Abstract
Inflammatory recruitment of leukocytes into the cerebrospinal fluid (CSF) during bacterial meningitis has been shown to contribute significantly to the neurological damage commonly associated with this serious disease. In this study we tested whether or not inhibition of leukocyte rolling, a precondition for firm leukocyte adhesion to vascular endothelium in vivo, may reduce CSF leukocyte recruitment and associated inflammatory changes in rabbits with experimental meningitis. As documented by intravital microscopy of small venules in the rabbit mesentery and tenuissimus muscle, leukocyte rolling was rapidly and profoundly reduced by intravenous treatment with the polysaccharide fucoidin, a homopolymer of sulfated L-fucose known to block the function of the leukocytic "rolling receptor" L-selectin. Moreover, fucoidin treatment dramatically reduced the accumulation of both leukocytes and plasma protein in the CSF of rabbits challenged intrathecally with pneumococcal antigen. These main findings thus illustrate that inhibition of leukocyte rolling, an early and obligatory step in the process of leukocyte extravasation, may be an effective therapeutic approach to attenuate leukocyte-dependent central nervous system damage in bacterial meningitis.
Full text
PDF

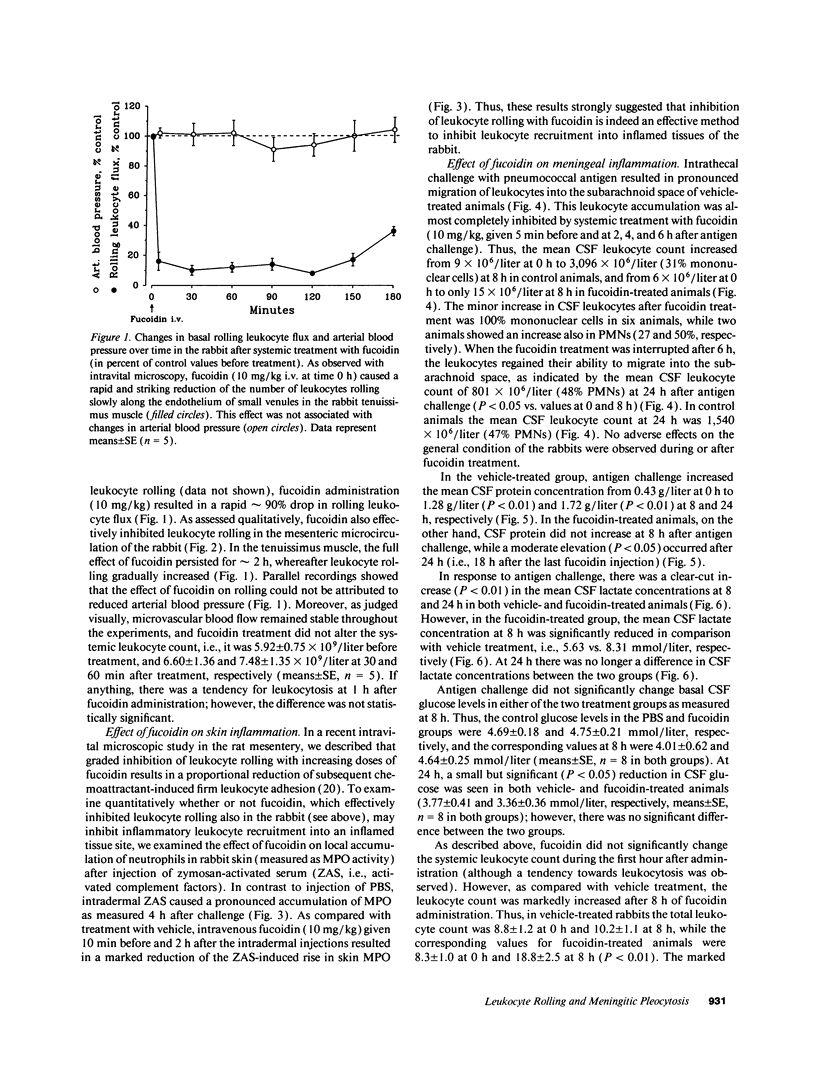

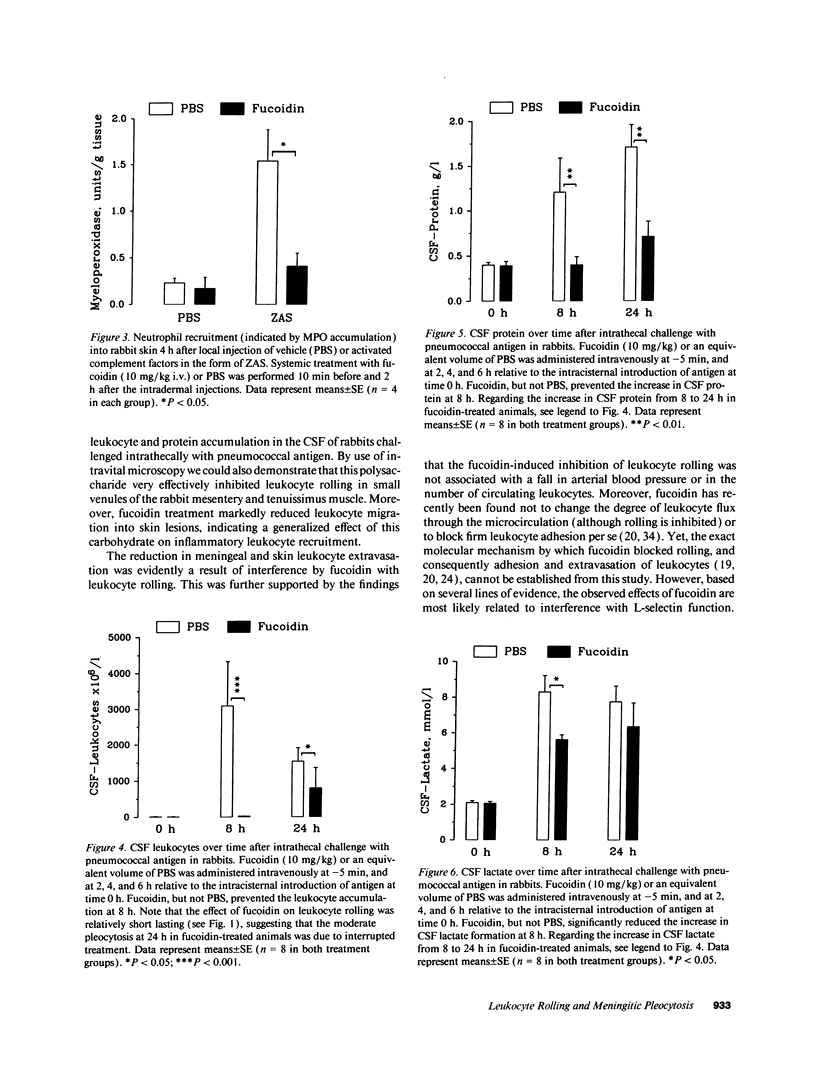



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arfors K. E., Lundberg C., Lindbom L., Lundberg K., Beatty P. G., Harlan J. M. A monoclonal antibody to the membrane glycoprotein complex CD18 inhibits polymorphonuclear leukocyte accumulation and plasma leakage in vivo. Blood. 1987 Jan;69(1):338–340. [PubMed] [Google Scholar]
- Argenbright L. W., Letts L. G., Rothlein R. Monoclonal antibodies to the leukocyte membrane CD18 glycoprotein complex and to intercellular adhesion molecule-1 inhibit leukocyte-endothelial adhesion in rabbits. J Leukoc Biol. 1991 Mar;49(3):253–257. doi: 10.1002/jlb.49.3.253. [DOI] [PubMed] [Google Scholar]
- Atherton A., Born G. V. Quantitative investigations of the adhesiveness of circulating polymorphonuclear leucocytes to blood vessel walls. J Physiol. 1972 Apr;222(2):447–474. doi: 10.1113/jphysiol.1972.sp009808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua M. P. Endothelial-leukocyte adhesion molecules. Annu Rev Immunol. 1993;11:767–804. doi: 10.1146/annurev.iy.11.040193.004003. [DOI] [PubMed] [Google Scholar]
- Bevilacqua M. P., Nelson R. M. Selectins. J Clin Invest. 1993 Feb;91(2):379–387. doi: 10.1172/JCI116210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronstein B. N., Kimmel S. C., Levin R. I., Martiniuk F., Weissmann G. A mechanism for the antiinflammatory effects of corticosteroids: the glucocorticoid receptor regulates leukocyte adhesion to endothelial cells and expression of endothelial-leukocyte adhesion molecule 1 and intercellular adhesion molecule 1. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):9991–9995. doi: 10.1073/pnas.89.21.9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings R. D., Smith D. F. The selectin family of carbohydrate-binding proteins: structure and importance of carbohydrate ligands for cell adhesion. Bioessays. 1992 Dec;14(12):849–856. doi: 10.1002/bies.950141210. [DOI] [PubMed] [Google Scholar]
