Abstract
Objective
This study tested the hypothesis that preterm infants who had a blood gas derangement on at least 2 of the first 3 postnatal days are at increased risk for more severe retinopathy of prematurity (ROP).
Method
1,042 infants born before 28 weeks’ gestational age (GA) were included. An infant was considered to be exposed if his/her blood gas measure was in the highest or lowest quartile for GA on at least 2 of the first 3 postnatal days.
Results
Multivariable models adjusting for confounders indicate that exposure to a PCO2 in the highest quartile predicts ROP (stage 3, 4 or 5: OR = 1.6, 95% CI = 1.1–2.3); zone 1: 2.0, 1.1–3.6; prethreshold/threshold: 1.9, 1.2–3.0; plus disease: 1.8, 1.1–2.9). Estimates are similar for a low pH for zone 1 (2.1, 1.2–3.8), prethreshold/threshold (1.8, 1.1–2.8), but did not quite achieve statistical significance for ROP stage 3, 4, or 5 (1.4, 0.9–2.0) and plus disease (1.5, 0.9–2.4). A PaO2 in the highest quartile for GA on at least 2 of the first 3 postnatal days was associated with a doubling of the risk of ROP in zone 1 (2.5, 1.4–4.4) and of prethreshold/threshold disease (2.1, 1.4–3.3), a 70% risk increase for plus disease (1.7, 1.04–2.8), while a 40% risk increase for ROP stage 3 or higher did not achieve statistical significance (1.4, 0.96–2.0).
Conclusion
Infants exposed to high PCO2, low pH and high PaO2 appear to be at increased risk of more severe ROP.
Key Words: Retinopathy of prematurity, Hypercapnia, Hyperoxemia, Acidemia, Extremely low gestational age
Introduction
More than half a century ago, prolonged exposure to high oxygen concentration was identified as an important contributor to retrolental fibroplasia, the potentially blinding disorder now known as retinopathy of prematurity (ROP) [1, 2]. Despite efforts to reduce oxygen exposure, ROP remains a common disorder among low gestational age (GA) newborns [3,4,5].
As neonatologists try to achieve blood oxygen concentrations that minimize risks of damage to brain, lung and retina, controversy continues about how low blood oxygen levels can/should be without damaging any organ [6,7,8,9]. In this report, we explore the relationships between blood oxygen, carbon dioxide, and pH levels during the first 3 postnatal days and severe ROP in a contemporary cohort.
Methods
The ELGAN Study was designed to identify characteristics and exposures that increase the risk of structural and functional neurologic disorders in extremely low gestational age newborns (ELGANs) [10,11,12,13,14,15,16]. During the years 2002–2004, women delivering before 28 weeks’ gestation at one of 14 participating institutions in 11 cities in 5 states were asked to enroll in the study. The enrollment and consent processes were approved by the individual institutional review boards.
Mothers were approached for consent either upon antenatal admission or shortly after delivery, depending on clinical circumstance and institutional preference. 1,249 mothers of 1,506 infants consented. A total of 464 of these infants either died, did not have blood gas assessments on at least 2 of the first 3 postnatal days, or did not have a retinal examination. The remaining 1,042 infants compose the sample for this report.
Demographic and Pregnancy Variables
After the infant's delivery, a trained research nurse interviewed each mother in her native language using a structured data collection and following procedures contained in a manual. Shortly after the mother's discharge, the research nurse reviewed the maternal chart using a second structured data collection form. The medical record was relied on for events following admission. The clinical circumstances that led to each maternal admission and ultimately to each preterm delivery were operationally defined using both data from the maternal interview and data abstracted from the medical record [14].
Newborn Variables
GA estimates were based on a hierarchy of the quality of available information. Most desirable were estimates based on the dates of embryo retrieval or intrauterine insemination or fetal ultrasound before the 14th week (62%). When these were not available, reliance was placed sequentially on a fetal ultrasound at 14 or more weeks (29%), last menstrual period without fetal ultrasound (7%), and GA recorded in the log of the neonatal intensive care unit (1%). The birth weight Z-score is the number of standard deviations the infant's birth weight is above or below the median weight of infants at the same GA in a standard dataset [17].
Placentas
Placentas were placed in a sterile examination basin and transported to a sampling room. 82% of the samples were obtained within 1 h of delivery. The microbiologic and histologic procedures are described elsewhere in detail [18, 19].
Blood Gases
On postnatal days 1, 2, and 3, we collected information about the lowest, modal, and highest PaO2, PCO2, and pH. Only arterial blood was used for oxygen measurements (indicated by the ‘a’ in PaO2). Measurements of PCO2 and pH were arterial except for 7 ELGANs on day 1, 15 on day 2, and 60 infants on day 3, all of whom had these measurements made on venous blood. We calculated quartiles of PCO2 and pH separately for arterial and venous measures, assigned a child's measure to the upper quartile based on whether her measure was arterial or venous, and combined the resulting dichotomous values.
