Abstract
Artificial percepts (phosphenes) can be induced by applying transcranial magnetic stimulation (TMS) over human visual cortex. Although phosphenes have been used to study visual awareness, the neural mechanisms generating them have not yet been delineated. We directly tested the two leading hypotheses of how phosphenes arise. These hypotheses correspond to the two competing views of the neural genesis of awareness: the early, feedforward view and the late, recurrent feedback model. We combined online TMS and EEG recordings to investigate whether the electrophysiological correlates of conscious phosphene perception are detectable early after TMS onset as an immediate local effect of TMS, or only at longer latencies, after interactions of TMS‐induced activity with other visual areas. Stimulation was applied at the intensity threshold at which participants saw a phosphene on half of the trials, and brain activity was recorded simultaneously with electroencephalography. Phosphene perception was associated with a differential pattern of TMS‐evoked brain potentials that started 160–200 ms after stimulation and encompassed a wide array of posterior areas. This pattern was differentiated from the TMS‐evoked potential after stimulation of a control site. These findings suggest that conscious phosphene perception is not a local phenomenon, but arises only after extensive recurrent processing. Hum Brain Mapp, 2010. © 2010 Wiley‐Liss, Inc.
Keywords: phosphenes, transcranial magnetic stimulation, electroencephalography, evoked potentials, visual perception, visual cortex, awareness, consciousness
INTRODUCTION
Transcranial magnetic stimulation (TMS) is a technique that can be used to test whether a cortical area is necessary for a given function, by transiently interacting with the normal underlying pattern of neural activity and studying the consequences. For example, if TMS is applied to primary visual cortex (V1) then participants can report having perceived an artificial flash‐like visual percept, or “phosphene.” TMS‐induced phosphenes can be used to explore the neural dynamics underlying visual perception [Antal et al.,2003; Cowey and Walsh,2000; Gothe et al.,2002; Rauschecker et al.,2004; Walsh and Pascual‐Leone,2003]. Phosphenes have been found to show several properties: they occur more often as the intensity of stimulation is increased, and a threshold intensity of stimulation can be determined for individual participants at which phosphenes are elicited on about half of trials [Kammer et al.,2001; Stewart et al.,2001]. This phosphene threshold can be reduced (i.e., it becomes easier to elicit a phosphene) if TMS is first applied to connected areas such as posterior parietal cortex [Silvanto et al.,2009], or the frontal eye fields [Silvanto et al.,2006] where TMS can also facilitate perception of visual stimuli [Grosbras and Paus,2003]. During phosphene threshold stimulation, cortical excitability immediately before the pulse predicts whether or not a phosphene is elicited [Romei et al.,2008a], suggesting that the perceptual differences between consecutive stimulation trials are driven by inherent variability in the central nervous system. The presence of phosphenes demonstrates that artificial conscious percepts can be generated noninvasively in humans, but the time course and pattern of the neural activity that are responsible for such percepts are not yet known. Obtaining an electrophysiological measure of these artificial percepts would offer new insights into how and when during visual processing phosphenes are generated. This could enable a deeper interpretation of previous studies that have used phosphenes and provide a novel measure for future work to exploit.
We recorded the brain activity in response to occipital TMS by combining online TMS with electroencephalography (EEG). TMS intensity was adjusted to phosphene‐threshold so that a phosphene would be perceived on approximately half of all trials. TMS‐EEG can show causal interactions between brain areas with high temporal resolution [Driver et al.,2009; Ilmoniemi et al.,1997; Miniussi and Thut, in press; Taylor et al.,2008]. This method was applied here to characterize the TMS‐evoked potential (TEP) in response to occipital TMS by comparing electrical brain responses that were time‐locked to TMS onset on phosphene‐present and phosphene‐absent trials. The rationale of this design was that the subtraction of the TEP on phosphene‐absent from phosphene‐present trials would isolate the differential activity related to conscious perception of the phosphene and remove the nonspecific responses to the acoustic or somatosensory artifact that accompany TMS, but are identical across experimental conditions. To characterize the visual cortical response that is triggered by TMS, but is independent of conscious phosphene perception, we also compared TEPs triggered by occipital TMS to TEPs elicited by stimulation of a control site in parietal cortex.