- Ernst J. D., Decazes J. M., Sande M. A. Experimental pneumococcal meningitis: role of leukocytes in pathogenesis. Infect Immun. 1983 Jul;41(1):275–279. doi: 10.1128/iai.41.1.275-279.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra-Romero L., Tureen J. H., Täuber M. G. Pathogenesis of central nervous system injury in bacterial meningitis. Antibiot Chemother (1971) 1992;45:18–29. doi: 10.1159/000420998. [DOI] [PubMed] [Google Scholar]
- Hartnell A., Kay A. B., Wardlaw A. J. Interleukin-3-induced up-regulation of CR3 expression on human eosinophils is inhibited by dexamethasone. Immunology. 1992 Dec;77(4):488–493. [PMC free article] [PubMed] [Google Scholar]
- Imai Y., Singer M. S., Fennie C., Lasky L. A., Rosen S. D. Identification of a carbohydrate-based endothelial ligand for a lymphocyte homing receptor. J Cell Biol. 1991 Jun;113(5):1213–1221. doi: 10.1083/jcb.113.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai Y., True D. D., Singer M. S., Rosen S. D. Direct demonstration of the lectin activity of gp90MEL, a lymphocyte homing receptor. J Cell Biol. 1990 Sep;111(3):1225–1232. doi: 10.1083/jcb.111.3.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutila M. A., Rott L., Berg E. L., Butcher E. C. Function and regulation of the neutrophil MEL-14 antigen in vivo: comparison with LFA-1 and MAC-1. J Immunol. 1989 Nov 15;143(10):3318–3324. [PubMed] [Google Scholar]
- Kansas G. S., Spertini O., Stoolman L. M., Tedder T. F. Molecular mapping of functional domains of the leukocyte receptor for endothelium, LAM-1. J Cell Biol. 1991 Jul;114(2):351–358. doi: 10.1083/jcb.114.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto T. K., Warnock R. A., Jutila M. A., Butcher E. C., Lane C., Anderson D. C., Smith C. W. Antibodies against human neutrophil LECAM-1 (LAM-1/Leu-8/DREG-56 antigen) and endothelial cell ELAM-1 inhibit a common CD18-independent adhesion pathway in vitro. Blood. 1991 Aug 1;78(3):805–811. [PubMed] [Google Scholar]
- Lasky L. A. Selectins: interpreters of cell-specific carbohydrate information during inflammation. Science. 1992 Nov 6;258(5084):964–969. doi: 10.1126/science.1439808. [DOI] [PubMed] [Google Scholar]
- Lawrence M. B., Springer T. A. Leukocytes roll on a selectin at physiologic flow rates: distinction from and prerequisite for adhesion through integrins. Cell. 1991 May 31;65(5):859–873. doi: 10.1016/0092-8674(91)90393-d. [DOI] [PubMed] [Google Scholar]
- Lebel M. H. Dexamethasone therapy of bacterial meningitis. Antibiot Chemother (1971) 1992;45:169–183. doi: 10.1159/000421007. [DOI] [PubMed] [Google Scholar]