In our sample, the blood gas measurement that defined the highest and lowest quartile varied by GA and by postnatal day (table 1). We therefore classified infants by whether or not their extreme value each day was in the extreme quartile for their GA (23–24, 25–26, and 27 weeks). Because an extreme measure on 1 day could reflect a transient event, we required that an infant be in the extreme quartile on at least 2 of the 3 days to be considered ‘exposed’ to such extremes.
Table 1.
The values that define the highest and lowest quartiles of each blood gas displayed on the left on each day in each gestational age group
| Blood gas | Postnatal day | Gestational age, weeks |
||
|---|---|---|---|---|
| 23-24 | 25-26 | 27 | ||
| Lowest quartile | 1 | 40 | 42 | 40 |
| PaO2, mm Hg | 2 | 43 | 45 | 44 |
| 3 | 43 | 45 | 43 | |
| Highest quartile | 1 | 152 | 145 | 143 |
| PaO2, mm Hg | 2 | 98 | 100 | 92 |
| 3 | 103 | 95 | 93 | |
| Lowest quartile | 1 | 27 | 29 | 29 |
| PCO2 mm Hg | 2 | 33 | 35 | 35 |
| 3 | 33 | 33 | 35 | |
| Highest quartile | 1 | 65 | 67 | 63 |
| PCO2> mm Hg | 2 | 60 | 64 | 60 |
| 3 | 58 | 57 | 56 | |
| Lowest quartile pH | 1 | 7.15 | 7.20 | 7.22 |
| 2 | 7.14 | 7.17 | 7.22 | |
| 3 | 7.16 | 7.19 | 7.22 | |
Our not having blood gas measurements on all 3 of the first 3 postnatal days might be an example of informative missingness. In essence, children who did not have a blood gas on postnatal day 3 were more likely to be physiologically stable than infants who had a set of blood gas measurements that day. Children who did not have day 3 measurements are also much less likely than others to have postnatal day 2 measurements that are in any extreme quartile. We, therefore, considered it reasonable to assign these newborns to the group with non-extreme measurements on postnatal day 3. This allows us to include these children in this sample, and thereby avoids our inflating odds ratios inappropriately.
We collected the minimum and maximum blood gas values each day. Because we cannot tell if extreme pH and PCO2 measurements are paired, we did not calculate base excess. We did not require any blood gases to be taken as per protocol. All measurements were taken at the discretion of the attending physician. Thus, there is no standardized frequency of blood gas measurements in this study.
Eye Examinations
Participating ophthalmologists helped prepare a manual and data collection form, and then participated in efforts to minimize observer variability. Definitions of terms were those accepted by the International Committee for Classification of ROP [20].
ELGAN ophthalmologists used indirect ophthalmoscopy. Only one of our sites used RetCam with any frequency. In keeping with guidelines [21], the first ophthalmologic examination was within the 31st to 33rd postmenstrual week. In accordance with follow-up examination guidelines, infants at high risk of ROP were examined weekly, while those with no ROP present at first examination were re-examined every 2–3 weeks. Photographs were encouraged in case of doubt about classification. We did not include information about treatment because Early Treatment for ROP (ETROP) guidelines, including suggested policy changes, were released during the recruitment phase [22].
Data Analysis
The generalized form of the null hypothesis we evaluated is that children who have a blood gas in an extreme quartile on 2 of the first 3 postnatal days are not at higher risk of severe ROP than children who did not have that blood gas extreme on 2 of the first 3 postnatal days. We evaluated five blood gas extremes (lowest quartile of PaO2, highest quartile of PaO2, lowest quartile of PCO2, highest quartile of PCO2, lowest quartile of pH) in models that included the three of the other four blood gas extremes as well as other confounders. Confounders were selected by their association with a blood gas extreme and stage 3 or higher ROP, biologic plausibility or previous identification in the literature (table 2). All conditional logistic multivariable models included a hospital cluster term (to account for the possibility that infants born at a particular hospital are more like each other than like infants born at other hospitals) [23]. The contributions of relevant variables are presented as risk ratios with 95% confidence intervals.
Table 2.
