Summary
Many respiratory pathogens establish persistent infection or a carrier state in the human nasopharynx without overt disease symptoms but the presence of these in the lungs usually results in disease. Although the anatomy and microenvironments between nasopharynx and lungs are different, a virulence factor with an organ-specific function in the colonization of the nasopharynx is unknown. In contrast to the severity of pertussis and mortality in non vaccinated young children, Bordetella pertussis results in milder and prolonged cough in vaccinated adolescents and adults. Individuals harboring bacteria in the nasopharynx serve as reservoirs for intra-familial and nosocomial transmission. We show that the Bps polysaccharide of B. pertussis is critical for initial colonization of the mouse nose and the trachea but not of the lungs. Our data reveal a biofilm lifestyle for B. pertussis in the nose and the requirement of Bps in this developmental process. Bps functions as an adhesin by promoting adherence of B. pertussis and E. coli to human nasal but not to human lung epithelia. Patient serum specifically recognized Bps suggesting its expression during natural human infections. We describe the first bacterial factor that exhibits a differential role in colonization and adherence between the nasopharynx and the lungs.
Introduction
Bacterial pathogens utilize numerous strategies to initiate intimate association with their host organisms. These interactions can be beneficial, neutral or harmful to their hosts. Association with the respiratory mucosa is a major step that leads to successful colonization, multiplication and transmission. While studies of bacterial virulence factors have provided significant insights into the development of the disease state, little is known about mechanisms that allow bacteria to utilize the same hosts as reservoirs without causing disease and subsequent death of the host. Among the respiratory tract organs, the human nasopharyngeal cavity serves as the primary site for the development of persistent infections for a myriad of bacterial pathogens, including the Gram negative bacterium Bordetella pertussis (Hava et al., 2003, Mattoo & Cherry, 2005, Cole et al., 2001). The yearly number of pertussis cases worldwide has been estimated to be in the range of 20–40 million per year (Tan et al., 2005). While B. pertussis causes typical acute and life-threatening disease whooping cough in very young unvaccinated children (Carbonetti, 2007, Cherry, 2005), it can cause prolonged cough with milder symptoms in previously vaccinated adolescents and adults (Strebel et al., 2001, Wilson, 2006). The most notable shift in incidence of pertussis cases in many developed countries has been in individuals 15 or older and is estimated to exceed more than one million a year (Strebel et al., 2001). Family members including siblings and parents have been found to serve as sources of infection for unvaccinated young children (Raymond et al., 2007).
Despite the well-documented role of nasopharyngeal colonization in the development of bacterial disease and the establishment of persistent infections or the carrier state, the identity and the role of bacterial factors that promote naso-bacterial interactions are poorly understood (Hava et al., 2003, Foster, 2004). While it has been argued that differences in anatomical features and microenvironment between the nasopharynx and lungs necessitate a specific nasopharyngeal colonization factor (Hava et al., 2003), the existence of such a factor is not known.
In this report, we have identified one such bacterial factor, the Bps polysaccharide of B. pertussis. The Bordetella bps locus is critical for the synthesis of a polysaccharide that is similar to the poly-β-1,6-N-acetylglucosamine polysaccharides (PGA/PIA/PNAG) synthesized by multiple pathogens and other bacteria (Vuong et al., 2004b, Kropec et al., 2005, Choi et al., 2009, Wang et al., 2004, Izano et al., 2007b, Izano et al., 2007a, Hinnebusch et al., 1996, Parise et al., 2007). We show that Bps is essential for colonization of the mouse nose and the trachea. Strikingly, we found that it did not appear to be critical for early lung colonization. Consistent with these in vivo data, lack of Bps led to a deficiency in binding to the human nasal epithelial cells but not to the human lung epithelial cells. We also examined the ability of B. pertussis and the role of Bps in mediating the formation of biofilms, a developmental mode that is frequently associated with prolonged bacterial survival in hosts (Parsek & Singh, 2003, Hall-Stoodley & Stoodley, 2009). Data presented herein provide the first evidence of a biofilm-lifestyle for B. pertussis in the mammalian nose. We conclude that by functioning as an adhesin to the nasal-epithelial cells, Bps promotes the formation of biofilms at this site. Sera from patients that were culture positive or linked to a culture-positive individual recognized Bps, suggesting its expression during natural infection. These results highlight the importance of Bps in B. pertussis pathogenesis during human infections. We believe that Bps is the first bacterial factor that is essential for initial colonization of the nasal cavity but not of the lungs.
Results
The B. pertussis bps locus is required for the production of the Bps polysaccharide
The B. pertussis bpsABCD locus is homologous to bacterial loci involved in the synthesis of poly-β-1,6-N-acetylglucosamine polysaccharides (Vuong et al., 2004b, Kropec et al., 2005, Choi et al., 2009, Wang et al., 2004, Izano et al., 2007b, Izano et al., 2007a, Hinnebusch et al., 1996, Parise et al., 2007). Previously, we have shown that B. pertussis expresses a polysaccharide that is recognized by an antibody raised to a conjugate of deacetylated PNAG from S. aureus (Parise et al., 2007). To determine the genetic basis of the synthesis of this polysaccharide, we generated the Δbps strain, harboring a non-polar in-frame deletion of the bps locus (see Experimental Procedures). Since we have not yet been able to successfully raise antibodies against Bps, we utilized the anti-dPNAG antibody (Maira-Litran et al., 2005) in immunoblot assays to compare the mutant strain to Bp 536 for the production of a PNAG-like material. As shown in Fig. 1A, deletion of the bps locus resulted in the production of very low levels of the immuno-reactive material. Transformation of the Δbps strain with a broad host range plasmid containing the entire bps locus (Δbpscomp) resulted in both the restoration and hyperexpression of the immuno-reactive material. As expected, the Δbps strain containing the vector plasmid alone (Δbpsvec) produced little of the PNAG-like material. The residual immuno-recative material that is observed in the Δbps and the Δbpsvec strains probably represents some other material that is weakly cross-reactive with the dPNAG antibody. These results confirm the involvement of the bps locus in the synthesis of a polysaccharide that is antigenically similar to S. aureus dPNAG.
Fig. 1. Critical role of the bps locus in Bps synthesis and formation of biofilms on abiotic surfaces.

(A) Boiled EDTA surface extracts were treated with pronase and spotted onto a nitrocellulose membrane followed by detection using the anti-dPNAG antibody as described previously (Parise et al., 2007) and in the experimental procedures.
(B) and (C), Crystal violet assays were performed on surface-attached cells grown statically in 12 well tissue culture plates. The OD540 of solubilized crystal violet from surface-attached cells is shown on the y axis. Note that the time-course of biofilm formation was extended in C because of the slower growth of these strains in the presence of chloramphenicol. Each data point is the average of three wells and error bars indicate the standard deviation. Representative data from one of at least three independent experiments are shown. Asterisks designate a P<.05 (students t-test).
The bps locus is critical for B. pertussis biofilm development
We and others have previously shown that B. pertussis forms biofilms on abiotic surfaces (Serra et al., 2007, Mishra et al., 2005). No information is available on the factors that promote B. pertussis biofilm development. Based on the demonstrated role of bps-like loci in the growth and maturation of bacterial biofilms (Wang et al., 2004, Maira-Litran et al., 2005, Cramton et al., 1999, Vuong et al., 2004a, Hinnebusch et al., 1996, Choi et al., 2009, Parise et al., 2007), we utilized three independent assays to determine the contribution of this locus in B. pertussis biofilm formation on different abiotic surfaces (glass and plastic) and under different conditions (static vs. continuous flow).
Crystal violet assay
Bp 536 does not grow well in 96 well microtitre plates under shaking or static conditions (Mishra et al., 2005). In order to utilize the commonly used crystal violet microtitre plate assay (O'Toole & Kolter, 1998) to quantitate the biofilm biomass of B. pertussis, we cultured these strains in twelve well tissue culture plates and examined the adherence to the polystyrene surfaces in a time-dependent manner. Compared to Bp 536, the Δbps strain was defective in its ability to form biofilms at 24h (Fig. 1B). Even after 72h of growth in the culture plates, the biofilm biomass of the Δbps strain remained only slightly above the background level of staining. The reduction in biofilm biomass of the Δbps strain is not due to slower growth, since this strain grows similar to Bp 536 under both shaking and static conditions (Fig. S1 and data not shown).
We also examined the Δbpscomp and the Δbpsvec strains in the CV assay. These strains were cultured in the presence of chloramphenicol, since growth without this antibiotic caused plasmid loss in greater than 50% of the bacterial cells by 24h (data not shown). Surprisingly, we found that presence of chloramphenicol led to a slower growth of both these strains under shaking conditions (Fig. S1). When grown statically in tissues culture plates, these strains grew even slower (data not shown). Therefore, to demonstrate complementation of the biofilm defect observed in the Δbps strain, we directly compared biofilms formed by the Δbpsvec and the Δbpscomp strains. On culturing for extended periods (96 and 144h), while the biofilm biomass formed by the Δbpsvec strain did not increase significantly, a four fold increase was observed for the Δbpscomp strain (Fig. 1C), suggesting that complementation of the Δbps mutant restores biofilm formation in the 12 well plates. Note that the reduction in biofilm biomass of the Δbpsvec strain is not due to slower growth, since this strain grows similar to Δbpscomp under both shaking and static conditions (Fig. S1 and data not shown).
