Abstract
External ventricular drains (EVD) are associated with a high infection rate. Early detection of infection is frequently problematic due to a lack of clinical signs and the time period required for culturing. Bacterial biofilms have been suggested to play an important role in the infection of EVD, but direct evidence is as yet lacking. We report the case of a 17- year-old male with Dandy-Walker malformation who presented with headache, nausea and drowsiness; a CT scan revealed enlarged ventricles. The patient had a history of ventriculoperitoneal shunt revision 3 weeks prior to admission. The shunt was removed on suspicion of infection and an EVD placed. Daily surveillance cultures through the EVD were negative and the EVD was replaced on day 5. Examination of the initial EVD by confocal microscopy demonstrated clear intraluminal biofilm formation; molecular analysis by PCR identified Staphylococcus aureus resident on the catheter. To our knowledge, this is the first direct demonstration of an intraluminal biofilm compromising an EVD. Despite the presence of biofilm on this catheter, the patient demonstrated no clinical signs of infection, and the routine surveillance culture was negative. Undetected biofilm may pose a latent risk on EVD and other neurosurgical catheters.
Key Words: Biofilm, Confocal microscopy, External ventricular drain, Staphylococcus, Ventriculoperitoneal shunt
Introduction
External ventricular drains (EVD) are employed for temporary intracranial pressure monitoring and drainage of cerebrospinal fluid (CSF). A potentially life-threatening complication of EVD placement is ventriculitis resulting from microbial infection of these devices. Studies have reported EVD-related infection rates ranging from 5 to 20%, with Staphylococcus epidermidis and Staphylococcus aureus being the most common pathogens [1]. In a prospective study of 1,333 patients with EVD or lumbar drains, 26 patients (2%) developed meningitis, with coagulase-negative staphylococci (CoNS) presenting as the main pathogen (56%) followed by S. aureus (25%) [2]. A retrospective study of 595 patients with ventricular catheters reported that the infection rate increased significantly over the first 4 days before reaching a plateau with an overall 8.6% incidence [3]. The early diagnosis of EVD-related infection is confounded by the absence of a correlation between clear clinical signs such as inflammation [1], fever and Glasgow Coma Scale score [4].
Bacterial Biofilms
Bacterial biofilms are communities of bacteria which attach to and grow on the surface of abiotic materials as well as host tissues [5,6]. The bacteria live within an extracellular polymeric slime matrix which protects them from phagocytic cells [7]. Biofilm infections are often difficult to detect using conventional culturing methods and difficult to treat using conventional antibiotic therapy [8]. Bacterial biofilm formation is a recognized contributor to multiple scenarios of chronic or recurrent infection, particularly those associated with foreign bodies, prompting several groups to hypothesize that the high infection rate in neurosurgical devices might be biofilm-related [9,10,11,12,13]. The reduction in infection rates from 9.4 to 1.3% when antibiotic-impregnated EVD catheters were used [13] has been suggested to derive from an antibiofilm effect. Biofilm formation by CoNS to explain infections of ventriculoperitoneal (VP) shunts has been indirectly inferred on isolates that were positive for slime formation (by Congo red agar assay, PCR identification of the icaA gene) and an ability to form biofilms in vitro [10,11,14]. Biofilms have been reported in other neurosurgical devices as well: Walsh et al. [15] used scanning electron microscopy (SEM) to identify a fungal biofilm formed by Cryptococcus neoformans on a ventriculoatrial shunt. Davis et al. [16] used confocal microscopy and SEM to show a Coccidioides immitis fungal biofilm (identified by culture) in VP shunt tubing as the presumed cause of persistent meningitis, and Fux et al. [17] used SEM to demonstrate biofilms in VP shunts from 3 patients. In this last report, identification of bacterial species in the biofilm was attempted by PCR and sequencing of bacterial 16S rDNA. Although all 3 specimens were culture positive, only 1 of the 3 was positive by PCR. The authors attributed this to a possible interference due to prior formalin fixation.
