Summary
Accumulation of proteins in the endoplasmic reticulum (ER) triggers the unfolded protein response (UPR), comprising three signaling pathways initiated by Ire1, Perk and Atf6 respectively. UPR activation was compared in chemically stressed murine wildtype melanocytes and mutant melanocytes that retain tyrosinase in the ER. Thapsigargin, an ER stressor, activated all pathways in wildtype melanocytes, triggering Caspase 12-mediated apoptosis at toxic doses. Albino melanocytes expressing mutant tyrosinase showed evidence of ER stress with increased Ire1 expression; but the downstream effector, Xbp1, was not activated even following thapsigargin treatment. Attenuation of Ire1 signaling was recapitulated in wildtype melanocytes treated with thapsigargin for eight days, with diminished Xbp1 activation observed after four days. Atf6 was also activated in albino melanocytes, with no response to thapsigargin, while the Perk pathway was not activated and thapsigargin treatment elicited robust expression of the downstream effector CHOP. Thus, melanocytes adapt to ER stress by attenuating two UPR pathways.
Keywords: Unfolded protein response, melanocyte, tyrosinase, chemoresistance, ER stress
Introduction
Tyrosinase, the first and rate-limiting enzyme in melanin biosynthesis, undergoes an extended period of post-translational modification, including N-linked glycosylation (Gupta et al., 2009), and disulfide bond formation-dependent folding (Wang et al., 2005) in the endoplasmic reticulum (ER) in order to attain a functional tertiary structure (Ujvari et al., 2001). This process requires classical ER chaperones (Wang et al., 2005), as well as melanocyte-specific factors (Francis et al., 2003). Mutations at four loci, encoding melanocyte-specific genes have been shown to result in tyrosinase misfolding, namely tyrosinase itself (TYR) (Halaban et al., 2000), the oculocutaneous albinism type 2 gene (OCA2; Chen et al., 2002), tyrosinase-related protein 1 (TYRP1; Toyofuku et al., 2001b) and OCA4 (Costin et al., 2003). While misfolding due to point mutations at the tyrosinase locus can be readily explained due to changes in amino acid sequence, the precise cause/s for ER retention of tyrosinase observed in OCA2, OCA3 and OCA4 remain unclear.
Diseases that involve disruption of protein folding include lysosomal and neurological conditions such as Alzheimer and Parkinson diseases. Protein misfolding results in peptide retention in the ER, the primary cellular site for protein synthesis and folding. A complex quality control system ensures that only correctly folded proteins are transported to the Golgi for further modification or to their site of activity. Misfolded proteins are either refolded or targeted for proteasomal degradation. Accumulation of unfolded proteins in the ER exerts a stress on the organelle which if unchecked activates a signal cascade known as the unfolded protein response (UPR). The UPR ameliorates the effects of protein misfolding by transiently halting global translation, increasing expression of folding chaperones to augment the capacity of the ER to deal with unfolded proteins, and escalating proteolytic degradation of misfolded proteins (reviewed in Malhotra and Kaufman, 2007). In the event that the stress is not resolved, the UPR can induce apoptosis. This mechanism underlies progression to type 2 diabetes. Excessive insulin production in pancreatic β-cells leads to accumulation of the protein in the ER, thereby activating the UPR and subsequently apoptosis (Marciniak and Ron, 2006).
The UPR is a fundamental process that is highly conserved through evolution. It consists of three distinct arms triggered by the inositol-requiring protein 1 (Ire1), PKR-like endoplasmic reticulum kinase (Perk), and activating transcription factor (Atf)-6, respectively. These proteins reside in the membrane of the ER, with their lumenal domains bound to immunoglobulin-binding protein (BiP, also known as glucose-regulated protein 78/GRP78). BiP is sequestered to unfolded proteins as they accumulate and the UPR initiators are released and activated (Bertolotti et al., 2000).
Upon release, Ire1 is phosphorylated and its nuclease activated, leading to splicing of the X-box binding protein 1 (Xbp1) RNA. Spliced Xbp1 encodes a transcription factor that targets and induces expression of genes containing an unfolded protein response element (UPRE). These genes include ER chaperones, heat shock proteins and Xbp-1 itself (Calfon et al., 2002). In addition to the UPR-related genes, Xbp1 has recently been shown to play a role in DNA damage and repair pathways (Acosta-Alvear et al., 2007) suggesting a critical role for the protein in melanocyte survival in the skin. This hypothesis is further supported by the statistical association observed between Xbp-1 sequence variants and increased risk of developing vitiligo, a disease that results from localized death of skin melanocytes (Ren et al., 2009).
Perk undergoes phosphorylation and oligomerization following release from BiP, thereby activating a second UPR pathway. Perk phosphorylates the eukaryotic translation initiation factor 2-α (Eif2α), which leads to inhibition of cap-dependent translation and subsequently protein synthesis (Harding et al., 2000). Some mRNAs that contain a 5′ untranslated region with an internal ribosomal entry site (IRES), such as the one encoding activating transcription factor 4 (Atf4), can escape Perk-mediated repression. Atf4 regulates the expression of genes required for cell survival including some involved in redox reactions and amino acid metabolism (Harding et al., 2003). In addition, Atf4 activates expression of BiP and the transcription factor CCAAT-enhancer-binding protein homologous protein (CHOP). Although activated primarily via the Perk pathway, CHOP can also be activated by the Ire1 and Atf6 pathways (Fawcett et al., 1999). CHOP plays a major role in UPR-induced cell death, with deletion of the gene in mouse models of type 2 diabetes preventing phenotype onset (Song et al., 2008).