- Lebel M. H., Freij B. J., Syrogiannopoulos G. A., Chrane D. F., Hoyt M. J., Stewart S. M., Kennard B. D., Olsen K. D., McCracken G. H., Jr Dexamethasone therapy for bacterial meningitis. Results of two double-blind, placebo-controlled trials. N Engl J Med. 1988 Oct 13;319(15):964–971. doi: 10.1056/NEJM198810133191502. [DOI] [PubMed] [Google Scholar]
- Lewinsohn D. M., Bargatze R. F., Butcher E. C. Leukocyte-endothelial cell recognition: evidence of a common molecular mechanism shared by neutrophils, lymphocytes, and other leukocytes. J Immunol. 1987 Jun 15;138(12):4313–4321. [PubMed] [Google Scholar]
- Ley K., Cerrito M., Arfors K. E. Sulfated polysaccharides inhibit leukocyte rolling in rabbit mesentery venules. Am J Physiol. 1991 May;260(5 Pt 2):H1667–H1673. doi: 10.1152/ajpheart.1991.260.5.H1667. [DOI] [PubMed] [Google Scholar]
- Ley K., Gaehtgens P., Fennie C., Singer M. S., Lasky L. A., Rosen S. D. Lectin-like cell adhesion molecule 1 mediates leukocyte rolling in mesenteric venules in vivo. Blood. 1991 Jun 15;77(12):2553–2555. [PubMed] [Google Scholar]
- Ley K., Linnemann G., Meinen M., Stoolman L. M., Gaehtgens P. Fucoidin, but not yeast polyphosphomannan PPME, inhibits leukocyte rolling in venules of the rat mesentery. Blood. 1993 Jan 1;81(1):177–185. [PubMed] [Google Scholar]
- Lindbom L., Lundberg C., Prieto J., Raud J., Nortamo P., Gahmberg C. G., Patarroyo M. Rabbit leukocyte adhesion molecules CD11/CD18 and their participation in acute and delayed inflammatory responses and leukocyte distribution in vivo. Clin Immunol Immunopathol. 1990 Oct;57(1):105–119. doi: 10.1016/0090-1229(90)90026-m. [DOI] [PubMed] [Google Scholar]
- Lindbom L., Tuma R. F., Arfors K. E. Blood flow in the rabbit tenuissimus muscle. Influence of preparative procedures for intravital microscopic observation. Acta Physiol Scand. 1982 Jan;114(1):121–127. doi: 10.1111/j.1748-1716.1982.tb06960.x. [DOI] [PubMed] [Google Scholar]
- Lindbom L., Xie X., Raud J., Hedqvist P. Chemoattractant-induced firm adhesion of leukocytes to vascular endothelium in vivo is critically dependent on initial leukocyte rolling. Acta Physiol Scand. 1992 Dec;146(4):415–421. doi: 10.1111/j.1748-1716.1992.tb09442.x. [DOI] [PubMed] [Google Scholar]
- Lindquist L., Lundbergh P., Hedström K. G., Hansson L. O., Hultman E. Experimental bacterial meningitis in the rabbit: cerebrospinal fluid changes and its relation to leukocyte response. Scand J Infect Dis. 1987;19(2):263–270. doi: 10.3109/00365548709032409. [DOI] [PubMed] [Google Scholar]
- Lundberg C., Arfors K. E. Polymorphonuclear leukocyte accumulation in inflammatory dermal sites as measured by 51Cr-labeled cells and myeloperoxidase. Inflammation. 1983 Sep;7(3):247–255. doi: 10.1007/BF00917262. [DOI] [PubMed] [Google Scholar]
- McEver R. P. Selectins: novel receptors that mediate leukocyte adhesion during inflammation. Thromb Haemost. 1991 Mar 4;65(3):223–228. [PubMed] [Google Scholar]