In vitro studies clearly demonstrated that biofilms can form on neurosurgical materials [18]; however, there has not as yet been any direct demonstration of biofilms on a clinically explanted EVD. We have developed a combination of viability staining by confocal microscopy and RT-PCR to demonstrate the presence of metabolically active staphylococcal biofilms in orthopedic joint infections, eliminating the reliance on culture, and also to demonstrate that otitis media with effusion is a biofilm disease [6,19,20]. In this report, we describe a case where we have employed micrographic and molecular techniques to identify a staphylococcal biofilm partially occluding the lumen of an EVD from a patient suffering from Dandy-Walker syndrome and with a long history of VP shunt failure despite repeated negative cultures.
Case Report
Presentation and Patient History
The patient was a 17-year-old white male who had suffered intraventricular hemorrhage as a premature infant and had been diagnosed with Dandy-Walker malformation. The patient had already undergone 31 prior surgeries for VP shunt revisions or replacements, the most recent of which had occurred just 3 weeks prior to his presentation. He now presented with headache, nausea and drowsiness, and a CT scan revealed enlarged ventricles. Although 5-day cultures of CSF performed at the time of his recent VP revision were negative (no growth), an infectious etiology for the shunt failure was suspected due to the temporal proximity of the previous revision surgery. The VP shunt was accordingly removed and an EVD placed for daily surveillance (with marked improvement in the patient's symptoms).
Over the ensuing 5 days, daily cultures of CSF collected via the EVD showed no growth and the patient remained afebrile. No antibiotics were administered at this time. The EVD was removed on the 5th day and specimens were sterilely collected in the operating room; some material was sent for standard microbiological culture, and the remaining sections of the EVD for micrographic and molecular analysis were placed aseptically in sterile phosphate buffer salts (PBS) or sterile RNAlater® (Ambion) and maintained at 4°C. A portion of the tip and a subcutaneous (tunneled) segment of the explanted catheter were positive for CoNS in broth only after 5 days of culture. Minimal inhibitory concentrations could not be determined due to the fastidious nature of the isolate.
At the time of explantation of the initial EVD, a second EVD was placed to allow for continued drainage while awaiting final culture results. When the positive broth culture results were discovered, the patient was placed on intravenous nafcillin for 2 weeks. The CSF cultures from this second EVD taken on days 5, 6 and 22 were negative (no growth). The EVD was therefore removed and another VP shunt device placed, which remained in place for 9 months.
Specimens were collected and analyzed with the patient's consent and institutional review board approval.
Confocal Laser Scanning Microscopy and Viability Staining
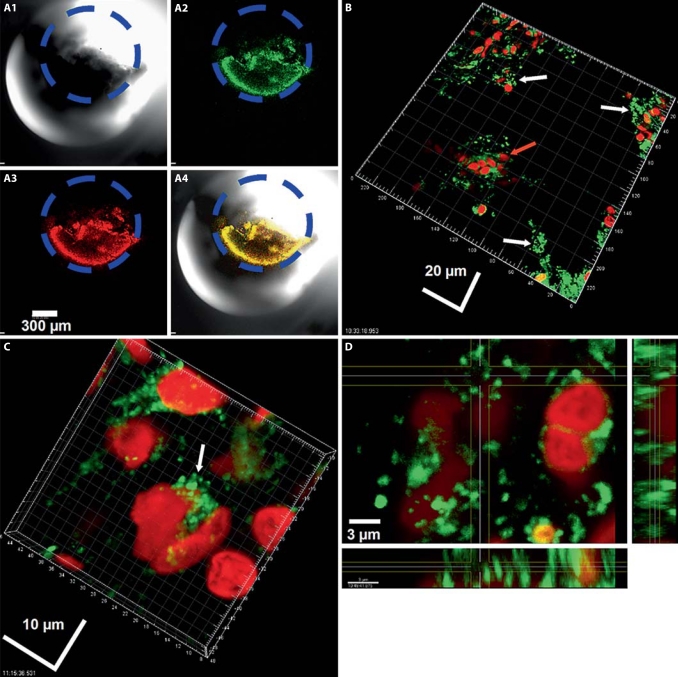
A 3-mm-long section was cut from the midpart of the EVD and placed on a 100-mm Petri dish using Lubriseal stopcock grease (Thomas Scientific) for immobilization. The EVD was stained using a BacLight Live/Dead kit (Molecular Probes, Eugene, Oreg., USA) by drop-pipetting the stain directly into the lumen at the manufacturer's recommended concentration. The Petri dish was closed and the specimens were incubated for 15 min in the dark at room temperature. The sample was kept moist by the inclusion of a water-soaked tissue in the Petri dish during incubation. After incubation, excess stain was rinsed away by flooding