Abstract
Background
Mitral regurgitation (MR) doubles mortality following myocardial infarction (MI). We have demonstrated that MR worsens remodeling after MI, and that early correction reverses remodeling. SERCA2a is downregulated in this process. We hypothesized that upregulating SERCA2a may inhibit remodeling in a surgical model of apical MI (no intrinsic MR) with independent MR-type flow.
Methods and Results
In 12 sheep, percutaneous gene delivery was performed using a validated protocol to perfuse both LAD and circumflex coronary arteries with occlusion of venous drainage. We administered adeno-associated virus 6 (AAV6) carrying SERCA2a under CMV promoter control in 6 sheep, and a reporter gene in 6 controls. After 2 weeks, standardized apical MI was created, and a shunt implanted between the LV and LA, producing regurgitant fractions of ~30%. Animals were compared at baseline, 1 and 3 months using 3D echo, Millar hemodynamics and biopsies. The SERCA2a group had well-maintained preload-recruitable stroke work at 3 months (decrease by 8±10% vs. 42±12% with reporter gene controls (p<0.001)). LV dP/dt followed the same pattern (no change vs. 55% decrease, p<0.001). LVESV was lower with SERCA2a (82.6±9 6 vs 99.4±9.7 ml, p=0.03); LVEDV, reflecting volume overload, was not significantly different (127.8±6.2 vs 134.3±9.4 ml). SERCA2a sheep showed 15% rise in anti-apoptotic pAkt vs. 30% reduction with reporter gene (P<0.001). Pro-hypertrophic activated STAT3 was also 41% higher with SERCA2a than in controls (p<0.001). Pro-apoptotic activated caspase-3 rose over 5-fold over 1 month in both SERCA2a and controls (p=NS), and decreased by 19% at 3 months, remaining elevated in both groups.
Conclusions
In this controlled model, upregulating SERCA2a induces better function and lesser remodeling, with improved contractility, smaller volume and activation of pro-hypertrophic/anti-apoptotic pathways. Although caspase-3 remains activated in both arms, SERCA2a sheep had increased molecular anti-remodeling “tone”. We therefore conclude that upregulating SERCA2a inhibits MR-induced post-MI remodeling in this model, and thus may constitute a useful approach to reduce the vicious cycle of remodeling in ischemic MR.
Keywords: mitral regurgitation, valvular heart disease, echocardiography, remodeling
Expansion of infarcted tissue begins acutely after myocardial infarction (MI), but a more gradual remodeling process also involves noninfarcted areas;1 initially compensatory, this process becomes maladaptive, as the ventricle enlarges and contracts poorly2 with reduced survival.3
MI also causes ischemic mitral regurgitation (MR) by altering ventricular geometry and function,4, 5 doubling the risk of death. Severe non-ischemic MR has been shown to promote LV remodeling and reduce survival.6–8 We have previously demonstrated 9 that moderate MR, simulated by an LV-to-LA shunt, added to a small antero-apical MI (causing no intrinsic MR) causes greater ventricular remodeling than a comparable infarction alone, with an earlier transition to a failure phenotype. We have also shown that repairing the regurgitant-type flow at an early stage after the MI reverses the remodeling-related processes.10 Whole heart changes parallel cellular and molecular abnormalities in the non-infarcted myocardium that reflect the complex remodeling process. These molecular events also progress differently with MR than with comparable infarction alone, with an initial rise in pro-hypertrophic and anti-apoptotic signals followed by their exhaustion.
Most experimental models of post-MI remodeling use infero-posterior MIs,11, 12 but this necessarily links the MI-induced remodeling to the development of MR. The shunt model allows MR to be varied independently in the presence of MI and without interventions such as infarct patching13 that might themselves influence remodeling.
Upregulating genes encoding for proteins of interest has been demonstrated to be an effective approach to modulate and treat heart failure. One candidate for such gene therapy is the sarcoplasmic (SR) Ca+2-ATPase (SERCA2a), which is down-regulated in that model, and plays a pivotal role in the regulation of intracellular Ca2+ in cardiomyocytes14. Calcium entry into the cytosol during systole induces Ca2+ release from the SR through the ryanodyne receptor, coupling excitation and contraction. During relaxation, Ca2+ is returned to the SR by the SR Ca2+-ATPase (SERCA2a). Some is also extruded by the sarcolemmal Na+/Ca2+ exchanger (NCX), which is upregulated in cardiac hypertrophy and failure.15, 16 Because SERCA2a is the major determinant of the amount of Ca2+ available to be released during the upcoming systole, changes in SERCA function significantly affect cardiac excitation-contraction coupling. SERCA2a activity also has a major influence on myocardial relaxation,17, 18 Ca2+ extrusion via SERCA2a being more efficient energetically than the alternative NCX pathway.19 SERCA2a mRNA levels are reduced in failing hearts.20 Using a gene therapy approach, up-regulating SERCA2a levels in different models of heart failure resulted in improvement in systolic21–23 and diastolic function,17 as well as improving metabolism, 24, 25 potentially reducing arrhythmias26, 27 and improving survival.25 We have demonstrated that SERCA2A is down-regulated in the remote zones of post-MI remodeling ventricles- significantly more so when MR was also present, accompanied reduction in contractility of the whole ventricle and of isolated cells, and reduction in single cell calcium transients.9 Pathways involved in the compensatory hypertrophic response in were initially up-regulated, only to fall below baseline at 3 months, when severe dilatation and failure were present. As repairing MR in the early phase, before these processes have been activated, induces reversal of remodeling,10 SERCA2a may have a unique role in determining this reversibility. This is emphasized by the recently reported effects of SERCA2a up-regulation in an MR-only pig model of heart failure,28 where it induced inhibition of ventricular enlargement and myocardial dysfunction apparent in the control animals.
This study aims to apply the gene therapy approach in a clinically relevant large-animal model of actively evolving remodeling induced by the combination of ischemic and valvular lesions in which a biphasic pattern of compensatory and decompensatory changes has been demonstrated. An intriguing question to address in this model is whether the potentially beneficial effects of SERCA2a gene therapy are accompanied by molecular changes typical of compensated hypertrophy9 as seen early in the course of MR-augmented remodeling, or only by measurable reductions in ventricular volumes and improvements in contractile dysfunction without molecular changes in other aspects of the remodeling processes. Genetic modification of such a key pathway can thereby help dissect its contribution to the entire disease process,
Thus, we hypothesized that up-regulating SERCA2a levels by gene delivery using a viral vector may reverse the remodeling process in our model of “ischemic-type” MR. that is, MR associated with myocardial infarction.9 We also hypothesized that this reversal will be manifest both in ventricular volumes and function, and in persistent activation of pro-hypertrophic and anti-apoptotic pathways.
In this context, prolonged and sustained expression of the transgene is critical, as is the lack of host immune response to the vector. Adeno-associated vector has been demonstrated to confer prolonged and sustained expression of myocardial transgenes, while lacking immunogenic and cardiotoxic effects,29 and was therefore used in this study.
