temperature. The volatiles were then evaporated and saturated NaHCO3 solution was added. It was then extracted with dichloromethane dried over Na2SO4, filtered, and concentrated. The crude residue was then taken up in EtOAc, at which time ethereal HCl was added, and the crude salt was filtered and dried over vacuum oven. The crude salt was then recrystalized in ethanol to yield 9.5 g as white solid (yield 65%) and used in the subsequent transformations. 1H NMR (free base) (400 MHz, CDCl3) 0.91–0.95 (t, 3H, J = 7.6 Hz), 1.38 (bs, 1H), 1.48–1.60 (m, 3H), 2.04–2.09 (m, 1H), 2.54–2.62 (m, 2H), 2.67–2.71 (t, 3H, J = 7.6 Hz), 2.88–2.92 (m, 2H), 2.97–3.04 (m, 1H), 3.81 (s, 3H), 6.60–6.61 (dd, 1H, J = 1.6 Hz), 6.65–6.78 (m, 1H), 6.95–6.98 (d, 1H, J = 8.8 Hz).
Synthesis of (5-Methoxy-1,2,3,4-tetrahydro-naphthalen-2-yl)-propyl-amine(10b)
Compound 10b was prepared following Procedure H from 5-methoxy 2-tetralone (64%). 1H NMR (free base) (400 MHz, CDCl3) 0.92–0.96 (t, 3H, J = 7.6 Hz), 1.39 (bs, 1H), 1.49–1.61 (m, 3H), 2.05–2.10 (m, 1H), 2.53–2.62 (m, 2H), 2.66–2.70 (t, 3H, J = 7.6 Hz), 2.87–2.94 (m, 2H), 2.98–3.03 (m, 1H), 3.81 (s, 3H), 6.65–6.67 (d, 1H, J = 8 Hz), 6.96–6.71 (d, 1H, J = 8 Hz), 7.07–7.11 (t, 1H, J = 7.2 Hz).
Procedure I. Resolution of 5-Methoxy-N-propyl-1,2,3,4-tetrahydronaphthalen-2-amine for preparation of 11c and 11d
Racemic (±)−10b was resolved into its (+) and (−) isomers by using the both (−) and the (+) isomers of the synthetic resolving agent 4-(2-chlorophenyl)-5,5-dimethyl-2-hydroxy-1,3,2-dioxaphosphorinane 2-oxide. This optically active resolving agents were prepared according to the published procedure.20 10b (free base 14.77 g, 67.36 mmol) and (+)-4-(2-chlorophenyl)-5,5-dimethyl-2-hydroxy-1,3,2-dioxaphosphorinane 2-oxide (20.5 g, 74.1 mmol) were dissolved by warming in 100 ml of ethanol. The solution was cooled to room temperature and then at 0°C. The precipitated crystals were filtered off, washed with cold ether to yield 17.4 g of the salt ([α] D= (−)1.2°, c =1 in methanol). Further recrystallization two times from hot ethanol yielded the salt (12.9 g, [α] D= (−) 14.1°, c =1 in methanol). Further crystallization of the salt from hot ethanol did not change the optical rotation to a significant extent. The salt was then hydrolyzed in presence of 20% NaOH solution in water under stirred condition for 2 h at room temperature. The aqueous layer was extracted with dichloromethane (3×100 ml), dried over Na2SO4 and evaporated to dryness to yield 11c (5.8 g, [α] D of the white solid HCl salt of 11c = (−)71.5°, (c =1 in methanol) Yield. 78.5 %.
(±)−10b (18.5 g, 84.35 mmol) was similarly treated using (−)-4-(2-chlorophenyl)-5,5- dimethyl-2-hydroxy-1,3,2-dioxaphosphorinane 2-oxide (24.5 g, 88.57 mmol). Recrystallization from hot ethanol yielded the salt (16.2 g, [α] D = (+)−13.0, c = 1 in methanol). Yield is 78%. Further crystallization of the salt from hot ethanol did not change the optical rotation to a significant extent. Hydrolysis of the chlocyphos salt following above mentioned procedure yielded 11d white solid hydrochloride salt, [α]D of the HCl salt is (+)−69.8°, c =1 in methanol).
Resolution of 7-methoxy-N-propyl-1,2,3,4-tetrahydronaphthalen-2-amine for preparation of 11a and 11b
This resolution was done by using the above Procedure I. (±)−10a (5.99 g, 27.31 mmol) was similarly treated using (−)-4-(2-chlorophenyl)-5,5-dimethyl-2-hydroxy-1,3,2-dioxaphosphorinane 2-oxide (7.93 g, 28.68 mmol). Recrystallization from hot ethanol yielded the salt (5.4 g, [α] D = (+)−12.9°, c = 1 in methanol). Yield is 80 %. Further crystallization of the salt from hot ethanol did not change the optical rotation to a significant extent. Hydrolysis of the chlocyphos salt following above mentioned procedure yielded 11a as white solid hydrochloride salt, [α]D of the HCl salt is (+)−71.1° (c =1 in methanol).
(±)−10a was similarly treated using (+)-4-(2-chlorophenyl)-5,5-dimethyl-2-hydroxy-1,3,2- dioxaphosphorinane 2-oxide to afford 11b as white solid hydrochloride salt, [α]D of the HCl salt is (−) −69.8°, c =1 in methanol).
Procedure J. Synthesis of (+)-2-chloro-N-(7-methoxy-1,2,3,4-tetrahydronaphthalen-2-yl)-N-propylacetamide (12a)
Compound 11a (1.1 g, 5.01 mmol) and Et3N (3.5 ml, 25.05 mmol) was stirred at 0° C in CH2Cl2 (25 ml) for 15 min. Chloroacetylchloride (1.0 ml, 12.54 mmol) was added drop wise and the resulting solution was stirred at room temperature for 20 min. The reaction mixture was poured into a 1M solution of NaOH (25 ml) and the product was extracted with dichloromethane, dried (Na2SO4), filtered, and concentrated. The crude material was purified by column chromatography (Hex:EtOAc, 3:1) to give 12a as thick transparent liquid (1.26 g, 93.4 %): 1H NMR (400 MHz, CDCl3) ) δ 0.90–0.98 (m, 3H), 1.64–1.72 (m, 2H), 1.83–2.12 (m, 2H), 2.58–2.70 (m, 1H), 2.84–2.89 (dd, 1H, J1 = 16.0 Hz, J2 = 4.8 Hz), 3.00–3.10 (m, 2H), 3.15–3.26 (m, 2H), 3.82 (s, 3H), 3.95–4.03 (m, 1H), 4.08–4.12 (m, 2H), 6.61–6.62 (dd, 1H, J1 = 1.6 Hz, J2 = 4.8 Hz), 6.64–6.77 (m, 1H), 6.96–6.99 (d, 1H, J = 8.8 Hz).