We tested two contrasting hypotheses with respect to the time course of neural activity that is related to phosphene perception. The “early” hypothesis predicts that the TEP on phosphene‐present trials should start to differ from the TEP on phosphene‐absent trials within a very short time interval (i.e. several tens of milliseconds) after the TMS pulse. Such a result would suggest that variations in the initial response of the visual areas directly stimulated by the TMS pulse determine whether or not a phosphene is perceived. In other words, phosphenes are perceived whenever the stimulated area itself is in a particularly excitable state at the time of the TMS pulse, and phosphene perception does not require any interactions with other areas. Such early effects on cortico‐spinal excitability have been demonstrated after TMS of the primary motor cortex M1 [Hess et al.,1987] and might be predicted if all areas respond to TMS in a similar fashion such that the motor‐evoked potential can be regarded as a direct (albeit peripheral) analog of the occipital TEP. By contrast, an alternative “late” theory predicts that differences between the pattern of neural activity on phosphene‐present and phosphene‐absent trials, as reflected by TEPs, will not emerge immediately after TMS onset, but are instead observed with a considerable delay (e.g., 150 or 200 ms after the TMS pulse). According to this account, conscious phosphene perception is not simply determined by differences in local cortical excitability, but is instead linked to interactions between a wider range of visual areas. For example, phosphene perception and phosphene‐related potentials might only arise after the feedforward activation of higher‐order visual areas, followed by recurrent loops that involve the stimulated site. Such additional processing from recurrent loops of activation has been suggested to be a critical prerequisite for phosphenes [Pascual‐Leone and Walsh,2001] and for normal conscious visual perception [Lamme and Roelfsema,2000]. Additional electrophysiological evidence consistent with this “late” theory comes from recent work where ERPs were measured in response to backward‐masked visual stimuli that were presented at perceptual threshold [Del Cul et al.,2007]. In that study, only broad and relatively late ERP modulations (positive deflections in the P3 component starting 270–300ms poststimulus) correlated with whether or not the participant perceived the target.
While the “early” hypothesis suggests that phosphene‐related potentials after occipital TMS are functionally analogous to motor‐evoked potentials following M1 TMS, the “late” hypothesis claims that conscious phosphene perception and its associated phosphene‐related potentials are similar to the conscious perception of external visual stimuli and its electrophysiological correlates. Our results demonstrate that differential ERP modulations associated with conscious phosphene perception emerge relatively late (160–200 ms after TMS onset) and over a wide region of areas, a pattern unlike the immediate effects of M1 TMS. However, phosphene‐related potentials still emerged substantially earlier than the ERP correlates of conscious visual perception previously observed.
MATERIALS AND METHODS
Participant Screening
All participants were right‐handed and gave informed consent for a TMS protocol approved by the Birkbeck College Psychology School Ethics Committee. Previous studies have elicited phosphenes from approximately half of participants without extensive training [Romei et al.,2008a] and here potential participants were screened as to whether or not they were saw phosphenes during right occipital TMS. The screening task was similar to that used in the main experiment (Fig. 1, described in the next section). A gray fixation point was presented on a cathode ray tube monitor 150 cm from the participant. TMS was applied at a variable random delay of 1,000–1,500 ms after the onset of the fixation point, which then remained on‐screen for a further 1,000 ms. This was followed by a 2,000‐ms intertrial interval, during which the monitor was blank and the participants were to report verbally whether a phosphene was present or absent (note that manual responses were used in the main experiment, see below). This task was designed to provide an epoch for event‐related potential (ERP) analysis comprising only a measure of the brain response to the TMS pulse that did not include any eye movements, blinks, or manual responses. Participants were therefore instructed to fixate with eyes open while the fixation point was presented and to respond as accurately as possible (and to blink or move their eyes) only during the 2,000‐ms blank intertrial interval after fixation offset.
Figure 1.
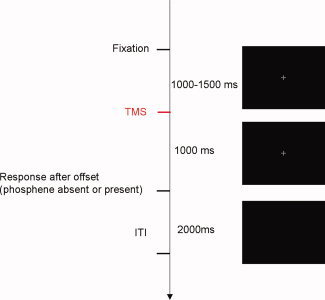
Task. TMS was applied either over early visual cortex or over a parietal control site while participants fixated centrally: phosphene present/absent judgments were made 1,000 ms after the TMS pulse, during the intertrial interval. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
For TMS, we used a figure‐8 flat coil with an internal diameter of 70 mm (Magstim Rapid2 Machine, Whitland, Wales, United Kingdom). Single pulses of TMS were applied over the right occipital lobe, with the initial stimulation site 2 cm dorsal and 1 cm lateral of the inion (electrode position Iz) [Kamitani and Shimojo,1999; Silvanto et al.,2005]. To ensure that the current experiment would be comparable to previous phosphene studies the coil was placed flush with the electrode cap directly on the scalp and not on top of any EEG electrodes, necessitating removing electrode Oz from the electrode array (see “Event‐Related Potentials” below). The coil was held with the handle pointing towards the right (and the current therefore flowing lateral‐to‐medial, from right to left), an efficient coil orientation for inducing phosphenes [Kammer et al.,2001]. During screening, stimulation intensity was increased from 50% of maximal stimulator output in 5–10% steps up until 90% output was reached or a phosphene was reported, whichever occurred first. Participants were excluded if no phosphenes had been reported after 10 consecutive stimulation trials using 90% stimulation over each of nine points spanning a 2 cm × 2 cm grid centered on the starting point. Fifteen of twenty‐seven subjects failed this criterion leaving 12 to participate in the study (mean age: 24 years; age range: 19–33; eight were female).