METHODS
Animal studies
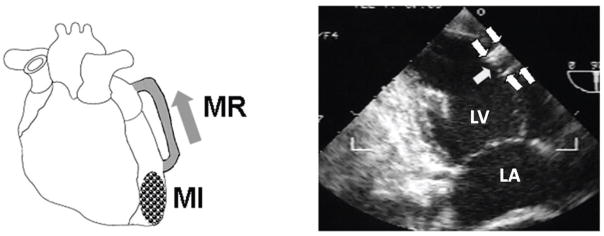
A total of 12 male Dorsett hybrid sheep (20–30 kg) were included. Our established model of independent MI and MR-type flow9, 10 was implemented using an 8-cm long, 8-mm diameter reinforced Teflon (PTFE) graft (Edwards, cross-sectional area 0.50 cm2) implanted under sterile conditions into the mid-lateral LV and LA appendage with intramuscular portions stiffened with epoxy resin (Figure 1). The regurgitant flow was confirmed during each thoracotomy using a Transonic flow probe and color Doppler. The standardized shunt diameter and length consistently produced moderate MR (regurgitant fractions of ~30%30). Animals were treated with heparin (3 days) and then oral aspirin.
Figure 1.
Model of apical MI and independent MR: LV-to-LA shunt (arrows).
Vector design
Vector production, harvest, purification, and testing were done as previously described.31 The rAAV6.SERCA2a vector used in this study contains an AAV serotype 6 viral capsid and a single-stranded ~4.5 kb DNA containing the human SERCA2a cDNA driven by a CMV immediate-early promoter/enhancer, a hybrid intron, and a bovine growth hormone poly-adenylation signal, all flanked by 145 nt AAV2 inverted terminal repeat sequences necessary for replication and packaging of the vector DNA in the capsid. The vector was manufactured using standard calcium phosphate transfection methods in adherent 293 cells. Three plasmids were used, 1 containing helper functions from adenovirus, 1 containing the AAV rep2 and cap1 genes, and the third containing the vector genome. Final vector preparations were more than 95% pure as judged by SDS-PAGE (Invitrogen, Carlsbad, California).
Gene delivery
Two weeks prior to the first thoracotomy (in order to obtain significant gene expression at model creation), antegrade coronary arterial injection with concomitant great cardiac vein blockade was performed with AAV6 as a vehicle for the reporter gene β-galactosidase (β-gal-control) and SERCA2a, each at a titer of 5×1014 genomes/ml. The great cardiac vein was cannulated via internal jugular access and occluded with a standard balloon-tipped catheter. The left anterior descending coronary artery (LAD) was cannulated via the femoral artery and occluded with a standard angioplasty balloon before the first diagonal branch. With both arterial and venous balloons transiently inflated for 2 minutes, intracoronary adenosine was administered to increase permeability and prolong dwell time32, followed by 5×1012 genomes of either AAV6.βgal or AAV6.SERCA2a (six sheep each). This sequence was repeated for the left circumflex (LCX) artery. Previous work has shown increased tissue expression in the whole adult heart using this delivery method33.
Model creation
Sheep were loaded for 3 days with amiodarone (200 mg PO BID), anesthetized with thiopentothal (0.5 ml/kg), intubated and ventilated at 15 ml/kg with 2% isoflurane-oxygen, receiving glycopyrrolate (0.4 mg IV) and prophylactic vancomycin (0.5 g IV) and amiodarone (150 mg IV drip). Surface ECG was monitored and a sterile left thoracotomy performed with pericardial cradle creation. A high-fidelity micromanometer-tipped catheter (Millar, Houston, TX) was placed into the LV. After baseline 2D and 3D echo imaging, a septal MI was produced by ligating the mid- to distal left anterior descending coronary artery, known to produce substantial MIs without MR.34 2D echo confirmed that wall motion abnormality involved approximately one-third of the anteroseptum from apex to base for standardization. In addition to analgesia, propranolol, 1 mg IV in two doses, was given for evident stress and tachycardia (>150) upon extubation. Antibiotics (Cephapirin, 0.5 gm IV) and analgesics (Buprenorphine, 0.3 mg BID) were administered for 5 days, and oral amiodarone (200 mg BID) for three.
During repeat sterile thoracotomy at day 30, 3D echo evaluated LV remodeling and function, with directed TruCut needle biopsies of the noninfarcted myocardium near and remote from the border zone. At day 90, 3D echo and blood sampling were repeated at thoracotomy, followed by euthanasia. Animal studies conformed to NIH guidelines (National Research Council, Washington, DC, 1996) and were IRB-approved.
3D echo and LV function.35
Rotated apical images were obtained at 10-degree intervals with an epicardial 5MHz TEE probe (Sonos 7500, Philips, Andover, MA), rotated by software and gated to ECG and respiration. Digital images were analyzed on a workstation with custom programs, by an operator blinded to treatment assignment. Endocardial surfaces were traced to calculate LV volumes validated against a 36-crystal sonomicrometer array. Remodeling was quantified in terms of increasing LV volumes. Regurgitant fraction was calculated as MR-equivalent flow by Transonic flow-meter, divided by LV ejection volume, with verification of MR flow by pulsed Doppler time-velocity integral of shunt flow multiplied by shunt cross-sectional area. LV pressure-volume loops at the initial and final thoracotomies were obtained using Millar catheters and subendocardial crystals (Sonometrics, London, ON, Canada), with IVC occlusion to obtain pressure-volume curves and derive preload-recruitable stroke work.36 Crystals were not placed at day 30 to maximize sterility and survival. Maximal systolic dP/dt was obtained by the high-fidelity Millar catheter.
Molecular assays
We measured levels of several molecular species associated with remodeling that modulate cell hypertrophy and death and are responsible for extracellular matrix turnover.9, 10 All protein assays were performed for each treatment group and stage on each individual sheep separately, and the results were averaged.
Calcium cycle
Sarcoplasmic reticulum (SR) membrane was obtained using sucrose gradient centrifugation.37 Proteins were separated and an immunoblot using monoclonal anti-SERCA2 and anti-phospholamban (Santa Cruz Biotechnology, Santa Cruz, CA) was performed, normalized to total protein. Na+/Ca2+ exchanger (NCX) levels were measured by Western blot, using monoclonal anti-NCX antibodies (Santa Cruz Biotechnology, Santa Cruz, CA).
Pro-hypertrophic and pro-apoptotic cascades
We measured levels of Akt (protein kinase B) and gp130, which are both at their respective levels (cytosol and membrane) important crossroads in pro-hypertrophic signaling; phosphorylated (activated) STAT3, an important downstream effector of gp130; and activated caspase-3, the final common pathway for intra-cellular apoptosis signaling. Western blot analysis was performed on cell lysates from biopsies at baseline and days 30 and 90. Anti-gp130, anti-phosphoAkt, anti-phospho STAT3 and anti-activated caspase-338 (Santa Cruz Biotech-nology, Santa Cruz, CA) were detected with peroxidase-conjugated anti-mouse IgG and chemiluminescence, with α-actin as housekeeping control. Integrated blot pixel density was assessed using standard software (ImageJ, NIH) by an operator blinded to treatment assignments.
