Abstract
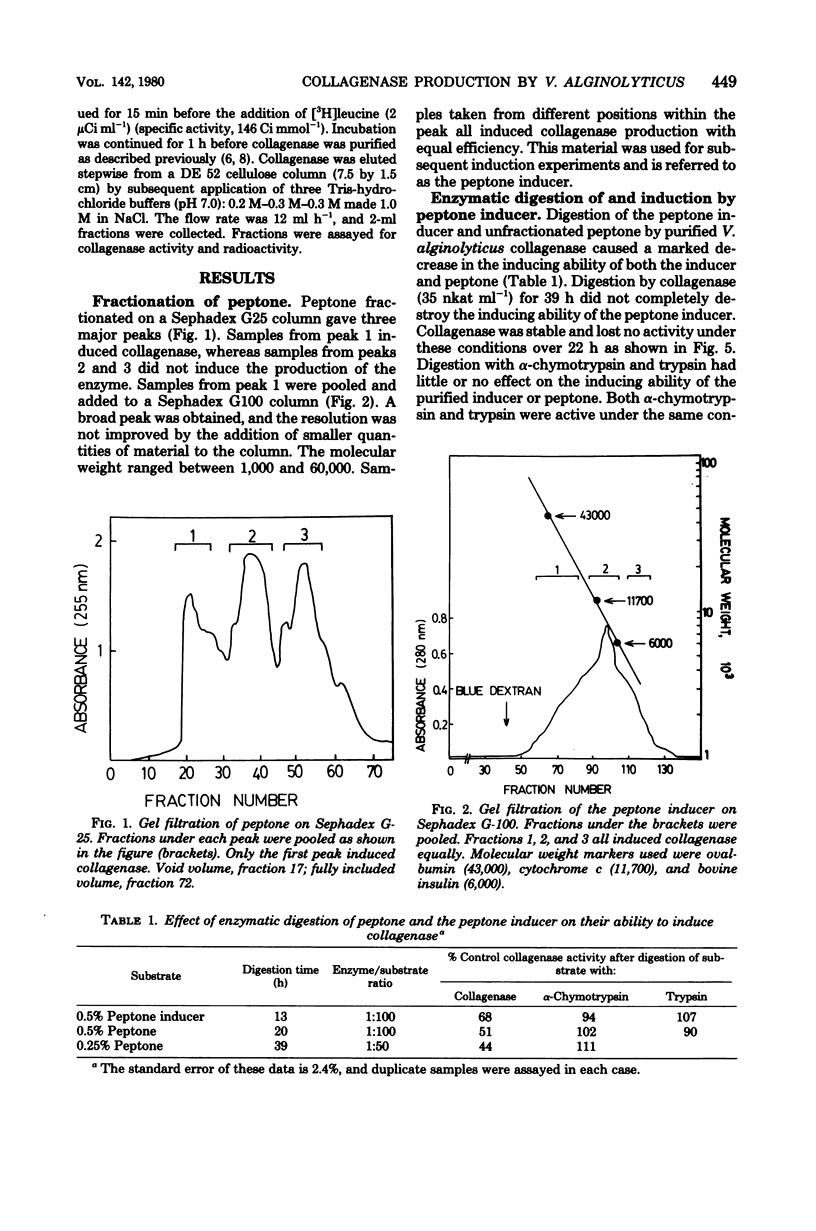
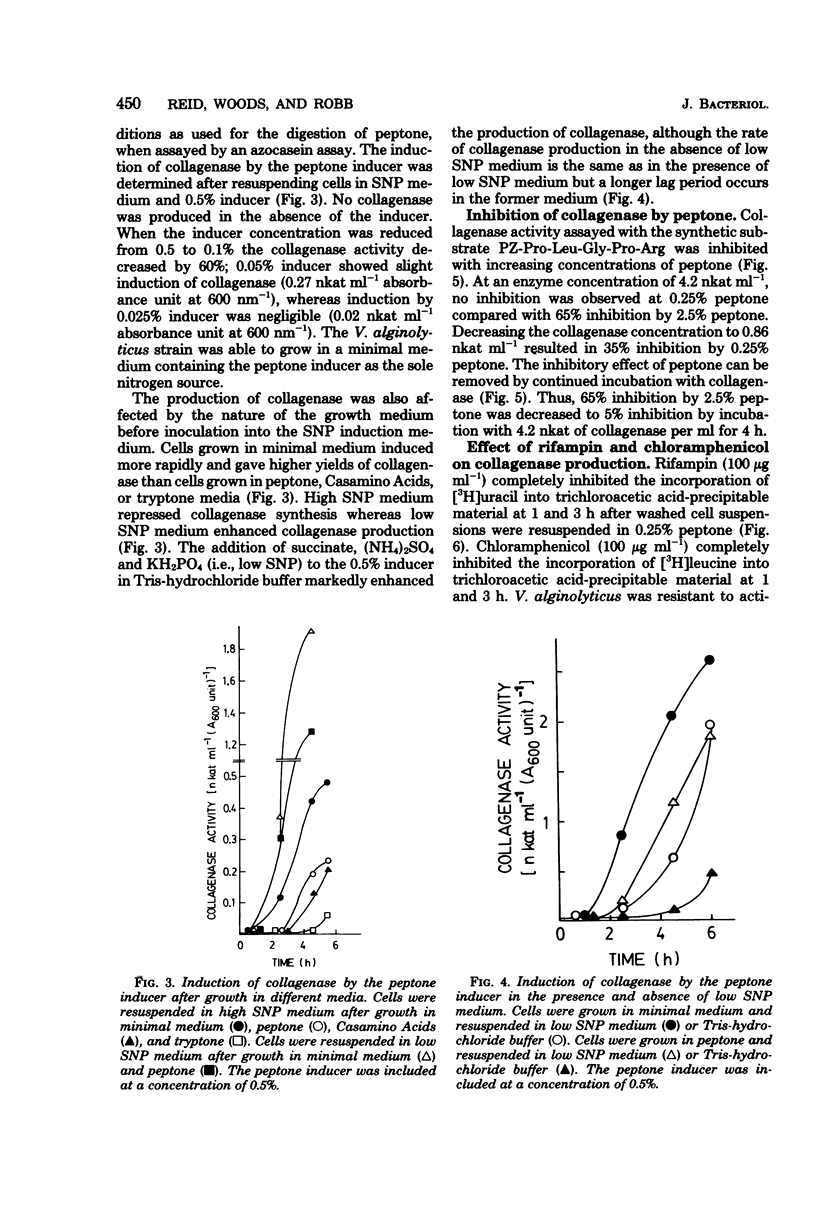
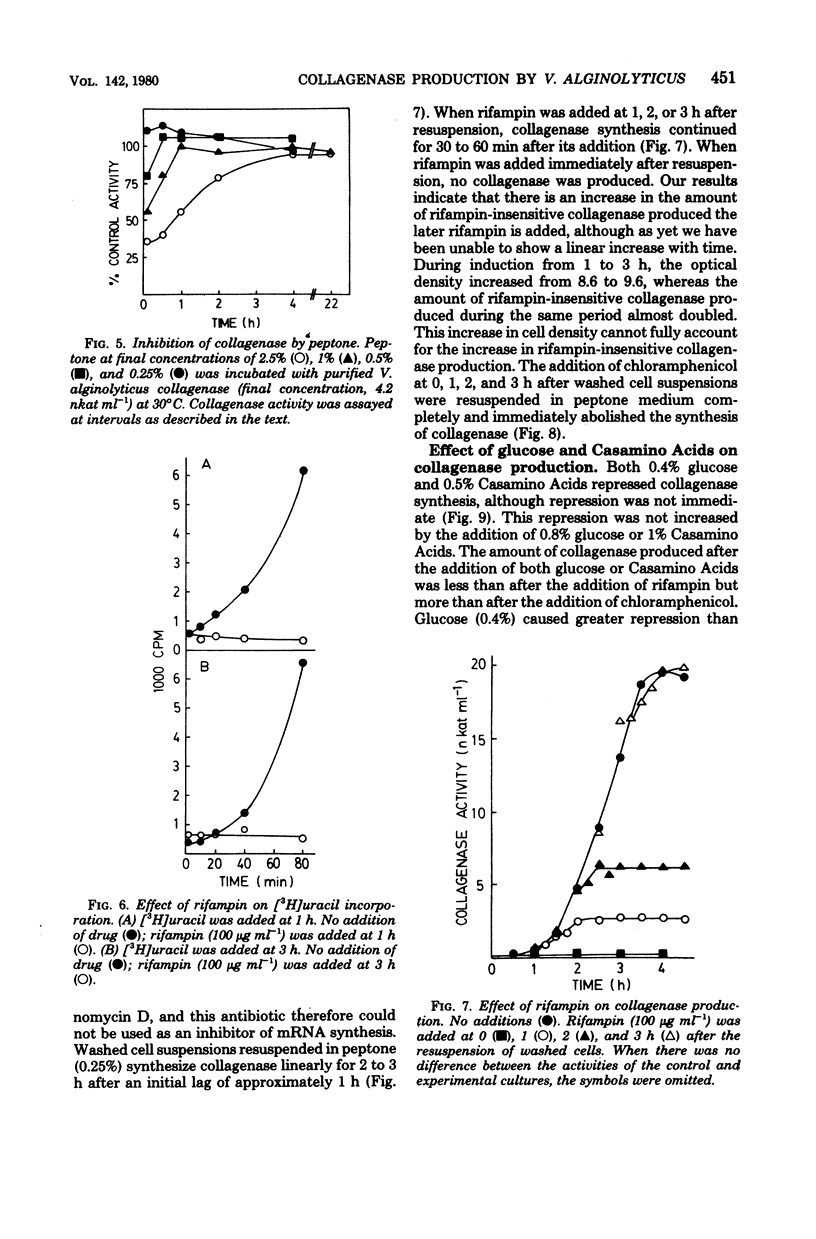
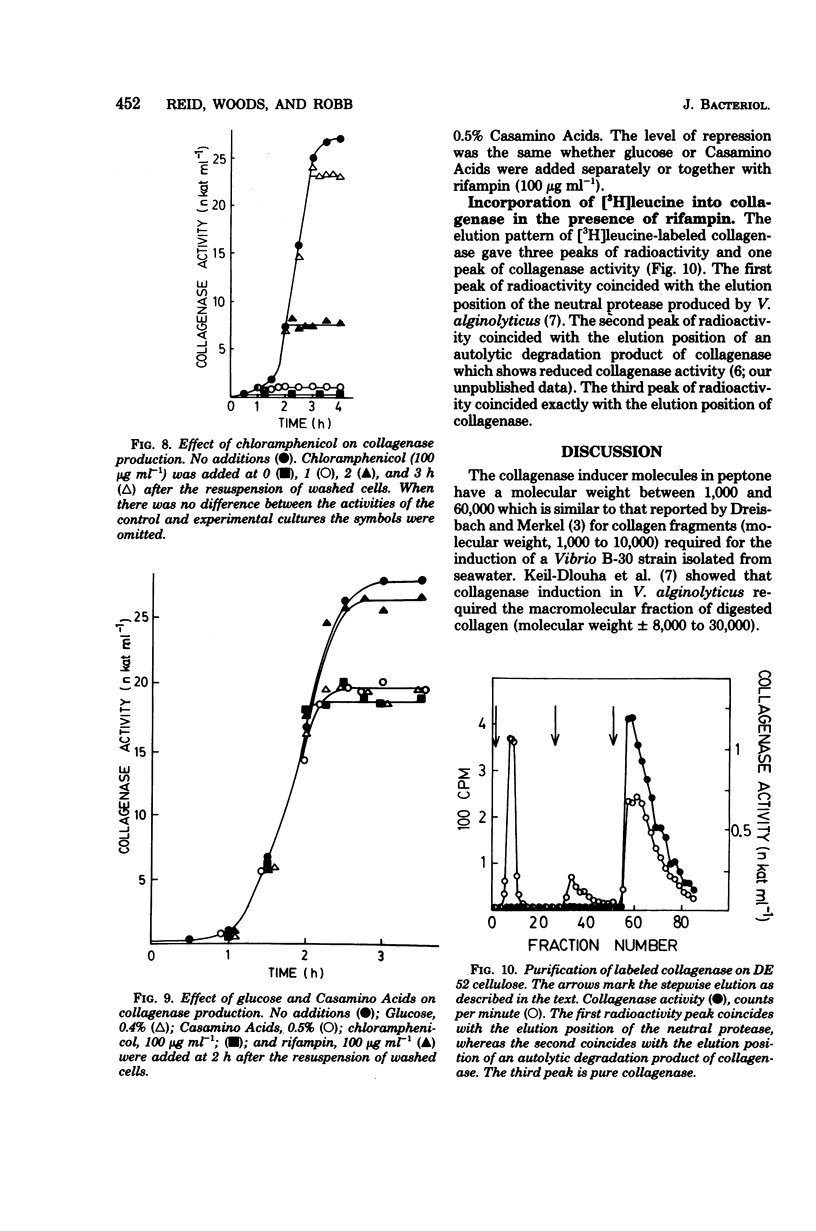
Vibrio alginolyticus produces an extracellular collagenase which requires specific induction by collagen or its high-molecular-weight fragments. Peptone also induces collagenase during the late exponential and early stationary growth phases. The peptone inducers have been shown to have a broad molecular weight range between 1,000 and 60,000. The peptone inducers supported slow growth of V. alginolyticus when supplied as the sole nitrogen source in minimal medium. Digestion of the peptone inducers with purified V. alginolyticus collagenase resulted in a decrease in their inducing ability, whereas digestion with trypsin or α-chymotrypsin did not. This indicated that induction by the inducers required the presence of collagenase-sensitive bonds. Prolonged digestion of the inducers with collagenase did not completely eliminate the inducing ability of the inducers. The peptone inducers acted as inhibitors of collagenase. A minimal medium induction system has been developed which involves resuspending cells at high density in a medium containing succinate, (NH4)2SO4, KH2PO4, and the peptone inducer. Cells grown in minimal medium induce earlier