Abstract
Epithelial-mesenchymal interactions are important in wound healing and scarring, but are difficult to study in vitro. We have previously reported on an in vitro keratinocyte-fibroblast coculture system exploring these interactions and found that coculture modifies the levels of cytokines they secrete. The same coculture model was used to study changes in MMP- and TIMP-activity. We hypothesized that the previously shown decrease of collagen is partly due to increased MMPs.
Adult human cutaneous keratinocytes and fibroblasts were cocultured under serum-free conditions. Keratinocytes were either kept at the air-liquid-interface or hydrated. The conditioned medium was submitted to a multiplex sandwich enzyme-linked immunosorbent assay including gelatinases, collagenases, stromelysins, and tissue inhibitors of metalloproteinases. Collagen content was determined by western blot. Zymography depicted the gelatinases in conditioned media. For confirmation of the coculture results fibroblasts were treated with conditioned media from keratinocyte monocultures as well.
MMP-1, MMP-9, and MMP-10 were mainly secreted by keratinocytes, whereas MMP-2, TIMP-1 and -2 by fibroblasts. MMP-13 was secreted by both cell types at comparable levels. Collagenases, gelatinases, MMP-3, and TIMPs increased significantly in cocultures compared to monocultures. Hydration of keratinocytes revealed a significant increase of MMP-3 and MMP-2, and a decrease of TIMP-2.
Paracrine interactions between keratinocytes and fibroblasts modify strongly MMPs and TIMPs, whereas hydration of keratinocytes had a smaller impact in this context. The observed changes may be in part responsible for reduced collagen in coculture conditioned media. The present coculture experiments reemphasize the role of epidermis in controlling scarring.
Keywords: MMP-3, MMP-2, TIMP-2, MMP-1, hydration, hypertrophic scar
Introduction
Cutaneous wound healing is a finely controlled dynamic event involving multiple cell types, extracellular matrix, an array of soluble cytokines, and proteases. At the end of the remodeling phase the wound resolves in most cases to a normal barely visible scar without functional consequences. Hypertrophic scar formation represents wound healing where the control mechanisms are abnormal and excessive collagen has been deposited [1,2]. Clinical observations suggest that wounds with delayed epithelialization have excess dermal collagen deposition, and thus form hypertrophic scars.
Regulation of collagen synthesis and deposition is a direct approach to control scar tissue formation. Type I collagen is the major collagen of the extracellular matrix and the major structural protein of scar tissue [1]. Its metabolism is modulated by numerous cytokines including the transforming growth factor-β superfamily, tumor necrosis factor-α (TNF-α) and interleukin-1 (IL-1) [3].
Experimental observations support the hypothesis that keratinocytes strongly contribute to the modulation of dermal fibroblast collagen production and degradation [4,5]. Detailed mechanisms of paracrine interactions between keratinocytes and fibroblasts have partly been investigated [6]. We have previously shown keratinocytes-fibroblast dependent production of TNF-α and IL-1 influencing collagen secretion in an in vitro coculture model [7].
Collagen degradation happens through cleavage by matrix metalloproteinases (MMPs). Another line of evidence has implicated the epidermis to be also an important factor in regulating MMP activity [8,9].
In cutaneous wounds MMPs are synthesized by keratinocytes, fibroblasts, macrophages, and endothelial cells [10]. They are up-regulated within hours after injury [11].
During wound healing the collagenases, gelatinases, and stromelysins are of particular relevance [10,12]: MMP-1, MMP-3, MMP-9 and MMP-10 contribute to keratinocyte migration during epithelialization. MMP-2, together with MMP-3 and MMP-13, is important in collagen degradation. MMPs also contribute to morphogenesis, angiogenesis, growth factor release, processing of precursor proteins, and to cell-cell as well as cell-matrix interactions [12].
However, the secretory behaviour of primary human cutaneous keratinocytes and fibroblasts concerning the different types of MMPs and TIMPs has not fully been elucidated. An exhaustive literature search is not giving the answer on the MMP profile secreted by keratinocytes and fibroblasts. No comparison of the whole array of MMPs that are employed during wound healing has been made in a single connected experiment.
The purpose of this study was to examine in vitro the effects of paracrine epithelial-mesenchymal interactions on MMPs and TIMPs that are assumed most relevant to wound healing and scarring by using a keratinocyte-fibroblast coculture system.
Primary adult human epidermal keratinocytes and dermal fibroblasts were cocultured under serum-free conditions. A comparison was made between monocultures and cocultures on the level of the released collagenases, gelatinases, stromelysins, tissue inhibitors of metalloproteinases, and the content of collagen I in conditioned media. In addition we wished to elucidate the impact of keratinocyte hydration on MMP- and TIMP-activity as we had previously demonstrated its importance in the regulation of keratinocyte cytokine expression [7].
Material and Methods
Cell Culture
Isolation of the primary human cells was performed with the approval of the Northwestern University Institutional Review Board.
Keratinocytes
Epidermal keratinocyte cultures were isolated from normal skin from three healthy female donors (21-30 years) undergoing breast reduction. The samples were placed in sterile culture plates and washed with Ca2+-, Mg2+-free PBS containing penicillin-streptomycin. The tissue was cut into 1 cm2 pieces. The dermis was removed using dispase. The epidermal sheets were digested with trypsin. The released keratinocytes were cultured in Defined Keratinocyte-SFM (a serum- and BPE-free medium with low calcium (<0.1 mM), containing a growth supplement, Invitrogen, Carlsbad, CA) and penicillin-streptomycin (humidified, 5% CO2, 37°C) [13]. Cells were passaged during amplification at max. 80% confluence and used between passages 4-7.