Statistics
All values are reported as mean±SD. Statistical analysis used 2-tailed Student’s t-test for continuous variables compared at specific time-points; the Bonferroni correction was applied when appropriate. Repeated measures over time were analyzed with repeated-measures ANOVA (JMP 8, SAS Institute). P<0.05 was considered significant. Inter- and intra-observer variability for 3D echo-measured LV volumes in our lab were 3.5% as previously reported.9
RESULTS
Infarct size, traced and integrated by 3D echo, was 12–22% of the endocardial surface area, with a mean of 17±3% (n=12).
Function and volumes
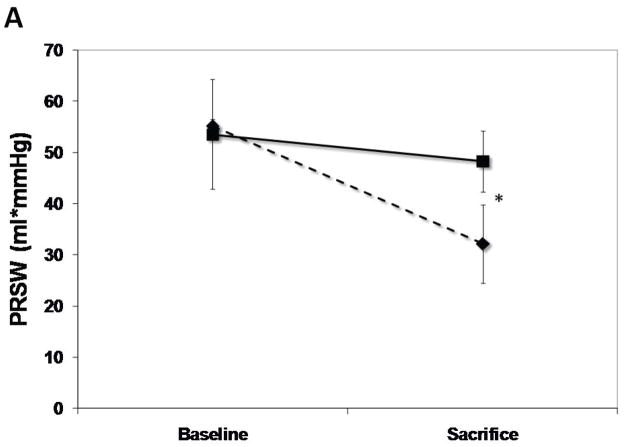
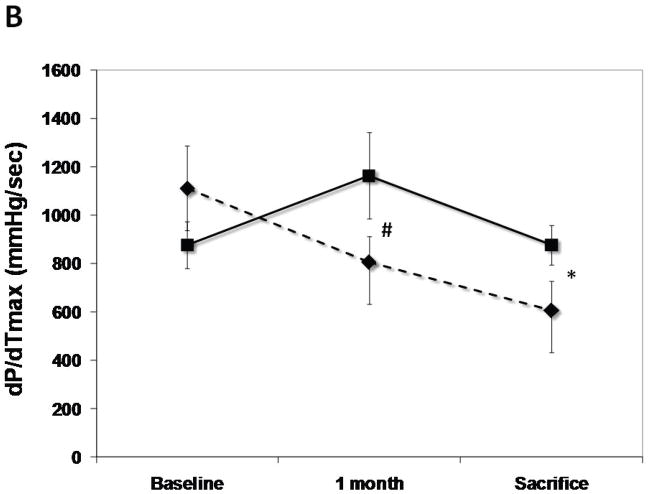
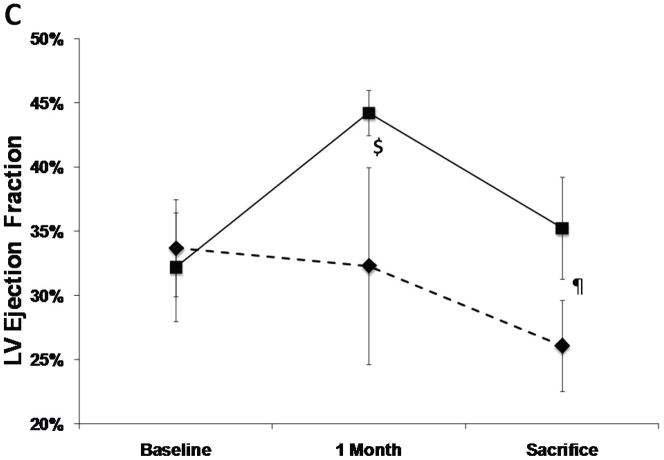
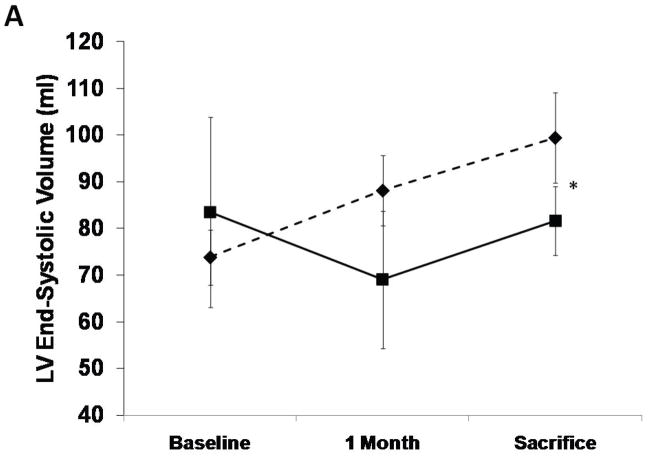
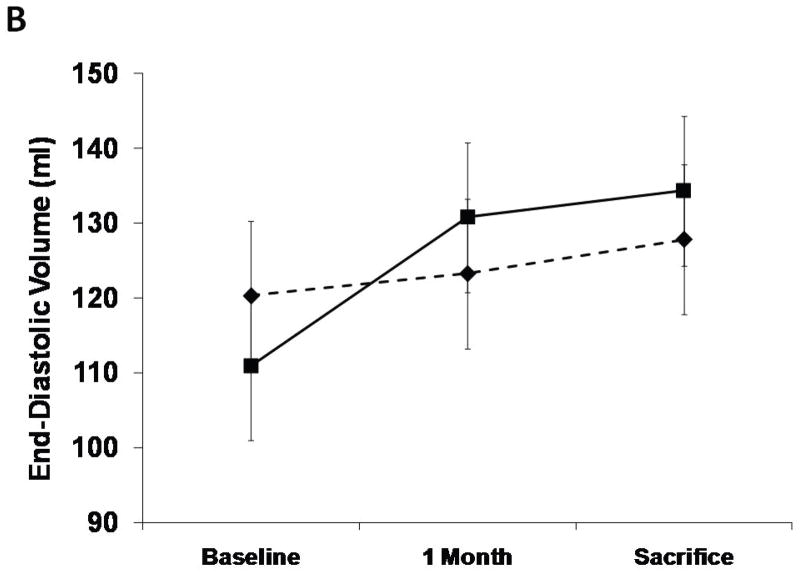
The SERCA2a group had well-maintained preload-recruitable stroke work at 3 months sacrifice (decrease by 8±10%) vs. a 42±12% decrease with reporter gene controls (p<0.001, Fig. 2). Peak systolic LV dP/dt followed the same pattern (no change vs. 55% decrease, p<0.001, Fig. 2). Although 3D echo-derived LVEF was decreased in both groups beginning with the post-MI baseline, it was better maintained at sacrifice with SERCA2a (35.2±4.0% vs. 26.1±3.5%, p=0.01, Fig. 2). This was accompanied by a lower LVESV with SERCA2a (82.6±9.6 ml vs 99.4±9.7 ml, p=0 03, Fig. 3); LVEDV, reflecting the volume overload, was not significantly different at sacrifice (127.8±6.2 ml vs 134.3±9.4 ml, p=NS, Fig. 3).
Figure 2.