The third arm of the UPR is regulated by Atf6, which is trafficked to the Golgi following release from BiP (Shen et al., 2002). There, Atf6 is cleaved from a 90kDa membrane protein to a soluble 50kDa form (Atf6s), which is translocated to the nucleus (Yoshida et al., 2000) and induces expression of ER chaperones such as BiP (Yoshida et al., 1998) as well as CHOP and Xbp-1 (Lee et al., 2003).
In the event that UPR activation is not sufficient to balance the homeostasis of the ER, apoptosis is initiated by Perk/Atf4-mediated activation of Noxa and Bim (Harding et al., 2003), pro-apoptotic members of the Bcl2 protein family (Fribley et al., 2006). In addition to diabetes, the UPR has also been implicated in retinitis pigmentosa (RP), which results from apoptosis induced by accumulation of misfolded, mutated rhodopsin in the retina (Lin et al., 2007). There are also instances in which sustained UPR activation does not result in apoptosis. For example, the UPR is triggered during B-cell differentiation, however while Ire1 expression is sustained, CHOP expression is down-regulated and cells do not undergo apoptosis (Todd et al., 2008). Cells can therefore adapt to chronic UPR activation via what is likely a cell-specific mechanism.
In this study, we sought to determine if accumulation of tyrosinase in the melanocyte ER resulted in activation of the UPR. To further characterize this process, we also evaluated melanocyte responses to treatment with the chemical stressors thapsigargin and tunicamycin. Thapsigargin disrupts ER homeostasis by inhibition of sarco/endoplasmic reticulum calcium ATPases and thus calcium concentrations, while tunicamycin inhibits N-glycosylation of newly synthesized peptides causing the proteins to be retained in the ER.
Characterizing the mechanisms involved in UPR activation in melanocytes will further our understanding of this recently-identified fundamental cellular stress response. In addition, the melanocyte would provide a useful model for investigation of the UPR and potential chemical mediators of the pathway. The UPR has also been suggested as a target for melanoma therapeutics and characterization of the pathway in melanocytes may allow for refinement of this approach (Chen et al., 2007, Jiang et al., 2007).
Results
Wildtype mouse melanocytes (WT/melan-a) were treated with thapsigargin and tunicamycin to chemically induce the UPR. Activation and expression levels of key UPR components were also compared in WT cells, in which tyrosinase is efficiently folded and removed from the ER, and in melanocytes that carry mutations at the tyrosinase (Tyr-mut/melan-c) or Tyrp1 (Tyrp1-mut/melan-b) loci that both result in retention of misfolded tyrosinase in the ER (Toyofuku et al., 2001b).
Ire1-mediated UPR activation in melanocytes is attenuated with sustained ER stress
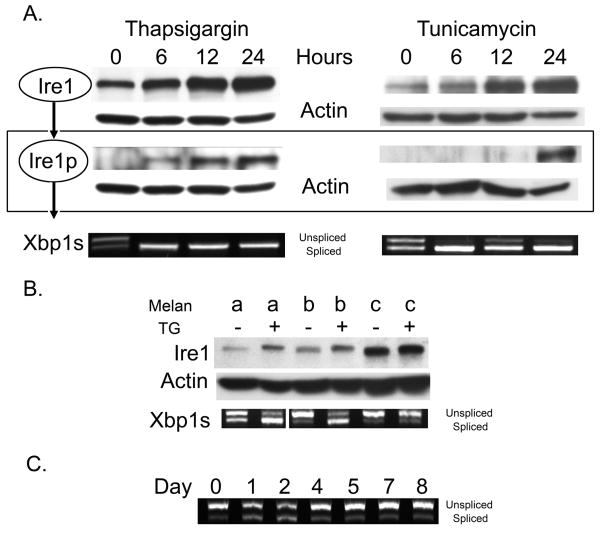
We performed Western blot analysis to assess Ire1 expression and phosphorylation following treatment of WT melanocytes with either 150nM thapsigargin or 10μg/mL tunicamycin (Figure 1A). A robust increase in Ire1 expression and phosphorylation was observed in WT cells with increasing time of exposure to thapsigargin concomitant with Xbp-1 splicing, indicating UPR activation within six hours of treatment. In comparison, tunicamycin induced Xbp-1 splicing that decreased slightly at 12 hrs and increased, together with Ire1 phosphorylation, at 24 hours of treatment initiation.
Figure 1.
(A) WT melanocytes were treated with 150nM thapsigargin or 10μg/mL tunicamycin for the indicated times, (B) mutant cells were treated with 150nM thapsigargin for 24 hours and (C), and WT melanocytes were treated with 15nM thapsigargin over an eight day period. Cells were lysed, proteins and RNA harvested and samples normalized for protein/RNA concentration prior to analysis. Ire1 expression and phosphorylation was monitored by Western blot analysis and Xbp1 splicing by RT-PCR as previously reported (Lin et al., 2007). Actin is shown as a loading control.
Ire1 expression was increased in Tyr-mutant cells and to a lesser degree in Tyrp1-mutant cells when compared to WT cells (Figure 1B). Dosing with thapsigargin for six hours resulted in increased Ire1 expression and Xbp1 splicing in Tyrp1-mutant but not Tyr-mutant cells. Tyr-mutant cells express significantly higher levels of ER-retained tyrosinase than do Tyrp1-mutant cells (Toyofuku et al., 2001b), accounting for the increased UPR activation. The lack of response to thapsigargin in Tyr-mutant cells suggested adaptation to ER stress. In order to determine if sustained ER stress altered melanocyte activation of the UPR, WT cells were dosed daily with 15nM thapsigargin for an eight day period and Xbp-1 splicing monitored (Figure 1C). The reduced thapsigargin dose was used because the 150nM dose over 48 hours was toxic. Increased Xbp-1 splicing was observed until day 3, after which splicing returned to levels that matched those of the vehicle-treated control. Thus, melanocytes appear to attenuated UPR activation when subjected to mild and sustained ER stress.