Distribution of ROP stage 3, 4 or 5 and infants who had a blood gas extreme (defined as a Pa02, PC02, or pH in the highest or lowest quartile for gestational age on at least 2 of the first 3 postnatal days) listed at the top of each column on within strata of potential confounders, listed on the left (these are row percents)
| Potential confounder | Blood gas extreme |
||||||
|---|---|---|---|---|---|---|---|
| lowest PaO2 | highest PaO2 | lowest PCO2 | highest PCO2 | lowest pH | ROP stage 3 or higher | Row n | |
| Pre-pregnancy Body Mass Index | |||||||
| <18.5 | 22 | 19 | 25 | 28 | 28 | 30 | 67 |
| ≥18.5, <25 | 24 | 19 | 22 | 22 | 20 | 30 | 514 |
| ≥25, <30 | 18 | 27 | 21 | 18 | 21 | 32 | 204 |
| ≥30 | 22 | 22 | 23 | 24 | 25 | 35 | 207 |
| Aspirin | |||||||
| Yes | 32 | 15 | 23 | 23 | 25 | 49 | 43 |
| No | 21 | 22 | 22 | 22 | 22 | 30 | 955 |
| Highest WBC1 | |||||||
| >20,000 | 26 | 21 | 19 | 21 | 16 | 38 | 210 |
| ≤20,000 | 21 | 21 | 23 | 22 | 23 | 30 | 812 |
| Initiator of delivery | |||||||
| Preeclampsia | 26 | 28 | 22 | 32 | 28 | 38 | 138 |
| Fetal indication | 38 | 18 | 11 | 31 | 36 | 36 | 45 |
| Spontaneous | 21 | 20 | 23 | 20 | 20 | 31 | 857 |
| Decidual hemorrhage/fibrin deposition | |||||||
| Yes | 20 | 14 | 20 | 23 | 22 | 37 | 158 |
| No | 22 | 22 | 22 | 21 | 21 | 31 | 797 |
| Gestational age | |||||||
| 23-24 weeks | 21 | 20 | 22 | 24 | 23 | 51 | 255 |
| 25-26 weeks | 22 | 21 | 22 | 20 | 20 | 33 | 488 |
| 27 weeks | 22 | 22 | 22 | 23 | 23 | 13 | 299 |
| Birth weight Z-score2 | |||||||
| <-2 | 23 | 22 | 25 | 27 | 39 | 45 | 64 |
| ≥-2, <-l | 28 | 27 | 25 | 33 | 28 | 41 | 150 |
| ≥-l | 21 | 20 | 21 | 20 | 19 | 29 | 828 |
| Overall row percent | 22 | 21 | 22 | 22 | 22 | 32 | |
| Maximum column number | 227 | 221 | 230 | 229 | 225 | 331 | 1,042 |
White blood cell count (WBC) within the interval before delivery to 48 h post-delivery
Birth weight Z-scores based on standard of Yudkin et al. [17].
In this sample, children who had a PCO2 in the highest quartile tended to have a pH in the lowest quartile more commonly than expected if their co-occurrence was independent of the other (data not shown). As expected, models with both of these variables provided evidence that these two variables shared discriminating information. Consequently, we present two multivariable models for each ROP classification. Model 1 includes a variable for high PCO2, but not for low pH. Model 2, on the other hand, has a variable for low pH, but not for high PCO2.
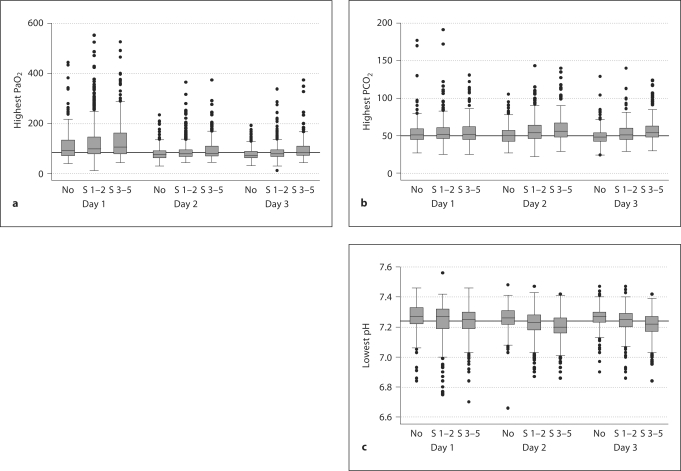
We summarize some of our data with box-and-whisker displays of the central tendency and dispersion of blood gases in ROP groups (fig. 1). The central tendency is indicated by the line close to the middle of the box, which is the median, and by the top and bottom of each box, which indicate the 25th and 75th centiles. The dispersion of blood gases is indicated by the length of the vertical lines that emanate from the box, as well as by the black dots, which identify outliers.
Fig. 1.
Box-and-whisker plots of highest PaO 2 (a), highest PCO 2 (b), and lowest pH (c) on each postnatal day of infants with no ROP, stage 1-2 ROP, and stage 3-5 ROP. The median is indicated by the line closest to the middle of the box, while the top and bottom of the box indicate the 25th and 75th centiles. The dispersion of blood gases is indicated by the length of the vertical lines emanating from each box, as well as the black dots, which identify outliers.