the plate with PBS, then aspirated. Finally, the specimen was submerged in PBS before microscopic examination using a Leica DM RXE upright microscope attached to a TCS SP2 AOBS confocal system (Leica Microsystem, Exton, Pa., USA); this allowed visualization of the specimen in a fully hydrated state. The specimen was examined using either a ×10 air objective or a ×63 long-working-distance water immersion objective. The 488-nm line of the Kr/AG laser was used as the excitation wavelength and the detector wavelength windows set such that the ‘live’ stain (SYTO 9) appeared green and the ‘dead’ stain (propidium iodide) appeared red. The wall of the catheter was visualized using transmitted microscopy. There was a ‘deposit’ within the lumen of the EVD which stained with both live and dead stains. The deposit occluded approximately two thirds of the luminal cross-sectional area in this section of the EVD (fig. 1A). Higher magnification demonstrated that the deposit contained live clusters of cocci in biofilm configuration, interspersed with host cells (fig. 1B–D). Host nuclei and fibrous material were stained red with propidium iodide. The distribution of biofilm was patchy, with some sites displaying large bacterial aggregations, while other regions were devoid of microorganisms.
Fig. 1.
Confocal images of the catheter lumen stained by Live/Dead kit. A Low-power image showing occlusion of the lumen. Dashed gray circle, blue circle online: approximate circumference of the catheter lumen. A1 Transmitted image. A2 SYTO 9 ‘live’ green staining. A3 Propidium iodide red staining. A4 Overlay. B Higher magnification showing that the material occluding the catheter consisted of biofilm cell clusters of live cocci (white arrows) interspersed with host cells (gray arrow, red arrow online: representative cell). It should be noted that the BacLight Live/Dead kit cannot be used as an indicator of the viability of human cells since even viable human cells rapidly take up propidium iodide and the nuclei turn from green to red within minutes of staining. C Even higher magnification showing clumps of single cocci and clumps of cocci (arrow). D Plane view (main panel) and sagittal sections through a biofilm cluster (panel left; panel below). The cocci were presumed to be held together by the extracellular polymeric slime matrix, which was not stained by nucleic acids and thus was invisible in these micrographs.
Nucleic Acid Isolation and PCR
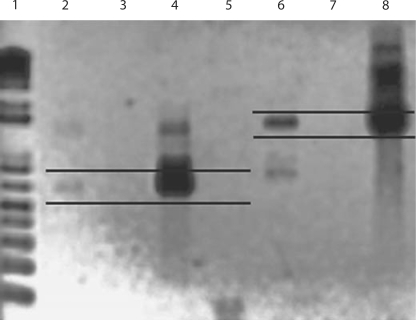
Two sections of explanted EVD were transferred to sterile microfuge tubes containing RNAlater and stored at 4°C prior to extraction. Isolation of nucleic acids from these specimens was carried out using a hot phenol extraction protocol as previously described [19,20]. To identify S. aureus, we used the Sau327/Sau1645 primer set for 23S rDNA [21] and the Sau562/Sau1155 primer set for the hutH housekeeping gene. PCR reactions were performed as described [19,20]. Amplimer products were visualized on 1% agarose gels stained with ethidium bromide (fig. 2). PCR analysis of the nucleic acids demonstrated the presence of S. aureus DNA in 1 of the 2 sections assayed. Amplimers of the expected molecular weight were detected, using both 23S rDNA and hutH primer sets, in catheter section 1 but not in section 2 (fig. 2), signifying that S. aureus bacteria were present in the former but not the latter. These results are congruent with the patchy and inconsistent nature of the biofilm observed by confocal laser scanning microscopy. S. epidermidis-specific primers tested against the same catheter-extracted DNA resulted in no amplification, signifying the absence of S. epidermidis in these samples (not shown).