Microscopic analyses of B. pertussis biofilms on abiotic surfaces
Scanning electron microscopy (SEM) was used to analyze the impact of Bps on B. pertussis biofilm architecture. Bacterial strains were cultured on glass coverslips and the kinetics of biofilms formed at the air liquid interface was examined. After 24h of growth, both Bp 536 and the Δbps strains were present as scattered single cells (Figs. 2A and 2B, respectively). At 96h, while Bp 536 displayed a complex multi-layered three dimensional structure that was characteristic of bacterial biofilms (Fig. 2C), the mutant strain continued to remain as single cells with large regions of the coverslip remaining unoccupied (Fig. 2D). As expected, SEM of biofilms formed by the Δbpscomp strain resulted in the restoration of the multicellular appearance of the bacterial cells as observed for the wild type strain (Fig. 2E), while the Δbpsvec strain was observed on the coverslips as single cells (data not shown).
Fig. 2. SEM analysis of the role of Bps in B. pertussis biofilm formation.

SEM of biofilms formed at the air-liquid interface on glass coverslips. Bp 536 (left panels) grown for 24h (A) and 96h (C); Δbps mutant grown for 24h (B) and 96h (D); Δbpscomp strain grown for 96h (E). Bar, 10µm.
To further examine how Bps influences the biofilm architecture, biofilms formed in flow cells under constant medium flow were examined. The advantage of a flow cell system is that it allows for the continuous analyses of live fully hydrated biofilms grown on glass coverslips by confocal microscopy (Heydorn et al., 2000). After injection of equal numbers of either Bp 536 or the Δbps cells harboring the GFP plasmid pGB5P1, flow cells were incubated for 12h to allow efficient adherence to the glass cover slips. The flow of the growth medium was then resumed and the dynamics of biofilm development was visualized by confocal microscopy over a period of 72h.
After 24h of flow, there were no significant differences between Bp 536 and the Δbps strains in either the biofilm thickness or the confluence of the cells (Fig. 3). At this time point, both these strains existed in the form of a monolayer across the cover slip with no evidence of a structured biofilm. At later time points (48 and 72h after the initiation of the flow), Bp 536 continued to increase in thickness and density resulting in a highly structured biofilm. In contrast, the Δbps strain displayed a severe defect in biofilm formation. Notably, after 72h of continuous flow, there were very few cells of the mutant strain that remained adherent to the glass slide (Fig. 3, compare top right panel with the bottom right panel). This demonstrates that while the Δbps strain is still able to attach to the glass surface at early time points, it may be a transient interaction that prevents stable biofilm formation. Collectively, these results suggest that B. pertussis Bps functions to promote the complex architecture and stability of the biofilm.
Fig. 3. Bps is crucial for biofilm development under flow conditions.

Confocal scanning laser micrographs of biofilms formed in flow cells by Bp 536 (top panels) and the Δbps mutant (bottom panels). Strains were inoculated directly in the flow cell and visualized in situ every 24h. For each micrograph, the middle panel represents the x–y plane, and the adjacent top and side panels represent the x–z and y–z planes, respectively. For each strain, images were taken from at least eight areas and the experiment was repeated three times. A representative CSLM image for each sample is shown. Bar, 50µm.
B. pertussis exists as a community in the mouse nose
Despite widespread and very efficient vaccination, B. pertussis is found to colonize the nasopharynx of older children and adults (Strebel et al., 2001, Wilson, 2006). We hypothesized that the continued survival of B. pertussis in the human nasopharynx is due to the formation of biofilms. To investigate this form of existence in an experimental mouse model of infection (Carbonetti et al., 2005, Kirimanjeswara et al., 2005), nasal septa from mice inoculated intranasally with PBS, Bp 536 or with the Δbps strain were probed for B. pertussis and the respiratory epithelium followed by visualization by CSLM (See Experimental Procedures). As shown in Fig. 4A, Bp 536 existed as distinct clusters of cells resembling towers or pillars on the apical surface of the epithelium, which are similar to bacterial biofilms formed on abiotic surfaces (Kuchma & O'Toole, 2000). Although the majority of the epithelium stained positive for B. pertussis, the height and the distribution of the tower-like structures varied considerably suggesting the formation of focal biofilms. Sera from PBS-infected mice or the secondary antibody alone did not cross-react with nasal tissues (data not shown), confirming the identity of the biofilms as Bordetella. Nasal septa from mice infected with PBS or with the Δbps strain displayed reactivity to actin only (Fig. 4A).
Fig. 4. B. pertussis forms biofilms in the mouse nose.
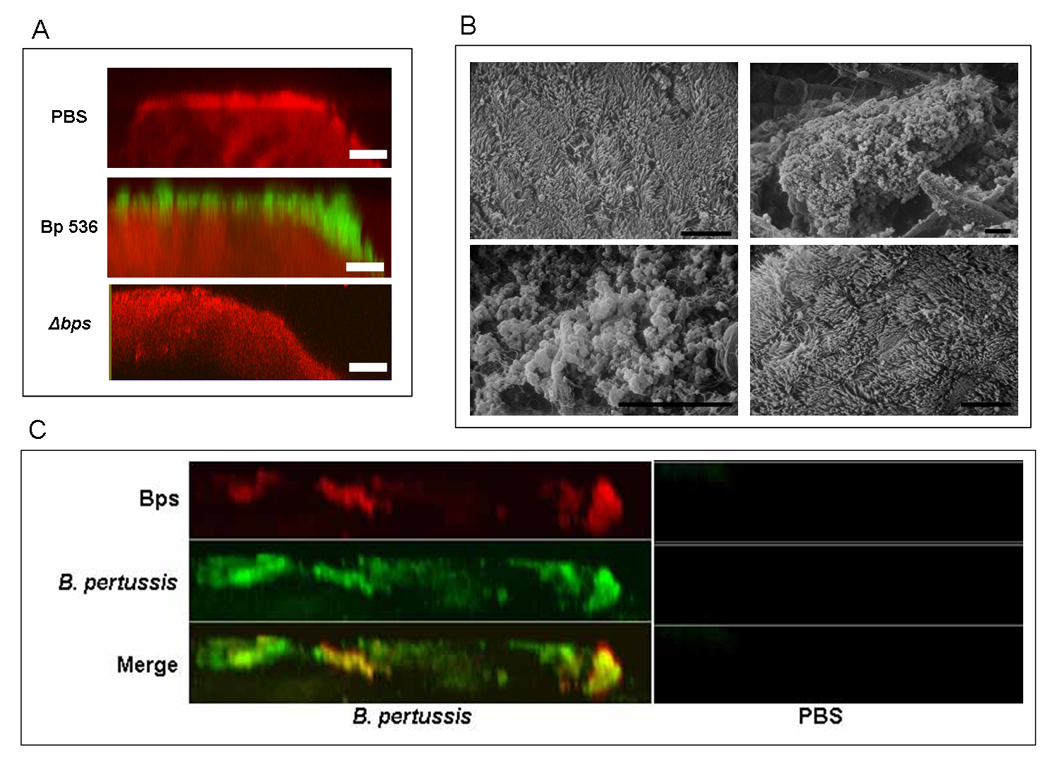
(A) CSLM of biofilms formed within the murine nasal cavity by B. pertussis. C57BL/6 mice were inoculated with PBS, Bp 536, or the Δbps strain. Seven days post-inoculation, nasal septa were harvested, immediately fixed, and probed with rat anti-Bordetella serum followed by a secondary anti-rat antibody conjugated to Alexa Fluor 488 (which stains bacteria green). To determine the localization of the host epithelium, specimens were stained for F-actin using phalloidin conjugated to Alexa Fluor 633 (which stains the epithelium red) and visualized with CSLM. Each micrograph represents an x–z reconstruction. For each specimen, images were obtained from at least five areas of the nasal septum and from at least three independent animals. Bar, 10µm.
(B) SEM of B. pertussis biofilm formation on nasal septa. Specimens were collected from animals 7 days post-inoculation with PBS (top left), Bp 536 (top right and bottom left), Δbps mutant (bottom right), directly fixed, and processed for SEM. Scale bars, 10 µm.
(C) Bps co-localizes with nasal biofilms. Nasal septa were harvested from C57BL/6 mice inoculated with PBS or Bp 536. Samples were collected 7 days post-inoculation and were stained for Bordetella (green) as described in the legend to Fig. 4A. To detect Bps production (red), specimens were stained using goat anti-dPNAG, followed by anti-goat conjugated to Alexa Fluor 633. Yellow staining indicates co-localization of B. pertussis and Bps. Micrographs are x–z reconstructions and are representative of at least three independently harvested tissues.