Once the participants had been selected, the next stage of experimental preparation was to optimize the TMS sites used. The active TMS site was defined functionally as the point on the 2 cm × 2 cm grid, which produced the strongest phosphenes, as reported verbally by the participant. At the end of each session, the location of the stimulated sites was plotted using Brainsight stereotactic infrared registration to each subject's structural MRI scan. The active TMS sites, which were defined functionally, were nevertheless clustered tightly around the classical occipital phosphene site in the calcarine sulcus (see Fig. 2). The TMS intensity was also chosen functionally and tailored to each participant so that there would be high enough numbers of both phosphene‐present and also phosphene‐absent trials to derive robust ERP averages, i.e., approximately half of each. To determine the phosphene threshold, participants performed sequences of ten consecutive trials using each candidate stimulation intensity, reporting phosphene presence or absence verbally during the intertrial interval. If three or more trials elicited phosphenes in each sequence of ten, then the stimulation intensity was reduced by 2% of the maximal stimulator output and the procedure repeated; if not, then the previous intensity that had been tried was used from the start of the experiment. The mean phosphene threshold intensity was 70% of maximal stimulation output (range 55–80%).
Figure 2.
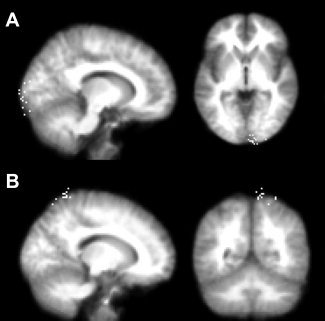
TMS sites. (A) Right occipital TMS. The dots represent the MNI coordinates of each site in each participant superimposed over their average structural brain image. The locations of TMS sites were recorded using infrared stereotactic registration to every participant's structural MRI scan. TMS sites are tightly centered in the right occipital lobe (mean, x = 9, y = −81, z = −19). (B) Control site TMS. The sites are clustered in the right superior medial parietal cortex (mean, x = 13, y = −37, z = 57).
A control site was also stimulated to control for the acoustic, somatosensory, and electrical artifacts that accompany TMS of any area on the scalp and which are nonspecific to the site stimulated, and at the same lateral distance from the midline as the occipital TMS site in each individual participant, ensuring no differences in the extent to which the active and control TMS pulses could have acted as cues to orient spatial attention to one side of space. The control site was also plotted to be level with electrode position CPz in the anterior–posterior direction so that the distance and relative position between the control TMS site and midline electrode Cz would be approximately similar to that between the phosphene site and midline electrode POz, allowing us to display whether TMS effects on the ERP differed over different sites. The control sites clustered around superior medial parietal cortex (see Fig. 2). In addition, this site does not overlie any cortical area that has been associated in previous studies with phosphenes, motor‐evoked potentials, or the control of visual spatial attention.
Main Experiment
The main experiment comprised 16 blocks of 40 trials. The task, fixation point, and timing were the same as during screening (see Fig. 1), but rather than using verbal reports, participants executed manual button presses made with the right hand, using the index or third finger to indicate phosphene presence or absence, respectively. Block order was pseudorandomized and counterbalanced: each group of four consecutive blocks included two blocks with occipital TMS on every trial, one with control site TMS, and one without any TMS. Participants were instructed to respond manually to the presence or absence of a phosphene in the intertrial interval—even with control site TMS or without TMS, when phosphenes were not predicted. The rationale of using control TMS and no TMS conditions was as follows. Similarities between the TEPs on phosphene‐absent trials during active TMS or control TMS would consist of the ERP response to the somatosensory and auditory stimulation accompanying TMS and any commonalities in how the two stimulated sites respond to TMS. The control site TMS condition was included to find these similarities. Any differences between the TEPs on phosphene‐absent control TMS and phosphene‐absent occipital TMS trials would therefore be attributable to differences in how the two areas react to the interference of neural activity caused by TMS. This would then enable determination of which parts of the TEP from occipital TMS phosphene‐absent trials were due to the somatosensory‐acoustic artifact and which were due to effects of TMS on visual cortex. The no TMS block was included simply to demonstrate conclusively that in the absence of TMS, and before participants reported the absence of a phosphene, there was little or no change in the ERP and that any activity changes present in the EEG after TMS of the control site were driven by the TMS. Overall, participants reported that phosphenes were present on approximately half (45%) of occipital TMS trials. There were phosphenes reported on only 8% of control site trials: six participants reported the presence of phosphenes on a small number of control site trials. Two of those participants reported a phosphene on only one trial (out of a total of 160 control site trials), and the other four had scores of 7, 9, 36, or 80 trials. In these participants, the control site may have been near a recently reported parietal phosphene area [Marzi et al.,2009]: note that trial numbers on which the participants reported a phosphene at this control site were too low to form a reliable grand average and therefore only phosphene‐absent trials were included in the current analysis.