Synthesis of (−)-2-chloro-N-(5-methoxy-1,2,3,4-tetrahydronaphthalen-2-yl)-N-propylacetamide (12b)
Compound 12b was prepared by following similar conditions as reported in the Procedure J. Compound 11c (HCl salt, 6.0 g, 23.46 mmol) was treated with Chloroacetylchloride (5.6 mL, 70.37 mmol) at 0° C in CH2Cl2 (100 ml) for 15 min to afford the optically pure 12b as a viscous oil (6.52 g, 94%): 1H NMR (400 MHz, CDCl3) δ ppm 0.92–0.96 (t, 3H, J = 8 Hz), 1.64–1.72 (m, 2H), 1.83–2.12 (m, 2H), 2.58–2.70 (m, 1H), 2.84–2.89 (dd, 1H, J1 = 16.0 Hz, J2 = 4.8 Hz), 3.00–3.10 (m, 2H), 3.19–3.27 (m, 2H), 3.86 (s, 3H), 3.95–4.03 (m, 1H), 4.08–4.12 (m, 2H), 6.61–6.68 (m, 2H), 7.07–7.11 (t, 1H, J = 8 Hz).
Synthesis of 2-chloro-N-(7-methoxy-1,2,3,4-tetrahydronaphthalen-2-yl)-N-propylacetamide (12c)
Compound 12c was prepared by following the similar conditions as reported in Procedure J. Compound 10a (HCl salt, 3.11 g, 12.18 mmol) was treated with Chloroacetylchloride (1.94 ml, 24.37 mmol) at 0° C in CH2Cl2 (100 ml) for 15 min to afford the racemic 12c as a viscous oil (3.42 g, 95%). 1H NMR (400 MHz, CDCl3) ) δ ppm 0.90–0.98 (m, 3H), 1.64–1.72 (m, 2H), 3.19–3.27 (m, 2H), 4.00 (s, 3H), 6.61–6.62 (dd, 1H, J = 1.6 Hz), 6.64–6.77 (m, 1H), 6.96–6.99 (d, 1H, J = 8.8 Hz).
Synthesis of tert-butyl 4-(4-iodophenyl)piperazine-1-carboxylate (14)
To a stirring solution of 1-(4-iodophenyl)piperazine, 13 (3.0 g, 9.24 mmol) in dichloromethane (25 mL), di-tert-butyl dicarbonate (2.42 g, 11.09 mmol) and triethylamine (3.84 mL, 27.73 mmol) were added at 0 °C and stirring was continued for another 4 h. The brine was added to the reaction mixture and extracted with dichloromethane and concentrated under vacuum which was then purified by column chromatography using ethylacetate: methanol: Et3N (80:15:5) to get pure compound 14 as yellow solid (2.44 g, 68%). 1H NMR (400 MHz, CDCl3) δ ppm 1.38 (s, 9H); 3.33–3.35 (t, 8H, J = 4 Hz); 6.53 (d, 2H, J = 6 Hz); 7.48 (m, 2H).
Procedure K. Synthesis of pyridin-3-ylboronic acid (16a)
A 100 mL three-neck flask was charged with toluene (17 mL) and cooled below −60 °C, and a solution of n-BuLi (2.85 M in hexanes, 6.1 mL, 17.4 mmol) was added dropwise over 10 min. After the internal temperature reached −60 °C, a solution of 3-bromopyridine (1.6 mL, 15.8 mmol) in toluene (8 mL) was added drop wise to keep the internal temperature below −50 °C. A brownish black solid precipitated, and the resultant slurry was stirred for 20 min. THF (10 mL) was added drop wise to keep the internal temperature below −50 °C, and the resultant slurry was stirred for 15 min. To the slurry was added triisopropyl borate (4.37 mL, 19 mmol) in one portion via syringe. The solution was warmed to −15 °C, the reaction was quenched with HCl (aq) (2.7 N, 14 mL), and the solution was transferred to a separatory funnel. The aqueous layer was collected, the organic layer was washed with water (10 mL), and the combined aqueous layers were neutralized to pH 7 with NaOH (aq) (10 N) and extracted with THF (30 mL × 3). The combined organic layers were concentrated in vacuo, and the residue was dissolved in THF/CH3OH (1:1, 30 mL), filtered, and diluted to 30 mL with CH3CN. The solvent was switched to CH3CN by distillation and concentrated to 20 mL. The solids were collected by filtration to afford the title compound 16a as a solid (0.98 g, 50 % yield). 1H NMR (400 MHz, CDCl3) δ ppm 7.71–7.74 (t, 1H, J = 6 Hz); 8.43–8.45 (d, 1H, J = 8 Hz); 8.51–8.53 (d, 1H, J = 8 Hz); 8.58 (s, 1H).
Synthesis of pyridin-4-ylboronic acid (16b)
This compound was prepared by following the procedure K using n-BuLi (2.85 M in hexanes, 13.5 mL, 38.57 mmol), a slury of hydrochloride salt of 4-bromopyridine (3.0 g, 15.4 mmol) in toluene (24.64 mL) and triisopropyl borate (5.3 mL, 23.14 mmol) to afford compound 16b as a solid (1.13 g, 60 % yield). 1H NMR (400 MHz, CDCl3) δ ppm 7.78–7.80 (d, 2H, J = 8 Hz); 8.32–8.34 (d, 2H, J = 8 Hz).
Procedure L. Synthesis of tert-butyl 4-(4-(pyridin-3-yl)phenyl)piperazine-1-carboxylate (17a)
Into a glass vial containing a magnetic stir bar is added the tert-butyl 4-(4-iodophenyl)piperazine-1-carboxylate (1.65 g, 4.26 mmol), and the vial is purged with argon. In to the vial was added a solution of tetrakis(triphenylphosphine) palladium(0) (0.32 g, 0.27 mmol) in dimethoxyethane (6.6 mL) and sodium carbonate (aq) (2 M, 5.53 mL, 11.06 mmol), and the vial was once again purged with argon. The resultant solution was stirred at room temperature for 5 min when the slurry of pyridin-3-ylboronic acid, 16a (0.68 g, 5.53 mmol) in ethanol (6.6 mL) was added, the vial was purged with argon and capped, and the mixture was heated to 90 °C and stirred for 1 h. The solution was cooled to room temperature and filtered through a pad of Celite (washed with dichloromethane) into a flask containing anhydrous magnesium sulfate. The solution was dried and filtered through filter paper and the solvent was removed in vacuo to afford the crude product, which was chromatographed on silica gel using ethylacetate/hexane (15:85) solvent system to afford pure compound 17a (0.85 g, 45%). 1H NMR (400 MHz, CDCl3) δ ppm 1.48 (s, 9H); 3.23–3.25 (t, 4H, J = 4 Hz); 3.56–3.60 (t, 4H, J = 8 Hz); 7.08–7.10 (d, 2H, J = 8 Hz); 7.45–7.48 (dd, 1H, J1 = 5.2 Hz, J2 = 8 Hz); 7.56–7.58 (m, 2H); 8.02–8.05 (d, 1H, J = 12 Hz); 8.41–8.44 (d, 1H, J = 12 Hz); 8.75 (s, 1H).