Fig. 2.
Agarose gel electrophoresis of PCR amplimers. Lanes 2 and 6 were from DNA extracted directly from 1 catheter section (section 1); lanes 3 and 7 were extracted from a second section (section 2) taken from the same catheter. Lanes 2, 3, 4 and 5 are amplimers from the S. aureus hutH primer set Sau562/Sau1155; lanes 6, 7 and 8 are from the S. aureus 23S Sau327/Sau1645 primer set. Parallel black lines: expected amplicon size for the 2 primer sets. Catheter section 1 demonstrated amplimers of the expected molecular weight for both primer sets, indicating a positive identification for S. aureus. However, catheter section 2 was negative. Lanes 4 and 8 were the positive controls from DNA extracted from S. aureus ATCC No. 25923. DNA extracted from S. epidermidis ATCC No. 35984 was used as a negative control against the hutH primer set in lane 5; the absence of any amplimer demonstrates that the primer set tested is actually S. aureus-specific. Lane 1 is a ladder of molecular weight markers using the Track-It™ 1 Kb Plus DNA Ladder (Invitrogen, Carlsbad, Calif., USA).
Discussion
To our knowledge, this case represents the first demonstration that an EVD can provide an abiotic surface for biofilm formation, that a substantial biofilm can develop even in the lumen of the device and, importantly, that the presence of a biofilm infection can remain undetected by standard surveillance culture of CSF drawn via the EVD device. In this case, routine surveillance cultures performed daily were uniformly negative, and only the final broth culture on the explanted EVD was positive at 5 days. In fact, the organism isolated by broth culture (CoNS) was not consistent with the organisms identified by PCR within the catheter (S. aureus), and it is not certain whether the CoNS was indeed present on the catheter or may represent an artifactual or contaminating finding. Given the direct micrographic and molecular evidence for the presence of S. aureus within the catheter, this case highlights the inadequacy of the routine culture of sampled CSF as a reliable surveillance tool. There are multiple possible reasons for the culture insensitivity of biofilm bacteria. Although CSF withdrawn from the EVD was necessarily passing over the biofilm, sufficient bacteria may not have been detaching from the biofilm foci so as to reach the level of detectability by culture. It is also possible that the biofilm in this case was producing slow-growing ‘small colony variants’. In orthopedic infections, the presence of such staphylococcal small colony variants has been identified as a possible explanation for the high rate of false negatives associated with conventional culture techniques [22].
It remains unclear whether the explanted VP shunt that necessitated the placement of this EVD was itself infected since it was not retained for confocal or molecular evaluation. It is possible that bacteria shed from the VP shunt colonized and propagated within the EVD; indeed, given that no other cause for the shunt failure was immediately apparent, this would seem to be the most parsimonious explanation, but direct evidence is lacking. The absence of culture positivity from the explanted VP shunt does not exclude a biofilm resident on the shunt, which would echo the culture negativity of the EVD despite a clearly demonstrable biofilm therein.
In this patient, fortuitously, a positive late culture finding at the time of EVD removal (even though it may have been artifactual) prompted treatment with intravenous nafcillin prior to placement of yet another VP shunt (during which time a replacement EVD was in place). This new VP shunt remained in place and functional for some 9 months. It appears, therefore, that the antibiosis administered may have been effective in inhibiting transmission of the biofilm bacteria to the new foreign body. Any biofilm that had formed on the (replacement) EVD may have been removed with the drain, and any bacteria shed in the CSF may have proven more susceptible to antibiotics in planktonic form.
Our findings in this case largely fulfill the Parsek-Singh criteria for the diagnosis of biofilm infection. Briefly, these include: (1) the presence of pathogenic bacteria associated with a surface, (2) the presence of bacteria aggregated in cell clusters, and (3) the infection being localized, with dissemination being a rare event [5,8]. Given that the patient exhibited no meningeal signs while the infected EVD was in place, the infection can be considered to have remained localized to the EVD and clinically silent although the potential for acute exacerbation and dissemination was present. A fourth criterion, that the biofilm infection may be impossible to eradicate by antibiosis, is untested in this case since the foreign body hosting the biofilm (the infected EVD) was explanted, thereby physically removing the nidus of infection, while any planktonic bacteria shed by the biofilm would likely prove more susceptible to antibiosis. Both micrographic and molecular analyses in this instance showed that a clinical biofilm need not be uniform; thus, failure to detect bacteria from clinical specimens may be due to sampling error as well as other factors (e.g. simple physical dislodgment of an attached biofilm in the process of removing the device or tissue).