Fibroblasts
Dermal fibroblast cultures were isolated from normal skin from three female donors (20-26 years) undergoing breast reduction. De-epithelialized dermal tissue pieces were incubated at 37°C in collagenase type II (200 U/ml; Invitrogen, Carlsbad, CA) in serum-free Dulbecco's modified Eagle's medium (DMEM, Invitrogen, Carlsbad, CA). After inactivation of the collagenase by addion of DMEM containing 10% fetal bovine serum (FBS; Invitrogen, Carlsbad, CA) fibroblasts were released by trituration of the remaining tissue fragments. The released cells were cultured in fibroblast growth media (DMEM supplemented with 10% FBS and 1% penicillin-streptomycin) under standard culture conditions (humidified, 5% CO2, 37°C). Cells were passaged 1:3 upon confluence and used between passages 3-5.
Keratinocyte-Fibroblast Coculture
A coculture model was used to study paracrine interactions between keratinocytes and fibroblasts as previously described [7,14]. The model consists of two chambers separated by a semi-permeable 3.0 μm pore-size polyethylene terepthalate membrane (Fig. 1).
Figure 1. Experimental setup.
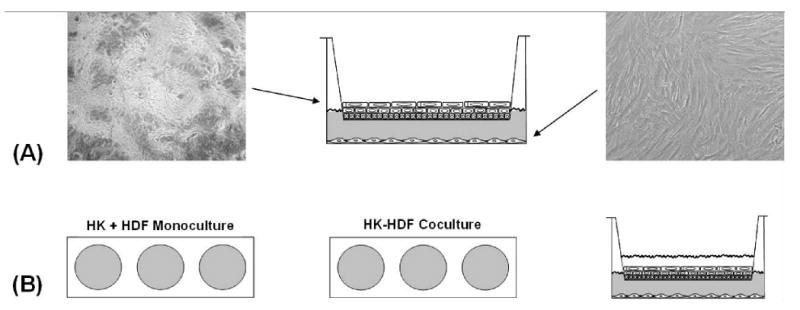
(A) Adult human epidermal keratinocytes (HK, left) were cultured submerged in inserts until confluent and stratifying and then set to the air-liquid-interface for 7 days. Adult human dermal fibroblasts (HDF, right) were cultured in 6-well-plates until confluent. Images were captured at ×100 magnification and are representative of all the cell cultures used in this study. (B) Hydration experiment. Keratinocytes (HK) and fibroblasts (HDF) were cocultured for 72 hours as in (B) except the keratinocytes were hydrated with culture medium. The monocultured controls were exposed to the same conditions.
After reaching a high proliferative activity, the keratinocytes were passaged at a cell density of 50,000 cells per cm2 into 6-well-inserts (4.65 cm2, BD Falcon™, San Jose, CA) at the presence of Defined K-SFM and remained submerged under serum-free conditions at low calcium (<0.1 mM) until confluence was complete and stratification became apparent. At this point the keratinocytes were exposed to air by keeping them at the air-liquid interface in Defined K-SFM supplemented with 10% FBS.
Meanwhile the fibroblasts were passed into 6-well plates (9.6 cm2, BD Falcon™, San Jose, CA) at a density of 40,000 cells per cm2 and cultured until confluent.
To initiate coculture both cell types were washed with sterile PBS and the keratinocyte-populated inserts were placed together with the confluent fibroblasts in the presence of DMEM supplemented with 1% BSA (bovine serum albumin, Sigma Chemical Co., St. Louis, MO), 1% ITS (insulin, transferrin and selenium, Sigma Chemical Co., St. Louis, MO), 1% penicillin-streptomycin and 50μg/ml vitamin C under hydrating conditions. In one group keratinocytes remained at the air-liquid interface in another they were submerged by media. The monocultured controls were treated equally. After 72 hours conditioned media was collected and after centrifugation at 3000 rpm for 15 minutes used for analysis or for further experiments.
Fibroblast Treatment with Conditioned Media from Keratinocytes
Fibroblasts were passed into 6-well plates at a density of 40,000 cells per cm2. When confluent they were washed with sterile PBS and treated with conditioned media from either air-exposed or hydrated monocultured keratinocytes (see coculture experiment). The controls were treated with conditioned media obtained from the control fibroblasts treated with the same experimental media. After 48 hours conditioned media was collected, centrifuged at 3000 rpm for 15 minutes and used for analysis.
Enzyme-Linked Immunosorbent Assay (ELISA)
The concentrations of MMP-1, MMP-8, MMP-13, MMP-2, MMP-9, MMP-3, MMP-10, TIMP-1 and TIMP-2 in conditioned media were analyzed by SearchLight Human MMP Array 1 (Pierce, Rockford, IL) as described previously [23].
Each well of the microplate provided was pre-spotted with MMP- and TIMP-specific antibodies that capture the active form of these proteins in the standards and samples added to the plate. After washing away of unbound proteins the biotinylated detecting antibodies were added. After washing away of excess detecting antibody streptavidin-horseradish peroxidase was added. The latter reacted with SUPERSIGNAL ELISA Femto Chemiluminescent Substrate (Patent 6,432,662) to produce a luminescent signal that is detected with a SEARCHLIGHT CCD Imaging and Analysis system. The amount of signal produced was proportional to the amount of each protein in the original sample. Each sample was assayed 2 times and the mean value of both measurements was used.