Accumulation of tyrosinase does not result in Perk-mediated UPR activation
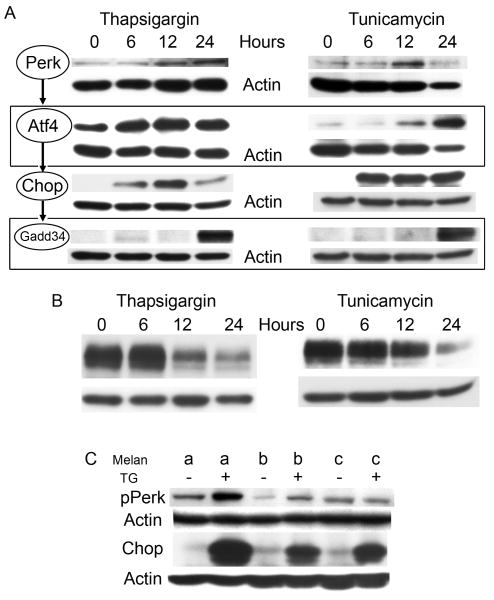
The Perk pathway was upregulated by WT melanocytes in response to chemical-induced ER stress (Figure 2A). Thapsigargin and tunicamycin each induced increased expression of Perk, which peaked at 24 hours in thapsigargin treated cells and 12 hours in tunicamycin treated cells. Activation of the Perk pathway was demonstrated by increased expression of downstream mediators Atf4 and CHOP. Diminished ER stress was observed within 24 hours with increased expression of Gadd34, which prevents phosphorylation of Eif2α and allows resumption of global transcription. Expression of tyrosinase was downregulated in response to thapsigargin, confirming reduced protein synthesis in response to UPR activation (Figure 2B). The Perk pathway was not activated in untreated Tyr-mutant and Tyrp1-mutant cells, but was functional as indicated by Perk phosphorylation in Tyrp1-mutants and robust expression of CHOP in both cell types following treatment with thapsigargin (Figure 2C).
Figure 2.
(A, B) WT melanocytes were treated with either 150nM thapsigargin or 10μg/mL tunicamycin and (C) mutant melanocytes with 150nM thapsigargin for 24 hours. Cells were lysed, and proteins harvested at the indicated time points. Extracts were normalized for protein concentration prior to analysis. Actin is shown as a loading control. Expression of (A) Expression of Perk and its downstream effectors Atf4, CHOP and Gadd34, as well as (B) tyrosinase were monitored by Western blot analysis in WT cells, while (C) Perk phosphorylation and CHOP expression were monitored in mutant cells.
Atf6 is cleaved in response to tyrosinase accumulation and chemical-induced ER stress
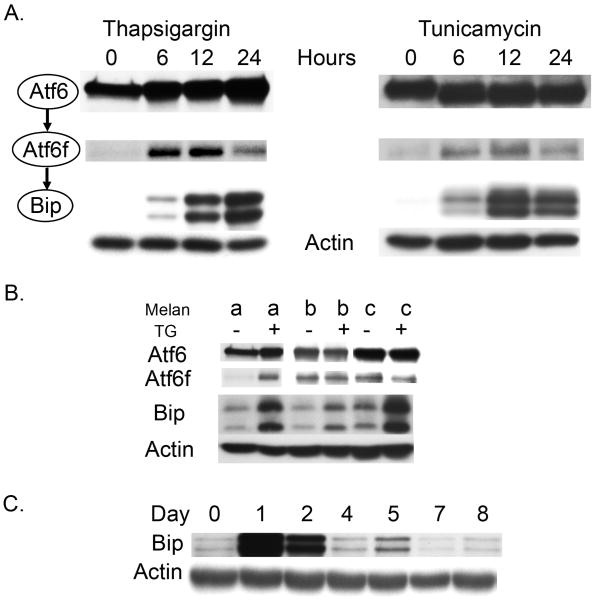
Cleavage of Atf6 was monitored following transfection of an HA-tagged-Atf6 protein (Figure 3). Both thapsigargin and tunicamycin induced Atf6 cleavage in WT melanocytes within 6 hours of treatment. The Atf6 pathway is the most direct trigger of increased BiP expression, which continues to rise at 24 hours in thapsigargin-treated cells and 12 hours in tunicamycin-treated cells (Figure 3A). Atf6 is cleaved in untreated Tyrp1-mutant and Tyr-mutant cells, suggesting that tyrosinase accumulation is sufficient to activate the pathway; however, mutant cells do not demonstrate increased expression of BiP. Both Tyr-mutant and Tyrp1-mutant cells respond to thapsigargin treatment with increased BiP expression (Figure 3B). Expression of Bip was increased at days 1 and 2 in WT melanocytes treated with 15nM thapsigargin, but was found to diminish in WT cells after two days of an eight day regimen of daily dosing (Figure 3C).
Figure 3.
WT and mutant melanocytes were transfected with an expression plasmid encoding an HA-tagged Atf6 protein for the purpose of monitoring Golgi-based protein cleavage. (A) Transfected WT melanocytes were subjected to either 15nM thapsigargin or 10μg/mL tunicamycin, while (B) transfected mutant cells were dosed with thapsigargin for 24 hours. Western blot analysis was used to determine Atf6 cleavage and expression of its downstream target BiP. (C) WT cells were treated with 15mM thapsigargin over an eight day period and BiP expression was monitored by Western blot analysis.