Independent evidence of nasal biofilms was obtained by SEM. Nasal septa harvested from Bp 536-infected mice revealed adherent micro-colony structures consistent with bacterial forms which were encased in a matrix-like material (Fig. 4B, top right and bottom left). Similar to that observed with CSLM, these bacterial clusters were interspersed. On some regions of the nasal septum, the bacterial microcolonies were separated, while at other regions confluent layers of bacteria obscured the underlying cilia. The inability to visualize cilia in the B. pertussis-infected mice is probably due to dense coverage by the bacterial cells and the associated matrix material. Consistent with this, during infection of organ cultures of human nasal turbinates, Bp 536 obscured cilia (Soane et al., 2000). Another possibility is that Bordetella-specific factors like tracheal cytotoxin and pertussis toxin (PT) are resulting in the senescence of the ciliated cells (Heiss et al., 1993).
Micro or macro colony like structures were not observed on nasal septa harvested from mice inoculated either with PBS (Fig. 4B, top left panel) or infected with the Δbps strain (Fig. 4B, bottom right panel). The nasal epithelia harvested from these animals had a tissue surface mainly composed of ciliated cells. The lack of damage to the host epithelium in Δbps-infected animals probably reflects the failure to detect any bacteria associated with the nasal tissue.
Production of Bps by B. pertussis during mouse infection
A defining characteristic of mature bacterial biofilms is the presence of an extracellular matrix (Branda et al., 2005, Parsek & Singh, 2003). We reasoned that if the observed micro and macro-colonies of B. pertussis were truly in the form of biofilms, then Bps will be produced within these biofilms. We stained infected mouse nasal septa for B. pertussis and Bps. Our results demonstrate that the Bps stain (red) co-localized with the majority of B. pertussis biofilm cells (green) (Fig. 4C). The intensity of the Bps stain was higher in areas of the nasal septum where the bacterial cells were present in the form of microcolonies or in a community-like structure. In contrast, the intervening areas corresponding to diffused cells showed very little to no staining. Most importantly, the detection of an extruded polysaccharide along with the observation of distinct architectural features of the bacterial cells further confirmed the biofilm nature of B. pertussis in the nasal cavity. Tissues from mice inoculated with PBS (Fig. 4C, right panel) or the Δbps strain displayed little cross reactivity to Bps (and data not shown). Sera from PBS-inoculated mice, heterologous goat sera or the secondary antibody alone did not cross-react with nasal tissues (data not shown). Taken together, data from Fig. 4 confirm the biofilm nature of B. pertussis in the mouse respiratory tract.
Role of the B. pertussis bps locus in colonization of the mouse respiratory tract
Based on the essential requirement of Bps in the development of nasal biofilms, we hypothesized that Bps will contribute to the colonization of the mouse nares. By utilizing the intranasal inoculation protocol that leads to the seeding of the entire respiratory tract (Carbonetti et al., 2005, Kirimanjeswara et al., 2005), we also assessed the colonization of the trachea and the lungs along with that of the nasal cavity. Compared to Bp 536, the Δbps strain was obtained from the nose and the trachea at lower numbers for all time-points examined (Fig. 5). Even as early as six hours post-inoculation, the numbers of the Δbps strain in the nose and the trachea were either at or below the lower limit of detection. While there was a slight increase in the cfus of this strain at 24h and 3 days in the trachea, it was rapidly cleared from the nose at these time-points. Although for the first three days there was a statistically significant reduction in the numbers of bacteria harvested from the lungs of mice infected with the Δbps strain, greater than 104 cfus of the mutant strain were still detected in the lungs. Strikingly, at seven days post-inoculation, the mutant strain colonized the lungs at numbers that were similar to that of the wild type strain (Fig. 5). Overall these data suggest that while B. pertussis Bps is crucial for initial colonization of the mouse nose and trachea, it is not essential in early stages of lung colonization.
Fig. 5. Bps is essential for nasal colonization.

Colonization of the mouse respiratory tract by Bp 536 and the Δbps mutant. Groups of five 6-week-old C57BL/6 mice were intranasally inoculated with 50 µl containing 5 × 105 cfus of either the Bp 536 or Δbps strain. At the designated time points, mice were sacrificed and the nasal septum, trachea, and lungs removed, homogenized and plated for enumeration of the resident bacteria. Statistically significant differences were observed at all time points and tissues except at 7 days in the lungs. Error bars represent standard deviation. *P<0.05, students t-test.
Bps functions as a nasal adhesin
One mechanism by which Bps may lead to initial colonization and subsequently promote biofilm formation in the nose is by mediating attachment of B. pertussis to the nares. On the basis of the mouse colonization data, we further hypothesized that while Bps will promote attachment of B. pertussis to nasal epithelial cells, it will have a minor role in adherence to lung epithelial cells. Adherence assays were carried out with immortalized epithelial cell-lines originating from the human nasal septum (RPMI 2650) and human lungs (A549). The nasal cell line RPMI 2650 has several characteristics that are closely related to those of the normal human nasal epithelium and has been used in a number of model systems including binding of bacteria to epithelial cells (Merkle et al., 1998). The Δbps mutant adhered at significantly lower numbers to RPMI 2650 than did Bp 536 (Fig. 6A). In contrast, there were no statistically significant differences in the adherence of either of these strains to A549 or to the rat lung epithelial cell line, L2 (Fig. 6A). Both L2 and A549 have previously been used to study Bordetella attachment to epithelial cells and also for other aspects of pathogenesis (Kuwae et al., 2003, Cotter et al., 1998, Lamberti et al., 2009). These data indicate that while Bps is essential for promoting attachment to nasal epithelial cells, it is not required for binding to the lung epithelial cells. It is also apparent from Fig. 6A, that both Bp 536 and the Δbps mutant exhibited a higher degree of adherence to the human epithelial cell lines than to L2 cells. This is consistent with the demonstrated tropism in adherence of Bordetella spp. to respiratory cells. It has been previously shown that while B. pertussis and B. parapertussis adhere better to cell originating from humans, B. bronchiseptica demonstrates preferential adherence to nonhuman cells (Tuomanen et al., 1983).
Fig. 6. Bps functions as a nasal adhesin.

(A) Attachment assays were conducted with L2 (rat lung), A549 (human lung) and RPMI 2650 (human nasal septum) epithelial cell lines. Bp 536 or the Δbps strain was incubated at a multiplicity of infection of 10. Results are expressed as adherent cfus. Each data point is the average for six wells, and error bars indicate the standard deviation. Representative data from one of at least five independent experiments are shown. Asterisks designate a P<0.05 (students t-test).
(B and C) RPMI 2650 cells were used for attachment assays. Bp 536 was pre-incubated with various concentrations of the anti-dPNAG antibody (B) or the goat IgG antibody (C). Each data point is the average for three wells, and error bars indicate the standard deviation. Representative data from one of at least three independent experiments are shown. Asterisks designate a P<0.05 (students t-test).
(D) Attachment assays were conducted with A549 cell line as above except that Bp 536 was pre-incubated with various concentrations of the anti-dPNAG antibody or goat IgG control. Each data point is the average for three wells, and error bars indicate the standard deviation. Representative data from one of at least three independent experiments are shown.
If Bps has a specific role in attachment to the nasal epithelial cell line, then it should be possible to block adherence of Bp 536 to RPMI 2650 using the anti-dPNAG antibody that specifically recognizes Bps (Fig. 1A). Pre-incubation of Bp 536 with this antibody resulted in an inhibition in the attachment of B. pertussis to RPMI 2650 cells (Fig. 6B) whereas the isotype antibody control or non-specific sera did not have any significant effect (Fig. 6C, and data not shown). The somewhat modest reduction in the attachment (40–50%) of Bp 536 to RPMI 2650, despite an increase in the antibody concentration from 1% to 10%, is probably because of the inability of the heterologous antibody raised against S. aureus dPNAG to completely inhibit attachment. In contrast to that observed for RPMI 2650, prior incubation of Bp 536 with the anti-dPNAG antibody or the goat IgG control failed to significantly block the adherence to A549 (Fig. 6D).
To further define the role of Bps as a nasal adhesin, we conducted adherence assays with nasal septa extracted from naïve mice. Compared to Bp 536, which attached at an efficiency approximating 70% of the inoculum, only 22% of the mutant bacteria bound to the nasal septum. The presence of the plasmid containing the bps locus ameliorated the attachment defect of the Δbps strain, whereas the presence of the vector plasmid had no significant effect (Fig. 7).
Fig. 7. Attachment assays with nasal explants.

Similar sized mouse nasal septa were excised from naïve mice and incubated with the indicated strains as described in experimental procedures. Results are expressed as adherent cfu from 5–6 nasal septa. Error bars represent standard deviation. *P<0.05, students t-test.