Results
On each postnatal day, we observed an upward trend for highest oxygen and carbon dioxide with increasing ROP severity (fig. 1). Infants with more severe ROP had lower pH levels on each postnatal day.
Identification of Confounders (table 2)
We created table 2 to identify confounders, those variables associated with a blood gas extreme, and with stage 3 or higher ROP. Our comments about the tables are limited to potential confounders.
Maternal consumption of aspirin was associated with both high and low extremes of PaO2 as well as with stage 3 or higher ROP. Infants whose mother presented with preeclampsia were at increased risk of hypercapnia and severe (i.e. stages 3, 4 and 5) ROP, while newborns delivered for fetal indications were at increased risk of hypoxemia, hypercapnia and acidemia, as well as severe ROP.
Decidual hemorrhage or fibrin deposition was associated with a small risk increase for severe ROP, but not with blood gas extremes.
Newborns in the lowest birth weight Z-score category (i.e. <–2) were at the highest risk of acidemia and stage 3 or higher ROP. Although the risk of severe ROP increased dramatically with progressively lower GA, blood gas extremes did not (largely because we defined the boundaries for blood gas extremes within GA groups) (see table 1).
We did not see an effect of sex on ROP risk or on the association between blood gases and ROP.
ROP Severity Classifications and Blood Gases (table 3)
Table 3.
Percentages of children classified by their ROP status (on the left) who satisfied the blood gas criterion at the top of each column (these are row percents)
| ROP severity | Blood gas extreme |
|||||
|---|---|---|---|---|---|---|
| lowest quartile PaO2 | highest quartile PaO2 | lowest quartile PCO2 | highest quartile PCO2 | lowest quartile pH | row n | |
| Stage 3, 4, or 5 | ||||||
| Yes | 20 | 27 | 24 | 26 | 25 | 331 |
| No | 23 | 19 | 21 | 20 | 20 | 711 |
| Zone 1 | ||||||
| Yes | 20 | 37 | 19 | 30 | 35 | 89 |
| No | 22 | 20 | 22 | 21 | 20 | 953 |
| Prethreshold or thre | :shold | |||||
| Yes | 25 | 31 | 21 | 33 | 32 | 174 |
| No | 21 | 19 | 22 | 20 | 20 | 868 |
| Plus disease | ||||||
| Yes | 28 | 28 | 22 | 36 | 31 | 134 |
| No | 21 | 20 | 22 | 20 | 20 | 908 |
| Overall | 22 | 21 | 22 | 22 | 22 | |
| Column number | 227 | 221 | 230 | 229 | 225 | 1,042 |
Infants with stage 3 or higher ROP were more likely than their peers to be exposed to a high PaO2. Further, infants with plus disease were more likely than their peers to be exposed to a high PCO2or low pH. Stratifying newborns by the presence/absence of zone 1 or prethreshold/threshold disease resulted in even more prominent differences in hyperoxemia frequency, as well as prominent differences in frequencies of hypercapnia and acidemia. This increased difference is probably due to the fact that ‘stage 3 or higher’ includes ROP in all zones, thereby diluting the effect.
Multivariable Analyses (table 4)
Table 4.
Odds ratios1 (and 95% CI) of the association between blood gas extremes (defined as a blood gas measure in the highest or lowest quartile for gestational age) and the risk of ROP (the referent group for each set of analyses consists of all children who did not have the ROP classification listed at the top of each set of columns)
| Blood gas | Stage 3, 4, or 5 |
Zone 1 |
||
|---|---|---|---|---|
| model 1 | model 2 | model 1 | model 2 | |
| Lowest PaO2 | 0.9 (0.6,1.4) | 1.0 (0.7,1.5) | 0.6(0.3,1.1) | 0.6 (0.3, 1.2) |
| Highest PaO2 | 1.4(0.95,2.0) | 1.4(0.96,2.0) | 2.5(1.5,4.5) | 2.5(1.4,4.4) |
| Lowest PCO2 | 0.8 (0.6, 1.2) | 0.8 (0.6, 1.2) | 0.7(0.4,1.5) | 0.7(0.4,1.4) |
| Highest PCO2 | 1.6(1.1,2.3) | – | 2.0(1.1,3.6) | – |
| Lowest pH | – | 1.4(0.9,2.0) | – | 2.1 (1.2,3.8) |
| Blood gas | Prethreshold or threshold |
Plus disease |
||
|---|---|---|---|---|
| model 1 | model 2 | model 1 | model 2 | |
| Lowest PaO2 | 1.1 (0.7,1.7) | 1.1 (0.7,1.8) | 1.4(0.9,2.3) | 1.5(0.9,2.4) |
| Highest PaO2 | 2.1 (1.3,3.3) | 2.1 (1.4,3.3) | 1.7(1.02,2.7) | 1.7(1.04,2.8) |
| Lowest PCO2 | 0.8 (0.5, 1.3) | 0.8 (0.5, 1.3) | 0.8 (0.5, 1.4) | 0.8 (0.5, 1.4) |
| Highest PCO2 | 1.9(1.2,3.0) | – | 1.8(1.1,2.9) | – |
| Lowest pH | – | 1.8(1.1,2.8) | – | 1.5(0.9,2.4) |
All models are adjusted for maternal Body Mass Index >30, maternal use of aspirin during pregnancy, WBC >20,000 within 48 h of delivery, delivery for preeclampsia or fetal indication, decidual hemorrhage/fibrin deposition in placenta, gestational age (23-24,25-26,27 weeks) and birth weight Z-score <-1, definite early sepsis, definite late sepsis and all of the other blood gas extremes in the model. These models include a hospital cluster term to account for the possibility that infants born at a particular hospital are more like each other than like infants born at other hospitals.