UPR-mediated apoptosis is mediated by phosphorylation of Bcl2 and cleavage of Caspase-12
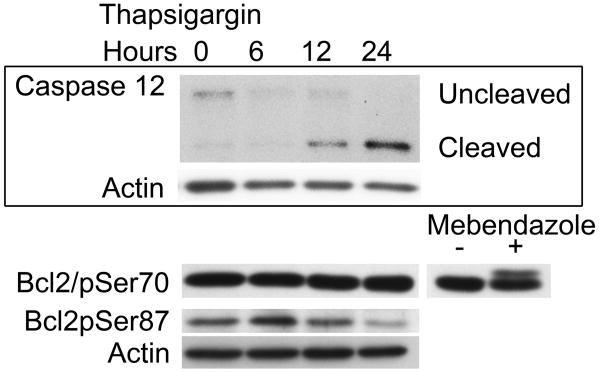
Despite an attenuated response to chronic exposure to low doses of thapsigargin, melanocyte viability was compromised by exposure to 150nM thapsigargin for extended periods of time (greater than 48 hours). In order to determine the pathway responsible for melanocyte death, we monitored phosphorylation of Bcl2 as well as cleavage of Caspases 7 and 12 which have been implicated in UPR-mediated apoptosis previously. Bcl2 was found to be transiently phosphorylated at Serine 87 but not at Serine 70. Only Caspase 12 was found to be cleaved in response to thapsigargin treatment (Figure 4).
Figure 4.
WT melanocytes were treated with 150nM thapsigargin. Cells were lysed and proteins harvested for analysis at the indicated time points. Samples were normalized for protein concentration prior to analysis and subjected to Western blot analysis to detect expression and phosphorylation of Bcl2 and Caspase 12 cleavage. Actin is shown as a loading control. Mebendazole, previously shown to trigger phosphorylation of Bcl2 at Serine 70 was used as a positive control (Doudican et al., 2008).
Discussion
In this study we investigated activation of the unfolded protein response in melanocytes either following treatment with chemical ER stressors or as a consequence of accumulation of tyrosinase in the ER due to mutations in either the tyrosinase or Tyrp1 genes. Wildtype melanocytes demonstrated a robust response to treatment with either thapsigargin or tunicamycin, both of which disturb the ability of proteins to fold correctly in the ER. All three of the pathways that constitute the UPR are activated within 6 hours of treatment, and the response begins to subside within 24 hours. At toxic doses of thapsigargin, apoptosis is initiated via Bcl2 phosphorylation and cleavage of Caspase 12. Thus, melanocytes respond to transient ER stress by activating a canonical UPR.
Bcl2 was found to be phosphorylated at Serine 87 rather than the more frequently reported site, Serine 70. Bcl2 phosphorylation has been shown to modulate calcium levels in the ER and result in increased activity of pro-apoptotic proteins, however the effect of phosphorylation site on activity is less well understood (Bassik et al., 2004). It has been shown that there may be an additive effect of phosphorylation at multiple sites (Deng et al., 2004) and that phosphorylation at Serine 70 appears to be more common following exposure to chemotoxins (Haldar et al., 1998). Thus Serine 87 phosphorylation following thapsigargin treatment may be a marker of UPR-mediated apoptosis.
Chronic UPR activation and subsequent initiation of apoptosis can lead to disease. For example, Charcot-Marie-Tooth Syndrome 1B results from mutations in the Myelin protein zero gene (Mpz). The resulting protein is misfolded and retained in the ER and the UPR is triggered, resulting in apoptosis of Schwann cells and ultimately loss of motor function in a mouse model (Pennuto et al., 2008). Conversely, albino melanocytes that express a mutant tyrosinase protein (Tyr-mutant), that fails to fold correctly due to an amino acid substitution, (Toyofuku et al., 2001a), remain viable and can be cultured, undergoing spontaneous immortalization similar to wildtype cells (Bennett et al., 1989). Tyr-mutant melanocytes demonstrate upregulation of the UPR, with increased expression of the initiator Ire1 and cleavage of Atf6, which initiates a second UPR pathway. However, Xbp1, the downstream target of Ire1, is not spliced and activated. A second UPR pathway, initiated by Perk phosphorylation, is not upregulated, which may account for the viability of these cells.
Chondrocytes have been shown to evade UPR-mediated apoptosis via Akt-1 which promotes Caspase 3 inactivation and BclXL expression (Price et al.). This mechanism may also play a role in melanocyte survival, given that Akt has been shown to play a role in melanocyte survival following UV exposure (Oka et al., 2004, Kadekaro et al., 2005). Alternatively, Bcl2 expression and Nrf2 regulation have both been implicated in resistance to UPR-mediated cell death (Gomez et al., 2007, Cullinan and Diehl, 2004). These proteins also play a role in melanocyte survival following challenge by ultraviolet light-induced damage (Kokot et al., 2009, Hornyak et al., 2009). There are thus several mechanisms through which melanocytes may adapt to sustained ER stress.
Tyr-mutant melanocytes demonstrate an attenuated response to thapsigargin-induced ER stress. Unlike wildtype melanocytes, Tyr-mutant melanocytes fail to increase expression of Ire1 and Xbp-1 splicing, cleavage of Atf6 or phosphorylation of Perk. There is however increased expression of Bip and CHOP, suggesting that there is partial activation of the UPR. Previous studies have shown that sustained Perk activation leads to apoptosis while Ire1 signals could be sustained without causing cell death (Lin et al., 2009). Since wildtype melanocytes, subjected to thapsigargin for extended periods of time, display a similar reduction in UPR signaling, melanocytes may adapt to ER stress, allowing continued survival despite increased tyrosinase levels in the ER.