LV functional parameters: Preload recruitable stroke-work (PRSW), maximal systolic derivative of pressure development (max dP/dT) and 3D echo-derived LV ejection fraction (LVEF). A significantly better global LV function is noted in the SERCA (solid lines) group as compared with controls (dashed lines) (p<0.001 by repeated-measures ANOVA), which is apparent already at 1 month follow-up (*:P<0.001, #:P=0.002, ¶:P=0.003, $:P=0.01).
Figure 3.
LV end-systolic and end-diastolic volumes from 3D echo analysis. There is a lower end-systolic volume at sacrifice in the SERCA group (solid lines, P=0.001 by repeated measures ANOVA) as compared with controls (dashed lines) while no difference was found in end-diastolic volumes. (*=P:0.03)
Although no quantitative assessment of animal well-being could be performed, the animals in the control group were less active and seemed more short of breath.
Molecular pathways of remodeling
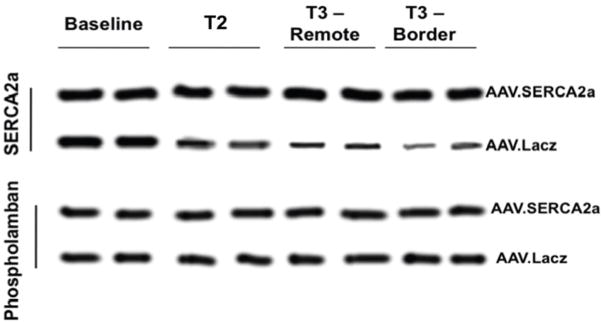
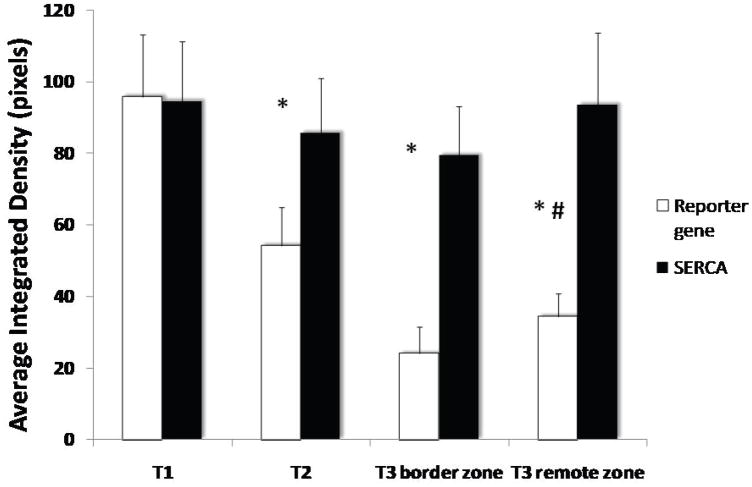
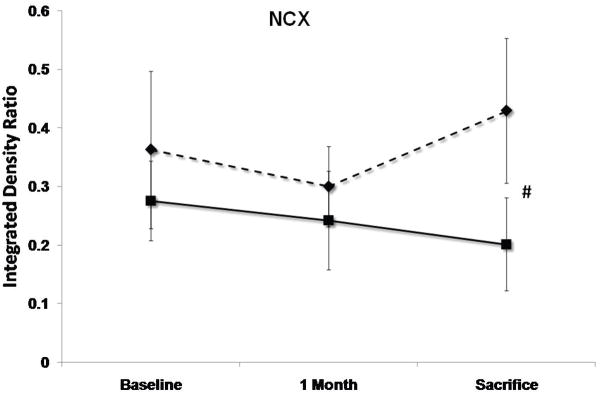
As expected, in the SERCA group there was a very significant increase in SERCA2a protein levels in both remote and border zones at sacrifice, which was already apparent at 1 month, follow up, while control sheep demonstrated a sharp reduction in SERCA2a levels at 1 month follow-up (average integrated density 85.6±15.2 vs 54.2±10.8 p<0.001, Fig. 4) and even more so at sacrifice (average integrated density 93.6±20.1 vs 34.2±6.3, p<0.001, Fig. 4). Of note, no significant change was noted in regulatory phospholamban levels (Fig. 4). NCX levels were significantly more elevated in the control sheep as compared with the SERCA sheep at sacrifice, consistent with a more active remodeling process39 (P=0.025, Fig. 5).
Figure 4.
Enhanced widespread expression of SERCA2a protein in AAV. SERCA2a (black) sheep at 1 month follow-up and 3 months sacrifice as compared with reduced expression in controls (white). Note that there was no change in phospholamban levels in both groups. (*:p<0.0005 vs baseline, #:p<0.0001 vs SERCA group at 3 months)
Figure 5.
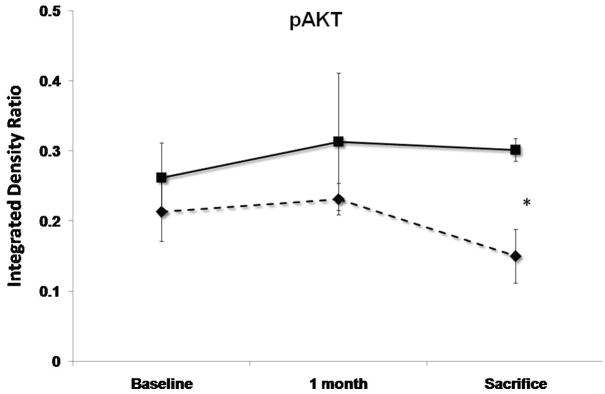
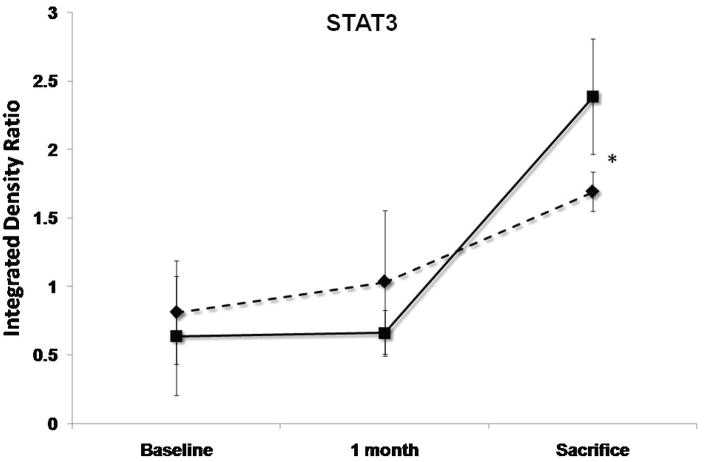
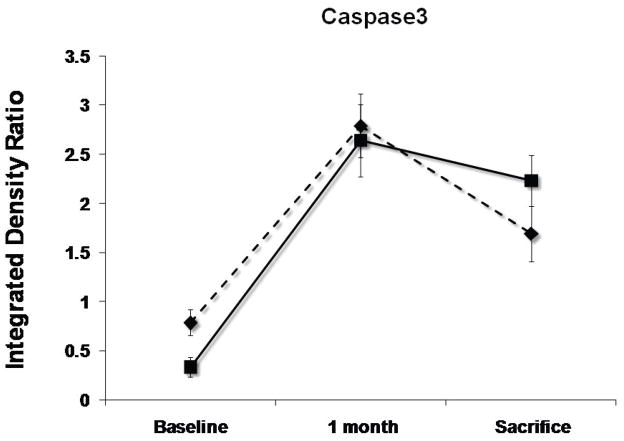
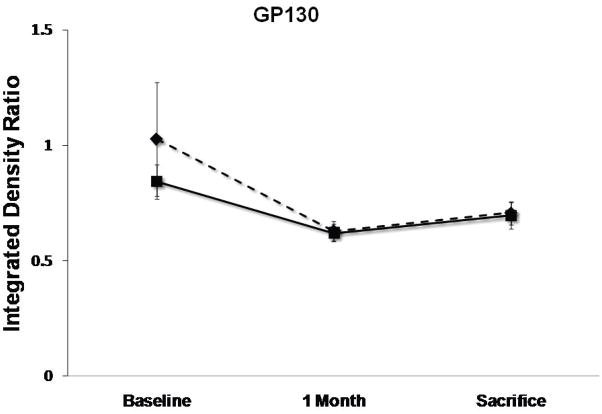
Levels of pAKT, STAT3, Caspase3, gp130 and Na+/Ca2+ exchanger (NCX) at baseline, 1 month follow-up and 3 months sacrifice at the remote zone. Note that pAKT and STAT3 levels are significantly more elevated at sacrifice in the SERCA2a group (solid lines) as compared with controls (dashed lines), while NCX levels are more elevated significantly in the control group (P<0.05 by repeated-measures ANOVA). No significant difference between the groups in caspase3 and gp130 mean levels was detected. (*:P<0.001, #:P<0.03)
SERCA2a sheep showed at 3 months’ sacrifice a 15% rise in anti-apoptotic phospho-Akt vs. 30% reduction with reporter gene (P<0.001, Fig. 5). The sample mean of STAT3 was also 41% higher at sacrifice with SERCA2a than reporter gene (p<0.001, Fig. 5). In contrast, gp130 fell by 25%–26% in both groups (p=NS by repeated-measures ANOVA), raising the possibility that improved contractility blunted the stimulus for pro-hypertrophic compensation, or alternatively, that SERCA2a over-expression compensates for but does not entirely eliminate the remodeling drive. Pro-apoptotic activated caspase-3 rose over 5-fold and to a comparable extent over 1 month in both SERCA2a and reporter gene animals (p=NS, Fig. 5), and decreased by only 19% from 1 to 3 months, remaining elevated in both groups at sacrifice.
DISCUSSION