- Mustafa M. M., Ramilo O., Olsen K. D., Franklin P. S., Hansen E. J., Beutler B., McCracken G. H., Jr Tumor necrosis factor in mediating experimental Haemophilus influenzae type B meningitis. J Clin Invest. 1989 Oct;84(4):1253–1259. doi: 10.1172/JCI114292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odio C. M., Faingezicht I., Paris M., Nassar M., Baltodano A., Rogers J., Sáez-Llorens X., Olsen K. D., McCracken G. H., Jr The beneficial effects of early dexamethasone administration in infants and children with bacterial meningitis. N Engl J Med. 1991 May 30;324(22):1525–1531. doi: 10.1056/NEJM199105303242201. [DOI] [PubMed] [Google Scholar]
- PETERSDORF R. G., LUTTRELL C. N. Studies on the pathogenesis of meningitis. I. Intrathecal infection. J Clin Invest. 1962 Feb;41:311–319. doi: 10.1172/JCI104484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry M. A., Granger D. N. Role of CD11/CD18 in shear rate-dependent leukocyte-endothelial cell interactions in cat mesenteric venules. J Clin Invest. 1991 May;87(5):1798–1804. doi: 10.1172/JCI115200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picker L. J., Warnock R. A., Burns A. R., Doerschuk C. M., Berg E. L., Butcher E. C. The neutrophil selectin LECAM-1 presents carbohydrate ligands to the vascular selectins ELAM-1 and GMP-140. Cell. 1991 Sep 6;66(5):921–933. doi: 10.1016/0092-8674(91)90438-5. [DOI] [PubMed] [Google Scholar]
- Price T. H., Beatty P. G., Corpuz S. R. In vivo inhibition of neutrophil function in the rabbit using monoclonal antibody to CD18. J Immunol. 1987 Dec 15;139(12):4174–4177. [PubMed] [Google Scholar]
- Raud J., Lindbom L. Leukocyte rolling and firm adhesion in the microcirculation. Gastroenterology. 1993 Jan;104(1):310–314. doi: 10.1016/0016-5085(93)90866-b. [DOI] [PubMed] [Google Scholar]
- Saukkonen K., Sande S., Cioffe C., Wolpe S., Sherry B., Cerami A., Tuomanen E. The role of cytokines in the generation of inflammation and tissue damage in experimental gram-positive meningitis. J Exp Med. 1990 Feb 1;171(2):439–448. doi: 10.1084/jem.171.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simberkoff M. S., Moldover N. H., Rahal J., Jr Absence of detectable bactericidal and opsonic activities in normal and infected human cerebrospinal fluids. A regional host defense deficiency. J Lab Clin Med. 1980 Mar;95(3):362–372. [PubMed] [Google Scholar]
- Spangrude G. J., Braaten B. A., Daynes R. A. Molecular mechanisms of lymphocyte extravasation. I. Studies of two selective inhibitors of lymphocyte recirculation. J Immunol. 1984 Jan;132(1):354–362. [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Sáez-Llorens X., Jafari H. S., Severien C., Parras F., Olsen K. D., Hansen E. J., Singer I. I., McCracken G. H., Jr Enhanced attenuation of meningeal inflammation and brain edema by concomitant administration of anti-CD18 monoclonal antibodies and dexamethasone in experimental Haemophilus meningitis. J Clin Invest. 