In comparison, Tyrp1-mutant cells, which also accumulate tyrosinase in the ER (Toyofuku et al., 2001b), do not exhibit increased Ire1 expression. However there is increased Perk phosphorylation and cleavage of Atf6. The milder effects observed in these cells may be due to a smaller proportion of tyrosinase being present in the ER. Tyrp1-mutant cells, although less pigmented than wildtype melanocytes, continue to produce melanin at low concentrations suggesting that some tyrosinase does in fact mature and is transported successfully to melanosomes.
Adaptation of melanocytes to sustained UPR activation may be an important consideration in development of therapies for melanoma. Disruption of ER homeostasis due to hypoxia or hypoglycemia has been proposed as a driver of adaptation, producing cells highly resistant to stress, including chemotherapeutics, and resulting in the extreme drug resistance observed in malignant melanoma (Hersey and Zhang, 2008). For example, Xbp-1 can activate the phosphoinositide 3-kinase/Akt pathway in melanoma cells, leading to docetaxel and vincristine resistance (Jiang et al., 2009). This phenomenon has also been demonstrated in breast cancer, where activation of the UPR induces resistance to doxorubicin and 5-fluorouracil (Scriven et al., 2009). Additionally, the UPR has been proposed as a target for the development of therapies for melanoma (Chen et al., 2007). Given the resistance to UPR-mediated death we observed in melanocytes, it will be necessary to characterize the mechanism/s that allow for adaptation prior to development of such drugs.
UPR–related genes have also been shown to play a role in vitiligo, a common condition that results from premature melanocyte death. A sequence variant of the XBP1 gene that confers stronger promoter activity was found to be associated with increased risk of developing vitiligo, and increased expression of XBP1 was observed in areas where melanocyte death occurred (Ren et al., 2009). Characterizing the melanocyte-specific UPR will be critical in developing and evaluating the efficacy of response modifying compounds that can be used to prevent melanocyte death in vitiligo. At least one modulator, irestatin, has been shown to be effective in reducing UPR-meditated apoptosis (Feldman and Koong, 2007). Evaluation of such compounds will be useful in developing treatments for vitiligo. In addition, deletion of CHOP can prevent β-cell death in mouse models of diabetes (Song et al., 2008), suggesting that the Perk pathway may also provide therapeutic targets to down-regulate the UPR.
In summary, accumulation of tyrosinase in the ER of melanocytes leads to ER stress and activation of the UPR. Unlike certain other cell types, melanocytes do not undergo UPR-mediated apoptosis under conditions of chronic ER stress, suggesting that they are able to adapt to this stress. This ability may be a crucial factor in the development of melanomas, which are usually extremely resistant to stress, and vitiligo, which results from compromised melanocyte viability. Further studies are required to characterize the processes leading to adaptation, which may identify novel therapeutic targets for melanoma and vitiligo.
Methods
Experiments were performed in triplicate and representative results are shown.
Cell culture
Melan-a (WT) is an immortalized melanocyte line derived from C57B/6J mice, wild-type at both the tyrosinase and Tyrp1 loci (Bennett et al., 1987). Melan-b (Tyrp1-mutant) and melan-c (Tyr-mutant) are immortalized melanocyte lines from mice homozygous for Tyrp1 and tyrosinase mutations respectively. These lines were obtained from the Wellcome Trust Functional Genomics Cell Bank. Cells were cultured in RPMI supplemented with 10% fetal bovine serum, 1% sodium pyruvate, 1% nonessential amino acids, 1% glutamate, 5 units/mL penicillin, 5 μg/mL streptomycin, and 200 nM 12-O-tetradecanoylphorbol-13-acetate at 37°C in a 10% CO2 humidified incubator.
To test the effects of thapsigargin and tunicamycin, cells were cultured until 80-90% confluent. The culture medium was then replaced with medium containing 0 (vehicle control), 15nM, or 150nM thapsigargin (Sigma, St. Louis, MO) or 10μg/mL tunicamycin (Sigma, St. Louis, MO). These doses result in less than 10% loss of viability following 12 hours of treatment and less than 20% loss after 24 hours of treatment (data not shown). After 6, 12, and 24 hours, media and cells were collected for analysis. MTT assays demonstrated that cell viability was not compromised at 24 hours with either dose. For extended dosing regimens, cells were treated with 15nM thapsigargin as the 150nM dose was toxic. Cells were dosed daily with thapsigargin for eight days prior to protein harvesting.
pCGN-Atf6 HA plasmid 11974 (Addgene, Cambridge, MA) was delivered into cells by electroporation using the Amaxa Nucleofector System Kit L (Lonza Walkersville Inc., Walkersville, MD) according to the manufacturer's instructions. Briefly, 2×106 WT cells that were 70-80% confluent were resuspended in 100 μL nucleofector L solution and 2ug of indicated plasmid was added. The suspension was transferred to a cuvette and nucleofected with an Amaxa nucleofector apparatus using the U-20 pulsing parameter. After electroporation, cells were transferred into wells containing culture medium pre-warmed to 37°C in six-well plates for thapsigargin and tunicamycin dosing and subsequent protein analysis.
Protein Extraction and Western Blotting
Cells were washed with ice-cold PBS and harvested in extraction buffer [1% Triton X-100, 50 mmol/L Tris, 2 mmol/L EDTA, 150 mmol/L NaCl (pH 7.5)] containing protease inhibitors (Roche Applied Science, Indianapolis, IN). The lysates were centrifuged at 10,000×g, 4°C for 20min in a microcentrifuge and protein concentrations of the supernatants were measured with BCA protein assay (Thermo Scientific Pierce, Rockford, IL) and BSA as a standard control. 25-30μg protein was loaded onto 8-12% SDS-PAGE gels and transferred onto polyvinylidene difluoride membranes (Polyscreen, Perkin-Elmer, Waltham, MA). Antibodies used included: Atf4, Calnexin, CHOP, GADD34, Perk (Santa-Cruz 200, Santa Cruz, CA); BiP, Caspase 12, Ire1 (Cell Signaling, Danvers, MA); phospho-Ire1 (Novus Biologicals, Littleton, CO) and anti-tyrosinase (αPep7, a gift from Dr. V. Hearing, NIH, Bethesda, MD). Anti-actin (Sigma, St.Louis, MO) was used as a loading control. The immunoreactive bands were detected using an enhanced chemiluminescence detection kit (Perkin-Elmer, Waltham, MA) and X-OMAT processing.