Synthesis of tert-butyl 4-(4-(pyridin-4-yl)phenyl)piperazine-1-carboxylate (17b)
Compound 17b was prepared according to the procedure L using tert-butyl 4-(4-iodophenyl)piperazine-1-carboxylate (0.22 g, 0.57 mmol), a solution of tetrakis(triphenylphosphine) palladium(0) (0.047g, 0.041mmol) in dimethoxyethane (1.0 mL) and sodium carbonate(aq) (2 M, 0.8 mL, 1.63 mmol), and the slurry of pyridin-4yl boronic acid, 16b (0.22 g, 5.53 mmol) in ethanol (1.0 mL) to afford pure compound 17b (0.10 g, 43%). 1H NMR (400 MHz, CDCl3) δ ppm 1.48 (s, 9H); 3.22–3.23 (t, 4H, J = 2 Hz); 3.58–3.60 (t, 4H, J = 4 Hz); 6.98–6.99 (d, 2H, J = 4 Hz); 7.45–7.46 (d, 2H, J = 4 Hz); 7.57–7.58 (d, 2H, J = 4 Hz); 8.58–8.59 (d, 2H, J = 4 Hz).
Procedure M. Synthesis of 1-(4-(pyridin-3-yl)phenyl)piperazine (18a)
Into the solution of tert-butyl 4-(4-(pyridin-3-yl)phenyl)piperazine-1-carboxylate (0.85 g, 2.5 mmol) in 10 mL of dry CH2Cl2 was added trifluoroacetic acid (10 mL) dropwise at room temperature. Reaction mixture was stirred at room temperature for 6 h. The reaction mixture was concentrated under reduced pressure. The trifluoroacetate salt was recrystalized from ethanol and the pure compound was made free base using sodium bicarbonate to afford sufficiently pure compound 18a as semi solid (0.55 g, 93%). 1H NMR (400 MHz, CDCl3) δ ppm 3.02–3.04 (t, 4H, J = 4 Hz); 3.23–3.26 (t, 4H, J = 6 Hz); 7.08–7.10 (d, 2H, J = 8 Hz); 7.45–7.48 (dd, 1H, J1 = 5.2 Hz, J2 = 8 Hz); 7.56– 7.58 (m, 2H); 8.02–8.05 (d, 1H, J = 12 Hz); 8.41–8.44 (d, 1H, J = 12 Hz); 8.75 (s, 1H).
Synthesis of 1-(4-(pyridin-4-yl)phenyl)piperazine (18b)
Compound 17b (0.86 g, 2.5 mmol) was deprotected by trifluoroacetic acid in dichloromethane (1:1) to yield compound 18b (procedure M) as semi solid (0.55 g, 91 %).1H NMR (400 MHz, CDCl3) δ ppm 3.29–3.32 (m, 8H); 7.08–7.10 (d, 2H, J = 8 Hz); 7.65–7.71 (m, 4H); 8.48–8.49 (d, 2H, J = 4 Hz). Synthesis of 2-chloro-1-(4-(4-(pyridin-3-yl)phenyl)piperazin-1-yl)ethanone (19a). Compound 19a was prepared under similar conditions as reported in procedure J. Compound 18a (0.60 g, 2.5 mmol) was treated with chloroacetylchloride (0.4 ml, 5.0 mmol) at 0° C in CH2Cl2 (25 ml) for 15 min to afford the 19a as thick yellow liquid (0.72 g, 91%). 1H NMR (400 MHz, CDCl3) δ ppm 3.23–3.25 (t, 4H, J = 4 Hz); 3.56–3.60 (t, 4H, J = 8 Hz); 4.08–4.12 (m, 2H); 7.08–7.10 (d, 2H, J = 8 Hz); 7.45–7.48 (dd, 1H, J1 = 5.2 Hz, J2 = 8 Hz); 7.56–7.58 (m, 2H); 8.02–8.05 (d, 1H, J = 12 Hz); 8.41–8.44 (d, 1H, J = 12 Hz); 8.75 (s, 1H).
Synthesis of 2-chloro-1-(4-(4-(pyridin-4-yl)phenyl)piperazin-1-yl)ethanone (19b)
Compound 19b was prepared under similar conditions as reported in procedure J. Compound 18b (0.48 g, 2.03 mmol) was treated with chloroacetylchloride (0.2 ml, 2.44 mmol) at 0° C in CH2Cl2 (10 ml) for 15 min to afford the 19b as thick yellow liquid (0.22 g, 44 %). 1H NMR (400 MHz, CDCl3) δ ppm 3.29–3.32 (m, 8H); 3.61–3.66 (m, 2H) 7.08–7.10 (d, 2H, J = 8 Hz); 7.65–7.71 (m, 4H); 8.48–8.49 (d, 2H, J = 4 Hz).
Procedure N. Synthesis of 2-((7-methoxy-1,2,3,4-tetrahydronaphthalen-2-yl)(propyl)amino)-1-(4-(4-(pyridin-3-yl)phenyl)piperazin-1-yl)ethanone 20a
This was made following the general procedure for N-alkylation where compound 19a (0.72 g, 2.28 mmol) was refluxed with 10a (0.58 g, 2.28 mmol) in CH3CN (50 ml) in presence of K2CO3 (0.947 g, 6.85 mmol) for 1h to furnish 20a as semi solid (0.61 g, 53.5 %). 1H NMR (400 MHz, CDCl3) δ ppm 0.80–0.84 (t, 3H, J = 8 Hz); 1.38–1.48 (m, 1H); 1.90–1.98 (m, 1H); 2.46–2.49 (t, 2H, J = 6 Hz); 2.62–2.76 (m, 4H); 2.82–2.88 (m, 2H); 2.94–2.99 (bs, 1H); 3.16–3.18 (t, 4H, J = 4 Hz); 3.41 (s, 2H); 3.63–3.83 (m, 4H); 3.91 (s, 3H); 6.61 (s, 1H); 6.65–6.67 (d, 1H, J = 8 Hz); 6.82–6.85 (d, 1H, J = 12 Hz); 6.93–6.95 (m, 2H); 7.26–7.29 (dd, 1H, J1 = 5.2 Hz, J2 = 8.4 Hz); 7.44–7.46 (d, 2H, J = 8 Hz); 7.77–7.80 (m, 1H); 8.45–8.46 (d, 1H, J = 4 Hz); 8.75 (s, 1H).