1991 Dec;88(6):2003–2011. doi: 10.1172/JCI115527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunkel A. R., Scheld W. M. Pathogenesis and pathophysiology of bacterial meningitis. Clin Microbiol Rev. 1993 Apr;6(2):118–136. doi: 10.1128/cmr.6.2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuomanen E. I., Saukkonen K., Sande S., Cioffe C., Wright S. D. Reduction of inflammation, tissue damage, and mortality in bacterial meningitis in rabbits treated with monoclonal antibodies against adhesion-promoting receptors of leukocytes. J Exp Med. 1989 Sep 1;170(3):959–969. doi: 10.1084/jem.170.3.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuomanen E. Adjunctive therapy of experimental meningitis: agents other than steroids. Antibiot Chemother (1971) 1992;45:184–191. doi: 10.1159/000421008. [DOI] [PubMed] [Google Scholar]
- Tuomanen E., Hengstler B., Rich R., Bray M. A., Zak O., Tomasz A. Nonsteroidal anti-inflammatory agents in the therapy for experimental pneumococcal meningitis. J Infect Dis. 1987 May;155(5):985–990. doi: 10.1093/infdis/155.5.985. [DOI] [PubMed] [Google Scholar]
- Täuber M. G., Borschberg U., Sande M. A. Influence of granulocytes on brain edema, intracranial pressure, and cerebrospinal fluid concentrations of lactate and protein in experimental meningitis. J Infect Dis. 1988 Mar;157(3):456–464. doi: 10.1093/infdis/157.3.456. [DOI] [PubMed] [Google Scholar]
- Wagner J. L., Hugli T. E. Radioimmunoassay for anaphylatoxins: a sensitive method for determining complement activation products in biological fluids. Anal Biochem. 1984 Jan;136(1):75–88. doi: 10.1016/0003-2697(84)90308-7. [DOI] [PubMed] [Google Scholar]
- Watson S. R., Fennie C., Lasky L. A. Neutrophil influx into an inflammatory site inhibited by a soluble homing receptor-IgG chimaera. Nature. 1991 Jan 10;349(6305):164–167. doi: 10.1038/349164a0. [DOI] [PubMed] [Google Scholar]
- Watson S. R., Imai Y., Fennie C., Geoffroy J. S., Rosen S. D., Lasky L. A. A homing receptor-IgG chimera as a probe for adhesive ligands of lymph node high endothelial venules. J Cell Biol. 1990 Jun;110(6):2221–2229. doi: 10.1083/jcb.110.6.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. J. Tissue destruction by neutrophils. N Engl J Med. 1989 Feb 9;320(6):365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- Yednock T. A., Butcher E. C., Stoolman L. M., Rosen S. D. Receptors involved in lymphocyte homing: relationship between a carbohydrate-binding receptor and the MEL-14 antigen. J Cell Biol. 1987 Mar;104(3):725–731. doi: 10.1083/jcb.104.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Andrian U. H., Chambers J. D., McEvoy L. M., Bargatze R. F., Arfors K. E., Butcher E. C. Two-step model of leukocyte-endothelial cell interaction in inflammation: distinct roles for LECAM-1 and the leukocyte beta 2 integrins in vivo. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7538–7542. doi: 10.1073/pnas.88.17.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]