Ectopic expression of Bps in E. coli confers the ability to attach to nasal epithelia
We also expressed Bps in an E. coli strain that lacks the pga locus, which is a homolog of the bps locus. Expression of Bps by the Δpga strain led to a significantly higher level of attachment to RPMI 2650 compared to that containing the expression vector alone (Fig. 8). However, there were no significant differences between these two E. coli strains in adherence to A549 cells (Fig. 8). These data clearly demonstrate that while Bps directly mediated adherence to nasal epithelial cells, its expression alone was not sufficient to increase the binding of E. coli to lung epithelial cells.
Fig. 8. Ectopic expression of Bps confers adherence.

Attachment assays were conducted with the Δpga strain of E. coli containing the empty pBBR1MCS vector or the pMM11 plasmid that expresses Bps. Each data point is the average for six wells, and error bars indicate the standard deviation. Representative data from one of at least five independent experiments are shown. *P<0.05, students t-test.
Bps binds to human nasal epithelial cells
To demonstrate direct binding of Bps to the nasal epithelial cells, we incubated RPMI 2650 with Bps followed by detection of bound Bps by confocal microscopy. We observed that Bps bound to the surface of RPMI 2650 cells in a predominantly punctuate manner, whereas a mock-purified preparation did not show any binding. Consistent with the above results which showed that lack of Bps from B. pertussis or heterologous expression of Bps in E. coli had no effect on adherence to the lung epithelial cell line, incubation of A549 cells with the Bps preparation revealed no significant binding (Fig. 9). These results further substantiate a cell-specific interaction between Bps and nasal epithelia. In combination, results from the above experiments strongly suggest that Bps imparts a nasal epithelial adhesive function to B. pertussis.
Fig. 9. The Bps polysaccharide binds to RPMI 2650 but not to A549 cells.

Epithelial cells adherent to glass coverslips were treated with purified Bps preparation from the Δbpscomp strain or a mock-purified preparation from the Δbpsvec strain. Bound Bps was detected by anti-dPNAG antibody followed by secondary antibody coupled to Alexa-fluor 488. Epithelial cells were counter stained with rhodamine labeled phalloidin. Yellow staining denotes co-localization of Bps with the RPMI 2650 cells (indicated by arrows).
Expression of Bps during natural human infections
The experiments presented above clearly demonstrate that Bps is expressed in the mouse nasal cavity and promotes colonization of the nose and the trachea. B. pertussis is a strict human pathogen with no other reservoir and mice are not natural hosts for B. pertussis. To determine the expression of Bps in human hosts, we screened sera obtained from people either culture positive or epidemiologically linked to a culture positive contact for antibody reactivity to Bps. Immunoblot analyses using the Bps preparation revealed that ten of eleven human serum samples tested, reacted with a high molecular weight material that barely entered the resolving gel during extended electrophoresis (representative results from three individuals are shown in Fig. 10). The anti-dPNAG antibody also recognized a similarly sized band (Fig. 10). Lack of detection of this material from mock purified preparation (Fig. 10, mock lanes) by either the human sera or the anti-dPNAG antibody further confirms the identity of this band as Bps. We also examined normal human sera for reactivity against Bps and against B. pertussis antigens in an immunoblot assay. While five of these sera displayed high cross-reactivity against multiple B. pertussis antigens and Bps, three were found to have very low reactivity against pertussis antigens and correspondingly did not react with Bps (data not shown). Our results are similar to a recently published report where some of the sera from normal donors recognized B. pertussis antigens (Brickman et al., 2008). Presently, it is not possible to completely exclude that the observed recognition of Bps was because of cross-reactivity from similar polysaccharides from other bacteria that may have infected these individuals. Nonetheless, the detection of Bps by human sera provides reasonable evidence that Bps is expressed during human infections.
Fig. 10. Bps is expressed during human infections.

Immunoblot analysis of Bps reactivity with the human sera. Bps or the mock-purified preparation were separated by SDS-PAGE and after transfer onto nitrocellulose were probed with either anti-dPNAG antibody or human serum. The separation of the stacking and the resolving gel is indicated by the arrow on the left.
Discussion
A detailed knowledge of the adherence mechanisms to host surfaces is a prerequisite towards an advanced understanding of bacterial colonization and ultimately the establishment of persistent infections or the carrier state. The human nasopharyngeal cavity serves as a major reservoir for many bacterial pathogens (Murphy et al., 2009, Foster, 2004). Although colonization of the nasopharynx by these bacteria mainly leads to asymptomatic infections, it is often a major risk factor for the pathogenesis of infections. We are studying the members of the Bordetella species with a goal towards understanding the role of bacterial factors in promoting respiratory tract survival. Several recent studies have documented that B. pertussis can be isolated from the nasopharynx of children and adults (Strebel et al., 2001, Wilson, 2006). Adults and adolescents harboring B. pertussis in the nasopharynx are responsible for familial transmission to infants and young children, in whom the disease is severe and sometimes lethal (Raymond et al., 2007). In human subjects and organ cultures of human tissues (Soane et al., 2000), B. pertussis is found to mainly colonize the ciliated epithelial cells of the nares. In rodent infections, while many B. pertussis virulence factors are essential for colonization of the lower respiratory tract, none of these factors have been demonstrated to have a marked impact on colonization of the nose (Carbonetti et al., 2005, Harvill et al., 1999, Alonso et al., 2002, Geuijen et al., 1997).
In this report, we show that the B. pertussis Bps polysaccharide is essential for colonization of the mouse nose and the trachea. In contrast, Bps plays a non-essential role in lung colonization. It is generally believed that colonization of the nose and the trachea is a prerequisite for both initiation and perpetuation of infection in the lungs. Thus our finding of that Bps is not required for initial colonization of the lungs compared to that of the nose and the trachea is quite unusual and has not been observed in case of respiratory pathogens. For bacterial pathogens that are frequent colonizers of the nose, while many factors have been shown to be critical for nasal colonization, their role in promoting lung colonization or attachment to the lung epithelia is not known (Cole et al., 2001, Weidenmaier et al., 2004). In case of S. aureus, while multiple surface-exposed factors like Wta (wall teichoic acid), clumping factor B, and iron regulated surface protein A are critical for colonization of rodent nasopharynx, their role in lung colonization has not been examined (Corrigan et al., 2009, Schaffer et al., 2006). Similarly, while type IV pili of Moraxella catarrhalis has been shown to promote colonization of the chinchilla nasopharynx, its role in lung colonization is not known (Luke et al., 2007). In instances where such a differential role has been experimentally determined, these factors have also been found to be essential for efficient lung colonization and adherence. Mutants lacking pneumococcal surface protein A and pneumolysin were found to be deficient in colonization of both the nasopharynx and the lungs (Ogunniyi et al., 2007). Similarly, a S. aureus mutant defective in the production of Wta attached less efficiently to both nasal and lung epithelial cells (Weidenmaier et al., 2004). Thus, we believe that Bps represents the first bacterial factor that is essential for promoting efficient early stage colonization of the nose but not the lungs.
Bps is a surface polysaccharide of B. pertussis and one mechanism by which it may confer the observed cellular tropism between nose and the lungs is by promoting differential adherence to the respective respiratory epithelia. The bps mutant, when compared to Bp 536, was found to be highly attenuated in attachment to the human nasal epithelial cell line, RPMI 2650. In contrast, there were no significant differences in the binding of these strains to lung epithelial cell lines of rat and human origin. Adherence assays conducted with nasal explants further strengthen the adhesive function of Bps for the nasal epithelial cells. In the present study, we further demonstrate a direct interaction between Bps and nasal epithelial cells, since Bps bound to the nasal but not to the lung epithelial cell line. One determinant of the observed tropism in epithelial cell adherence could be that nasal epithelial cells exclusively express a Bps-binding surface receptor that is not expressed on lung epithelial cells. We are currently exploring the presence of a Bps-specific nasal receptor. Identification of such a mammalian receptor will enable a better understanding of the interactions between B. pertussis and the nasopharynx.
Despite the unequivocal importance of chronic bacterial infections in disease pathogenesis, there is a dearth of knowledge on the mechanisms that lead to their establishment and maintenance. Our data provide the first potential mechanistic explanation for the continued presence of B. pertussis in the human nasopharynx. We show that in an experimental infection setting, B. pertussis exists in the form of biofilms in the mouse nose. The in vivo biofilm state has been defined by us and others as a surface-adherent community of bacteria that are covered by a matrix material and are resistance to host clearance (Sloan et al., 2007, Parsek & Singh, 2003, Hall-Stoodley & Stoodley, 2009). We have found clear evidence by two independent techniques of the presence of Bordetella communities attached to the nasal epithelium. We also show that these biofilm-like structures are characterized by the production of the Bps polysaccharide, which co-localized with the bacterial cells. Consistent with the essential role of Bps in attachment to the nasal septum, the bps mutant failed to display any evidence of biofilms in the nose.
The production of the biofilm matrix consisting of Bps and the development of sporadic microcolonies in the form of focal biofilms may allow Bordetella to develop specific areas of residence in the nasopharynx thereby allowing for increased survival. In the intranasal mouse model of infection utilized here, the Bp 536 strain of B. pertussis is not completely cleared from the nose until a month following inoculation. Additionally, the biofilm-borne bacteria possess physicochemical properties distinct from those of the planktonic cells. When in the biofilm mode, bacteria are protected from host defenses and become resistant to multiple antimicrobial agents, e.g., antibiotics, reactive oxygen species, and detergents (Anderson & O'Toole, 2008, Lewis, 2007). Thus, we propose that the biofilm-like attributes observed for B. pertussis in the mouse nose afford resistance to clearance by host immune mechanisms.