Western Blot Analysis
Collagen content in conditioned media was measured by western blot analysis as described previously [11]. Duplicate samples were subjected to 4 to 12% acrylamide gradient SDS-polyacrylamide gel electrophoresis. The proteins were transferred to PVDF (320 mA for 150 minutes in a Western blotting apparatus cooled to 4°C in the cold room; Bio-Rad Laboratories, Hercules, CA), blocked with 5% powdered milk in PBS with 0.1% Tween 20, and reacted with LF67 at a 1:20,000 dilution (a rabbit antihuman collagen-I antibody, generously provided by Dr. L. W. Fisher, National Institutes of Health [24]). The blots reacted with goat anti-rabbit horseradish peroxidase (Pierce Chemical Co., Rockford, IL), developed for chemiluminescence using enhanced chemiluminescence reagents (Amersham, England) and analyzed with a digital scanner EPI-CHEMI II Darkroom (UVP, Upland, CA) using UVP Labworks 4.0 software. Band intensities were normalized to total protein content and expressed as a stimulation index.
Zymography
Gelatinase activities present in conditioned media were visualized by gelatine zymography as described previously [15]. Duplicate samples of conditioned media were subjected to 10-well 10% Tris-Glycine gels containing copolymerized gelatine (Invitrogen, Carlsbad, CA) and electrophoretically separated under non-reducing conditions. Following electrophoresis, gels were washed with 2.5% Triton X-100 and incubated in 0.1 M glycine, 10 mM CaCl2, 1 μM ZnCl2, pH 8.3 for 24 hours prior to staining with Coomassie Blue. The zymograms were used for qualitative comparison (Fig. 2).
Figure 2. Representative gelatine zymograms.
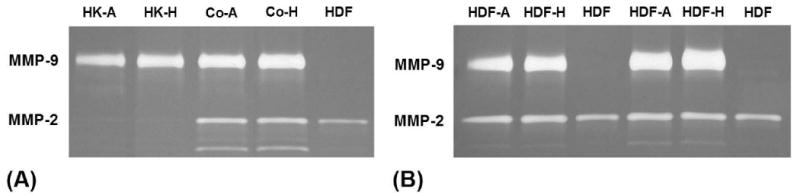
(A) Conditioned media from the coculture experiment (HK-A, air-treated keratinocytes; HK-H, hydrated keratinocytes; Co, coculture, A, air-treated; H, hydrated) in comparison to the conditioned media of monocultured keratinocytes and fibroblasts (HDF, control fibroblasts). One representative experiment. (B) Conditioned media from the conditioned media experiment (HDF-A, fibroblasts treated with conditioned media from air-treated keratinocytes; HDF-H, fibroblasts treated with conditioned media from hydrated keratinocytes; (HDF, control fibroblasts). Two representative experiments.
Statistical Analysis
Cells from three different donors were used for three independent experiments; the results were uniformly consistent, although the magnitudes varied. Each experiment including various treatments was performed in triplicate for each cell strain and repeated three times. Individual cell strain responses to treatments were quantified, averaged and compared to control treatments using the Student's t-test for paired groups. The level of significance was set to p values less than 0.05 in all analyses. Data are presented as mean ± SEM of percent change.
Results
Primary adult human epidermal keratinocytes and primary adult dermal fibroblasts released matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) at differing intensity. The various experimental conditions lead to significant changes of the measured MMP- and TIMP-activities (Table 1).
Table 1.
Concentrations of MMP and TIMP [ng/ml] secreted by primary adult human epidermal keratinocytes and primary adult human dermal fibroblasts respectively under various conditions – the values are depicted as average of the measured concentrations.
| [ng/ml] | Air-treated Keratinocyte Monoculture | Hydrated Keratinocyte Monoculture | Air-treated HK/HDF Coculture | Hydrated HK/HDF Coculture | Fibroblast Monoculture |
|---|---|---|---|---|---|
| Collaqenases | |||||
| MMP-1 | 92.45 | 93.04 | 807.22 | 802.79 | 21.99 |
| MMP-8 | 0.145 | 0.122 | 0.517 | 0.543 | 0.103 |
| MMP-13 | 0.350 | 0.337 | 0.493 | 0.511 | 0.361 |
| Gelatinases | |||||
| MMP-2 | 16.78 | 14.81 | 385.43 | 379.80 | 135.66 |
| MMP-9 | 16.61 | 23.61 | 60.97 | 61.48 | 1.38 |
| Stromelvsins | |||||
| MMP-3 | 1.49 | 1.27 | 3283.67 | 3657.78 | 672.18 |
| MMP-10 | 29.04 | 30.20 | 42.51 | 44.17 | 0.37 |
| TIMPs | |||||
| TIMP-1 | 17.46 | 20.83 | 520.13 | 469.95 | 133.64 |
| TIMP-2 | 7.59 | 7.28 | 66.64 | 59.25 | 28.99 |
Keratinocyte-Fibroblast Coculture
Collagenase-1 (MMP-1) was secreted by monocultured keratinocytes and monocultured fibroblasts, though at lower levels by the latter. Coculture increased the content of MMP-1 in conditioned media up to ninefold. Collagenase-2 (MMP-8) and collagenase-3 (MMP-13) were secreted at lower concentrations than MMP-1, but at comparable levels by monocultured keratinocytes and monocultured fibroblasts. Coculture increased MMP-8 more than threefold, to a lesser degree MMP-13. The measured concentrations of MMP-1, MMP-8, and MMP-13 were within the range from 17.76-827.04 ng/ml, 91.02-788.67 pg/ml and 157.61-597.43 pg/ml respectively (Fig. 3).
Figure 3. Coculture-Experiment.