Synthesis of (−)-2-((7-methoxy-1,2,3,4-tetrahydronaphthalen-2-yl)(propyl)amino)-1-(4-(4-(pyridin-4-yl)phenyl)piperazin-1-yl)ethanone. (−) −20b
This was made by following the procedure N when compound 19b (0.25 g, 0.79 mmol) was reacted with 11b (0.17 g, 0.79 mmol) in CH3CN (25 ml) to furnish 20b as semi solid (0.12 g, 32 %). 1H NMR (400 MHz, CDCl3) δ ppm 0.88–0.91 (t, 3H, J = 6 Hz); 1.48–1.54 (m, 3H); 2.0 (bs, 1H); 2.55–2.59 (t, 2H, J = 8 Hz); 2.79–2.83 (m, 4H), 2.99–3.01 (m, 1H); 3.21–3.31 (m, 6H); 3.50 (s, 2H); 3.7 (s, 3H); 3.78–3.81 (t, 2H, J = 6 Hz); 6.61–6.64 (m, 2H); 6.91–6.93 (d, 1H, J = 8 Hz); 7.04–7.06 (d, 2H, J = 8 Hz); 7.62–7.68 (m, 4H); 8.46–8.47 (d, 2H, J = 1 Hz).
Synthesis of (−)-2-((5-methoxy-1,2,3,4-tetrahydronaphthalen-2-yl)(propyl)amino)-1-(4-(4-(pyridin-4-yl)phenyl)piperazin-1-yl)ethanone. (−) −20c
This was made by following the procedure N when compound 19b (0.22 g, 0.71 mmol) was reacted with 11c (0.133 g, 0.61 mmol) in CH3CN (15 ml) to furnish 20c as semisolid (0.12 g, 41.7 %). 1H NMR (400 MHz, CDCl3) δ ppm 0.91–0.93 (t, 3H, J = 4 Hz); 1.42–1.56 (m, 3H); 2.18 (bs, 1H); 2.46–3.12 (m, 13H); 3.32–3.34 (t, 4H, J = 4 Hz); 3.86 (s, 3H); 6.61–6.67 (m, 3H); 7.01–7.06 (m, 2H); 7.62–7.67 (m, 4H); 8.51–8.53 (d, 2H, J = 8 Hz)
Synthesis of (+)-2-((7-methoxy-1,2,3,4-tetrahydronaphthalen-2-yl)(propyl)amino)-1-(4-(4-(pyridin-4-yl)phenyl)piperazin-1-yl)ethanone. (+)-20d
This was made by following the procedure N when compound 19b (0.25 g, 0.78 mmol) was reacted with 11a (0.17 g, 0.78 mmol) in CH3CN (20 ml) to furnish 20d as semi solid (0.13 g, 33.7 %). 1H NMR (400 MHz, MeOH-d4) δ ppm 0.89–0.93 (t, 3H, J = 7.2 Hz); 1.46–1.68 (m, 3H); 2.0 (br s, 1H); 2.57–2.61 (t, 2H, J = 7.6 Hz); 2.72–2.84 (m, 4H), 2.99– 3.01 (m, 1H); 3.22–3.31 (m, 6H); 3.53 (s, 2H); 3.72 (s, 3H); 3.78–3.82 (m, 2H); 6.61–6.65 (m, 2H); 6.93–6.95 (d, 1H, J = 8 Hz); 7.07–7.09 (d, 2H, J = 8.8 Hz); 7.62–7.70 (m, 4H); 8.48–8.50 (d, 2H, J = 5.6 Hz).
Synthesis of 7-methoxy-N-propyl-N-(2-(4-(4-(pyridin-3-yl)phenyl)piperazin-1-yl)ethyl)-1,2,3,4-tetrahydronaphthalen-2-amine (21a)
Compound 20a (0.61 g, 1.22 mmol) was reacted with LiAlH4 (0.23 g, 6.1 mmol) in THF (20 mL) by following the procedure F to furnish 21a as oil (0.302 g, 51 %). 1H NMR (400 MHz, CDCl3) δ ppm 0.80–0.84 (t, 3H, J = 8 Hz); 1.38–1.48 (m, 1H); 1.90–1.98 (m, 1H); 2.46–2.49 (t, 2H, J = 6 Hz); 2.62–2.76 (m, 4H); 2.82–2.88 (m, 2H); 2.94–2.99 (bs, 1H); 3.16–3.18 (t, 4H, J = 4 Hz); 3.41 (s, 2H); 3.63–3.83 (m, 6H); 3.91 (s, 3H); 6.61 (s, 1H); 6.65–6.67 (d, 1H, J = 8 Hz); 6.82–6.85 (d, 1H, J = 12 Hz); 6.93–6.95 (m, 2H); 7.266–7.299 (dd, 1H, J1 = 5.2 Hz, J2 = 8.4 Hz); 7.44–7.46 (d, 2H, J = 8 Hz); 7.77–7.80 (m, 1H); 8.45–8.46 (d, 1H, J = 4 Hz); 8.75 (s, 1H).
Synthesis of (−)-7-methoxy-N-propyl-N-(2-(4-(4-(pyridin-4-yl)phenyl)piperazin-1-yl)ethyl)-1,2,3,4-tetrahydronaphthalen-2-amine. (−) −21b
Compound 20b (0.07 g, 0.15 mmol) was reacted with LiAlH4 (0.06 g, 1.5 mmol) in THF (20 mL) by following the procedure F to furnish 21b as oil (0.041 g, 56.2 %). 1H NMR (400 MHz, CDCl3) δ ppm ppm 0.94–0.96 (t, 3H, J = 4 Hz); 1.58–1.63 (m, 2H); 2.15–2.18 (m, 1H); 2.61–2.94 (m, 12H); 3.12 (bs, 1H); 3.28–3.31 (t, 4H, J = 6 Hz); 3.86 (s, 3H); 6.61 (s, 1H); 6.65–6.67 (d, 1H, J = 8 Hz); 6.84–6.86 (d, 2H, J = 8 Hz); 6.96–6.98 (d, 1H, J = 8 Hz);7.63–7.68 (m, 4H); 8.50–8.52 (d, 2H, J = 8 Hz).