The direct documentation of infection within the catheter despite culture negativity has important clinical implications and especially highlights the need for more reliable diagnostic measures that can offer better clinical guidance. Confocal microscopy is unlikely to become a standard diagnostic in its present format: the preparation and examination of specimens are labor-intensive and can only be performed after a shunt or drain has been removed. However, the PCR-based detection of nucleic acids from pathogens in the CSF offers the ability to detect minute quantities of biofilm bacteria despite their low cultivability and deserves further evaluation. One report comparing PCR detection of bacterial DNA with cultural results in CSF from shunt/ventriculostomy patients with clinically suspected infection has demonstrated that PCR significantly increases the number of patients found to have evidence of bacteria in the CSF [23].
Our data support the hypothesis by Stevens et al. [11] that a major contributor to infection associated with EVD may be biofilm formation. Hayhurst et al. [24] reported that, of 27 pediatric patients with EVD, 4 EVD were infected (by clinical signs and apparent response to treatment) even though all patients had sterile CSF throughout surveillance. Occult infection has also been associated with peritoneal catheters: Gorman et al. [25] examined 32 peritoneal catheters from patients with a history of peritonitis by electron and confocal microscopy. Bacteria were cultured after removal by sonication and vortexing. Biofilms were found in 4 of 4 catheters removed for peritonitis, but were also observed in 17 of 21 catheters removed for renal transplant from patients with no diagnosis of clinical infection; 41% of the infected catheters had mixed biofilms of CoNS and S. aureus. Biofilm formation in the intraluminal compartment might also explain the absence of early clinical signs of infection. In the early stages of biofilm formation, an intraluminal biofilm would be sequestered from the host, which might explain the limited inflammation associated with EVD-related infections [1].
The recognition of a biofilm involvement has significance for the design of devices specifically designed to prevent shunt (or EVD) infection (and presumably biofilm formation). Several studies have examined whether antibiotic-impregnated shunts can reduce the rate of infection with somewhat encouraging results. Hayhurst et al. [24] and Parker et al. [26] reported that antibiotic-impregnated shunts reduced the incidence of CSF infection in pediatric patients. However, it was noted that when S. aureus was cultured from patients with antibiotic-impregnated shunts, the strains were more likely to be oxacillin-resistant than from patients with conventional shunts[26], illustrating the potential danger of selecting for antibiotic resistance by this strategy. Lastly, it has been noted that the simple placement of an EVD, whether culture-positive or -negative, is itself a risk factor for the infection even of a subsequent antibiotic-impregnated shunt [25]. These results again suggest that a culture-negative EVD may still host a hard-to-detect biofilm infection which may transfer to a subsequent device, again reinforcing the need for better methods for the detection and combat of biofilm.
Acknowledgements
The authors thank Mary O’Toole for help in the preparation of the manuscript. This work was supported by Allegheny General Hospital, Allegheny-Singer Research Institute, and grants from the National Institute of Health, DE 014780 (S.K.), DC 02148 (G.D.E.), DC 04173 (G.D.E.), and DC 05659 (J.C.P.), the Pittsburgh Tissue Engineering Initiative, and the Health Resources and Services Administration of the Department of Health and Human Services.