We have previously examined the function of Bps in biofilm development and respiratory tract colonization of B. bronchiseptica, an animal pathogen and an evolutionary progenitor of B. pertussis (Parise et al., 2007, Sloan et al., 2007). Comparison of the results from these studies with that of the current study, suggests that with respect to biofilm formation on abiotic surfaces, Bps performs similar functions. In both these species, Bps is not essential at the initial stages of biofilm formation but is required for the stability and the maintenance of the complex architecture of biofilms. However, with respect to host colonization and biofilm development in the nose, Bps appears to function in a mechanistically different manner. In contrast to the presently observed role in early nasal colonization of B. pertussis, Bps promotes persistent colonization of B. bronchiseptica in the mouse nose. B. pertussis has evolved from B. bronchiseptica through genome decay resulting in a loss of nearly 1 Mb of genome and inactivation of a large number of ORFs (Parkhill et al., 2003). We hypothesize that if Bps has a role in early respiratory colonization and cell attachment of B. bronchiseptica, we were unable to resolve this in our assays because of the presence of multiple or redundant adhesins. Consistent with this hypothesis, it has been shown that FHA of B. bronchiseptica plays a role in the colonization of non-ciliated respiratory epithelia (Irie & Yuk, 2007). In addition, we have previously shown that two outer membrane proteins of B. bronchiseptica, BipA and BcfA, have a combinatorial role in colonization of the rat trachea (Sukumar et al., 2007).
The ability of B. pertussis to form biofilms in mice implicates a role for this mode of existence during human infections. We hypothesize that biofilm formation in the human nasopharynx allows B. pertussis to escape immune defenses and ultimately serve as reservoirs for transmission of the organism to unvaccinated infants and children (Tan et al., 2005). A principal impediment towards the development of improved vaccines for B. pertussis and other bacterial pathogens is a gap in our understanding of factors that promote colonization in the nasopharynx. A potential reduction in nasal colonization will disrupt the cycle of transmission and thereby the incidence of infection and disease. For B. pertussis, while the current pertussis vaccines have been remarkably successful in the prevention of the severe disease, these do not prevent colonization of the nasopharynx. The finding in the present study that Bps is expressed during human infection leads us to believe that therapeutic interventions aimed at targeting or inhibiting Bps synthesis will eliminate B. pertussis from asymptomatic carriers and will have profound impact on the familial and adult transmission to infants and young children.
In conclusion, we have identified a novel function for the Bps polysaccharide in adherence to the nasopharynx, a conserved site of chronic residence for a multitude of bacterial pathogens like Streptococcus pneumoniae, S. aureus and Haemophilus influenzae (Murphy et al., 2009, Foster, 2004). The production of Bps-like polysaccharides and a functional role for this family of polysaccharides in biofilm development is conserved in a number of bacterial pathogens unrelated to Bordetella spp., (Wang et al., 2004, Maira-Litran et al., 2005, Cramton et al., 1999, Vuong et al., 2004a, Hinnebusch et al., 1996, Choi et al., 2009, Parise et al., 2007, Sloan et al., 2007) thus suggesting not only a common mechanism of pathogenesis but potentially the development of widely applicable preventive or prophylactic measures.
Experimental procedures
Bacterial strains and growth conditions
Bp 536 was utilized as the parental wild type strain of B. pertussis (Mishra et al., 2005). The E. coli strain TRXWMG1655 containing a deletion in the pga locus (Wang et al., 2004) was used as the surrogate host for attachment assays.
B. pertussis strains were maintained on Bordet-Gengou agar (BG) supplemented with 7.5% defibrinated sheep blood. Liquid cultures were grown in Stainer-Scholte (SS) broth with supplement and heptakis (2,6-di-O-methyl-β-cyclodextrin) as described (Parise et al., 2007). E. coli strains were grown in Luria-Bertani medium. As necessary, the various growth media were supplemented with the appropriate antibiotics, chloramphenicol (10µg ml−1), kanamycin (25µg ml−1) and streptomycin (50µg ml−1).
Construction of the Δbps strain
An in-frame non-polar deletion of the entire bpsABCD locus (ORFs; Bp1941–Bp1944) was constructed using allelic exchange as previously described by utilizing the plasmid pGP8 (Parise et al., 2007, Sukumar et al., 2007). This plasmid was constructed by ligating upstream and downstream regions flanking 5’ to the bpsA and 3’ to the bpsD ORFs for allelic exchange. The upstream region was amplified using primers GP1 (ctagtctagaggcgaaattataccgcgtt) and GP2 (cccaagcttccccgccaccagcagccgagt) while the downstream region was amplified using primers GP3 (cccaagcttcagcggcaacccgacggacgcat) and GP4 (cggggtaccgggcgcggctgctgctgcagg). Allelic exchange utilizing the resultant plasmid led to an in-frame deletion of the entire bps locus except the first 20 codons of the bpsA ORF and the last 20 codons of the bpsD ORF. The plasmid pGP8 was transformed into the E. coli strain SM10λpir and mobilized into Bp 536. Exoconjugates were selected on BG agar containing chloramphenicol and streptomycin. Colonies that underwent second recombination events were selected on BG agar containing 7.5% sucrose as described previously (Sukumar et al., 2007). The genotype of the Δbps strain was confirmed by PCR and DNA sequencing of the PCR product.
Genetic complementation of the bpsABCD locus in B. pertussis and E. coli
The plasmid pMM11(Parise et al., 2007) containing the entire bps locus was utilized for complementation. Cognate strains containing the parent vector plasmid pBBR1MCS were used as negative controls.
Construction of GFP strains
The previously described GFP plasmid pGB5P1 (Weingart et al., 1999) was transformed into either Bp 536 or the Δbps strains by electroporation and the recombinants were selected on BG agar containing kanamycin. Randomly picked colonies containing pGB5P1 were grown in SS broth with kanamycin and were analyzed for GFP expression utilizing a Nikon Eclipse TE300 inverted microscope. One of the GFP-expressing clones corresponding to each of the strains was chosen for experimental analysis. Comparison of the GFP-expressing strains with the respective parental strains not containing the plasmids revealed no differences in growth in batch cultures or colony morphology on BG agar containing blood.
Detection of Bps by Immunoblot
Crude exopolysaccharide extracts were prepared using a previously described method for purification of PNAG in Staphylococcus species (Kropec et al., 2005). Approximately, 5×109 cells of different strains grown for three-four days at 37°C in broth culture were harvested by centrifugation, resuspended in 100 µl of 0.5M EDTA and boiled for 5 min at 100°C. Cells were removed by centrifugation and the supernatant was treated with 1mg ml−1 of pronase for 3h at 37°C. At the end of the incubation period, samples were heated to 85°C for 15 min to inactivate the pronase. 5µl of the extract was spotted on a nitrocellulose membrane and allowed to dry overnight. The membrane was blocked with 5% nonfat milk and probed with a 1:5000 dilution of a goat antibody raised against S. aureus dPNAG conjugated to diphtheria toxoid (Maria-Litran 2005). A secondary mouse anti-goat IgG antibody conjugated to horseradish peroxidase (Pierce) was used at a concentration of 1:20,000 for detection in conjunction with the Amersham ECL (enhanced chemiluminescence) Western blotting system.
Crystal Violet Assay
B. pertussis strains were inoculated at an OD600 of 0.1 into 12 well tissue culture plates containing 1.5ml of supplemented SS broth and heptakis and incubated at 37°C under static conditions. At each time point, the nonattached and loosely adherent bacteria were removed by discarding the media and the wells were vigorously washed three times with water. Adherent cells were then stained with a 0.1% solution of crystal violet (CV) incubated at room temperature for 30 min and the washing process was repeated. The CV staining the cells was solubilized with 95% ethanol. Biofilm formation was quantitated by measuring the OD540 for each well by transferring 100 µl of the solubilized CV stain to a fresh polystyrene microtiter dish.
Scanning Electron Microscopy
B. pertussis strains were inoculated at an OD600 of 0.1 into the wells of 12 well tissue culture plates, each containing 1.5ml of supplemented SS broth and heptakis. A sterilized glass coverslip was suspended vertically against the walls of each well and incubated at 37°C. After incubating for the designated time points, the coverslips were removed, washed gently with sterile PBS and fixed with 2.5% glutaraldehyde for SEM processing as previously described (Parise et al., 2007, Swords et al., 2004).
A similar procedure was used for visualizing biofilms formed in vivo. Nasal septa were harvested from mice infected with PBS-inoculated or Bordetella-infected animals, washed with PBS and processed for SEM.