After adjustment for confounders, our multivariable models indicated several significant relationships between blood gas extremes and ROP, which were more prominent when ROP was dichotomized by zone or prethreshold/threshold than by stage. First, higher arterial O2 levels were significantly associated with zone 1, pre- and threshold ROP, and the presence of plus disease, while the association with stage 3 or higher disease did not reach statistical significance (both models). Second, high PCO2 levels were invariably significantly associated with increased risk of ROP stage 3 or higher, zone 1, prethreshold and threshold ROP and the presence of plus disease (model 1). Third, very similar effects were present with exposure to low pH (model 2).
Discussion
This is the first large-scale epidemiologic study of the relationship between blood gas extremes and ROP risk in ELGANs. Our main finding is that high blood concentrations ofcarbon dioxide and oxygen, and low blood pH in the first 3 postnatal days are significantly associated with an increased risk of severe ROP. These relationships remain statistically significant after adjustment for each of the other blood gases, as well as non-blood gas confounders.
Low GA and low birth weight are the strongest risk factors for ROP [3,4]. Other known antecedents include low insulin-like growth factor 1 [24], varying vascular endothelial growth factor levels [25], systemic infection [26], inflammation [27], and genetic predisposition [28,29]. Intraventricular hemorrhage appears to be associated with ROP, probably via shared risk factors [30, 31].
Hypercarbia and acidosis increase retinal neovascularization in animal models [32, 33]. The presumed sequence is that hypercarbia promotes vessel dilation, which leads to increased oxygenation and increased blood flow, which in turn contributes to abnormal retinal vascularization [5].
Prior smaller clinical studies yielded inconsistent results [34, 35]. In a study of 91 mechanically-ventilated infants of mean GA 29 weeks, a measurement of hypercarbia or hypocarbia on the first 3 postnatal days was not associated with an increased risk of ROP [35]. The authors suggested that cumulative exposure integrated over time might be a better way to capture potentially adverse events. Similar ‘negative’ results were found in another underpowered study, of 25 infants less than 30 weeks GA, in which neither carbon dioxide tension nor duration of hypo-/hypercarbia correlated with ROP [36]. Investigators of both studies suggested further investigation in larger cohorts.
In contrast to the above studies, we defined exposure as a blood gas measure in the highest or lowest quartile for GA on 2 of the first 3 postnatal days, rather than using a mean value or an upper limit. In this way, we are better able to perform risk analyses, while internallyadjusting for GA.
In our study the infants exposed to PCO2 values in the highest quartile for their GA experienced carbon dioxide levels that exceed those defined as hypercarbia in previous studies [35]. In contrast, we found that a pH in the lowest quartile was associated with an increased risk of severe ROP.
Finally, we found that exposure to higher arterial oxygen levels in the first 3 postnatal days is associated with more severe ROP. This is consistent with previous studies and current clinical practice advocating the benefits of lower blood oxygen saturation during the first few weeks of life [37, 38]. Further, lowering oxygen alarm set-points appears to lead to a decreased risk of severe ROP according to a structured formal review performed by our own group [39], and in recently published studies of policy changes [40,41,42,43]. This is currently the topic of ongoing randomized trials.