Event‐Related Potentials
EEG was DC‐recorded continuously at 1,000 Hz with a TMS‐compatible ERP amplifier (BrainAmp DC, Brainproducts, Germany) capable of recording a veridical EEG without TMS or recharging artifacts within ∼50 ms of a TMS pulse [Veniero et al.,2009]. EEG was recorded with minimal filtering (DC‐450Hz, no notch) from a whole‐head montage of custom‐built Ag‐AgCl electrodes (each with a built‐in 5‐kΩ‐resistor) at positions C3, C4, CP5, CP6, Cz, F3, F4, F7, F8, FC5, FC6, FPz, Fz, P3, P4, P7, P8, PO3, PO4, PO7, PO8, POz, Pz, T7, and T8. Horizontal EOG was recorded from the left and right temples. The ground was at AFz and the active reference on the left earlobe. Electrode impedance was kept below 10 kΩ. Data were rereferenced to the average of the left and right earlobes, and an HEOG signal was formed from linear derivation of the left and right EOG electrodes. Data were then epoched to form 5,600‐ms segments containing the whole trial. Then the TMS artifact was removed from the data through linear interpolation of the data between 5 ms before and 40 ms after the TMS pulse [Fuggetta et al.,2006; Taylor et al.,2008]. Filtering used a notch 50‐Hz filter and then a Butterworth zero phase filter with low cutoff of 0.01 Hz and high cutoff of 40 Hz (12 dB/octave). Data were then epoched into 600‐ms periods, starting 100 ms before the time of TMS onset. Baseline correction used the 100 ms prior to the TMS pulse. Automated ERP artifact rejection removed trials with eye movements by eliminating trials where the HEOG signal exceeded ±30 μV. Blinks were removed by deleting trials if the signal at FPz exceeded ±60 μV, and other movement‐related artifacts were removed by eliminating any trials where the signal from any electrode exceeded ±80 μV. A minimum criterion of 30 trials per condition was set to ensure a sufficiently high signal‐to‐noise ratio of the ERP averages, which were time‐locked to TMS onset. No blocks fell below this level. The mean number of trials per condition per participant was as follows: occipital TMS phosphene present, 132; occipital TMS phosphene absent, 109; control site TMS phosphene absent, 102; no TMS phosphene absent, 111.
ERP effects were characterized statistically by comparing the mean TEP amplitude within three different time windows centered on successive TMS‐evoked components (70–140, 160–200, 280–400 ms) across conditions. The phosphene effect was analyzed by comparing phosphene‐present and phosphene‐absent trials during occipital TMS. A second analysis compared the phosphene‐absent trials during occipital and control‐site TMS to determine any effect of TMS site. For both analyses, the spatial distribution of any effect was investigated by pooling electrodes into three groups comprising a posterior group over visual cortex (electrodes PO3, P3, POz, Pz, PO4, P4, PO8), a central group (T7, CP5, C3, Cz, C4, CP6, T8), and a frontal group (FC5, F7, F3, Fz, F4, FC6, F8). To determine effects at the individual electrode level and with finer temporal resolution, each time window was divided into sequential 20‐ms time bins, comparing the means between conditions with Student t‐tests, and correcting for multiple comparisons by adopting the criterion that significant differences should last for at least two consecutive 20‐ms time bins.
RESULTS
The TMS‐Evoked potential
TMS was applied at the threshold for phosphene perception either to occipital cortex or to a control parietal site (Figs. 1 and 2), and TMS of either site produced TEPs of ∼10 μV amplitude (see Fig. 3). The TEP profile consisted of an early frontocentral negativity from 70 to 120 ms, a centroparietal positivity from 140 to 220 ms, and a posterior positivity over visual areas that was apparent from 240 ms after TMS onset. The first two components that were present both for occipital and control‐site TMS primarily reflect sensory‐specific auditory and tactile ERP components triggered by TMS. The third posterior component was much more pronounced for occipital relative to control‐site TMS. Two sets of analyses were performed. The main analyses tested which aspects of the TEP were sensitive to phosphene perception by comparing ERPs in response to occipital TMS on phosphene‐present and phosphene‐absent trials. A second set of analyses investigated the impact of stimulation site on TEPs by comparing ERPs triggered when TMS was applied to the occipital site or to the centroparietal control site.
Figure 3.
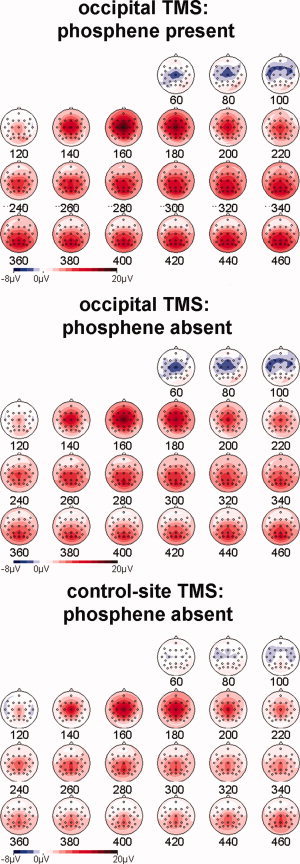
The mean amplitude of the ERP after occipital TMS on phosphene‐present (top), phosphene‐absent trials (middle), and control site phosphene‐absent trials (bottom) calculated in 20‐ms time bins. The nonspecific TMS artifact (i.e., the ERP response to somatosensory or acoustic stimulation accompanying TMS) is evident as a central positivity in all sites.