Synthesis of (−)-5-methoxy-N-propyl-N-(2-(4-(4-(pyridin-4-yl)phenyl)piperazin-1-yl)ethyl)-1,2,3,4-tetrahydronaphthalen-2-amine. (−) −21c
Compound 20c (0.125 g, 0.25 mmol) was reacted with LiAlH4 (0.095 g, 2.5 mmol) in THF (15 mL) by following the procedure F to furnish 21c as oil (0.70 g, 72 %). 1H NMR (400 MHz, CDCl3) δ ppm 0.91–0.93 (t, 3H, J = 4 Hz); 1.42–1.56 (m, 3H); 2.18 (bs, 1H); 2.46–3.12 (m, 15H); 3.32–3.34 (t, 4H, J = 4 Hz); 3.86 (s, 3H); 6.61–6.67 (m, 3H); 7.01–7.06 (m, 2H); 7.62–7.67 (m, 4H); 8.51–8.53 (d, 2H, J = 8 Hz)
Synthesis of (+)-7-methoxy-N-propyl-N-(2-(4-(4-(pyridin-4-yl)phenyl)piperazin-1-yl)ethyl)-1,2,3,4-tetrahydronaphthalen-2-amine. (+)−21d
Compound 20d (0.12 g, 0.24 mmol) was reacted with LiAlH4 (0.09 g, 2.4 mmol) in THF (15 mL) by following the procedure F to furnish 21d as oil (0.072 g, 62.1 %). 1H NMR (400 MHz, MeOH-d4) δ ppm 0.93–0.96 (t, 3H, J = 7.2 Hz); 1.55–1.67 (m, 3H); 2.01–2.15 (m, 1H); 2.57–2.91 (m, 16H); 3.00–3.07 (m, 2H); 3.57 (m, 1H); 3.73 (s, 3H); 6.64-6-67 (m, 2H); 6.94–6.96 (d, 1H, J = 8 Hz); 7.04–7.06 (d, 2H, J = 8.8 Hz); 7.63–7.68 (m, 4H); 8.46–8.48 (d, 2H, J = 6 Hz).
Synthesis of 7-(propyl(2-(4-(4-(pyridin-3-yl)phenyl)piperazin-1-yl)ethyl)amino)-5,6,7,8-tetrahydronaphthalen-2-ol (22a)
Compound 21a (0.302 g, 0.62 mmol) was reacted with 1 M BBr3/CH2Cl2 (2.5 mL, 2.5 mmol) in CH2Cl2 (20 mL) by following the procedure G to furnish 22a (0.181 g, 62 %): 1H NMR (400 MHz, CDCl3) δ ppm 0.87–0.91 (t, 3H, J = 8 Hz); 1.46–1.58 (m, 2H); 1.97–2.00 (bs, 1H); 2.53–2.79 (m, 15H); 2.97 (bs, 1H); 3.27–3.30 (t, 4H, J = 6 Hz); 6.52 (s, 1H), 6.59–6.61 (d, 1H, J = 8 Hz); 6.88–6.90 (d, 1H, J = 8 Hz); 6.98–7.00 (d, 2H, J = 8 Hz); 7.316–7.349 (dd, 1H, J1 = 5.6 Hz, J2 = 8.88 Hz); 7.48–7.50 (d, 2H, J = 8 Hz), 7.83–7.86 (m, 1H); 8.50–8.515 (d, 1H, J = 6 Hz); 8.81 (s, 1H).
The product was converted into the corresponding oxalate salt as white solid; mp is 161–163 °C. Anal. Calcd for (C36H44N4O13, 0.5H2O) C, H, N.
Synthesis of (−)-7-(propyl(2-(4-(4-(pyridin-4-yl)phenyl)piperazin-1-yl)ethyl)amino)-5,6,7,8-tetrahydronaphthalen-2-ol (−)−22b
Compound 21b (0.04 g, 0.083 mmol) was reacted with 1 M BBr3/CH2Cl2 (0.41 mL, 0.41 mmol) in CH2Cl2 (10 mL) by following the procedure G to furnish 22b (0.02 g, 59 %): 1H NMR (400 MHz, CDCl3) δ ppm 0.94–0.96 (t, 3H, J = 4 Hz); 1.58–1.63 (m, 2H); 2.15–2.18 (m, 1H); 2.61–2.94 (m, 12H); 3.12 (bs, 1H); 3.28–3.31 (t, 4H, J = 6 Hz); 6.60 (s, 1H); 6.66–6.68 (d, 1H, J = 8 Hz); 6.84–6.86 (d, 2H, J = 8 Hz); 6.98–7.00 (d, 1H, J = 8 Hz); 7.63–7.68 (m, 4H); 8.50–8.52 (d, 2H, J = 8 Hz). [α]25D= (−) −22.4°, c = 0.5 in MeOH. The product was converted into the corresponding tetrahydrochloride salt as white solid; mp is 227–230 °C. Anal. Calcd for (C30H42N4Cl4O, 2H2O) C, H, N.
Synthesis of (−)-6-(propyl(2-(4-(4-(pyridin-4-yl)phenyl)piperazin-1-yl)ethyl)amino)-5,6,7,8-tetrahydronaphthalen-1-ol (−) −22c
Compound 21c (0.07 g, 0.14 mmol) was reacted with 1 M BBr3/CH2Cl2 (0.72 mL, 0.72 mmol) in CH2Cl2 (15 mL) by following the procedure G to furnish 22c (0.05 g, 81 %): 1H NMR (400 MHz, CDCl3) δ ppm 0.91–0.94 (t, 3H, J = 6 Hz); 1.48–1.65 (m, 3H); 2.08–2.13 (bs, 1H); 2.46–3.03 (m, 15H); 3.26–3.30 (t, 4H, J = 8 Hz); 6.53–6.57 (m, 2H); 6.87–6.90 (t, 1H, J = 6 Hz); 7.03–7.05 (d, 2H, J = 8 Hz); 7.62–7.67 (m, 4H); 7.45–8.47 (d, 2H, J = 8 Hz). [α]25D= (−) −37.5°, c = 1 in MeOH. The product was converted into the corresponding oxalate salt as white solid; mp : 162–165 °C. Anal. Calcd for (C36H44N4O13, 0.5C4H10O) C, H, N.