Our study included 1,042 ELGANs, of whom 800 (77%) developed stage 1 or higher ROP, 331 (32%) developed stages 3–5 ROP, 89 (9%) developed zone 1 ROP, and 174 (17%) developed prethreshold/threshold disease. Compared to previous studies with samples of fewer than 100 infants, this is an appreciable increase in sample size, and an appreciable increase in power. While most prior studies did not fully address confounding variables, we created multivariable models adjusting for a host of confounders. Consequently, our findings have the potential to improve our understanding of the relationship between blood gas extremes and severity of ROP.
The weaknesses of our study are those of all observational studies. Further, we assume that a blood gas derangement on 2 of the first 3 postnatal days constitutes an ‘exposure’. We used this operationalized definition since we had no access to continuous blood gas measurements. Thus, we did not see any way of modeling fluctuations of blood gases in a consistent and thus meaningful fashion. Finally, we are unable to differentiate between respiratory and metabolic acidosis, a clinically relevant distinction that might deserve further investigation.
In light of the biologic plausibility of our findings, we suggest that blood-gas-associated ROP risk be considered in discussions of clinical practice. While oxygen saturation has dominated most of the recent ROP literature, the role of hypercarbia and acidosis in severe ROP may warrant further clinical consideration and study. This is especially important in light of recommendations about the safety and efficacy of ‘permissive hypercarbia’ [44]. Our findings, however, justify concerns raised in current discussions [45].
Conclusion
In summary, we found that repeatedly high blood concentrations of oxygen and carbon dioxide and low blood pH are associated with increased risk of severe ROP. Further studies might help better characterize the relationships of oxygenation, ventilation and acidosis with risk of ROP development in ELGANs.
Acknowledgements
This research was supported by a cooperative agreement with the National Institute of Neurological Disorders and Stroke (5U01NS040069-05), a grant from the National Eye Institute (1R21EY019253-01), a program project grant from the National Institute of Child Health and Human Development (NIH-P30-HD-18655), a Yale University School of Medicine Student Research Fellowship, and the Richard Saltonstall Charitable Foundation.
References
- 1.Campbell K. Intensive oxygen therapy as a possible cause of retrolental fibroplasia: a clinical approach. Med J Aust. 1951;2:48–50. [PubMed] [Google Scholar]
- 2.Patz A, Hoeck LE, De La Cruz E. Studies on the effect of high oxygen administration in retrolental fibroplasia. I. Nursery observations. Am J Ophthalmol. 1952;35:1248–1253. doi: 10.1016/0002-9394(52)91140-9. [DOI] [PubMed] [Google Scholar]
- 3.Gibson DL, Sheps SB, Uh SH, Schechter MT, McCormick AQ. Retinopathy of prematurity-induced blindness: birth weight-specific survival and the new epidemic. Pediatrics. 1990;86:405–412. [PubMed] [Google Scholar]
- 4.Palmer EA, Flynn JT, Hardy RJ, et al. Incidence and early course of retinopathy of prematurity. The Cryotherapy for Retinopathy of Prematurity Cooperative Group. Ophthalmology. 1991;98:1628–1640. doi: 10.1016/s0161-6420(91)32074-8. [DOI] [PubMed] [Google Scholar]
- 5.McColm JR, Fleck BW. Retinopathy of prematurity: causation. Semin Neonatol. 2001;6:453–460. doi: 10.1053/siny.2001.0079. [DOI] [PubMed] [Google Scholar]
- 6.Cole CH, Wright KW, Tarnow-Mordi W, Phelps DL, Pulse Oximetry Saturation Trial for Prevention of Retinopathy of Prematurity Planning Study Group Resolving our uncertainty about oxygen therapy. Pediatrics. 2003;112:1415–1419. doi: 10.1542/peds.112.6.1415. [DOI] [PubMed] [Google Scholar]
- 7.Anderson CG, Benitz WE, Madan A. Retinopathy of prematurity and pulse oximetry: a national survey of recent practices. J Perinatol. 2004;24:164–168. doi: 10.1038/sj.jp.7211067. [DOI] [PubMed] [Google Scholar]
- 8.Wright KW, Sami D, Thompson L, Ramanathan R, Joseph R, Farzavandi S. A physiologic reduced oxygen protocol decreases the incidence of threshold retinopathy of prematurity. Trans Am Ophthalmol Soc. 2006;104:78–84. [PMC free article] [PubMed] [Google Scholar]