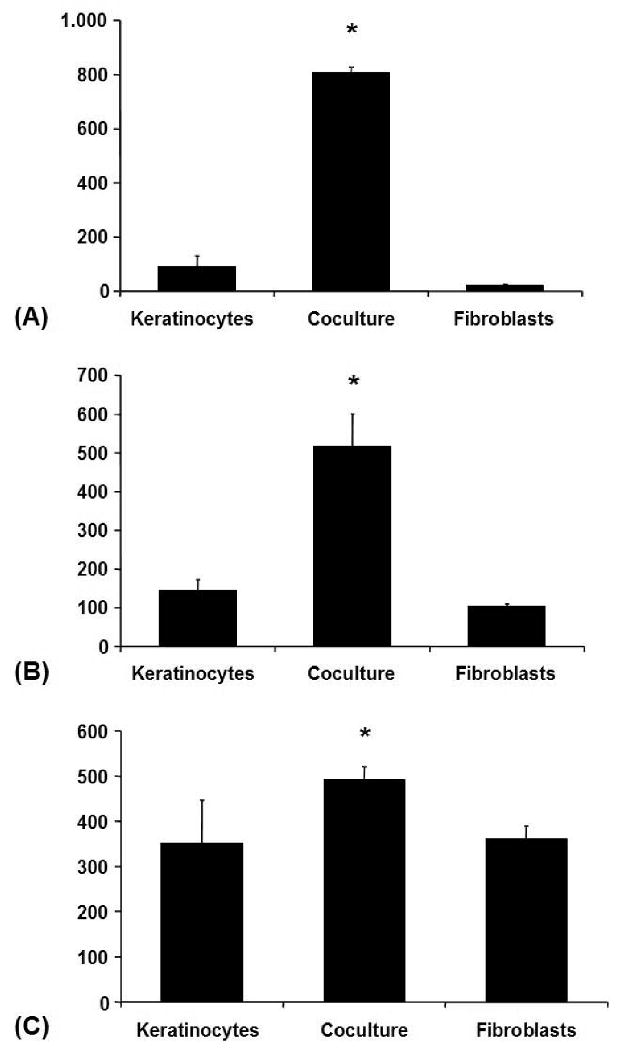
(A) MMP-1 (Collagenase-1) in conditioned media from the coculture experiment (ELISA): Averaged absolute values in [ng/ml] ± SEM, *p < 0.05. (B) MMP-8 (Collagenase-2) in conditioned media from the coculture experiment (ELISA): Averaged absolute values in [pg/ml] ± SEM, *p < 0.05. (C) MMP-13 (Collagenase-3) in conditioned media from the coculture experiment (ELISA): Averaged absolute values in [pg/ml] ± SEM, *p < 0.05.
Gelatinase A (MMP-2) was secreted in monoculture by both cell types, but at very low levels by keratinocytes in comparison to fibroblasts. There was a more than twofold increase of MMP-2 under coculture conditions. Gelatinase B (MMP-9) was mainly secreted by keratinocytes and only at very low levels by fibroblasts. Coculture increased MMP-9 by more than twofold. The measured concentrations of MMP-2 and -9 were within the range from 14.81-451.49 ng/ml and 1.19-75.74 ng/ml respectively (Fig 4).
Figure 4. Coculture-Experiment.
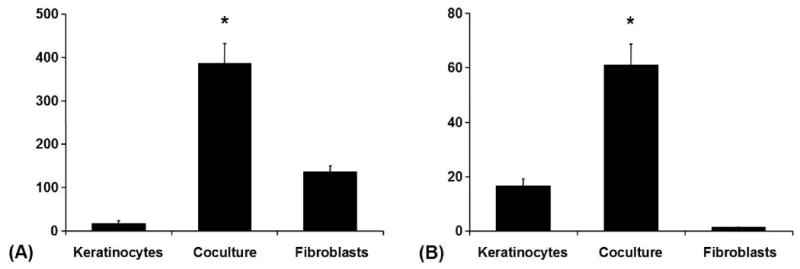
(A) MMP-2 (Gelatinase-A) in conditioned media from the coculture experiment (ELISA): Avaraged absolute values in [ng/ml] ± SEM, *p < 0.05. (B) MMP-9 (Gelatinase-B) in conditioned media from the coculture experiment (ELISA): Avaraged absolute values in [ng/ml] ± SEM, *p < 0.05.
Stromelysin-1 (MMP-3) was secreted predominantly by fibroblasts. It was also detectable at very low levels in conditioned media from keratinocyte monocultures. Coculture increased the content of MMP-3 in conditioned media fivefold. Stromelysin-2 (MMP-10) was secreted mainly by keratinocytes and was only detectable at very low levels in conditioned media from fibroblast monocultures. In conditioned media from cocultures MMP-10 showed a tendency to higher levels. The measured concentrations of MMP-3 and -10 were within the range from 58.71-5555.5 ng/ml and 0.32-61.05 ng/ml respectively (Fig. 5).
Figure 5. Coculture-Experiment.
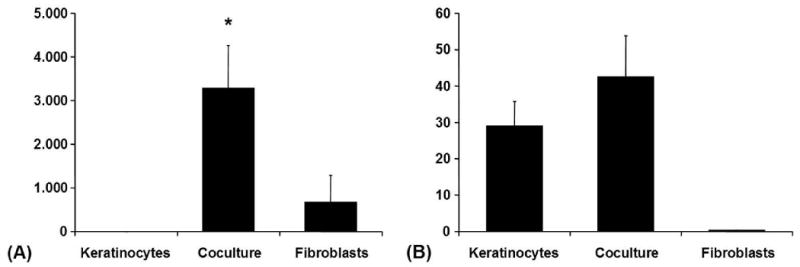
(A) MMP-3 (Stromelysin-1) in conditioned media from the coculture experiment (ELISA): Averaged absolute values in [ng/ml] ± SEM, */**p < 0.05. (B) MMP-10 (Stromelysin-2) in conditioned media from the coculture experiment (ELISA): Averaged absolute values in [ng/ml] ± SEM.