References
- 1.Beer R, Lackner P, Pfausler B, Schmutzhard E. Nosocomial ventriculitis and meningitis in neurocritical care patients. J Neurol. 2008;255:1617–1624. doi: 10.1007/s00415-008-0059-8. [DOI] [PubMed] [Google Scholar]
- 2.Scheithauer S, Bürgel U, Ryang YM, Haase G, Schiefer J, Koch S, Häfner H, Lemmen S. Prospective surveillance of drain-associated meningitis/ventriculitis in a neurosurgery and a neurologic intensive care unit. J Neurol Neurosurg Psychiatry. 2009;80:1381–1385. doi: 10.1136/jnnp.2008.165357. [DOI] [PubMed] [Google Scholar]
- 3.Park P, Garton HJ, Kocan MJ, Thompson BG. Risk of infection with prolonged ventricular catheterization. Neurosurgery. 2004;55:594–599. doi: 10.1227/01.neu.0000134289.04500.ee. [DOI] [PubMed] [Google Scholar]
- 4.Muttaiyah S, Ritchie S, Upton A, Roberts S. Clinical parameters do not predict infection in patients with external ventricular drains: a retrospective observational study of daily cerebrospinal fluid analysis. J Med Microbiol. 2008;57:207–209. doi: 10.1099/jmm.0.47518-0. [DOI] [PubMed] [Google Scholar]
- 5.Hall-Stoodley L, Stoodley P. Evolving concepts in biofilm infections. Cell Microbiol. 2009;11:1034–1043. doi: 10.1111/j.1462-5822.2009.01323.x. [DOI] [PubMed] [Google Scholar]
- 6.Hall-Stoodley L, Hu FZ, Gieseke A, Nistico L, Nguyen D, Hayes J, Forbes M, Greenberg DP, Dice B, Burrows A, Wackym Pa, Stoodley P, Post JC, Ehrlich GD, Kerschner JE. Direct detection of bacterial biofilms on the middle-ear mucosa of children with chronic otitis media. JAMA. 2006;296:202–211. doi: 10.1001/jama.296.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leid JG, Shirtliff ME, Costerton JW, Stoodley P. Human leukocytes adhere, penetrate, and respond to Staphylococcus aureus biofilms. Infect Immun. 2002;70:6339–6345. doi: 10.1128/IAI.70.11.6339-6345.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parsek MR, Singh PK. Bacterial biofilms: an emerging link to disease pathogenesis. Annu Rev Microbiol. 2003;57:677–701. doi: 10.1146/annurev.micro.57.030502.090720. [DOI] [PubMed] [Google Scholar]
- 9.Braxton EE, Jr, Ehrlich GD, Hall-Stoodley L, Stoodley P, Veeh R, Fux C, Hu FZ, Quigley M, Post JC. Role of biofilms in neurosurgical device-related infections. Neurosurg Rev. 2005;28:249–255. doi: 10.1007/s10143-005-0403-8. [DOI] [PubMed] [Google Scholar]
- 10.Sandoe JA, Longshaw CM. Ventriculoperitoneal shunt infection caused by Staphylococcus lugdunensis. Clin Microbiol Infect. 2001;7:385–387. doi: 10.1046/j.1198-743x.2001.00268.x. [DOI] [PubMed] [Google Scholar]
- 11.Stevens NT, Tharmabala M, Dillane T, Greene CM, O'Gara JP, Humphreys H. Biofilm and the role of the ica operon and aap in Staphylococcus epidermidis isolates causing neurosurgical meningitis. Clin Microbiol Infect. 2008;14:719–722. doi: 10.1111/j.1469-0691.2008.02012.x. [DOI] [PubMed] [Google Scholar]
- 12.Wong GK, Poon WS, Wai S, Yu LM, Lyon D, Lam JM. Failure of regular external ventricular drain exchange to reduce cerebrospinal fluid infection: result of a randomised controlled trial. J Neurol Neurosurg Psychiatry. 2002;73:759–761. doi: 10.1136/jnnp.73.6.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zabramski JM, Whiting D, Darouiche RO, Horner TG, Olson J, Robertson C. Efficacy of antimicrobial-impregnated external ventricular drain catheters: a prospective, randomized, controlled trial. J Neurosurg. 2003;98:725–730. doi: 10.3171/jns.2003.98.4.0725. [DOI] [PubMed] [Google Scholar]