RT-PCR analysis of Xbp-1 splicing
Xbp-1 splicing was assessed by semi-quantitative RT-PCR as described previously (Lin et al., 2007). In short, cells were lysed and total RNA was isolated with the RNeasy Mini kit (Qiagen, Valencia, CA). cDNA was produced using ImProm-II Reverse Transcription System (Promega, Madison, WI). The primers, which spanned the fragment of Xbp-1 that contained the intron targeted by Ire1α, were: 5′-TACGGGAGAAAACTCACGGC-3′ and 5′-GGGTCCAACTTGTCCAGAATGC-3′. The thermal PCR cycling conditions were: 95°C for 5 min, 95°C for 1 min, 58°C for 30 sec, 72°C for 30 sec, and 72°C for 5 min with 35 cycles of amplification. The PCR products were separated on a 2.0% agarose/1×TAE gel and then visualized by ethidium bromide staining. The unspliced Xbp-1 produced a 289bp fragment while the spliced Xbp-1 generated a 263bp fragment.
Cell Proliferation Assay
Briefly, 5,000-6,000 cells per well were plated in 96-well culture plates. After 24-48 hours of incubation, the cells were treated with 15-700nM thapsigargin for 24-48 hours so as to maintain a total incubation period of 72 hours. A tetrazolium dye reduction assay (CellTiter 96 AQueous Non-Radioactive Cell Proliferation Assay, Promega, Madison, WI) was performed to assess cellular proliferation according to the manufacturer's instructions. Absorbance was measured using a microplate spectrophotometer (495nm).
Acknowledgments
The work was supported by NIH Grant AR41880 (SJO), a Dermatology Foundation Science of Human Appearance Career Development Award (PM) and an American Skin Association Medical Student Grant (SB).
Footnotes
SIGNIFICANCE: Sustained UPR activation can trigger apoptosis and lead to disease. For example, insulin overproduction can cause UPR-mediated β-cell death and subsequent diabetes. Melanocytes however have the capacity to adapt to chronic ER stress, which may contribute to melanoma resistance to chemotherapy. Hypoxic conditions during transformation can induce ER stress, and it has been proposed that subsequent adaptation of melanoma cells renders ER-targeted chemotherapeutics ineffective. Furthermore, the UPR-related gene Xbp-1 has been associated with decreased melanocyte viability in vitiligo. Thus, investigation of the UPR is critical for elucidation of the pathogenesis of disorders affecting melanocyte number, function and transformation.
References
- Acosta-Alvear D, Zhou Y, Blais A, Tsikitis M, Lents NH, Arias C, Lennon CJ, Kluger Y, Dynlacht BD. XBP1 controls diverse cell type- and condition-specific transcriptional regulatory networks. Mol Cell. 2007;27:53–66. doi: 10.1016/j.molcel.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Bassik MC, Scorrano L, Oakes SA, Pozzan T, Korsmeyer SJ. Phosphorylation of BCL-2 regulates ER Ca2+ homeostasis and apoptosis. EMBO J. 2004;23:1207–16. doi: 10.1038/sj.emboj.7600104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DC, Cooper PJ, Dexter TJ, Devlin LM, Heasman J, Nester B. Cloned mouse melanocyte lines carrying the germline mutations albino and brown: complementation in culture. Development. 1989;105:379–385. doi: 10.1242/dev.105.2.379. [DOI] [PubMed] [Google Scholar]
- Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol. 2000;2:326–32. doi: 10.1038/35014014. [DOI] [PubMed] [Google Scholar]
- Calfon M, Zeng H, Urano F, Till JH, Hubbard SR, Harding HP, Clark SG, Ron D. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature. 2002;415:92–6. doi: 10.1038/415092a. [DOI] [PubMed] [Google Scholar]
- Chen K, Manga P, Orlow SJ. Pink-eyed Dilution Protein Controls the Processing of Tyrosinase. Mol Biol Cell. 2002;13:1953–64. doi: 10.1091/mbc.02-02-0022.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LH, Jiang CC, Kiejda KA, Wang YF, Thorne RF, Zhang XD, Hersey P. Thapsigargin sensitizes human melanoma cells to TRAIL-induced apoptosis by up-regulation of TRAIL-R2 through the unfolded protein response. Carcinogenesis. 2007;28:2328–36. doi: 10.1093/carcin/bgm173. [DOI] [PubMed] [Google Scholar]
- Costin GE, Valencia JC, Vieira WD, Lamoreux ML, Hearing VJ. Tyrosinase processing and intracellular trafficking is disrupted in mouse primary melanocytes carrying the underwhite (uw) mutation. A model for oculocutaneous albinism (OCA) type 4. J Cell Sci. 2003;116:3203–12. doi: 10.1242/jcs.00598. [DOI] [PubMed] [Google Scholar]
- Cullinan SB, Diehl JA. PERK-dependent activation of Nrf2 contributes to redox homeostasis and cell survival following endoplasmic reticulum stress. J Biol Chem. 2004;279:20108–17. doi: 10.1074/jbc.M314219200. [DOI] [PubMed] [Google Scholar]
- Deng X, Gao F, Flagg T, May WS., Jr Mono- and multisite phosphorylation enhances Bcl2's antiapoptotic function and inhibition of cell cycle entry functions. Proc Natl Acad Sci U S A. 2004;101:153–8. doi: 10.1073/pnas.2533920100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doudican N, Rodriguez A, Osman I, Orlow SJ., Jr Mebendazole induces apoptosis via Bcl-2 inactivation in chemoresistant melanoma cells. Mol Cancer Res. 2008;6:1308–1315. doi: 10.1158/1541-7786.MCR-07-2159. [DOI] [PubMed] [Google Scholar]