- 9.Vento M, Moro M, Escrig R, et al. Preterm resuscitation with low oxygen causes less oxidative stress, inflammation, and chronic lung disease. Pediatrics. 2009;124:e439–e449. doi: 10.1542/peds.2009-0434. [DOI] [PubMed] [Google Scholar]
- 10.Kuban K, Adler I, Allred EN, et al. Observer variability assessing US scans of the preterm brain: the ELGAN Study. Pediatr Radiol. 2007;37:1201–1208. doi: 10.1007/s00247-007-0605-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laughon M, Bose C, Allred E, et al. Factors associated with treatment for hypotension in extremely low gestational age newborns during the first postnatal week. Pediatrics. 2007;119:273–280. doi: 10.1542/peds.2006-1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Shea TM, Kuban KC, Allred EN, et al. Neonatal cranial ultrasound lesions and developmental delays at 2 years of age among extremely low gestational age children. Pediatrics. 2008;122:e662–e669. doi: 10.1542/peds.2008-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuban KC, Allred EN, O'Shea M, et al. An algorithm for identifying and classifying cerebral palsy in young children. J Pediatr. 2008;153:466–472. doi: 10.1016/j.jpeds.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McElrath TF, Hecht JL, Dammann O, et al. Pregnancy disorders that lead to delivery before the 28th week of gestation: an epidemiologic approach to classification. Am J Epidemiol. 2008;168:980–989. doi: 10.1093/aje/kwn202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laughon M, Allred EN, Bose C, et al. Patterns of respiratory disease during the first two postnatal weeks in extremely premature infants. Pediatrics. 2009;123:1124–1131. doi: 10.1542/peds.2008-0862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olomu IN, Hecht JL, Onderdonk AO, Allred EN, Leviton A, Extremely Low Gestational Age Newborn Study Investigators Perinatal correlates of Ureaplasma urealyticum in placenta parenchyma of singleton pregnancies that end before 28 weeks of gestation. Pediatrics. 2009;123:1329–1336. doi: 10.1542/peds.2008-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yudkin PL, Aboualfa M, Eyre JA, Redman CW, Wilkinson AR. New birthweight and head circumference centiles for gestational ages 24 to 42 weeks. Early Hum Dev. 1987;15:45–52. doi: 10.1016/0378-3782(87)90099-5. [DOI] [PubMed] [Google Scholar]
- 18.Onderdonk AB, Hecht JL, McElrath TF, et al. Colonization of second-trimester placenta parenchyma. Am J Obstet Gynecol. 2008;199:52.e1–52.e10. doi: 10.1016/j.ajog.2007.11.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hecht J, Allred EN, Kliman H, et al. Histologic characteristics of singleton placentas delivered before the 28th week of gestation. Pathology. 2008;40:372–376. doi: 10.1080/00313020802035865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The International Committee for the Classification of the Late Stages of Retinopathy of Prematurity An international classification of retinopathy of prematurity. II. The classification of retinal detachment. Arch Ophthalmol. 1987;105:906–912. [PubMed] [Google Scholar]
- 21.American Academy of Pediatrics Section on Ophthalmology: Screening examination of premature infants for retinopathy of prematurity. Pediatrics. 2001;108:809–811. doi: 10.1542/peds.108.3.809. [DOI] [PubMed] [Google Scholar]
- 22.Early Treatment for Retinopathy of Prematurity Cooperative Group Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol. 2003;121:1684–1694. doi: 10.1001/archopht.121.12.1684. [DOI] [PubMed] [Google Scholar]
- 23.Begg MD, Parides MK. Separation of individual-level and cluster-level covariate effects in regression analysis of correlated data. Stat Med. 2003;22:2591–2602. doi: 10.1002/sim.1524. [DOI] [PubMed] [Google Scholar]
- 24.Hellstrom A, Engstrom E, Hard AL, et al. Postnatal serum insulin-like growth factor I deficiency is associated with retinopathy of prematurity and other complications of premature birth. Pediatrics. 2003;112:1016–1020. doi: 10.1542/peds.112.5.1016. [DOI] [PubMed] [Google Scholar]
- 25.Young TL, Anthony DC, Pierce E, Foley E, Smith LE. Histopathology and vascular endothelial growth factor in untreated and diode laser-treated retinopathy of prematurity. J AAPOS. 1997;1:105–110. doi: 10.1016/s1091-8531(97)90008-2. [DOI] [PubMed] [Google Scholar]
- 26.Bharwani SK, Dhanireddy R. Systemic fungal infection is associated with the development of retinopathy of prematurity in very low birth weight infants: a meta-review. J Perinatol. 2008;28:61–66. doi: 10.1038/sj.jp.7211878. [DOI] [PubMed] [Google Scholar]