A large number of patients with MI develop MR and progress to congestive heart failure. Our previous results9 have shown that, for a comparable infarct size, the presence of MR-type volume over-load leads to greater LV dilatation and dysfunction and to more severe changes at a cellular and molecular level. Molecular changes are biphasic, with initial upregulation and subsequent exhaustion of pro-hypertrophic and anti-apoptotic pathways that otherwise remain elevated when MI is not accompanied by MR. Maintained elevation of caspase-3 and extracellular matrix turnover lead to a failure phenotype with abnormal cellular morphology, decreased calcium cycling, and reduced sarcoplasmic reticulum Ca+2-ATPase (SERCA2a).9 This reduction was more pronounced in the border zones of the infarction, reflecting a probable larger element of cell loss through ischemic damages, but was also significant in the remote zones, possibly reflecting stretch-induced activation of the fetal program in these myocytes, resulting in diminution of SERCA2a levels. We have also demonstrated the corollary that early repair of such moderate MR-type volume overload reverses these progressive remodeling processes, and activates intracellular signals promoting hypertrophy, opposing apoptosis, and inhibiting matrix proteolysis.10 Manipulating the expression of key proteins and the activity of specific down-stream signaling pathways involved in cardiac hypertrophy and failure will allow us to understand their contribution to the disease process.
AAV (adeno-associated virus) is a gene therapy vector that provides gene expression lasting more than a year in muscle and brain with little or no immune reaction.40–42 SERCA2a was chosen as transgene because its expression is reduced in our MI+MR model9, and its overexpression improves contractility21, 23, 43 and might also decrease apoptosis by reducing intracellular diastolic Ca2+ concentrations.44, 45 In fact, a phase 1 clinical trial using AAV1.SERCA2a has been completed in patients with severe heart failure showing safety and positive biological effects (albeit in an open label trial) 46.
In our model, using a percutaneous delivery system for AAV6 encoding SERCA2a, we managed to secure robust transduction, as manifested by sustained elevation of SERCA2a levels as compared with a significant reduction in controls. We did not detect a compensatory increase in inhibitory pospholamban expression. This up-regulation translated into preserved LV contractility as measured by preload-recruitable stroke work, a relatively load-independent measure of LV function; LV dP/dt was also preserved, while these measurements where significantly depressed in the control animals. Morphologically, there was less evidence of remodeling in the SERCA animals, manifested as relatively preserved LV end-systolic volumes throughout the experiment. On the other hand, we did not detect a significant change in activated-caspase3 levels - suggesting that while the net tone in the cell is shifted to anti-apoptosis, as demonstrated by Akt and STAT3 activation, upregulating SERCA might not ablate all aspects of the intracellular remodeling cascade. One interesting aspect of the molecular changes was the effect on NCX expression in our model. An increase in NCX expression has been observed in a number of models of heart failure and has been associated with an increased risk of ventricular arrhythmias.47 In our model, NCX was increased in the control group but was remained at baseline levels with overexpression of SERCA2a. Likewise, we did not detect increased levels of gp130, as we have previously seen with MR repair;10 however, activated STAT3, downstream from gp130, was significantly increased, suggesting the possibility of greater activation of gp130-containing cytokine receptors, STAT3 activation by an alternative pathway, or decreased feedback inhibition. The results suggest that improving contractility and relaxation is insufficient to reverse the remodeling process completely at a molecular level. Nonetheless, the improvement in contraction, volumes and intracellular pro-hypertrophic pathways suggests that SERCA2a upregulation does at least strongly inhibit the remodeling process. As SERCA2a upregulation has been demonstrated, in different models, to improve function and retard progression to heart failure,17, 23, 26, 33, 43, 48, 49 our results are consistent with previously reported data.