- 14.Ziebuhr W, Dietrich K, Trautmann M, Wilhelm M. Chromosomal rearrangements affecting biofilm production and antibiotic resistance in a Staphylococcus epidermidis strain causing shunt-associated ventriculitis. Int J Med Microbiol. 2000;290:115–120. doi: 10.1016/S1438-4221(00)80115-0. [DOI] [PubMed] [Google Scholar]
- 15.Walsh TJ, Schlegel R, Moody MM, Costerton JW, Salcman M. Ventriculoatrial shunt infection due to Cryptococcus neoformans: an ultrastructural and quantitative microbiological study. Neurosurgery. 1986;18:373–375. doi: 10.1227/00006123-198603000-00025. [DOI] [PubMed] [Google Scholar]
- 16.Davis LE, Cook G, Costerton JW. Biofilm on ventriculo-peritoneal shunt tubing as a cause of treatment failure in coccidioidal meningitis. Emerg Infect Dis. 2002;8:376–379. doi: 10.3201/eid0804.010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fux CA, Quigley M, Worel AM, Post C, Zimmerli S, Ehrlich G, Veeh RH. Biofilm-related infections of cerebrospinal fluid shunts. Clin Microbiol Infect. 2006;12:331–337. doi: 10.1111/j.1469-0691.2006.01361.x. [DOI] [PubMed] [Google Scholar]
- 18.Livni G, Yuhas Y, Ashkenazi S, Michowiz S. In vitro bacterial adherence to ventriculoperitoneal shunts. Pediatr Neurosurg. 2004;40:64–69. doi: 10.1159/000078910. [DOI] [PubMed] [Google Scholar]
- 19.Stoodley P, Kathju S, Hu FZ, Erdös G, Levenson JE, Mehta NS, Dice B, Johnson S, Hall-Stoodley L, Nistico L, Sotereanos N, Sewecke J, Post JC, Ehrlich GD. Molecular and imaging techniques for bacterial biofilms in joint arthroplasty infections. Clin Orthop Relat Res. 2005;437:31–40. doi: 10.1097/01.blo.0000175129.83084.d5. [DOI] [PubMed] [Google Scholar]
- 20.Stoodley P, Nistico L, Johnson S, Carabin LA, Baratz M, Gahlot V, Ehrlich GD, Kathju S. Direct demonstration of viable Staphylococcus aureus biofilms in an infected total joint arthroplasty: a case report. J Bone Joint Surg Am. 2008;90:1751–1758. doi: 10.2106/JBJS.G.00838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riffon R, Sayasith K, Khalil H, Dubreuil P, Drolet M, Lagace J. Development of a rapid and sensitive test for identification of major pathogens in bovine mastitis by PCR. J Clin Microbiol. 2001;39:2584–2589. doi: 10.1128/JCM.39.7.2584-2589.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neut D, van der Mei HC, Bulstra SK, Busscher HJ. The role of small-colony variants in failure to diagnose and treat biofilm infections in orthopedics. Acta Orthop. 2007;78:299–308. doi: 10.1080/17453670710013843. [DOI] [PubMed] [Google Scholar]
- 23.Banks JT, Bharara S, Tubbs RS, Wolff CL, Gillespie GY, Markert JM, Blount JP. Polymerase chain reaction for the rapid detection of cerebrospinal fluid shunt or ventriculostomy infections. Neurosurgery. 2005;57:1237–1243. doi: 10.1227/01.neu.0000186038.98817.72. [DOI] [PubMed] [Google Scholar]
- 24.Hayhurst C, Cooke R, Williams D, Kandasamy J, O'Brien DF, Mallucci CL. The impact of antibiotic-impregnated catheters on shunt infection in children and neonates. Childs Nerv Syst. 2008;24:557–562. doi: 10.1007/s00381-007-0521-4. [DOI] [PubMed] [Google Scholar]
- 25.Gorman SP, Adair CG, Mawhinney WM. Incidence and nature of peritoneal catheter biofilm determined by electron and confocal laser scanning microscopy. Epidemiol Infect. 1994;112:551–559. doi: 10.1017/s0950268800051256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parker SL, Attenello FJ, Sciubba DM, Garces-Ambrossi GL, Ahn E, Weingart J, Carson B, Jallo GI. Comparison of shunt infection incidence in high-risk subgroups receiving antibiotic-impregnated versus standard shunts. Childs Nerv Syst. 2009;25:77–83. doi: 10.1007/s00381-008-0743-0. [DOI] [PubMed] [Google Scholar]