The Phosphene‐Evoked Potential
Figure 4 shows the TEP differences between phosphene‐present and phosphene‐absent trials following occipital TMS. When participants reported a phosphene, there was a greater visual positivity over a wide range of posterior but not frontal areas. To characterize this effect, the mean amplitudes of phosphene‐present and phosphene‐absent trials were compared across the three groups of electrodes (posterior, central, and frontal). Phosphene perception (the comparison between phosphene‐present and phosphene‐absent trials) did not affect the early phase of the TEP from 70 to 140 ms (no effect of phosphene perception and no interaction of phosphene perception with electrode group, all Fs ≤ 0.7, all Ps > 0.4). In contrast, brain activity between 160 and 200 ms after TMS onset was modulated by phosphene perception [F(1,11) = 11.0; P < 0.01]. The lack of any interaction between phosphene perception and electrode group showed that the phosphene effect was wide‐spread [F(2,22) = 0.3; P = 0.7]. The effect was also statistically significant at the individual electrode level and over a wide area, with all differences between 160 and 200 ms being driven by a relative positivity on phosphene‐present trials [C3, C4, CP5, Cz, F3, F4, FC5, Fz, P3, P4, PO4, POz, Pz: all ts (11) ≥ 2.2, Ps < 0.05]. The later phase of the TEP (280–400 ms) was also modulated by phosphene perception [F(1,11) = 10.9; P < 0.01]. Additionally, this later modulation was accompanied by effects of electrode group and an interaction of electrode group and phosphene perception (all Fs ≥ 4.5, all Ps < 0.05), indicating spatial heterogeneity across the different electrode groups. Phosphene effects were statistically significant within the posterior and central groups [posterior group: F(1,11) = 13.9; P < 0.01, central group: F(1,11) = 9.9; P < 0.01], but only marginally significant at the frontal group of electrodes [F(1,11) = 4.2; P = 0.066]. Follow‐up analysis conducted for individual electrodes between 280 and 400 ms showed significant differences between phosphene‐present and phosphene‐absent trials at most posterior and central electrodes [individual electrodes C3, C4, CP5, CP6, Cz, FC5, FC6, P3, P4, P8, PO3, PO4, PO7, PO8, POz, Pz, T8: all ts (11) ≥ 2.2, Ps < 0.05]. In contrast, no reliable differential effects of phosphene perception were observed at the frontal electrodes F3, F4, F7, F8, FZ, or FPz [all ts (11) < 2.0, Ps > 0.05].
Figure 4.
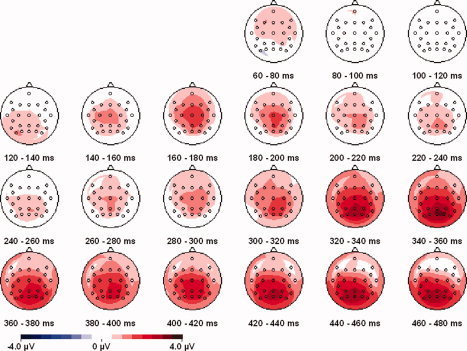
The phosphene effect. This is the difference in the mean amplitude of the ERP on phosphene‐present and phosphene‐absent trials during occipital TMS (the difference between the top two panels of Fig. 3). Effects reach statistical significance starting from 160 ms.
Effects of Stimulation Site on the TMS‐Evoked Potential
The second analysis compared the TEPs after TMS of the occipital site and the control parietal site, and only included phosphene‐absent trials, to avoid any confound with conscious phosphene perception. The control site was lateral to centro‐parietal electrode position CPz (see Materials and Methods and Fig. 2) and has not been reported to elicit phosphenes or motor twitches. Figure 5 shows the difference waveform obtained by subtracting the TEPs in response to control‐site TMS from TEPs following occipital TMS, for phosphene‐absent trials. An initial dipolar activity pattern over right posterior visual areas close to the occipital TMS location (right‐lateral positivity and central negativity) was apparent between 70 and 140 ms after TMS onset, although this differential ERP modulation was highly variable across participants and did not reach statistical significance (no effect of TMS site or interaction between site and electrode group: all Fs ≤ 0.7, all Ps > 0.4). At longer post‐TMS latencies, TEPs were more positive for occipital as compared to control site TMS, and this difference was broadly distributed. During the 160‐ to 200‐ms time window, the TEP after occipital TMS was more positive than the TEP following control site TMS [main effect of TMS site: F(1,11) = 8.4; P < 0.05] over a wide range of electrode locations [no interaction of TMS site and electrode group: F(2,22) = 0.5, P = 0.6]. In the later time window (280–400 ms), there were again effects of TMS site [F(1,11) = 46.2, P < 0.01], but in this case this effect differed across electrode groups [interaction between TMS site and electrode group: F(2,22) = 4.5, P < 0.05]. TMS site affected the ERP within every electrode group when analyzed separately [posterior group: F(1,11) = 68.8, P < 0.01, central group: F(1,11) = 21.3, P < 0.01, frontal group: F(1,11) = 15.5, P < 0.01] so that interaction (TMS site by electrode group) was driven by a difference in degree of the TMS site effect, with weaker effects frontally. To illustrate the time course and relative amplitude of these TEPs, Figure 6 shows TEPs for all trial conditions obtained at the posterior parieto‐occipital electrode POz where these late effects of phosphene perception and of TMS site were maximal.