- Fawcett TW, Martindale JL, Guyton KZ, Hai T, Holbrook NJ. Complexes containing activating transcription factor (ATF)/cAMP-responsive-element-binding protein (CREB) interact with the CCAAT/enhancer-binding protein (C/EBP)-ATF composite site to regulate Gadd153 expression during the stress response. Biochem J. 1999;339(Pt 1):135–41. [PMC free article] [PubMed] [Google Scholar]
- Feldman D, Koong AC. Identification of Irestatins: A Novel Class of Hypoxia Targeted Cancer Therapeutics. International Journal of Radiation Oncology*Biology*Physics. 2007;69:S149–S150. [Google Scholar]
- Francis E, Wang N, Parag H, Halaban R, Hebert DN. Tyrosinase maturation and oligomerization in the endoplasmic reticulum require a melanocyte-specific factor. J Biol Chem. 2003;278:25607–17. doi: 10.1074/jbc.M303411200. [DOI] [PubMed] [Google Scholar]
- Fribley AM, Evenchik B, Zeng Q, Park BK, Guan JY, Zhang H, Hale TJ, Soengas MS, Kaufman RJ, Wang CY. Proteasome inhibitor PS-341 induces apoptosis in cisplatin-resistant squamous cell carcinoma cells by induction of Noxa. J Biol Chem. 2006;281:31440–7. doi: 10.1074/jbc.M604356200. [DOI] [PubMed] [Google Scholar]
- Gomez BP, Riggins RB, Shajahan AN, Klimach U, Wang A, Crawford AC, Zhu Y, Zwart A, Wang M, Clarke R. Human X-box binding protein-1 confers both estrogen independence and antiestrogen resistance in breast cancer cell lines. Faseb J. 2007;21:4013–27. doi: 10.1096/fj.06-7990com. [DOI] [PubMed] [Google Scholar]
- Gupta G, Sinha S, Mitra N, Surolia A. Probing into the role of conserved N-glycosylation sites in the Tyrosinase glycoprotein family. Glycoconj J. 2009;26:691–5. doi: 10.1007/s10719-008-9213-x. [DOI] [PubMed] [Google Scholar]
- Halaban R, Svedine S, Cheng E, Smicun Y, Aron R, Hebert DN. Endoplasmic reticulum retention is a common defect associated with tyrosinase-negative albinism. Proc Natl Acad Sci U S A. 2000;97:5889–94. doi: 10.1073/pnas.97.11.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldar S, Basu A, Croce CM. Serine-70 is one of the critical sites for drug-induced Bcl2 phosphorylation in cancer cells. Cancer Res. 1998;58:1609–15. [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Bertolotti A, Zeng H, Ron D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell. 2000;5:897–904. doi: 10.1016/s1097-2765(00)80330-5. [DOI] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, Stojdl DF, Bell JC, Hettmann T, Leiden JM, Ron D. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell. 2003;11:619–33. doi: 10.1016/s1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- Hersey P, Zhang XD. Adaptation to ER stress as a driver of malignancy and resistance to therapy in human melanoma. Pigment Cell Melanoma Res. 2008;21:358–67. doi: 10.1111/j.1755-148X.2008.00467.x. [DOI] [PubMed] [Google Scholar]
- Hornyak TJ, Jiang S, Guzman EA, Scissors BN, Tuchinda C, He H, Neville JD, Strickland FM. Mitf dosage as a primary determinant of melanocyte survival after ultraviolet irradiation. Pigment Cell Melanoma Res. 2009;22:307–18. doi: 10.1111/j.1755-148X.2009.00551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang CC, Chen LH, Gillespie S, Kiejda KA, Mhaidat N, Wang YF, Thorne R, Zhang XD, Hersey P. Tunicamycin sensitizes human melanoma cells to tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis by up-regulation of TRAIL-R2 via the unfolded protein response. Cancer Res. 2007;67:5880–8. doi: 10.1158/0008-5472.CAN-07-0213. [DOI] [PubMed] [Google Scholar]
- Jiang CC, Yang F, Thorne RF, Zhu BK, Hersey P, Zhang XD. Human melanoma cells under endoplasmic reticulum stress acquire resistance to microtubule-targeting drugs through XBP-1-mediated activation of Akt. Neoplasia. 2009;11:436–47. doi: 10.1593/neo.09208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadekaro AL, Kavanagh R, Kanto H, Terzieva S, Hauser J, Kobayashi N, Schwemberger S, Cornelius J, Babcock G, Shertzer HG, Scott G, Abdel-Malek ZA. alpha-Melanocortin and endothelin-1 activate antiapoptotic pathways and reduce DNA damage in human melanocytes. Cancer Res. 2005;65:4292–9. doi: 10.1158/0008-5472.CAN-04-4535. [DOI] [PubMed] [Google Scholar]