This study has several limitations: Ischemic MR affecting a native valve often progressively increases,11, 50–52 but is inherently linked to the underlying MI and not standardized. Based on the study motivation, it was critical to separate the two processes of infarction and regurgitation to determine the incremental role of MR and to do so with a standardized orifice, which provided stable regurgitant fractions of ~30% throughout the study. In the clinical situation of the tethered mitral valve, SERCA2a may have an even more pronounced effect by reducing the severity of this dynamic MR: increased LV contractility and decreased LV volumes will increase the closing forces and decrease the tethering forces on the mitral valve, thereby improving coaptation and reducing MR.49 This will be examined in a separate study. We performed gene delivery 2 weeks before induction of MR+MI. This was done in order to have an established up-regulation of SERCA2a coincident with the initiation of the remodeling process, which starts immediately after infarction, to provide a proof of concept about the role of SERCA2a in this situation. Variations in timing of SERCA2a therapy relative to MR repair will also be assessed, for example, in the fully dilated remodeling state, as there may be a “point of no return” beyond which these interventions may be ineffective.53
In conclusion, we have demonstrated that up-regulating SERCA2a in a model of MR+MI may inhibit the remodeling process, as manifested by ventricular function, volumes, and intra-cellular pathways of hypertrophy. This may constitute a potentially useful approach to reduce the vicious cycle of remodeling in ischemic MR.
Acknowledgments
SOURCES OF FUNDING
Supported in part by grants R01 HL72265, R01 HL 078731, R01 HL080498, R01 HL083156 and K24 HL67434 from NIH/NHLBI, and grant 2005250 from the USA/Israel Binational Science Foundation (BSF). Dr Levine was also supported by grant 07CVD04 for the Transatlantic MITRAL Network, Leducq Foundation, Paris, France.
Footnotes
DISCLOSURES
None.
References
- 1.Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction: experimental observations and clinical implications. Circulation. 1990;81:1161–1172. doi: 10.1161/01.cir.81.4.1161. [DOI] [PubMed] [Google Scholar]
- 2.Picard MH, Wilkins GT, Ray PA, Weyman AE. Progressive changes in ventricular structure and function during the year after acute myocardial infarction. Am Heart J. 1992;124:24–31. doi: 10.1016/0002-8703(92)90916-j. [DOI] [PubMed] [Google Scholar]
- 3.Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling- concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. J Am Coll Cardiol. 2000;35:569–582. doi: 10.1016/s0735-1097(99)00630-0. [DOI] [PubMed] [Google Scholar]
- 4.Otsuji Y, Handshumacher MD, Schwammethal E, Jiang L, Song J-K, Guerrero JL, Vlahakes GJ, Levine RA. Insights from three-dimensional echocardiography into the mechanisms of functional mitral regurgitation: direct in vivo demonstration of altered leaflet geometry. Circulation. 1997;96:1999–2008. doi: 10.1161/01.cir.96.6.1999. [DOI] [PubMed] [Google Scholar]
- 5.Komeda M, Glasson JR, Bolger AF, Daughters GT, 2nd, Ingels NB, Jr, Miller DC. Papillary muscle-left ventricular “complex”. J Thorac Cardiovasc Surg. 1997;113:292–300. doi: 10.1016/s0022-5223(97)70326-x. [DOI] [PubMed] [Google Scholar]
- 6.Carabello BA, Nakano K, Corin W, Biederman R, Spann JF., Jr Left ventricular function in experimental volume overload hypertrophy. Am J Physiol. 1989;256:H974–981. doi: 10.1152/ajpheart.1989.256.4.H974. [DOI] [PubMed] [Google Scholar]
- 7.Spinale FG, Ishihra K, Zile MR, DeFryte G, Crawford FA, Carabello BA. Structural basis for changes in left ventricular function and geometry because of chronic mitral regurgitation and after correction of volume overload. J Thorac Cardiovasc Surg. 1993;106:1147–1157. [PubMed] [Google Scholar]
- 8.Ling LH, Enriquez-Sarano M, Seward JB, Tajik AJ, Schaff HV, Bailey KR, Frye RL. Clinical outcome of mitral regurgitation due to flail leaflet. N Engl J Med. 1996;335:1417–1423. doi: 10.1056/NEJM199611073351902. [DOI] [PubMed] [Google Scholar]
- 9.Beeri R, Yosefy C, Guerrero JL, Nesta F, Abeidat S, Chaput M, del Monte F, Handschumacher MD, Stroud R, Sullivan S, Pugatsch T, Gilon D, Vlahakes GJ, Spinale FG, Hajjar RJ, Levine RA. Mitral Regurgitation Augments Post-Myocardial Infarction Remodeling: Failure of Hypertrophic Compensation. J Am Coll Cardiol. 2008;51:476–486. doi: 10.1016/j.jacc.2007.07.093. [DOI] [PubMed] [Google Scholar]
- 10.Beeri R, Yosefy C, Guerrero JL, Abedat S, Handschumacher MD, Stroud RE, Sullivan S, Chaput M, Gilon D, Vlahakes GJ, Spinale FG, Hajjar RJ, Levine RA. Early repair of moderate ischemic mitral regurgitation reverses left ventricular remodeling: a functional and molecular study. Circulation. 2007;116:I288–293. doi: 10.1161/CIRCULATIONAHA.106.681114. [DOI] [PubMed] [Google Scholar]
- 11.Llaneras MR, Nance ML, Streicher JT, Lima JA, Savino JS, Bogen DK, Deac RF, Ratcliffe MB, Edmunds LH. Large animal model of ischemic mitral regurgitation. Ann Thorac Surg. 1994;57:432–439. doi: 10.1016/0003-4975(94)91012-x. [DOI] [PubMed] [Google Scholar]
- 12.Moainie SL, Guy TS, Gorman JH, Plappert T, Jackson BM, St John-Sutton MG, Edmunds LH, Gorman RC. Infarct restraint attenuates remodeling and reduces chronic ischemic mitral regurgitation after postero-lateral infarction. Ann Thorac Surg. 2002;74:444–449. doi: 10.1016/s0003-4975(02)03747-5. [DOI] [PubMed] [Google Scholar]
- 13.Hung J, Guerrero JL, Handschumacher MD, Supple G, Sullivan S, Levine RA. Reverse ventricular remodeling reduces ischemic mitral regurgitation: echo-guided device application in the beating heart. Circulation. 2002;106:2594–2600. doi: 10.1161/01.cir.0000038363.83133.6d. [DOI] [PubMed] [Google Scholar]