Figure 5.
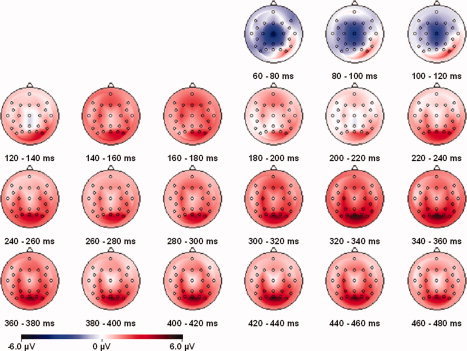
The topography of the difference between phosphene absent trials after occipital and control site TMS (active minus control: the difference between the lower two panels in Fig. 3).
Figure 6.
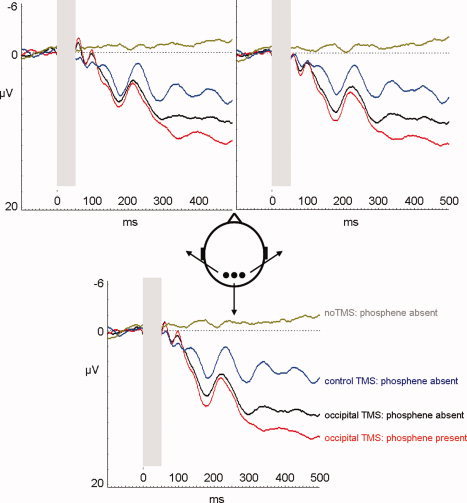
The time course of the phosphene effect in comparison to nonspecific TMS artifacts. This shows the ERPs from the electrodes nearest to the occipital TMS site (top left: PO3; top right: PO4; lower middle: POz; positive deflections plotted downwards). During occipital TMS, there is a relative positive deflection on phosphene‐present trials (red) starting at 160 ms. This effect is superimposed on the positive component, which is elicited by the somatosensory and acoustic stimulation that accompanies the TMS pulse and is present on all TMS trials. The gray‐shaded area shows the 50‐ms time window following the TMS pulse in which EEG recording is not possible.
DISCUSSION
Phosphene perception was linked to a differential TMS‐induced brain activation over central and posterior visual areas, with two positive components between 160 and 200 ms and 280 and 400 ms after TMS onset. This was shown by comparing the TEPs elicited during visual cortical stimulation at phosphene threshold intensity between trials where phosphenes were perceived and trials where no phosphenes were elicited. The relatively late onset and broad topography of this phosphene‐related potential, and the fact that electrical brain activity at earlier latencies was unrelated to phosphene perception, provide no support for the hypothesis that phosphenes are an immediate and local consequence of TMS‐induced activations of visual cortex, but are consistent with the “late” hypothesis.
Phosphene perception was linked to differential electrical activity that was measured over a broad area of parieto‐occipital, parietal, and central cortex. The effects immediately adjacent to the TMS coil were more short‐lived than those further away, and effects were generally earlier and more pronounced over the stimulated right hemisphere. The second positive wave (280–400 ms) was even more broadly distributed across posterior and central electrodes than the phosphene‐related potential measured between 160 and 200 ms after TMS onset. Even though the limited number of EEG recording sites used in this study precludes firm conclusions about the neural generator processes responsible for the phosphene‐related potential observed here, the overall topography of these effects suggests that the neural activation responsible started locally at the stimulated site and then spread to other areas. This greater spread of activation (even to the contralateral hemisphere) was then related to phosphene perception.
Comparing phosphene‐present and phosphene‐absent trials when stimulation parameters are constant controls for the somatosensory and acoustic artifacts accompanying TMS. On phosphene‐absent trials, TMS‐induced visual cortical activity was insufficient to induce phosphene perception. On phosphene‐present trials, TMS parameters were identical, but resulted in conscious phosphene perception. The TEP profiles on phosphene‐present and phosphene‐absent trials had a similar overall shape. Both included two discrete posterior components with a tendency toward right lateralization, with larger amplitudes on phosphene‐present trials. The general similarity of the morphology of phosphene‐absent and phosphene‐present TEPs and the link between TEP amplitudes and phosphene perception may point toward a threshold mechanism for the generation of conscious phosphene perception.