than cells grown on peptone, Casamino Acids, or tryptone. Collagenase production was shown to occur for 30 to 60 min in the presence of rifampin at levels which completely inhibit the incorporation of [3H]uracil into trichloroacetic acid-precipitable material. Chloramphenicol completely and immediately abolished collagenase production, which together with labeling studies has confirmed that collagenase production involves de novo synthesis of the enzyme. Both glucose and Casamino Acids repressed collagenase production, although synthesis of the enzyme continued for 30 to 60 min after their addition. The repression of collagenase production by glucose and Casamino Acids was more severe than the inhibition of enzyme formation due to addition of rifampin.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boethling R. S. Regulation of extracellular protease secretion in Pseudomonas maltophilia. J Bacteriol. 1975 Sep;123(3):954–961. doi: 10.1128/jb.123.3.954-961.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Both G. W., McInnes J. L., Hanlon J. E., May B. K., Elliott W. H. Evidence for an accumulation of messenger RNA specific for extracellular protease and its relevance to the mechanism of enzyme secretion in bacteria. J Mol Biol. 1972 Jun 20;67(2):199–217. doi: 10.1016/0022-2836(72)90236-7. [DOI] [PubMed] [Google Scholar]
- Dreisbach J. H., Merkel J. R. Induction of collagenase production in Vibrio B-30. J Bacteriol. 1978 Aug;135(2):521–527. doi: 10.1128/jb.135.2.521-527.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn A. R. Production of extracellular proteins by bacteria. Annu Rev Microbiol. 1976;30:41–62. doi: 10.1146/annurev.mi.30.100176.000353. [DOI] [PubMed] [Google Scholar]
- Keil-Dlouha V. Chemical characterization and study of the autodigestion of pure collagenase from Achromobacter iophagus. Biochim Biophys Acta. 1976 Mar 11;429(1):239–251. doi: 10.1016/0005-2744(76)90047-4. [DOI] [PubMed] [Google Scholar]
- Lecroisey A., Keil-Dlouha V., Woods D. R., Perrin D., Keil B. Purification, stability and inhibition of the collagenase from Achromobacter iophagus. FEBS Lett. 1975 Nov 15;59(2):167–172. doi: 10.1016/0014-5793(75)80367-x. [DOI] [PubMed] [Google Scholar]
- O'Connor R., Elliott W. H., May B. K. Modulation of an apparent mRNA pool for extracellular protease in Bacillus amyloliquefaciens. J Bacteriol. 1978 Oct;136(1):24–34. doi: 10.1128/jb.136.1.24-34.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priest F. G. Extracellular enzyme synthesis in the genus Bacillus. Bacteriol Rev. 1977 Sep;41(3):711–753. doi: 10.1128/br.41.3.711-753.