- 27.Dammann O, Brinkhaus MJ, Bartels DB, et al. Immaturity, perinatal inflammation, and retinopathy of prematurity: a multi-hit hypothesis. Early Hum Dev. 2009;85:325–329. doi: 10.1016/j.earlhumdev.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 28.Bizzarro MJ, Hussain N, Jonsson B, et al. Genetic susceptibility to retinopathy of prematurity. Pediatrics. 2006;118:1858–1863. doi: 10.1542/peds.2006-1088. [DOI] [PubMed] [Google Scholar]
- 29.Mohamed S, Schaa K, Cooper ME, et al. Genetic contributions to the development of retinopathy of prematurity. Pediatr Res. 2009;65:193–197. doi: 10.1203/PDR.0b013e31818d1dbd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Procianoy RS, Garcia-Prats JA, Hittner HM, et al. An association between retinopathy of prematurity and intraventricular hemorrhage in very low birthweight infants. Acta Paediatr Scand. 1981;70:473–477. doi: 10.1111/j.1651-2227.1981.tb05725.x. [DOI] [PubMed] [Google Scholar]
- 31.Lad EM, Nguyen TC, Morton JM, Moshfeghi DM. Retinopathy of prematurity in the United States. Br J Ophthalmol. 2008;92:320–325. doi: 10.1136/bjo.2007.126201. [DOI] [PubMed] [Google Scholar]
- 32.Holmes JM, Zhang S, Leske DA, Lanier WL. Carbon dioxide-induced retinopathy in the neonatal rat. Curr Eye Res. 1998;17:608–616. [PubMed] [Google Scholar]
- 33.Zhang S, Leske DA, Lanier WL, Berkowitz BA, Holmes JM. Preretinal neovascularization associated with acetazolamide-induced systemic acidosis in the neonatal rat. Invest Ophthalmol Vis Sci. 2001;42:1066–1071. [PubMed] [Google Scholar]
- 34.Saito Y, Omoto T, Cho Y, Hatsukawa Y, Fujimura M, Takeuchi T. The progression of retinopathy of prematurity and fluctuation in blood gas tension. Graefes Arch Clin Exp Ophthalmol. 1993;231:151–156. doi: 10.1007/BF00920938. [DOI] [PubMed] [Google Scholar]
- 35.Liao SL, Lai SH, Kuo CY. Effect of carbon dioxide tension in the first three days of life on the development of retinopathy of prematurity. Chang Gung Med J. 2000;23:755–760. [PubMed] [Google Scholar]
- 36.Gellen B, McIntosh N, McColm JR, Fleck BW. Is the partial pressure of carbon dioxide in the blood related to the development of retinopathy of prematurity? Br J Ophthalmol. 2001;85:1044–1045. doi: 10.1136/bjo.85.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tin W. Oxygen therapy: 50 years of uncertainty. Pediatrics. 2002;110:615–616. doi: 10.1542/peds.110.3.615. [DOI] [PubMed] [Google Scholar]
- 38.Higgins RD, Bancalari E, Willinger M, Raju TN. Executive summary of the workshop on oxygen in neonatal therapies: controversies and opportunities for research. Pediatrics. 2007;119:790–796. doi: 10.1542/peds.2006-2200. [DOI] [PubMed] [Google Scholar]
- 39.Chen ML, Guo L, Dammann CE, Dammann O. High or low oxygen saturation and severe retinopathy of prematurity: a meta-analysis. Pediatrics. 2010;125:e1483–e1492. doi: 10.1542/peds.2009-2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chow LC, Wright KW, Sola A, CSMC Oxygen Administration Study Group Can changes in clinical practice decrease the incidence of severe retinopathy of prematurity in very low birth weight infants? Pediatrics. 2003;111:339–345. doi: 10.1542/peds.111.2.339. [DOI] [PubMed] [Google Scholar]
- 41.Vanderveen DK, Mansfield TA, Eichenwald EC. Lower oxygen saturation alarm limits decrease the severity of retinopathy of prematurity. J AAPOS. 2006;10:445–448. doi: 10.1016/j.jaapos.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 42.Sears JE, Pietz J, Sonnie C, Dolcini D, Hoppe G. A change in oxygen supplementation can decrease the incidence of retinopathy of prematurity. Ophthalmology. 2009;116:513–518. doi: 10.1016/j.ophtha.2008.09.051. [DOI] [PubMed] [Google Scholar]
- 43.Finer N, Leone T. Oxygen saturation monitoring for the preterm infant: the evidence basis for current practice. Pediatr Res. 2009;65:375–380. doi: 10.1203/PDR.0b013e318199386a. [DOI] [PubMed] [Google Scholar]
- 44.Miller JD, Carlo WA. Safety and effectiveness of permissive hypercapnia in the preterm infant. Curr Opin Pediatr. 2007;19:142–144. doi: 10.1097/MOP.0b013e3280895e12. [DOI] [PubMed] [Google Scholar]
- 45.Jankov RP, Tanswell AK. Hypercapnia and the neonate. Acta Paediatr. 2008;97:1502–1509. doi: 10.1111/j.1651-2227.2008.00933.x. [DOI] [PubMed] [Google Scholar]