It has been suggested that phosphene perception can reflect transient changes in cortical excitability and that these changes can be detected even prior to TMS onset [Komssi and Kahkonen,2006; Romei et al.,2008a,b]. While such changes in excitability could have been linked to differences in brain activity between phosphene‐present and phosphene‐absent trials that are evident immediately after TMS onset, the early phase of the TEP in response to occipital TMS was unrelated to phosphene perception, with phosphene‐evoked activity only emerging 160–200 ms after TMS. This suggests that spontaneous changes in cortical excitability are not the sole factor involved in phosphene perception, or that their biasing effects on perceptual processing become effective only 160 ms after the initial TMS‐induced activation of visual cortex. This time course is consistent with a role of re‐entrant processes in phosphene perception. Recurrent loops of activation across subcortical or cortical regions would be one possible interpretation of the two discrete phases of phosphene‐related TEP modulations [Lamme and Roelfsema,2000; Pascual‐Leone and Walsh,2001]. It should however also be noted that the absence of ERP differences between phosphene‐present and phosphene‐absent trials in the first 160 ms after TMS onset does not necessarily imply that there was no differential neural activity at all during this time interval. Because the strength of EEG signals recorded at the scalp surface depends on the geometry and orientation of the underlying neural generator processes, it is possible in principle that some early phosphene‐related brain activity was present, but was not detected by EEG recordings.
Using a logic similar to the one employed here, a recent ERP study has investigated electrophysiological correlates of conscious visual perception in response to backward‐masked target stimuli presented at perceptual threshold [Del Cul et al.,2007]. An enhanced P3 component starting 270–300 ms after TMS was observed on trials where participants reported having seen the target, relative to trials where targets were not perceived. It is notable that in the present study, the first differential ERP effect that was linked to phosphene perception emerged ∼100 ms earlier than the awareness‐related P3 modulation observed by Del Cul et al. [2007]. Although it is difficult to directly compare a study where ERPs were triggered by external visual stimuli to a situation where electrical brain activity is elicited by TMS, this latency difference may reflect the different causal routes for conscious perception. Triggering phosphenes by directly stimulating occipital cortex obviously eliminates any involvement of prestriate stages of visual processing, such as the retinogeniculate pathway. If the activation of higher‐order visual areas is associated with the emergence of perceptual awareness, and if this activation requires a similar amount of time regardless of whether the initial occipital activation was produced as a consequence of retinal stimulation or directly via TMS, electrophysiological correlates of conscious perception should indeed be observed substantially earlier in response to occipital TMS than in response to external visual stimuli.
In addition to comparing the effects of TMS to occipital cortex with and without phosphenes, we were able to show that TMS to occipital cortex and to the control site (medial parietal cortex) both produced a large TEP consisting of an early central negativity (from 70 to 140 ms after the TMS pulse), a posterior positivity (160–200 ms), and a later and more broadly widespread positivity (280–400 ms), independently of phosphene perception (see Fig. 3). These TEPs are at least partly driven by that which is common to all conditions, namely, the somatosensory and acoustic stimulation that accompanied each TMS pulse. This site‐unspecific TEP profile is strikingly similar to the results of previous TMS‐EEG studies that have stimulated motor or frontal areas [Komssi and Kahkonen,2006; Nikouline et al.,1999], and which also reported strong positive central maxima at ∼175 ms after TMS, suggesting good replicability of nonspecific TMS‐induced ERP responses across participants, experimental paradigms, and stimulation/recording systems. However, there were also substantial differences between TEPs triggered in response to occipital versus control‐site TMS: electrodes nearest the control site showed a relative early negativity, whereas those nearest to the occipital site showed a later positivity, suggesting that TMS‐induced ERP modulations are not simply determined by the distance between the electrode and the TMS coil. TMS does not affect the electrical signal at adjacent electrodes in a uniform way, but rather depends on the properties of the stimulated cortex [Silvanto et al.,2008], suggesting that different areas will have different TEP profiles.
The current results not only show that TEPs triggered by occipital TMS are distinctive, but also that their modulation is linked to phosphene perception. Both observations may offer useful indices of visual connectivity for future studies of visual function and awareness. For example, the current demonstration of brain activity changes that are linked to phosphene perception could be extended in future work by testing whether, when and how phosphene‐related brain activity is influenced by top–down factors such as task set and spatial attention.