Synthesis of (+)-7-(propyl(2-(4-(4-(pyridin-4-yl)phenyl)piperazin-1-yl)ethyl)amino)-5,6,7,8-tetrahydronaphthalen-2-ol (+)−22d
A solution of compound 21d (0.06 g, 0.12 mmol) in 48% aq. HBr (3 mL) was refluxed for 2 h. The reaction mixture was cooled to room temperature and evaporated to give a solid. The resulting solid was neutralized by addition of saturated NaHCO3 solution at 0°C and extracted with dichloromethane, and concentrated under reduced pressure. The crude material was purified by column chromatography (DCM:MeOH, 9:1) to give compound 22d (0.035 g, 60 %): 1H NMR (400 MHz, MeOH-d4) δ ppm 0.96–1.00 (t, 3H, J = 7.2 Hz); 1.62–1.76 (m, 3H); 2.12–2.15 (m, 1H); 2.66–2.97 (m, 14H); 3.06 (m, 2H); 3.28–3.34 (m, 3H); 6.55–6.58 (m, 2H); 6.88–6.90 (d, 1H, J = 8 Hz); 7.04–7.06 (d, 2H, J = 8.8 Hz); 7.63–7.68 (m, 4H); 8.46–8.47 (d, 2H, J = 5.6 Hz). 13C NMR (400 MHz, MeOH-d4) δ ppm 10.65; 20.21; 25.39; 28.09; 30.92; 53.16; 53.54; 55.60; 59.34; 113.63; 115.19; 115.70; 120.79; 126.44; 127.53; 127.59; 129.28; 135.43; 149.08; 149.14; 152.31; 155.34. [α]25D= (+)−27.2°, c = 0.5 in MeOH. The product was converted into the corresponding tetrahydrochloride salt; mp is 132–134 °C. Anal. Calcd for (C30H42N4Cl4O, 2.3H2O) C, H, N
Synthesis of 2-(4-(4-iodophenyl)piperazin-1-yl)-N-(7-methoxy-1,2,3,4-tetrahydronaphthalen-2-yl)-N-propylacetamide (23)
This compound was prepared by following the N-alkylation procedure N. Compound 13 (HCl salt, 0.50 g, 1.39 mmol) was reacted with 12c (0.61 g, 2.08 mmol) in CH3CN (50 ml) to furnish 23 as semi solid (0.51 g, 67.4 %). 1H NMR (400 MHz, CDCl3) δ ppm 0.87–0.89 (t, 3H, J = 4 Hz); 1.60–1.66 (m, 3H); 1.98–2.04 (bs, 1H); 2.48–2.52 (t, 2H, J = 8 Hz); 2.69–2.81 (m, 4H); 2.96–3.01 (m, 1H); 3.10–3.14 (t, 4H, J = 8 Hz); 3.44–3.45 (d, 2H, J = 4 Hz); 3.65–3.86 (m, 4H); 3.91 (s, 3H); 6.61 (s, 1H); 6.65–6.66 (d, 1H, J = 4 Hz); 6.67–6.69 (d, 2H, J = 8 Hz); 6.96–6.98 (d, 1H, J = 8 Hz); 7.52–7.54 (d, 2H, J = 8 Hz).
Synthesis of N-(2-(4-(4-iodophenyl)piperazin-1-yl)ethyl)-7-methoxy-N-propyl-1,2,3,4-tetrahydronaphthalen-2-amine (24)
Compound 23 (0.51 g, 0.93 mmol) was reacted with LiAlH4 (0.17 g, 4.68 mmol) in THF (20 mL) by following the procedure F to furnish 24 as white semi solid (0.34 g, 68.5 %). 1H NMR (400 MHz, CDCl3) δ ppm 0.87–0.89 (t, 3H, J = 4 Hz); 1.60–1.66 (m, 3H); 1.98–2.04 (bs, 1H); 2.48–2.52 (t, 2H, J = 8 Hz); 2.69–2.81 (m, 4H); 2.96–3.01 (m, 3H); 3.10–3.14 (t, 4H, J = 8 Hz); 3.44–3.45 (d, 2H, J = 4 Hz); 3.65–3.86 (m, 4H); 3.91 (s, 3H); 6.61 (s, 1H); 6.65–6.66 (d, 1H, J = 4 Hz); 6.67–6.69 (d, 2H, J = 8 Hz); 6.96–6.98 (d, 1H, J = 8 Hz); 7.52–7.54 (d, 2H, J = 8 Hz).
Synthesis of 7-((2-(4-(4-iodophenyl)piperazin-1-yl)ethyl)(propyl)amino)-5,6,7,8-tetrahydronaphthalen-2-ol (25)
Compound 24 (0.30 g, 0.56 mmol) was reacted with 1 M BBr3/CH2Cl2 (2.25 mL, 2.25 mmol) in CH2Cl2 (20 mL) by following the procedure G to furnish 25 (0.15 g, 52 %): 1H NMR (400 MHz, CDCl3) δ ppm 0.87–0.90 (t, 3H, J = 6 Hz); 1.12–1.13 (t, 2H, J = 2 Hz); 1.48–1.54 (m, 3H); 1.95 (bs, 1H); 2.47–2.77 (m, 12H); 2.94 (bs, 1H); 3.17–3.19 (t, 4H, J = 4 Hz); 6.49 (s, 1H); 6.55–6.58 (d, 1H, J = 12 Hz); 6.64–6.68 (m, 2H); 6.89–6.90 (d, 1H, J = 4Hz); 7.48–7.50 (d, 2H, J = 8 Hz). The product was converted into the corresponding oxalate salt as yellowish solid; mp is 177–179 °C. Anal. Calcd for (C29H38N3O9I, 0.1C4H10O) C, H, N.
Synthesis of (+)-2-(4-(biphenyl-4-yl)piperazin-1-yl)-N-(7-methoxy-1,2,3,4-tetrahydronaphthalen-2-yl)-N-propylacetamide (26a)
Following the procedure N compound 12a (0.75 g, 2.5 mmol) and 2a (0.73 g, 3.04 mmol), K2CO3 (1.75 g, 12.68 mmol) were refluxed in CH3CN (25 ml) for 1h to get 0.77 g (61 %) of compound 26a as semi solid. 1H NMR (400 MHz, CDCl3) δ ppm 0.895–0.934 (t, 3H, J = 7.8 Hz); 1.64–1.71(m, 2H); 1.88–2.04 (m, 2H); 2.166–2.172 (d, 2H); 2.69–3.39 (m, 15H); 3.76 (s, 3H); 6.59 (s, 1H); 6.68–6.73 (t, 1H, J = 10 Hz); 6.97–7.03 (m, 3H); 7.26–7.30 (t, 1H, J = 8 Hz); 7.38–7.42 (m, 2H); 7.50–7.56 (m, 4H).