Both examined tissue inhibitors of metalloproteinases (TIMP-1 and TIMP-2) were secreted at lower levels by monocultured keratinocytes than by monocultured fibroblasts. Coculture increased the concentrations of TIMP-1 and TIMP-2 at least by a factor of two. The measured concentrations of TIMP-1 and -2 were within the range from 3.38-772.37 and 4.68-81.4 ng/ml respectively (Fig. 6).
Figure 6. Coculture-Experiment.
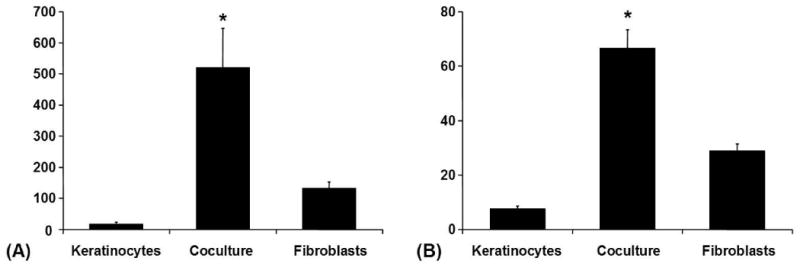
(A) TIMP-1 (Tissue inhibitor of metalloproteinases 1) in conditioned media from the coculture experiment (ELISA): Averaged absolute values in [ng/ml] ± SEM, *p < 0.05. (B) TIMP-2 (Tissue inhibitor of metalloproteinases 2) in conditioned media from the coculture experiment (ELISA): Averaged absolute values in [ng/ml] ± SEM, *p < 0.05.
These results were strengthened by the conditioned media experiment: when treated with conditioned media from air-treated keratinocytes fibroblasts secreted more collagenases, MMP-2, MMP-3, and more TIMPs than their controls.
Effects of Keratinocyte Hydration
Hydration of keratinocytes in the coculture experiment had a significant impact on MMP levels in conditioned media in the case of MMP 3 and TIMP-2. The already remarkable increase of MMP 3 in coculture media was further significantly intensified in the presence of hydrated keratinocytes whereas TIMP-2 decreased in this context (Fig. 7).
Figure 7. Hydration of Keratinocytes: Coculture-Experiment.
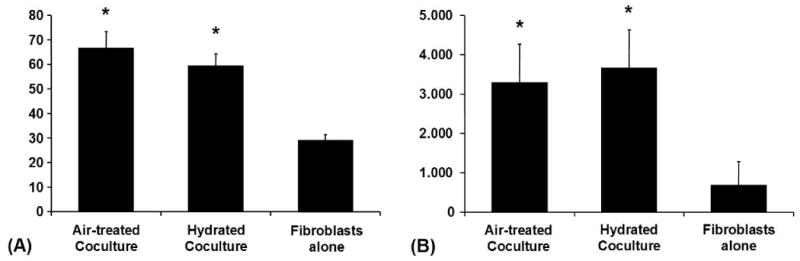
(A) MMP-3 in conditioned media from the coculture experiment (ELISA): Averaged absolute values in [ng/ml] ± SEM from various conditions, *p < 0.05. (B) TIMP-2 in conditioned media from the coculture experiment (ELISA): Averaged absolute values in [ng/ml] ± SEM from various conditions, *p < 0.05.
When adding conditioned media from hydrated keratinocytes to fibroblasts the activities of MMP-1, MMP-3, and MMP-2 increased by 10%, 17% and 24%. TIMP-2 decreased by 40% after addition of conditioned media from hydrated keratinocytes (Fig. 8).
Figure 8. Hydration of Keratinocytes: Treatment of HDFs with Conditioned Media from HKs.
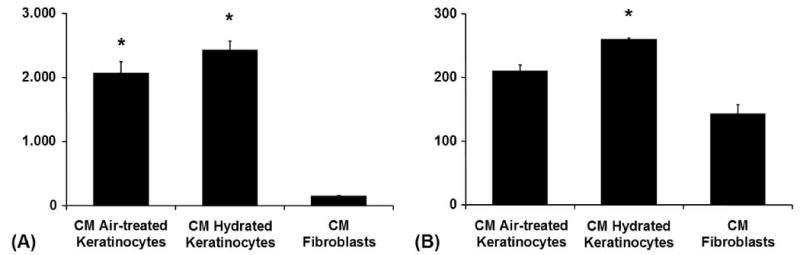
(A) MMP-3 (Stromelysin-1) in conditioned media from fibroblasts from the conditioned-media experiment (ELISA): Fibroblasts were treated with conditioned media from keratinocytes kept under different conditions. Absolute values in [ng/ml], */**p < 0.05. (B) MMP-2 (Gelatinase A) in conditioned media from fibroblasts from the conditioned-media experiment (ELISA): Fibroblasts were treated with conditioned media from keratinocytes kept under different conditions. Absolute values in [ng/ml], */**p < 0.05.
Collagen
Collagen I content in conditioned media decreased significantly when fibroblasts were cocultured with keratinocytes [11]. Hydration of the present keratinocytes decreased collagen further (Fig. 9).
Figure 9.