REFERENCES
- Antal A,Kincses TZ,Nitsche MA,Paulus W ( 2003): Manipulation of phosphene thresholds by transcranial direct current stimulation in man. Exp Brain Res 150: 375–378. [DOI] [PubMed] [Google Scholar]
- Cowey A,Walsh V ( 2000): Magnetically induced phosphenes in sighted, blind and blindsighted observers. Neuroreport 11: 3269–3273. [DOI] [PubMed] [Google Scholar]
- Del Cul A,Baillet S,Dehaene S ( 2007): Brain dynamics underlying the nonlinear threshold for access to consciousness. PLoS Biol 5: e260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driver J,Blankenburg F,Bestmann S,Vanduffel W,Ruff CC ( 2009): Concurrent brain‐stimulation and neuroimaging for studies of cognition. Trends Cogn Sci 13: 319–327. [DOI] [PubMed] [Google Scholar]
- Fuggetta G,Pavone EF,Walsh V,Kiss M,Eimer M ( 2006): Cortico‐cortical interactions in spatial attention: A combined ERP/TMS study. J Neurophysiol 95: 3277–3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gothe J,Brandt SA,Irlbacher K,Roricht S,Sabel BA,Meyer BU ( 2002): Changes in visual cortex excitability in blind subjects as demonstrated by transcranial magnetic stimulation. Brain 125( Pt 3): 479–490. [DOI] [PubMed] [Google Scholar]
- Grosbras MH,Paus T ( 2003): Transcranial magnetic stimulation of the human frontal eye field facilitates visual awareness. Eur J Neurosci 18: 3121–3126. [DOI] [PubMed] [Google Scholar]
- Hess CW,Mills KR,Murray NM ( 1987): Responses in small hand muscles from magnetic stimulation of the human brain. J Physiol 388: 397–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilmoniemi RJ,Virtanen J,Ruohonen J,Karhu J,Aronen HJ,Naatanen R,Katila T ( 1997): Neuronal responses to magnetic stimulation reveal cortical reactivity and connectivity. Neuroreport 8: 3537–3540. [DOI] [PubMed] [Google Scholar]
- Kamitani Y,Shimojo S ( 1999): Manifestation of scotomas created by transcranial magnetic stimulation of human visual cortex. Nat Neurosci 2: 767–771. [DOI] [PubMed] [Google Scholar]
- Kammer T,Beck S,Erb M,Grodd W ( 2001): The influence of current direction on phosphene thresholds evoked by transcranial magnetic stimulation. Clin Neurophysiol 112: 2015–2021. [DOI] [PubMed] [Google Scholar]
- Komssi S,Kahkonen S ( 2006): The novelty value of the combined use of electroencephalography and transcranial magnetic stimulation for neuroscience research. Brain Res Rev 52: 183–192. [DOI] [PubMed] [Google Scholar]
- Lamme VA,Roelfsema PR ( 2000): The distinct modes of vision offered by feedforward and recurrent processing. Trends Neurosci 23: 571–579. [DOI] [PubMed] [Google Scholar]
- Marzi CA,Mancini F,Savazzi S ( 2009): Interhemispheric transfer of phosphenes generated by occipital versus parietal transcranial magnetic stimulation. Exp Brain Res 192: 431–441. [DOI] [PubMed] [Google Scholar]
- Miniussi C,Thut G: Combining TMS and EEG offers new prospects in cognitive neuroscience. Brain Topogr (in press). [DOI] [PubMed] [Google Scholar]
- Nikouline V,Ruohonen J,Ilmoniemi RJ ( 1999): The role of the coil click in TMS assessed with simultaneous EEG. Clin Neurophysiol 110: 1325–1328. [DOI] [PubMed] [Google Scholar]
- Pascual‐Leone A,Walsh V ( 2001): Fast backprojections from the motion to the primary visual area necessary for visual awareness. Science 292: 510–512. [DOI] [PubMed] [Google Scholar]
- Rauschecker AM,Bestmann S,Walsh V,Thilo KV ( 2004): Phosphene threshold as a function of contrast of external visual stimuli. Exp Brain Res 157: 124–127. [DOI] [PubMed] [Google Scholar]
- Romei V,Brodbeck V,Michel C,Amedi A,Pascual‐Leone A,Thut G ( 2008a) Spontaneous fluctuations in posterior α‐band EEG activity reflect variability in excitability of human visual areas. Cereb Cortex 18: 2010–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romei V,Rihs T,Brodbeck V,Thut G ( 2008b) Resting electroencephalogram alpha‐power over posterior sites indexes baseline visual cortex excitability. Neuroreport 19: 203–208. [DOI] [PubMed] [Google Scholar]
- Silvanto J,Lavie N,Walsh V ( 2005): Double dissociation of V1 and V5/MT activity in visual awareness. Cereb Cortex 15: 1736–1741. [DOI] [PubMed] [Google Scholar]
- Silvanto J,Lavie N,Walsh V ( 2006): Stimulation of the human frontal eye fields modulates sensitivity of extrastriate visual cortex. J Neurophysiol 96: 941–945. [DOI] [PubMed] [Google Scholar]
- Silvanto J,Muggleton N,Walsh V ( 2008): State‐dependency in brain stimulation studies of perception and cognition. Trends Cogn Sci 12: 447–454. [DOI] [PubMed] [Google Scholar]
- Silvanto J,Muggleton N,Lavie N,Walsh V ( 2009): The perceptual and functional consequences of parietal top–down modulation on the visual cortex. Cereb Cortex 19: 327–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart LM,Walsh V,Rothwell JC ( 2001): Motor and phosphene thresholds: A transcranial magnetic stimulation correlation study. Neuropsychologia 39: 415–419. [DOI] [PubMed] [Google Scholar]
- Taylor PC,Walsh V,Eimer M ( 2008): Combining TMS and EEG to study cognitive function and cortico–cortico interactions. Behav Brain Res 191: 141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veniero D,Bortoletto M,Miniussi C ( 2009): TMS‐EEG co‐registration: On TMS‐induced artifact. Clin Neurophysiol 120: 1392–1399. [DOI] [PubMed] [Google Scholar]
- Walsh V,Pascual‐Leone A ( 2003): Transcranial Magnetic Stimulation: A Neurochromometrics of Mind. Cambridge: MIT Press. [Google Scholar]