Continuous flow confocal microscopy
Three-chambered flow cells were obtained as sterile units from Stovall. 500µl suspensions of B. pertussis strains at an OD600 of 0.5 were inoculated into the chambers using sterile 25 5/8 gauge needle. Cells were allowed to attach to the chamber without flow for 12h at 37 °C. After attachment, the chamber was inverted and medium flow (SS broth containing 25 µg ml−1 of kanamycin) was initiated at a rate of 0.5 ml min−1. Biofilms were observed every 24h using a Ziess LSM 510 confocal scanning laser microscope as described (Parise et al., 2007).
Animal Colonization
Six to eight week old female C57BL/6 mice obtained from Jackson laboratory were used for all experiments. Mice were infected intranasally with 5×105 cfus of Bp 536 or the Δbps mutant suspended in a 50µl droplet of PBS. At designated time points, mice were sacrificed and nasal septum, trachea, and two lobes of the lungs were harvested, homogenized and plated on BG agar containing 7.5% blood and streptomycin for colony counts. All animal procedures were conducted according to protocols approved by University Committee on the Care and Use of Animals at the Wake Forest University Health Sciences. Statistical significance was determined by the unpaired two tailed student’s t- test and data were determined to be significant if p<.05.
Confocal Microscopy
Nasal septa were harvested and placed immediately in 10% neutral buffered formalin for fixation overnight. Formalin was removed and the nasal septa were washed twice with PBS. Tissues were blocked for 30 min with 10% normal donkey serum (NDS), incubated with 1:200 dilution of rat anti-Bordetella serum at room temperature for 2h with shaking followed by extensive washing with PBS. The goat anti-rat IgG antibody conjugated to Alexa-flour 488 was added for 2h with shaking. The tissues were then washed with PBS as before and fixed with formalin for 30 min to prevent antibody separation. After removal of formalin, tissues were washed briefly with PBS to remove any traces of the fixative followed by the addition of 0.1% Triton X-100 for 5 min to permeabilize the tissues. A 1:40 dilution of Alexa fluor 633 labeled phalloidin, which binds to cellular actin was added, incubated for 1h with shaking followed by washing with PBS. The tissues were then mounted in ProLong Gold anti-fade reagent in four-chambered cover glass. Samples were viewed using Zeiss LSM 510 confocal scanning laser microscope.
A similar staining procedure was used for observing in vivo Bps production except that a 1:500 dilution of the anti-dPNAG antibody and an anti-goat IgG conjugated to Alexa-fluor 633 was utilized.
Attachment to immortalized cell lines and explants
A549, L2 and RPMI 2650 cells were grown in DMEM supplemented with 10% FBS and 4mM L-glutamine at 37°C under 5% CO2. A549 cells were originally isolated from a 58 year old cancer patient in 1972 and express numerous morphological characteristics similar to type II pneumocytes including lamellar body formation as well as expressing various cytokeratins and phospholipid markers traditionally expressed by lung epithelial cells (Nardone & Andrews, 1979). L2 cells are immortalized rat lung epithelial cells which also have shown numerous type II pneumocyte phospholipid markers and express copious amounts of surfactant (Douglas et al., 1983). RPMI 2650 cells were originally isolated from a human with squamous cell carcinoma of the nasal septum. These cells closely represent the human nasal epithelia in terms of their karyotype, cytokeratin expression profile, and their ability to excrete a mucin like material on the cell surfaces (Salib et al., 2005). Approximately, 2×105 cells were seeded in 24 well culture plates containing media and incubated overnight at 37°C under 5% CO2. The following day, 2×106 B. pertussis cells were added to the wells, centrifuged at 900 rpm for 5 min in order to facilitate contact between bacteria and epithelial cells. The plates were then incubated at 37°C for 15 min to allow bacterial attachment. The media was removed and the wells were washed four times with sterile PBS to remove any nonattached bacteria. The eukaryotic cells were then lysed with 0.05% saponin and the mixture was plated on BG-agar containing 10% blood and streptomycin for enumeration of attached bacteria. Note that saponin did not have any significant effect on the survival of any of the B. pertussis strains used in this study (data not shown).
Nasal septa were aseptically removed from naïve C57/BL6 mice and immediately placed in a 1.5ml tube containing 5×105 of B. pertussis cells in 1ml of DMEM followed by incubation at 37°C for 1.5h with gentle rocking. After incubation, the medium was removed, tissues were washed twice with sterile PBS to remove any nonattached bacteria followed by brief centrifugation at room temperature. 200µl of ice-cold PBS was then added to the tissues and homogenized. Serial dilutions were then plated to determine the numbers of bacteria attached to each tissue.
Inhibition of bacterial attachment
The anti-dPNAG, purified goat IgG and NDS were heat-inactivated at 55°C for 15 min. 2×106 B. pertussis cells were incubated under gentle rocking at 37°C for 30 min with the designated concentrations of the sera. The B. pertussis cells were then added to either RPMI 2650 or A549 cells and the attachment assay was performed as described above.
Bps purification
An exopoylsaccharide preparation enriched in Bps or a mock preparation was purified from the Δbpscomp or the Δbpsvec strains, respectively. Cells were grown in 10 liters of supplemented SS media with aeration at 37°C to 1 O.D. and harvested at 4°C. The cell pellet was resuspended in 30 ml of 0.5M EDTA, boiled for 5 min and centrifuged at 4°C to remove cell debris. The supernatant was extracted with phenol:chloroform to remove proteins. The aqueous phase was extracted with an equal volume of chloroform, precipitated using 100% ethanol overnight at 4°C and centrifugation. The pellet was washed with 70% ethanol and resuspended in 50ml of water. The solution was then treated with DNase I and RNase for 2h at 37°C to remove nucleic acids, followed by protease treatment to degrade any remaining proteins. A final phenol:chloroform extraction and ethanol precipitation were performed before the remaining solution was dialyzed against dH2O, lyophilized and stored at −80°C. Neutral-sugar content was determined using the phenol sulfuric acid assay (Wang et al., 2004). For assays utilizing the Bps and mock preparations, the samples were standardized by neutral-sugar content. Additionally, the two preparations contained similar amounts of LPS as determined by the Limulus Amebocyte Lysate assay (Cape Cod Inc.).
Bps binding to epithelial cell lines
Approximately 105 epithelial cells grown as above were added to tissue culture plates containing sterile small circular glass coverslips and cultured for two days. The coverslips containing the adhered cells were washed in PBS, transferred in fresh 35mm dishes and treated with 1% formalin for 10 min to prevent antigen internalization followed by brief washing with PBS. Next cells were incubated with either purified Bps or the mock purified preparation for 1h at 37°C with 1% BSA. The coverslips were then washed with PBS and treated with a 1:500 dilution of the anti-dPNAG antibody containing 1% BSA for 1h at 37°C. The coverslips were washed in PBS, incubated with an anti-goat Alexa-fluor 488 antibody at a dilution of 1:200 for 1h at 37°C, washed again in PBS and then fixed with 10% formalin for 30 min to prevent antigen/antibody separation. After a brief PBS wash the samples were permeabilized with 0.1% Triton X-100 for 5 min. Following another brief PBS wash, cells were counter stained with a 1:40 dilution of rhodamine labeled phalloidin for 1h. Subsequent to a final PBS wash, the coverslips were mounted in ProLong anti-fade gold reagent and viewed using a Zeiss LSM 510 confocal scanning laser microscope.
SDS-PAGE and immunoblot analysis
Purified Bps or the mock purified material was run on a 12% SDS-polyacrylamide gel (5% stacking) and transferred to a nitrocellulose membrane. The membranes were then probed with either the anti-dPNAG antibody as described (Parise et al., 2007) or various human sera. The human sera used were from individuals who were culture positive for B. pertussis or epidemiologically linked to a culture positive patient. A 1:1000 dilution of various human sera was used. A goat anti-human IgG conjugated to HRP (1:2000) was used as a secondary antibody.
Statistical Analysis
All statistics were performed using the student’s t-test and were determined to be significant if p<.05.
Acknowledgements
We are grateful to Dr. Gary N. Sanden and Dr. Lucia Pawloski for the human sera, Dr. Gerry B. Pier for the anti-dPNAG antibody and Dr. Allison Weiss for the GFP-expressing plasmid. We thank Dr. Manish Bharadwaj for the preparation of the Bps polysaccharide. We also acknowledge the generous help of Ken Grant and Dr. Purnima Dubey with microscopy and tissue culture, respectively. Research in the laboratory of R.D. is supported by funds from the NIH (grant no. 1R01AI075081), National Research Initiative Grant no. 2006-35604-16874 from the USDA National Institute of Food and Agriculture, Microbial Functional Genomics Program and the American Heart Association. M.C. is supported by a NIH predoctoral training grant, T32 AI07401.