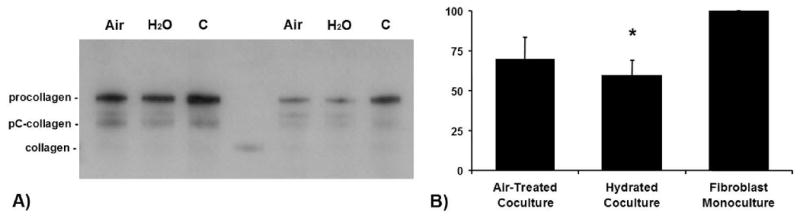
(A) Collagen-I-Content (Western blot): Two representative collagen-I Western blots of conditioned media from cocultures (Air, air-treatment; H2O, hydration) in comparison to the conditioned media of monocultured fibroblasts (C, control). LF67, rabbit antihuman collagen 1 (generously provided by Dr. L. W. Fisher, National Institutes of Health[24]), binds to the different stages of the post-translational biochemical modification of the secreted procollagen polypeptide. The fibroblasts secrete the soluble ‘procollagen’ which represents the major part in conditioned media. Outside the cell, procollagen peptidases cleave the registration peptides to form pC-collagen and pN-collagen. The LF67 antibody recognizes the pC-collagen as well. After 72 hours at which time the experiment ended only a small part of the secreted procollagen has been transformed to collagen. (B) Collagen-I-Content (IODs of Western blots): Relative collagen content in conditioned media [in %] between the two coculture groups in comparison to the conditioned media of monocultured fibroblasts, *p < 0.05. The IODs of the control were set to 100 %.
Discussion
MMPs are important in wound healing. They are needed for degradation of provisional matrix, angiogenesis, cell migration and extracellular matrix remodeling. Wound healing is impaired when MMPs are absent [16,17]. On the other hand excessive levels of MMPs impair wound healing or act destructive on normal tissue as in the case of rheumatoid arthritis or cardiovascular disease [18-20]. Premature collagen damage in photoaged skin is credited to MMPs induced by UV-light [21]. TIMPs are the natural inhibitors of MMPs. An imbalance between the activity of MMPs and TIMPs is stated to cause chronic non-healing wounds on one side and fibrotic disorders on the other [22,23].
In hypertrophic scars and keloids specific MMPs and TIMPs are still present in contrast to uninjured skin or normal scars [24,25]. The decreased production of MMP-1 in post-burn hypertrophic scar fibroblasts was stated to make these wounds prone to hypertrophic scars [26]. In an open wound, advancing epithelium results in apoptosis and reduction in inflammation in the underlying granulation tissue, and early grafting or addition of epithelial cells to resurface a wound result in improved scars [27,28]. These results suggest that part of the reason for improved scar and reduced collagen accumulation clinically could be due to increased MMP expression and increased collagen degradation, and support the observation that epithelial mesenchymal cell interactions enhance scar remodeling.
To date it cannot be said which MMP is more important for collagen degradation. A comparison of the entire array of MMPs and TIMPs all together in the context of wound healing and scarring is still missing. It is very likely that the activity of the different MMP groups make the sum together with their interplay with the TIMPs. The spectrum of substrates among the individual enzymes is quite similar though they act on them with different affinity. Nevertheless, the collagenases are noteworthy in particular as they control the process of collagen degradation. MMP-1 initiates matrix degradation by cleaving fibrillar native triple helical collagen and making it susceptible for the final break-down by the gelatinases and MMP-3. MMP-3 on the other side might play an important role in hypertrophic scars as it degrades proteoglycans [29]. Chondroitin-4-sulfate has been found to coat the whorl-like nodules in hypertrophic scars that contain collagen [30]. Its resistance to collagenases undoubtedly reduces the accessibility of these nodules to MMP degradation.
The collagen breakdown in a scar in vivo is impacted by which cells and where in the microenvironment these MMP are released than the affinity of the enzyme-substrate interactions. Epithelial-mesenchymal interactions are potentially important in this context, but teasing out these interactions in vivo is not feasible. Coculture of two different cells types in an insert system is a reliable method to investigate paracrine interactions in vitro. Our results add some new insights into this complex process.
In our experiments monocultured keratinocytes secreted mainly MMP-1, MMP-9, and MMP-10 whereas monocultured fibroblasts secreted predominantly MMP-2, MMP-3, TIMP-1, and TIMP-2. The collagenases MMP-8 and MMP-13 were secreted by both cell types at comparable low levels. Coculture of keratinocytes with fibroblasts had an overall and strong impact on the activity of MMPs and TIMPs: almost all MMPs and both TIMPs that were investigated in this study increased significantly and very consistently in conditioned media from cocultures. The conditioned media experiments strengthened these results: monocultured fibroblasts that were treated with conditioned media from air-exposed monocultured keratinocytes secreted more collagenases, more MMP-2, more MMP-3, and more TIMPs than their controls.
In coculture we saw an impressive fivefold increase of MMP-3. As a broad spectrum proteinase MMP-3 is important in degradation of major components of the extracellular matrix [29]. The gelatinases increased more than twofold and TIMP-1 and TIMP-2 increased up to fourfold and threefold respectively and confirmed in part a previous observation [9]. Our observed ninefold increase of MMP-1 in conditioned media from the coculture experiments matches findings from others [31].
Hydration of keratinocytes caused slight changes in most MMPs and TIMPs. They were significant in the case of MMP-3 and of TIMP-2 as well as of MMP-2. We had hypothesized a change in MMP activity under hydration, based on our previous observations on beneficial effects of epidermal hydration on scarring [32]. By restoration of the barrier function either by hydration or a mature stratum corneum the stimulus for epithelial proliferation is reduced – as a consequence less activated more differentiated keratinocytes are present and scarring is reduced [33].