Synthesis of (−)-2-(4-(biphenyl-4-yl)piperazin-1-yl)-N-(5-hydroxy-1,2,3,4-tetrahydronaphthalen-2-yl)-N-propylacetamide (26b)
Compound 12b (0.25 g, 0.85 mmol) was reacted with 2a (0.24 g, 1.01 mmol) in CH3CN (25 ml) by following the procedure N to furnish 26b as semi solid (0.24 g, 57 %). 1H NMR (400 MHz, CDCl3) δ ppm 0.89–0.93 (t, 3H, J = 8 Hz); 1.67–1.70 (m, 2H); 2.07–2.08 (bs, 1H); 2.54–2.60 (m, 3H); 2.66–2.69 (t, 4H, J = 6 Hz); 2.75–3.03 (m, 6H); 3.24–3.27 (t, 4H, J = 6 Hz); 3.68–3.71 (t, 1H, J = 6 Hz); 3.81 (s, 3H); 6.64–6.66 (d, 1H, J = 8 Hz); 6.71–6.73 (d, 1H, J = 8 Hz); 6.98–7.01 (d, 2H, J = 12 Hz); 7.07–7.11 (t, 1H, J = 8 Hz); 7.26–7.29 (t, 1H, J = 6 Hz); 7.38–7.42 (t, 2H, J = 8 Hz); 7.50–7.56 (m, 4H).
Synthesis of (+)-N-(2-(4-(biphenyl-4-yl)piperazin-1-yl)ethyl)-7-methoxy-N-propyl-1,2,3,4-tetrahydronaphthalen-2-amine (27a)
Compound 27a was prepared by using the procedure F where compound 26a (0.77 g, 1.55 mmol) in anhydrous THF (20 mL) was added dropwise into a suspension of lithium aluminum hydride (LiAlH4) (0.15 g, 4.07 mmol) in anhydrous THF (15 mL) at 0 °C under N2 atmosphere to afford compound 27a as semi solid (0.62 g, 84 %): 1H NMR (400 MHz, CDCl3) δ ppm 0.89–0.93 (t, 3H, J = 8 Hz); 1.49–1.69 (m, 2H); 2.03–2.05 (bs, 1H); 2.54–2.88 (m, 15H); 3.01 (bs, 1H); 3.25–3.27 (t, 4H, J = 4 Hz); 3.77 (3, 3H); 6.64 (s, 1H); 6.67–6.69 (d, 1H, J = 8 Hz); 6.98–7.00 (d, 3H, J = 8 Hz); 7.26–7.29 (t, 1H, J = 6 Hz); 7.38–7.42 (t, 2H, J = 8 Hz); 7.50–7.56 (m, 4H).
Synthesis of (−)-N-(2-(4-(biphenyl-4-yl)piperazin-1-yl)ethyl)-5-methoxy-N-propyl-1,2,3,4-tetrahydronaphthalen-2-amine (27b)
Compound 26b (0.24 g, 0.45 mmol) was reacted with LiAlH4 (0.14 g, 3.9 mmol) in THF (20 mL) by following the procedure F to furnish 27b as semi solid (0.18 g, 77 %). 1H NMR (400 MHz, CDCl3) δ ppm 0.89–0.93 (t, 3H, J = 8 Hz); 1.67–1.70 (m, 2H); 2.07–2.08 (bs, 1H); 2.54–2.60 (m, 3H); 2.66–2.69 (t, 4H, J = 6 Hz); 2.75–3.03 (m, 8H); 3.24–3.27 (t, 4H, J = 6 Hz); 3.68–3.71 (t, 1H, J = 6 Hz); 3.81 (s, 3H); 6.64–6.66 (d, 1H, J = 8 Hz); 6.71–6.73 (d, 1H, J = 8 Hz); 6.98–7.01 (d, 2H, J = 12 Hz); 7.07–7.11 (t, 1H, J = 8 Hz); 7.26–7.29 (t, 1H, J = 6 Hz); 7.38–7.42 (t, 2H, J = 8 Hz); 7.50–7.56 (m, 4H).
Synthesis of (+)-7-((2-(4-(biphenyl-4-yl)piperazin-1-yl)ethyl)(propyl)amino)-5,6,7,8-tetrahydronaphthalen-2-ol (+)−8a
Compound 27a (0.62 g, 1.28 mmol) was reacted with 1 M BBr3/CH2Cl2 (5.13 mL, 5.13 mmol) in CH2Cl2 (20 mL) by following the procedure G to furnish (+)−8a (0.46 g, 77.6 %): 1H NMR (400 MHz, CDCl3) δ ppm 0.88–0.92 (t, 3H, J = 8 Hz); 1.47–1.54 (m, 2H); 1.95–1.98 (bs, 1H); 2.55–2.73 (m, 15H); 2.95 (bs, 1H); 3.29 (m, 4H); 6.48 (s, 1H); 6.56–6.59 (d, 1H, J = 12 Hz); 6.88–6.90 (d, 1H, J = 8 Hz); 6.97–6.99 (d, 2H, J = 8 Hz); 7.26–7.30 (t, 1H, J = 8 Hz); 7.38–7.42 (t, 2H, J = 8 Hz); 7.50–7.56 (m, 4H). [α]25D= (+)−33.4°, c = 1 in MeOH. The product was converted into the corresponding trihydrochloride salt, white solid, mp is 190–192 °C. Anal. Calcd for (C31H42N3Cl3O, 1.2H2O) C, H, N.
Synthesis of (−)-6-((2-(4-(biphenyl-4-yl)piperazin-1-yl)ethyl)(propyl)amino)-5,6,7,8-tetrahydronaphthalen-1-ol (−) −28b
Compound 27b (0.18 g, 0.372 mmol) was reacted with 1 M BBr3/CH2Cl2 (1.9 mL, 1.9 mmol) in CH2Cl2 (20 mL) by following the procedure G to furnish 28b (0.13 g, 79 %): 1H NMR (400 MHz, CDCl3) δ ppm 0.88–0.92 (t, 3H, J = 8 Hz); 1.50 (m, 3H); 2.05 (bs, 1H); 2.46–2.73 (m, 13H); 2.91–2.95 (m, 2H); 3.29–3.30 (t, 4H, J = 2 Hz); 6.545–6.599 (dd, 2H, J1 = 8 Hz, J2 = 14.4 Hz); 6.94–7.00 (m, 3H); 7.26–7.30 (t, 1H, J = 8 Hz); 7.38–7.42 (t, 2H, J = 8 Hz); 7.50–7.56 (m, 4H). [α]25D= (−) −35.6 °, c = 1 in MeOH. The product was converted into the corresponding trihydrochloride salt, white solid, mp is 200–203 °C. Anal. Calcd for (C31H42N3Cl3O, H2O) C, H, N.
Biological experiments: potencies at dopamine D2 and D3 receptors
Compounds were tested for inhibition of radioligand binding to dopamine receptors as described in our previous studies.12, 21 Briefly, membranes from human embryonic kidney (HEK) 293 cells expressing rat D2 and D3 receptors were incubated with each test compound and [3H]spiperone (1.6 nM, 15 Ci/mmole, Perkin Elmer) for 1 h at 30°C in 50 mM Tris-HCl (pH 7.4), 0.9% NaCl, and 0.025% ascorbic acid. The final volume of the assay was 0.2 ml under conditions corresponding to our “high [radioligand] protocol” as described recently.21 (+)-Butaclamol (2 μM) was used to define nonspecific binding. Assays were terminated by filtration in the MACH 3–96 Tomtec harvester (Wallac, Gaithersburg, MD). Observed IC50 values were converted to inhibition constants (Ki) by the Cheng-Prusoff equation.20 In this conversion, the Kd values for [3H]spiperone binding were 0.057 nM for D2 receptors and 0.125 nM for D3 receptors.