REFERENCES
- Alonso S, Reveneau N, Pethe K, Locht C. Eighty-kilodalton N-terminal moiety of Bordetella pertussis filamentous hemagglutinin: adherence, immunogenicity, and protective role. Infect Immun. 2002;70:4142–4147. doi: 10.1128/IAI.70.8.4142-4147.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson GG, O'Toole GA. Innate and induced resistance mechanisms of bacterial biofilms. Curr Top Microbiol Immunol. 2008;322:85–105. doi: 10.1007/978-3-540-75418-3_5. [DOI] [PubMed] [Google Scholar]
- Branda SS, Vik S, Friedman L, Kolter R. Biofilms: the matrix revisited. Trends Microbiol. 2005;13:20–26. doi: 10.1016/j.tim.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Brickman TJ, Hanawa T, Anderson MT, Suhadolc RJ, Armstrong SK. Differential expression of Bordetella pertussis iron transport system genes during infection. Mol Microbiol. 2008;70:3–14. doi: 10.1111/j.1365-2958.2008.06333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonetti NH. Immunomodulation in the pathogenesis of Bordetella pertussis infection and disease. Curr Opin Pharmacol. 2007;7:272–278. doi: 10.1016/j.coph.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Carbonetti NH, Artamonova GV, Andreasen C, Bushar N. Pertussis toxin and adenylate cyclase toxin provide a one-two punch for establishment of Bordetella pertussis infection of the respiratory tract. Infect Immun. 2005;73:2698–2703. doi: 10.1128/IAI.73.5.2698-2703.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry JD. The epidemiology of pertussis: a comparison of the epidemiology of the disease pertussis with the epidemiology of Bordetella pertussis infection. Pediatrics. 2005;115:1422–1427. doi: 10.1542/peds.2004-2648. [DOI] [PubMed] [Google Scholar]
- Choi AH, Slamti L, Avci FY, Pier GB, Maira-Litran T. The pgaABCD locus of Acinetobacter baumannii encodes the production of poly-beta-1–6-N-acetylglucosamine, which is critical for biofilm formation. J Bacteriol. 2009;191:5953–5963. doi: 10.1128/JB.00647-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole AM, Tahk S, Oren A, Yoshioka D, Kim YH, Park A, Ganz T. Determinants of Staphylococcus aureus nasal carriage. Clin Diagn Lab Immunol. 2001;8:1064–1069. doi: 10.1128/CDLI.8.6.1064-1069.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigan RM, Miajlovic H, Foster TJ. Surface proteins that promote adherence of Staphylococcus aureus to human desquamated nasal epithelial cells. BMC Microbiol. 2009;9:22. doi: 10.1186/1471-2180-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter PA, Yuk MH, Mattoo S, Akerley BJ, Boschwitz J, Relman DA, Miller JF. Filamentous hemagglutinin of Bordetella bronchiseptica is required for efficient establishment of tracheal colonization. Infect Immun. 1998;66:5921–5929. doi: 10.1128/iai.66.12.5921-5929.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramton SE, Gerke C, Schnell NF, Nichols WW, Gotz F. The intercellular adhesion (ica) locus is present in Staphylococcus aureus and is required for biofilm formation. Infect Immun. 1999;67:5427–5433. doi: 10.1128/iai.67.10.5427-5433.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas WH, Sommers-Smith SK, Johnston JM. Phosphatidate phosphohydrolase activity as a marker for surfactant synthesis in organotypic cultures of type II alveolar pneumonocytes. J Cell Sci. 1983;60:199–207. doi: 10.1242/jcs.60.1.199. [DOI] [PubMed] [Google Scholar]
- Foster TJ. The Staphylococcus aureus "superbug". J Clin Invest. 2004;114:1693–1696. doi: 10.1172/JCI23825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuijen CA, Willems RJ, Bongaerts M, Top J, Gielen H, Mooi FR. Role of the Bordetella pertussis minor fimbrial subunit, FimD, in colonization of the mouse respiratory tract. Infect Immun. 1997;65:4222–4228. doi: 10.1128/iai.65.10.4222-4228.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall-Stoodley L, Stoodley P. Evolving concepts in biofilm infections. Cell Microbiol. 2009;11:1034–1043. doi: 10.1111/j.1462-5822.2009.01323.x. [DOI] [PubMed] [Google Scholar]
- Harvill ET, Cotter PA, Miller JF. Pregenomic comparative analysis between Bordetella bronchiseptica RB50 and Bordetella pertussis tohama I in murine models of respiratory tract infection. Infect Immun. 1999;67:6109–6118. doi: 10.1128/iai.67.11.6109-6118.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hava DL, LeMieux J, Camilli A. From nose to lung: the regulation behind Streptococcus pneumoniae virulence factors. Mol Microbiol. 2003;50:1103–1110. doi: 10.1046/j.1365-2958.2003.03764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiss LN, Flak TA, Lancaster JR, Jr, McDaniel ML, Goldman WE. Nitric oxide mediates Bordetella pertussis tracheal cytotoxin damage to the respiratory epithelium. Infect Agents Dis. 1993;2:173–177. [PubMed] [Google Scholar]
- Heydorn A, Ersboll BK, Hentzer M, Parsek MR, Givskov M, Molin S. Experimental reproducibility in flow-chamber biofilms. Microbiology. 2000;146(Pt 10):2409–2415. doi: 10.1099/00221287-146-10-2409. [DOI] [PubMed] [Google Scholar]
- Hinnebusch BJ, Perry RD, Schwan TG. Role of the Yersinia pestis hemin storage (hms) locus in the transmission of plague by fleas. Science. 1996;273:367–370. doi: 10.1126/science.273.5273.367. [DOI] [PubMed] [Google Scholar]
- Irie Y, Yuk MH. In vivo colonization profile study of Bordetellabronchiseptica in the nasal cavity. FEMS Microbiol Lett. 2007 doi: 10.1111/j.1574-6968.2007.00852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izano EA, Sadovskaya I, Vinogradov E, Mulks MH, Velliyagounder K, Ragunath C, Kher WB, Ramasubbu N, Jabbouri S, Perry MB, Kaplan JB. Poly-N-acetylglucosamine mediates biofilm formation and antibiotic resistance in Actinobacillus pleuropneumoniae. Microb Pathog. 2007a;43:1–9. doi: 10.1016/j.micpath.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izano EA, Sadovskaya I, Wang H, Vinogradov E, Ragunath C, Ramasubbu N, Jabbouri S, Perry MB, Kaplan JB. Poly-N-acetylglucosamine mediates biofilm formation and detergent resistance in Aggregatibacter actinomycetemcomitans. Microb Pathog. 2007b doi: 10.1016/j.micpath.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirimanjeswara GS, Agosto LM, Kennett MJ, Bjornstad ON, Harvill ET. Pertussis toxin inhibits neutrophil recruitment to delay antibody-mediated clearance of Bordetella pertussis. J Clin Invest. 2005;115:3594–3601. doi: 10.1172/JCI24609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropec A, Maira-Litran T, Jefferson KK, Grout M, Cramton SE, Gotz F, Goldmann DA, Pier GB. Poly-N-acetylglucosamine production in Staphylococcus aureus is essential for virulence in murine models of systemic infection. Infect Immun. 2005;73:6868–6876. doi: 10.1128/IAI.73.10.6868-6876.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchma SL, O'Toole GA. Surface-induced and biofilm-induced changes in gene expression. Curr Opin Biotechnol. 2000;11:429–433. doi: 10.1016/s0958-1669(00)00123-3. [DOI] [PubMed] [Google Scholar]
- Kuwae A, Ohishi M, Watanabe M, Nagai M, Abe A. BopB is a type III secreted protein in Bordetella bronchiseptica and is required for cytotoxicity against cultured mammalian cells. Cell Microbiol. 2003;5:973–983. doi: 10.1046/j.1462-5822.2003.00341.x. [DOI] [PubMed] [Google Scholar]
- Lamberti Y, Alvarez Hayes J, Perez Vidakovics ML, Rodriguez ME. Cholesterol-dependent attachment of human respiratory cells by Bordetella pertussis. FEMS Immunol Med Microbiol. 2009;56:143–150. doi: 10.1111/j.1574-695X.2009.00557.x. [DOI] [PubMed] [Google Scholar]
- Lewis K. Persister cells, dormancy and infectious disease. Nat Rev Microbiol. 2007;5:48–56. doi: 10.1038/nrmicro1557. [DOI] [PubMed] [Google Scholar]