Paracrine cross-talk between keratinocytes and fibroblasts influences the activity of various MMPs [8,34]. In the present study we examined in connected experiments the impact of these paracrine interactions on MMPs and tissue inhibitors of metalloproteinases (TIMPs) that are supposed to be involved in wound healing and are secreted by keratinocytes and fibroblasts. We chose the methodology of using primary cutaneous keratinocytes and fibroblasts at low passage numbers to model the in vivo wound, though recognizing the limitations of an in vitro system.
Consideration of the role of MMPs is critical to understanding scarring, as collagen deposition is a balance of synthesis and degradation. Our data strongly support the hypothesis that paracrine interactions between keratinocytes and fibroblasts direct the balance between proliferative and degradative processes related to collagen metabolism and, ultimately, scar formation. We were able to show that MMP activity is significantly induced when keratinocytes and fibroblasts were cocultured and that these increases were associated with decreased levels of collagen. These results provide further evidence for a paracrine interaction between keratinocytes and fibroblasts that appears to play an important role in the regulation of collagen metabolism.
Acknowledgments
There is no conflict of interest to disclose. This work was funded by a grant from the National Institution of Health, RO1 GM063825.
Footnotes
Subject Category: ‘Wound Healing / Plastic Surgery’
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Craig RD, Schofield JD, Jackson DS. Collagen biosynthesis in normal and hypertrophic scars and keloid as a function of the duration of the scar. Br J Surg. 1975;62:741–4. doi: 10.1002/bjs.1800620917. [DOI] [PubMed] [Google Scholar]
- 2.Ehrlich HP, Desmouliere A, Diegelmann RF, et al. Morphological and immunochemical differences between keloid and hypertrophic scar. Am J Pathol. 1994;145:105–13. [PMC free article] [PubMed] [Google Scholar]
- 3.Ghosh AK. Factors involved in the regulation of type I collagen gene expression: implication in fibrosis. Exp Biol Med (Maywood) 2002;227:301–14. doi: 10.1177/153537020222700502. [DOI] [PubMed] [Google Scholar]
- 4.Garner WL, Karmiol S, Rodriguez JL, Smith DJ, Jr, Phan SH. Phenotypic differences in cytokine responsiveness of hypertrophic scar versus normal dermal fibroblasts. J Invest Dermatol. 1993;101:875–9. doi: 10.1111/1523-1747.ep12371710. [DOI] [PubMed] [Google Scholar]
- 5.Bellemare J, Roberge CJ, Bergeron D, et al. Epidermis promotes dermal fibrosis: role in the pathogenesis of hypertrophic scars. J Pathol. 2005;206:1–8. doi: 10.1002/path.1737. [DOI] [PubMed] [Google Scholar]
- 6.Szabowski A, Maas-Szabowski N, Andrecht S, et al. c-Jun and JunB antagonistically control cytokine-regulated mesenchymal-epidermal interaction in skin. Cell. 2000;103:745–55. doi: 10.1016/s0092-8674(00)00178-1. [DOI] [PubMed] [Google Scholar]
- 7.Tandara AA, Kloeters O, Mogford JE, Mustoe TA. Hydrated keratinocytes reduce collagen synthesis by fibroblasts via paracrine mechanisms. Wound Repair Regen. 2007;15:497–504. doi: 10.1111/j.1524-475X.2007.00257.x. [DOI] [PubMed] [Google Scholar]
- 8.Nowinski D, Lysheden AS, Gardner H, et al. Analysis of gene expression in fibroblasts in response to keratinocyte-derived factors in vitro: potential implications for the wound healing process. J Invest Dermatol. 2004;122:216–21. doi: 10.1046/j.0022-202X.2003.22112.x. [DOI] [PubMed] [Google Scholar]
- 9.Sawicki G, Marcoux Y, Sarkhosh K, Tredget EE, Ghahary A. Interaction of keratinocytes and fibroblasts modulates the expression of matrix metalloproteinases-2 and -9 and their inhibitors. Mol Cell Biochem. 2005;269:209–16. doi: 10.1007/s11010-005-3178-x. [DOI] [PubMed] [Google Scholar]
- 10.Parks WC. Matrix metalloproteinases in repair. Wound Repair Regen. 1999;7:423–32. doi: 10.1046/j.1524-475x.1999.00423.x. [DOI] [PubMed] [Google Scholar]
- 11.Soo C, Shaw WW, Zhang X, et al. Differential expression of matrix metalloproteinases and their tissue-derived inhibitors in cutaneous wound repair. Plast Reconstr Surg. 2000;105:638–47. doi: 10.1097/00006534-200002000-00024. [DOI] [PubMed] [Google Scholar]
- 12.Ravanti L, Kahari VM. Matrix metalloproteinases in wound repair (review) Int J Mol Med. 2000;6:391–407. [PubMed] [Google Scholar]
- 13.Boyce ST, Ham RG. Calcium-regulated differentiation of normal human epidermal keratinocytes in chemically defined clonal culture and serum-free serial culture. J Invest Dermatol. 1983;81:33s–40s. doi: 10.1111/1523-1747.ep12540422. [DOI] [PubMed] [Google Scholar]
- 14.Katz AB, Taichman LB. Epidermis as a secretory tissue: an in vitro tissue model to study keratinocyte secretion. J Invest Dermatol. 1994;102:55–60. doi: 10.1111/1523-1747.ep12371732. [DOI] [PubMed] [Google Scholar]