- Kokot A, Metze D, Mouchet N, Galibert MD, Schiller M, Luger TA, Bohm M. Alpha-melanocyte-stimulating hormone counteracts the suppressive effect of UVB on Nrf2 and Nrf-dependent gene expression in human skin. Endocrinology. 2009;150:3197–206. doi: 10.1210/en.2008-1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AH, Iwakoshi NN, Glimcher LH. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol. 2003;23:7448–59. doi: 10.1128/MCB.23.21.7448-7459.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JH, Li H, Yasumura D, Cohen HR, Zhang C, Panning B, Shokat KM, Lavail MM, Walter P. IRE1 signaling affects cell fate during the unfolded protein response. Science. 2007;318:944–9. doi: 10.1126/science.1146361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JH, Li H, Zhang Y, Ron D, Walter P. Divergent effects of PERK and IRE1 signaling on cell viability. PLoS One. 2009;4:e4170. doi: 10.1371/journal.pone.0004170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra JD, Kaufman RJ. The endoplasmic reticulum and the unfolded protein response. Semin Cell Dev Biol. 2007;18:716–31. doi: 10.1016/j.semcdb.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marciniak SJ, Ron D. Endoplasmic reticulum stress signaling in disease. Physiol Rev. 2006;86:1133–49. doi: 10.1152/physrev.00015.2006. [DOI] [PubMed] [Google Scholar]
- Oka M, Kageyama A, Fukunaga M, Bito T, Nagai H, Nishigori C. Phosphatidylinositol 3-kinase/Akt-dependent and -independent protection against apoptosis in normal human melanocytes. J Invest Dermatol. 2004;123:930–6. doi: 10.1111/j.0022-202X.2004.23454.x. [DOI] [PubMed] [Google Scholar]
- Pennuto M, Tinelli E, Malaguti M, Del Carro U, D'antonio M, Ron D, Quattrini A, Feltri ML, Wrabetz L. Ablation of the UPR-mediator CHOP restores motor function and reduces demyelination in Charcot-Marie-Tooth 1B mice. Neuron. 2008;57:393–405. doi: 10.1016/j.neuron.2007.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price J, Zaidi AK, Bohensky J, Srinivas V, Shapiro IM, Ali H. Akt-1 mediates survival of chondrocytes from endoplasmic reticulum-induced stress. J Cell Physiol. 222:502–8. doi: 10.1002/jcp.22001. [DOI] [PubMed] [Google Scholar]
- Ren Y, Yang S, Xu S, Gao M, Huang W, Gao T, Fang Q, Quan C, Zhang C, Sun L, Liang Y, Han J, Wang Z, Zhang F, Zhou Y, Liu J, Zhang X. Genetic variation of promoter sequence modulates XBP1 expression and genetic risk for vitiligo. PLoS Genet. 2009;5:e1000523. doi: 10.1371/journal.pgen.1000523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scriven P, Coulson S, Haines R, Balasubramanian S, Cross S, Wyld L. Activation and clinical significance of the unfolded protein response in breast cancer. Br J Cancer. 2009 doi: 10.1038/sj.bjc.6605365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Chen X, Hendershot L, Prywes R. ER stress regulation of ATF6 localization by dissociation of BiP/GRP78 binding and unmasking of Golgi localization signals. Dev Cell. 2002;3:99–111. doi: 10.1016/s1534-5807(02)00203-4. [DOI] [PubMed] [Google Scholar]
- Song B, Scheuner D, Ron D, Pennathur S, Kaufman RJ. Chop deletion reduces oxidative stress, improves beta cell function, and promotes cell survival in multiple mouse models of diabetes. J Clin Invest. 2008;118:3378–89. doi: 10.1172/JCI34587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd DJ, Lee AH, Glimcher LH. The endoplasmic reticulum stress response in immunity and autoimmunity. Nat Rev Immunol. 2008;8:663–74. doi: 10.1038/nri2359. [DOI] [PubMed] [Google Scholar]
- Toyofuku K, Wada I, Spritz RA, Hearing VJ. The molecular basis of oculocutaneous albinism type 1 (OCA1): sorting failure and degradation of mutant tyrosinases results in a lack of pigmentation. Biochem J. 2001a;355:259–69. doi: 10.1042/0264-6021:3550259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyofuku K, Wada I, Valencia JC, Kushimoto T, Ferrans VJ, Hearing VJ. Oculocutaneous albinism types 1 and 3 are ER retention diseases: mutation of tyrosinase or Tyrp1 can affect the processing of both mutant and wild-type proteins. Faseb J. 2001b;15:2149–61. doi: 10.1096/fj.01-0216com. [DOI] [PubMed] [Google Scholar]
- Ujvari A, Aron R, Eisenhaure T, Cheng E, Parag HA, Smicun Y, Halaban R, Hebert DN. Translation rate of human tyrosinase determines its N-linked glycosylation level. J Biol Chem. 2001;276:5924–31. doi: 10.1074/jbc.M009203200. [DOI] [PubMed] [Google Scholar]
- Wang N, Daniels R, Hebert DN. The cotranslational maturation of the type I membrane glycoprotein tyrosinase: the heat shock protein 70 system hands off to the lectin-based chaperone system. Mol Biol Cell. 2005;16:3740–52. doi: 10.1091/mbc.E05-05-0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H, Haze K, Yanagi H, Yura T, Mori K. Identification of the cis-acting endoplasmic reticulum stress response element responsible for transcriptional induction of mammalian glucose-regulated proteins. Involvement of basic leucine zipper transcription factors. J Biol Chem. 1998;273:33741–9. doi: 10.1074/jbc.273.50.33741. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Okada T, Haze K, Yanagi H, Yura T, Negishi M, Mori K. ATF6 activated by proteolysis binds in the presence of NF-Y (CBF) directly to the cis-acting element responsible for the mammalian unfolded protein response. Mol Cell Biol. 2000;20:6755–67. doi: 10.1128/mcb.20.18.6755-6767.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]