- Luke NR, Jurcisek JA, Bakaletz LO, Campagnari AA. Contribution of Moraxella catarrhalis type IV pili to nasopharyngeal colonization and biofilm formation. Infect Immun. 2007;75:5559–5564. doi: 10.1128/IAI.00946-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maira-Litran T, Kropec A, Goldmann DA, Pier GB. Comparative opsonic and protective activities of Staphylococcus aureus conjugate vaccines containing native or deacetylated Staphylococcal Poly-N-acetyl-beta-(1–6)-glucosamine. Infect Immun. 2005;73:6752–6762. doi: 10.1128/IAI.73.10.6752-6762.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattoo S, Cherry JD. Molecular pathogenesis, epidemiology, and clinical manifestations of respiratory infections due to Bordetella pertussis and other Bordetella subspecies. Clin Microbiol Rev. 2005;18:326–382. doi: 10.1128/CMR.18.2.326-382.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkle HP, Ditzinger G, Lang SR, Peter H, Schmidt MC. In vitro cell models to study nasal mucosal permeability and metabolism. Adv Drug Deliv Rev. 1998;29:51–79. doi: 10.1016/s0169-409x(97)00061-6. [DOI] [PubMed] [Google Scholar]
- Mishra M, Parise G, Jackson KD, Wozniak DJ, Deora R. The BvgAS signal transduction system regulates biofilm development in Bordetella. J Bacteriol. 2005;187:1474–1484. doi: 10.1128/JB.187.4.1474-1484.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TF, Bakaletz LO, Smeesters PR. Microbial interactions in the respiratory tract. Pediatr Infect Dis J. 2009;28:S121–S126. doi: 10.1097/INF.0b013e3181b6d7ec. [DOI] [PubMed] [Google Scholar]
- Nardone LL, Andrews SB. Cell line A549 as a model of the type II pneumocyte. Phospholipid biosynthesis from native and organometallic precursors. Biochim Biophys Acta. 1979;573:276–295. doi: 10.1016/0005-2760(79)90061-4. [DOI] [PubMed] [Google Scholar]
- O'Toole GA, Kolter R. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol Microbiol. 1998;30:295–304. doi: 10.1046/j.1365-2958.1998.01062.x. [DOI] [PubMed] [Google Scholar]
- Ogunniyi AD, LeMessurier KS, Graham RM, Watt JM, Briles DE, Stroeher UH, Paton JC. Contributions of pneumolysin, pneumococcal surface protein A (PspA), and PspC to pathogenicity of Streptococcus pneumoniae D39 in a mouse model. Infect Immun. 2007;75:1843–1851. doi: 10.1128/IAI.01384-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parise G, Mishra M, Itoh Y, Romeo T, Deora R. Role of a putative polysaccharide locus in Bordetella biofilm development. J Bacteriol. 2007;189:750–760. doi: 10.1128/JB.00953-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhill J, Sebaihia M, Preston A, Murphy LD, Thomson N, Harris DE, Holden MT, Churcher CM, Bentley SD, Mungall KL, Cerdeno-Tarraga AM, Temple L, James K, Harris B, Quail MA, Achtman M, Atkin R, Baker S, Basham D, Bason N, Cherevach I, Chillingworth T, Collins M, Cronin A, Davis P, Doggett J, Feltwell T, Goble A, Hamlin N, Hauser H, Holroyd S, Jagels K, Leather S, Moule S, Norberczak H, O'Neil S, Ormond D, Price C, Rabbinowitsch E, Rutter S, Sanders M, Saunders D, Seeger K, Sharp S, Simmonds M, Skelton J, Squares R, Squares S, Stevens K, Unwin L, Whitehead S, Barrell BG, Maskell DJ. Comparative analysis of the genome sequences of Bordetella pertussis, Bordetella parapertussis and Bordetella bronchiseptica. Nat Genet. 2003;35:32–40. doi: 10.1038/ng1227. [DOI] [PubMed] [Google Scholar]
- Parsek MR, Singh PK. Bacterial biofilms: an emerging link to disease pathogenesis. Annu Rev Microbiol. 2003;57:677–701. doi: 10.1146/annurev.micro.57.030502.090720. [DOI] [PubMed] [Google Scholar]
- Raymond J, Armengaud JB, Cosnes-Lambe C, Chalumeau M, Bosdure E, Reglier-Poupet H, El Hajje MJ, Iniguez JL, Moulin F, Poyart C, Gendrel D. Pertussis in young infants: apnoea and intra-familial infection. Clin Microbiol Infect. 2007;13:172–175. doi: 10.1111/j.1469-0691.2006.01616.x. [DOI] [PubMed] [Google Scholar]
- Salib RJ, Lau LC, Howarth PH. The novel use of the human nasal epithelial cell line RPMI 2650 as an in vitro model to study the influence of allergens and cytokines on transforming growth factor-beta gene expression and protein release. Clin Exp Allergy. 2005;35:811–819. doi: 10.1111/j.1365-2222.2005.02258.x. [DOI] [PubMed] [Google Scholar]
- Schaffer AC, Solinga RM, Cocchiaro J, Portoles M, Kiser KB, Risley A, Randall SM, Valtulina V, Speziale P, Walsh E, Foster T, Lee JC. Immunization with Staphylococcus aureus clumping factor B, a major determinant in nasal carriage, reduces nasal colonization in a murine model. Infect Immun. 2006;74:2145–2153. doi: 10.1128/IAI.74.4.2145-2153.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra D, Bosch A, Russo DM, Rodriguez ME, Zorreguieta A, Schmitt J, Naumann D, Yantorno O. Continuous nondestructive monitoring of Bordetella pertussis biofilms by Fourier transform infrared spectroscopy and other corroborative techniques. Anal Bioanal Chem. 2007;387:1759–1767. doi: 10.1007/s00216-006-1079-9. [DOI] [PubMed] [Google Scholar]
- Sloan GP, Love CF, Sukumar N, Mishra M, Deora R. The Bordetella Bps Polysaccharide is Critical for Biofilm Development in the Mouse Respiratory Tract. J Bacteriol. 2007 doi: 10.1128/JB.00785-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soane MC, Jackson A, Maskell D, Allen A, Keig P, Dewar A, Dougan G, Wilson R. Interaction of Bordetella pertussis with human respiratory mucosa in vitro. Respir Med. 2000;94:791–799. doi: 10.1053/rmed.2000.0823. [DOI] [PubMed] [Google Scholar]
- Strebel P, Nordin J, Edwards K, Hunt J, Besser J, Burns S, Amundson G, Baughman A, Wattigney W. Population-based incidence of pertussis among adolescents and adults, Minnesota, 1995–1996. J Infect Dis. 2001;183:1353–1359. doi: 10.1086/319853. [DOI] [PubMed] [Google Scholar]
- Sukumar N, Mishra M, Sloan GP, Ogi T, Deora R. Differential Bvg phase-dependent regulation and combinatorial role in pathogenesis of two Bordetella paralogs, BipA and BcfA. J Bacteriol. 2007;189:3695–3704. doi: 10.1128/JB.00009-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swords WE, Moore ML, Godzicki L, Bukofzer G, Mitten MJ, VonCannon J. Sialylation of lipooligosaccharides promotes biofilm formation by nontypeable Haemophilus influenzae. Infect Immun. 2004;72:106–113. doi: 10.1128/IAI.72.1.106-113.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan T, Trindade E, Skowronski D. Epidemiology of pertussis. Pediatr Infect Dis J. 2005;24:S10–S18. doi: 10.1097/01.inf.0000160708.43944.99. [DOI] [PubMed] [Google Scholar]
- Tuomanen EI, Nedelman J, Hendley JO, Hewlett EL. Species specificity of Bordetella adherence to human and animal ciliated respiratory epithelial cells. Infect Immun. 1983;42:692–695. doi: 10.1128/iai.42.2.692-695.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuong C, Kocianova S, Voyich JM, Yao Y, Fischer ER, DeLeo FR, Otto M. A crucial role for exopolysaccharide modification in bacterial biofilm formation, immune evasion, and virulence. J Biol Chem. 2004a;279:54881–54886. doi: 10.1074/jbc.M411374200. [DOI] [PubMed] [Google Scholar]
- Vuong C, Voyich JM, Fischer ER, Braughton KR, Whitney AR, DeLeo FR, Otto M. Polysaccharide intercellular adhesin (PIA) protects Staphylococcus epidermidis against major components of the human innate immune system. Cell Microbiol. 2004b;6:269–275. doi: 10.1046/j.1462-5822.2004.00367.x. [DOI] [PubMed] [Google Scholar]
- Wang X, Preston JF, 3rd, Romeo T. The pgaABCD locus of Escherichia coli promotes the synthesis of a polysaccharide adhesin required for biofilm formation. J Bacteriol. 2004;186:2724–2734. doi: 10.1128/JB.186.9.2724-2734.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidenmaier C, Kokai-Kun JF, Kristian SA, Chanturiya T, Kalbacher H, Gross M, Nicholson G, Neumeister B, Mond JJ, Peschel A. Role of teichoic acids in Staphylococcus aureus nasal colonization, a major risk factor in nosocomial infections. Nat Med. 2004;10:243–245. doi: 10.1038/nm991. [DOI] [PubMed] [Google Scholar]
- Weingart CL, Broitman-Maduro G, Dean G, Newman S, Peppler M, Weiss AA. Fluorescent labels influence phagocytosis of Bordetella pertussis by human neutrophils. Infect Immun. 1999;67:4264–4267. doi: 10.1128/iai.67.8.4264-4267.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TR. Update on adolescent immunization: review of pertussis and the efficacy, safety, and clinical use of vaccines that contain tetanus-diphtheria-acellular pertussis. J Pediatr Health Care. 2006;20:229–237. doi: 10.1016/j.pedhc.2005.12.011. [DOI] [PubMed] [Google Scholar]