- 15.Fishman DA, Bafetti LM, Banionis S, et al. Production of extracellular matrix-degrading proteinases by primary cultures of human epithelial ovarian carcinoma cells. Cancer. 1997;80:1457–63. doi: 10.1002/(sici)1097-0142(19971015)80:8<1457::aid-cncr13>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 16.Bullard KM, Lund L, Mudgett JS, et al. Impaired wound contraction in stromelysin-1-deficient mice. Ann Surg. 1999;230:260–5. doi: 10.1097/00000658-199908000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beare AH, Krane SM, Ferguson MW. Variable impairment of wound healing in the heterozygous collagenase-resistant mouse. Wound Repair Regen. 2005;13:27–40. doi: 10.1111/j.1067-1927.2005.130105.x. [DOI] [PubMed] [Google Scholar]
- 18.Yager DR, Zhang LY, Liang HX, Diegelmann RF, Cohen IK. Wound fluids from human pressure ulcers contain elevated matrix metalloproteinase levels and activity compared to surgical wound fluids. J Invest Dermatol. 1996;107:743–8. doi: 10.1111/1523-1747.ep12365637. [DOI] [PubMed] [Google Scholar]
- 19.Burrage PS, Mix KS, Brinckerhoff CE. Matrix metalloproteinases: role in arthritis. Front Biosci. 2006;11:529–43. doi: 10.2741/1817. [DOI] [PubMed] [Google Scholar]
- 20.Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69:562–73. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 21.Rittie L, Fisher GJ. UV-light-induced signal cascades and skin aging. Ageing Res Rev. 2002;1:705–20. doi: 10.1016/s1568-1637(02)00024-7. [DOI] [PubMed] [Google Scholar]
- 22.Bou-Gharios G, Osman J, Black C, Olsen I. Excess matrix accumulation in scleroderma is caused partly by differential regulation of stromelysin and TIMP-1 synthesis. Clin Chim Acta. 1994;231:69–78. doi: 10.1016/0009-8981(94)90255-0. [DOI] [PubMed] [Google Scholar]
- 23.Saarialho-Kere UK. Patterns of matrix metalloproteinase and TIMP expression in chronic ulcers. Arch Dermatol Res. 1998;290(Suppl):S47–54. doi: 10.1007/pl00007453. [DOI] [PubMed] [Google Scholar]
- 24.Neely AN, Clendening CE, Gardner J, Greenhalgh DG, Warden GD. Gelatinase activity in keloids and hypertrophic scars. Wound Repair Regen. 1999;7:166–71. doi: 10.1046/j.1524-475x.1999.00166.x. [DOI] [PubMed] [Google Scholar]
- 25.Hembry RM, Ehrlich HP. Immunolocalization of collagenase and tissue inhibitor of metalloproteinases (TIMP) in hypertrophic scar tissue. Br J Dermatol. 1986;115:409–20. doi: 10.1111/j.1365-2133.1986.tb06235.x. [DOI] [PubMed] [Google Scholar]
- 26.Ghahary A, Shen YJ, Nedelec B, et al. Collagenase production is lower in post-burn hypertrophic scar fibroblasts than in normal fibroblasts and is reduced by insulin-like growth factor-1. J Invest Dermatol. 1996;106:476–81. doi: 10.1111/1523-1747.ep12343658. [DOI] [PubMed] [Google Scholar]
- 27.Brown DL, Kao WW, Greenhalgh DG. Apoptosis down-regulates inflammation under the advancing epithelial wound edge: delayed patterns in diabetes and improvement with topical growth factors. Surgery. 1997;121:372–80. doi: 10.1016/s0039-6060(97)90306-8. [DOI] [PubMed] [Google Scholar]
- 28.Wood FM, Kolybaba ML, Allen P. The use of cultured epithelial autograft in the treatment of major burn wounds: eleven years of clinical experience. Burns. 2006;32:538–44. doi: 10.1016/j.burns.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 29.Wilhelm SM, Collier IE, Kronberger A, et al. Human skin fibroblast stromelysin: structure, glycosylation, substrate specificity, and differential expression in normal and tumorigenic cells. Proc Natl Acad Sci U S A. 1987;84:6725–9. doi: 10.1073/pnas.84.19.6725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Linares HA, Larson DL. Proteoglycans and collagenase in hypertrophic scar formation. Plast Reconstr Surg. 1978;62:589–93. doi: 10.1097/00006534-197810000-00016. [DOI] [PubMed] [Google Scholar]
- 31.Moon SE, Bhagavathula N, Varani J. Keratinocyte stimulation of matrix metalloproteinase-1 production and proliferation in fibroblasts: regulation through mitogen-activated protein kinase signalling events. Br J Cancer. 2002;87:457–64. doi: 10.1038/sj.bjc.6600478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saulis AS, Chao JD, Telser A, Mogford JE, Mustoe TA. Silicone occlusive treatment of hypertrophic scar in the rabbit model. Aesthetic Surgery J. 2002;22:147–53. doi: 10.1067/maj.2002.123023. [DOI] [PubMed] [Google Scholar]
- 33.Tandara AA, Mustoe TA. The role of the epidermis in the control of scarring: evidence for mechanism of action for silicone gel. J Plast Reconstr Aesthet Surg. 2008;61:1219–25. doi: 10.1016/j.bjps.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 34.Ghahary A, Ghaffari A. Role of keratinocyte-fibroblast cross-talk in development of hypertrophic scar. Wound Repair Regen. 2007;15:S45–S53. doi: 10.1111/j.1524-475X.2007.00225.x. [DOI] [PubMed] [Google Scholar]