Functional activity of test compounds in activating dopamine hD2 and hD3 receptors expressed in CHO cells was measured by stimulation of binding of [35S]GTPγS (1250 Ci/mmole, Perkin-Elmer) in comparison to stimulation by the full agonist dopamine as described by us previously.12
Table 3.
Elemental Analysis of target compounds
| Compound | Elemental Analysis | |||||
|---|---|---|---|---|---|---|
| Calculated | Found | |||||
| C | H | N | C | H | N | |
| 8a | 62.93 | 7.39 | 7.10 | 63.11 | 7.46 | 6.70 |
| 8b | 52.47 | 7.15 | 10.20 | 52.42 | 7.26 | 9.92 |
| 8c | 52.37 | 6.24 | 8.14 | 52.05 | 6.23 | 8.54 |
| 8d | 54.47 | 7.85 | 10.59 | 54.07 | 7.48 | 10.20 |
| 8e | 56.34 | 6.48 | 12.17 | 56.08 | 6.49 | 12.00 |
| 22a | 57.67 | 6.05 | 7.47 | 57.46 | 6.06 | 7.33 |
| (−)-22b | 55.22 | 7.11 | 8.59 | 55.54 | 6.95 | 8.23 |
| (−)-22c | 58.68 | 6.35 | 7.20 | 58.95 | 6.73 | 6.90 |
| (+)-22d | 54.77 | 7.14 | 8.52 | 54.54 | 7.31 | 8.19 |
| 25 | 49.95 | 5.56 | 5.94 | 50.32 | 5.75 | 6.28 |
| (+)-8a | 61.99 | 7.45 | 7.00 | 61.92 | 7.21 | 6.90 |
| (−) −28b | 62.36 | 7.43 | 7.04 | 62.45 | 7.55 | 6.85 |
Acknowledgments
This work is supported by National Institute of Neurological Disorders and Stroke/ National Institute of Health (NS047198, AKD). We are grateful to Dr. K. Neve, Oregon Health and Science University, Portland, USA, for D2 and D3 expressing HEK cells. We are also grateful to Dr. J. Shine, Garvan Institute for Medical Research, Sydney, Australia, for D2 expressing CHO cells.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Emilien G, Maloteaux JM, Geurts M, Hoogenberg K, Cragg S. Pharmacology & Therapeutics. 1999;84:133. doi: 10.1016/s0163-7258(99)00029-7. [DOI] [PubMed] [Google Scholar]
- 2.Vallone D, Picetti R, Borrelli E. Neurosci Biobehav Rev. 2000;24:125. doi: 10.1016/s0149-7634(99)00063-9. [DOI] [PubMed] [Google Scholar]
- 3.Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Physiol Rev. 1998;78:189. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- 4.Zhang A, Neumeyer JL, Baldessarini RJ. Chem Rev. 2007;107:274. doi: 10.1021/cr050263h. [DOI] [PubMed] [Google Scholar]
- 5.Kuzhikandathil EV, Westrich L, Bakhos S, Pasuit J. Mol Cell Neurosci. 2004;26:144. doi: 10.1016/j.mcn.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 6.Beom S, Cheong D, Torres G, Caron MG, Kim KM. J Biol Chem. 2004;279:28304. doi: 10.1074/jbc.M403899200. [DOI] [PubMed] [Google Scholar]
- 7.Sokoloff P, Giros B, Martres MP, Andrieux M, Besancon R, Pilon C, Bouthenet ML, Souil E, Schwartz JC. Arzneimittelforschung. 1992;42:224. [PubMed] [Google Scholar]
- 8.Joyce JN, Millan MJ. Curr Opin Pharmacol. 2007;7:100. doi: 10.1016/j.coph.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Van Kampen JM, Eckman CB. J Neurosci. 2006;26:7272. doi: 10.1523/JNEUROSCI.0837-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boeckler F, Gmeiner P. Pharmacology & therapeutics. 2006;112:281. doi: 10.1016/j.pharmthera.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 11.Heidbreder CA, Newman AH. Ann N Y Acad Sci. 2010;1187:4. doi: 10.1111/j.1749-6632.2009.05149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Micheli F, Heidbreder C. Recent Pat CNS Drug Discov. 2006;1:271. doi: 10.2174/157488906778773634. [DOI] [PubMed] [Google Scholar]
- 13.Sartania N, Strange PG. J Neurochem. 1999;72:2621. doi: 10.1046/j.1471-4159.1999.0722621.x. [DOI] [PubMed] [Google Scholar]
- 14.Dutta AK, Venkataraman SK, Fei XS, Kolhatkar R, Zhang S, Reith ME. Bioorg Med Chem. 2004;12:4361. doi: 10.1016/j.bmc.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 15.Biswas S, Hazeldine S, Ghosh B, Parrington I, Kuzhikandathil E, Reith ME, Dutta AK. J Med Chem. 2008;51:3005. doi: 10.1021/jm701524h. [DOI] [PubMed] [Google Scholar]
- 16.Biswas S, Zhang S, Fernandez F, Ghosh B, Zhen J, Kuzhikandathil E, Reith ME, Dutta AK. J Med Chem. 2008;51:101. doi: 10.1021/jm070860r. [DOI] [PubMed] [Google Scholar]
- 17.Brown DA, Kharkar PS, Parrington I, Reith ME, Dutta AK. J Med Chem. 2008;51:7806. doi: 10.1021/jm8008629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghosh B, Antonio T, Zhen J, Kharkar P, Reith ME, Dutta AK. J Med Chem. 2010;53:1023. doi: 10.1021/jm901184n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frederic Paul JP, Hartwig John F. J Am Chem Soc. 1994;116:5969. [Google Scholar]
- 20.Hoeve WWH. J Org Chem. 1985;50:4508. [Google Scholar]
- 21.Jensen MS, Hoerrner RS, Li W, Nelson DP, Javadi GJ, Dormer PG, Cai D, Larsen RD. J Org Chem. 2005;70:6034. doi: 10.1021/jo050741o. [DOI] [PubMed] [Google Scholar]
- 22.Denton TT, Zhang X, Cashman JR. Journal of Medicinal Chemistry. 2005;48:224. doi: 10.1021/jm049696n. [DOI] [PubMed] [Google Scholar]