- 14.Gwathmey JK, Slawsky MT, Hajjar RJ, Briggs GM, Morgan JP. Role of intracellular calcium handling in force-interval relationships of human ventricular myocardium. J Clin Invest. 1990;85:1599–1613. doi: 10.1172/JCI114611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sipido KR, Volders PGA, Vos MA, Verdonck F. Altered Na/Ca exchange activity in cardiac hypertrophy and heart failure: a new target for therapy? Cardiovasc Res. 2002;53:782–805. doi: 10.1016/s0008-6363(01)00470-9. [DOI] [PubMed] [Google Scholar]
- 16.Hasenfuss G, Schillinger W, Lehnart SE, Preuss M, Pieske B, Maier LS, Prestle J, Minami K, Just H. Relationship between Na+-Ca2+-exchanger protein levels and diastolic function of failing human myocardium. Circulation. 1999;99:641–648. doi: 10.1161/01.cir.99.5.641. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt U, del Monte F, Miyamoto MI, Matsui T, Gwathmey JK, Rosenzweig A, Hajjar RJ. Restoration of diastolic function in senescent rat hearts through adenoviral gene transfer of sarcoplasmic reticulum Ca(2+)-ATPase. Circulation. 2000;101:790–796. doi: 10.1161/01.cir.101.7.790. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt U, Hajjar RJ, Helm PA, Kim CS, Doye AA, Gwathmey JK. Contribution of abnormal sarcoplasmic reticulum ATPase activity to systolic and diastolic dysfunction in human heart failure. J Mol Cell Cardiol. 1998;30:1929–1937. doi: 10.1006/jmcc.1998.0748. [DOI] [PubMed] [Google Scholar]
- 19.Sakata S, Lebeche D, Sakata N, Sakata Y, Chemaly ER, Liang LF, Takewa Y, Jeong D, Park WJ, Kawase Y, Hajjar RJ. Targeted gene transfer increases contractility and decreases oxygen cost of contractility in normal rat hearts. Am J Physiol Heart Circ Physiol. 2007;292:H2356–2363. doi: 10.1152/ajpheart.01310.2006. [DOI] [PubMed] [Google Scholar]
- 20.Gianni D, Chan J, Gwathmey JK, del Monte F, Hajjar RJ. SERCA2a in heart failure: role and therapeutic prospects. J Bioenerg Biomembr. 2005;37:375–380. doi: 10.1007/s10863-005-9474-z. [DOI] [PubMed] [Google Scholar]
- 21.del Monte F, Harding SE, Schmidt U, Matsui T, Kang ZB, Dec GW, Gwathmey JK, Rosenzweig A, Hajjar RJ. Restoration of contractile function in isolated cardiomyocytes from failing human hearts by gene transfer of SERCA2a. Circulation. 1999;100:2308–2311. doi: 10.1161/01.cir.100.23.2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hajjar RJ, Schmidt U, Matsui T, Guerrero JL, Lee KH, Gwathmey JK, Dec GW, Semigran MJ, Rosenzweig A. Modulation of ventricular function through gene transfer in vivo. Proc Natl Acad Sci U S A. 1998;95:5251–5256. doi: 10.1073/pnas.95.9.5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miyamoto MI, del Monte F, Schmidt U, DiSalvo TS, Kang ZB, Matsui T, Guerrero JL, Gwathmey JK, Rosenzweig A, Hajjar RJ. Adenoviral gene transfer of SERCA2a improves left-ventricular function in aortic-banded rats in transition to heart failure. Proc Natl Acad Sci U S A. 2000;97:793–798. doi: 10.1073/pnas.97.2.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sakata S, Lebeche D, Sakata N, Sakata Y, Chemaly ER, Liang LF, Tsuji T, Takewa Y, del Monte F, Peluso R, Zsebo K, Jeong D, Park WJ, Kawase Y, Hajjar RJ. Restoration of mechanical and energetic function in failing aortic-banded rat hearts by gene transfer of calcium cycling proteins. J Mol Cell Cardiol. 2007;42:852–861. doi: 10.1016/j.yjmcc.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.del Monte F, Williams E, Lebeche D, Schmidt U, Rosenzweig A, Gwathmey JK, Lewandowski ED, Hajjar RJ. Improvement in Survival and Cardiac Metabolism After Gene Transfer of Sarcoplasmic Reticulum Ca2+-ATPase in a Rat Model of Heart Failure. Circulation. 2001;104:1424–1429. doi: 10.1161/hc3601.095574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davia K, Bernobich E, Ranu HK, del Monte F, Terracciano CM, MacLeod KT, Adamson DL, Chaudhri B, Hajjar RJ, Harding SE. SERCA2A overexpression decreases the incidence of aftercontractions in adult rabbit ventricular myocytes. J Mol Cell Cardiol. 2001;33:1005–1015. doi: 10.1006/jmcc.2001.1368. [DOI] [PubMed] [Google Scholar]
- 27.del Monte F, Lebeche D, Guerrero JL, Tsuji T, Doye AA, Gwathmey JK, Hajjar RJ. Abrogation of ventricular arrhythmias in a model of ischemia and reperfusion by targetin myocardial calcium cycling. Proc Natl Acad Sci U S A. 2004;101:5622–5627. doi: 10.1073/pnas.0305778101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kawase Y, Ly HQ, Prunier F, Lebeche D, Shi Y, Jin H, Hadri L, Yoneyama R, Hoshino K, Takewa Y, Sakata S, Peluso R, Zsebo K, Gwathmey JK, Tardif J-C, Tanguay J-F, Hajjar RJ. Reversal of Cardiac Dysfunction After Long-Term Expression of SERCA2a by Gene Transfer in a Pre-Clinical Model of Heart Failure. J Am Coll Cardiol. 2008;51:1112–1119. doi: 10.1016/j.jacc.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 29.Palomeque J, Chemaly ER, Colosi P, Wellman JA, Zhou S, Del Monte F, Hajjar RJ. Efficiency of eight different AAV serotypes in transducing rat myocardium in vivo. Gene Ther. 2007;14:989–997. doi: 10.1038/sj.gt.3302895. [DOI] [PubMed] [Google Scholar]
- 30.Zoghbi WA, Enriquez-Sarano M, Foster E, Grayburn PA, Kraft CD, Levine RA, Nihoyannopoulos P, Otto CM, Quinones MA, Rakowski H, Stewart WJ, Waggoner A, Weissman NJ. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16:777–7802. doi: 10.1016/S0894-7317(03)00335-3. [DOI] [PubMed] [Google Scholar]
- 31.Sandalon Z, Bruckheimer EM, Lustig KH, Rogers LC, Peluso RW, Burstein H. Secretion of a TNFR:Fc Fusion Protein following Pulmonary Administration of Pseudotyped Adeno-Associated Virus Vectors. J Virol. 2004;78:12355–12365. doi: 10.1128/JVI.78.22.12355-12365.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beeri R, Guerrero JL, Supple G, Sullivan S, Levine RA, Hajjar RJ. New efficient catheter-based system for myocardial gene delivery. Circulation. 2002;106:1756–1759. doi: 10.1161/01.cir.0000035240.92015.e4. [DOI] [PubMed] [Google Scholar]
- 33.Kawase Y, Ly HQ, Prunier F, Lebeche D, Shi Y, Jin H, Hadri L, Yoneyama R, Hoshino K, Takewa Y, Sakata S, Peluso R, Zsebo K, Gwathmey JK, Tardif JC, Tanguay JF, Hajjar RJ. Reversal of cardiac dysfunction after long-term expression of SERCA2a by gene transfer in a pre-clinical model of heart failure. J Am Coll Cardiol. 2008;51:1112–1119. doi: 10.1016/j.jacc.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 34.Gorman JH, Gorman RC, Plappert T, Jackson BM, Hiramatsu Y, St John-Sutton MG, Edmunds LH. Infarct size and location determine development of mitral regurgitation in the sheep model. J Thorac Cardiovasc Surg. 1998;115:615–622. doi: 10.1016/S0022-5223(98)70326-5. [DOI] [PubMed] [Google Scholar]