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priest F. G. Typist: effect of glucose and cyclic nucleotides on the transcription of alpha-amylase mRHA in Bacillus subtilis. Biochem Biophys Res Commun. 1975 Apr 7;63(3):606–610. doi: 10.1016/s0006-291x(75)80427-x. [DOI] [PubMed] [Google Scholar]
- Semets E. V., Glenn A. R., May B. K., Elliott W. H. Accumulation of messenger ribonucleic acid specific for extracellular protease in Bacillus subtilis 168. J Bacteriol. 1973 Nov;116(2):531–534. doi: 10.1128/jb.116.2.531-534.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinson M. W., Merrick J. M. Extracellular enzyme secretion by Pseudomonas lemoignei. J Bacteriol. 1974 Jul;119(1):152–161. doi: 10.1128/jb.119.1.152-161.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WUENSCH E., HEIDRICH H. G. ZUR QUANTITATIVEN BESTIMMUNG DER KOLLAGENASE. Hoppe Seylers Z Physiol Chem. 1963;333:149–151. doi: 10.1515/bchm2.1963.333.1.149. [DOI] [PubMed] [Google Scholar]
- Welton R. L., Woods D. R. Collagenase production by Achromobacter iophagus. Biochim Biophys Acta. 1975 Mar 28;384(1):228–234. doi: 10.1016/0005-2744(75)90111-4. [DOI] [PubMed] [Google Scholar]
- Welton R. L., Woods D. R. Halotolerant collagenolytic activity of Achromobacter iophagus. J Gen Microbiol. 1973 Mar;75(1):191–196. doi: 10.1099/00221287-75-1-191. [DOI] [PubMed] [Google Scholar]