- 35.Handschumacher MD, Lethor J-P, Siu SC, Mele D, Rivera M, Picard MH, Weyman AE, Levine RA. A new integrated system for three-dimensional echocardiographic reconstruction: development and validation for ventricular volume with application in human subjects. J Am Coll Cardiol. 1993;21:743–753. doi: 10.1016/0735-1097(93)90108-d. [DOI] [PubMed] [Google Scholar]
- 36.Glower DD, Spratt JA, Snow ND, Kabas JS, Davis JW, Olsen CO, Tyson GS, Sabiston DC, Rankin JS. Linearity of the Frank-Starling relationship in the heart: the concept of preload recruitable stroke work. Circulation. 1985;71:994–1009. doi: 10.1161/01.cir.71.5.994. [DOI] [PubMed] [Google Scholar]
- 37.Chu A, Dixon MC, Saito A, Seiler S, Fleischer S. Isolation of sarcoplasmic reticulum fractions referable to longitudinal tubules and junctional terminal cisternae from rabbit skeletal muscle. Methods Enzymol. 1988;157:36–46. doi: 10.1016/0076-6879(88)57066-0. [DOI] [PubMed] [Google Scholar]
- 38.Molkentin JD, Dorn GW., II Cytoplasmic signaling pathways that regulate cardiac hypertrophy. Annu Rev Physiol. 2001;63:391–426. doi: 10.1146/annurev.physiol.63.1.391. [DOI] [PubMed] [Google Scholar]
- 39.Ito K, Yan X, Tajima M, Su Z, Barry WH, Lorell BH. Contractile Reserve and Intracellular Calcium Regulation in Mouse Myocytes From Normal and Hypertrophied Failing Hearts. Circ Res. 2000;87:588–595. doi: 10.1161/01.res.87.7.588. [DOI] [PubMed] [Google Scholar]
- 40.Malik AK, Monahan PE, Samulski RJ, Kurachi K. Kinetics of recombinant adeno-associated virus-mediated gene transfer. J Virol. 2000;74:3555–3565. doi: 10.1128/jvi.74.8.3555-3565.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Samulski RJ. AAV vectors, the future workhorse of human gene therapy. Ernst Schering Research Foundation Workshop; 2003. pp. 25–40. [DOI] [PubMed] [Google Scholar]
- 42.Young SM, Jr, McCarty DM, Degtyareva N, Samulski RJ. Roles of adeno-associated virus Rep protein and human chromosome 19 in site-specific recombination. J Virol. 2000;74:3953–3966. doi: 10.1128/jvi.74.9.3953-3966.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.del Monte F, Williams E, Lebeche D, Schmidt U, Rosenzweig A, Gwathmey JK, Lewandowski ED, Hajjar RJ. Improvement in survival and cardiac metabolism after gene transfer of sarcoplasmic reticulum Ca(2+)-ATPase in a rat model of heart failure. Circulation. 2001;104:1424–1429. doi: 10.1161/hc3601.095574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Faulk EA, McCully JD, Tsukube T, Hadlow NC, Krukenkamp IB, Levitsky S. Myocardial mitochondrial calcium accumulation modulates nuclear calcium accumulation and DNA fragmentation. Ann Thorac Surg. 1995;60:338–344. doi: 10.1016/0003-4975(95)00446-r. [DOI] [PubMed] [Google Scholar]
- 45.Sakata Y, Masuyama T, Yamamoto K, Nishikawa N, Yamamoto H, Kondo H, Ono K, Otsu K, Kuzuya T, Miwa T, Takeda H, Miyamoto E, Hori M. Calcineurin inhibitor attenuates left ventricular hypertrophy, leading to prevention of heart failure in hypertensive rats. Circulation. 2000;102:2269–2275. doi: 10.1161/01.cir.102.18.2269. [DOI] [PubMed] [Google Scholar]
- 46.Jaski BE, Jessup ML, Mancini DM, Cappola TP, Pauly DF, Greenberg B, Borow K, Dittrich H, Zsebo KM, Hajjar RJ. Calcium upregulation by percutaneous administration of gene therapy in cardiac disease (CUPID Trial), a first-in-human phase 1/2 clinical trial. J Card Fail. 2009;15:171–181. doi: 10.1016/j.cardfail.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pogwizd SM, Bers DM. Na/Ca exchange in heart failure: contractile dysfunction and arrhythmogenesis. Annals of the New York Academy of Sciences. 2002;976:454–465. doi: 10.1111/j.1749-6632.2002.tb04775.x. [DOI] [PubMed] [Google Scholar]
- 48.Sakata S, Lebeche D, Sakata N, Sakata Y, Chemaly ER, Liang LF, Takewa Y, Jeong D, Park WJ, Kawase Y, Hajjar RJ. Targeted gene transfer increases contractility and decreases oxygen cost of contractility in normal rat hearts. Am J Physiol Heart Circ Physiol. 2007;292:H2356–2363. doi: 10.1152/ajpheart.01310.2006. [DOI] [PubMed] [Google Scholar]
- 49.Sakata S, Lebeche D, Sakata Y, Sakata N, Chemaly ER, Liang L, Nakajima-Takenaka C, Tsuji T, Konishi N, del Monte F, Hajjar RJ, Takaki M. Transcoronary gene transfer of SERCA2a increases coronary blood flow and decreases cardiomyocyte size in a type 2 diabetic rat model. Am J Physiol Heart Circ Physiol. 2007;292:H1204–1207. doi: 10.1152/ajpheart.00892.2006. [DOI] [PubMed] [Google Scholar]
- 50.Liel-Cohen N, Guerrero JL, Otsuji Y, Handshumacher MD, Rudski LG, Hunziker PR, Tanabe H, Scherrer-Crosbie M, Sullivan S, Levine RA. Design of a new surgical approach for ventricular remodeling to relieve ischemic mitral regurgitation. Circulation. 2000;101:2756–2763. doi: 10.1161/01.cir.101.23.2756. [DOI] [PubMed] [Google Scholar]
- 51.Hung J, Papakostas L, Tahta SA, Hardy BG, Bollen BA, Duran CM, Levine RA. Mechanism of Recurrent Ischemic Mitral Regurgitation Post-Annuloplasty: Continued LV Remodeling as a Moving Target. Circulation. 2004;110:85–90. doi: 10.1161/01.CIR.0000138192.65015.45. [DOI] [PubMed] [Google Scholar]
- 52.Otsuji Y, Handschumacher MD, Liel-Cohen N, Tanabe H, Jiang L, Schwammethal E, Guerrero JL, Nicholls LA, Vlahakes GJ, Levine RA. Mechanism of ischemic mitral regurgitation with segmental left ventricular dysfunction: Three-dimensional echocardiographic studies in models of acute and chronic progressive regurgitation. J Am Coll Cardiol. 2001;37:641–648. doi: 10.1016/s0735-1097(00)01134-7. [DOI] [PubMed] [Google Scholar]
- 53.Enriquez-Sarano M, Loulmet DF, Burkhoff D. The conundrum of functional mitral regurgitation in chronic heart failure. J Am Coll Cardiol. 2008;51:487–489. doi: 10.1016/j.jacc.2007.09.046. [DOI] [PubMed] [Google Scholar]