Abstract
Background/aims
Serum high sensitivity C-reactive protein (hs-CRP) is a surrogate marker for cardiovascular disease risks and related mortality. However, the features of hs-CRP in chronic HCV infection (CHC) patients have not been fully addressed. This study aimed to elucidate the characteristics of hs-CRP and its correlation with clinical profiles in CHC patients.
Methods
Ninety-five CHC patients and 95 age- and sex-matched healthy controls were enrolled for serum hs-CRP level, biochemical, and metabolic profiles examinations. Sequential changes of hs-CRP levels in CHC patients receiving peginterferon/ribavirin combination therapy were also evaluated.
Results
The mean hs-CRP level of CHC patients was significantly higher than that of healthy controls (0.97 ± 0.11 vs. 0.24 ± 0.07 mg/L, P < 0.001). There was no significant correlation between hs-CRP and both virological and histological factors. CHC patients with a high LDL-C level had significantly higher mean hs-CRP (1.38 ± 0.20 mg/L) than that of patients without (0.59 ± 0.06 mg/L) (P < 0.001). Hs-CRP level was significantly decreased in 83 patients after peginterferon/ribavirin combination therapy (0.24 vs. 0.62 mg/L, P < 0.001), particularly in 68 patients achieving a sustained virological response (0.25 vs. 0.64 mg/L, P < 0.001).
Conclusion
CHC patients had a higher hs-CRP level than healthy controls which could be ameliorated after peginterferon/ribavirin combination therapy.
Keywords: High sensitivity C-reactive protein, Hepatitis C virus, Antiviral therapy
Introduction
Hepatitis C virus (HCV) infection is one of the most important causes of cirrhosis and hepatocellular carcinoma and has a strong impact on public health worldwide. HCV is lymphotropic as well as hepatotropic in nature [1]. Replication of HCV in diseased extrahepatic organs and tissues may have direct cytopathic effects, thus leading to a wide spectrum of extrahepatic manifestations, such as thyroid dysfunction, glucose abnormalities, lipid disorders, mixed cryoglobulinemia, and autoimmunity [2–8]. The related mechanisms in terms of liver injury and extrahepatic figures were not fully clarified. Systemic inflammatory response triggered by HCV per se and/or its subsequent immune cascades and cytokine storms may play a major role in it [9].
In the context of cytokines, high-sensitivity C-reactive protein (hs-CRP) has been shown to be closely related to the occurrence of systemic inflammatory response [10]. Serum hs-CRP levels were elevated in patients with insulin resistance and were correlated with metabolic syndrome [11, 12]. Moreover, hs-CRP has been shown to predict future risks for cardiovascular disease and related mortality [13, 14]. With respect to hepatological views, hs-CRP is a liver specific acute-phase protein, and its expression in hepatocytes is closely related with proinflammatory cytokines, such as tumor necrosis factor-alpha, interleukin-1, and interleukin-6 [15]. There were discrepant results of the association of CRP level and anti-HCV seropositivity in different study groups [16–21]. The feature of hs-CRP in chronic HCV infection (CHC) patients has not been fully addressed and remains a puzzle. Furthermore, study regarding the sequential changes of hs-CRP levels in terms of current standard-of-care antiviral therapy has never been evaluated.
The present study aimed to clarify the characteristics and the associated factors of hs-CRP in CHC patients. We also aimed to assess the impact of antiviral therapy on the sequential changes of hs-CRP levels.
Methods
The ethical committee of Kaohsiung Medical University Hospital approved the study before it began. All subjects gave written informed consent before enrollment.
CHC patients
Ninety-five treatment-naïve CHC patients were consecutively enrolled. All patients were seropositive for HCV antibodies (anti-HCV) and HCV RNA by polymerase chain reaction (PCR) for more than 6 months. All liver biopsies, which were obtained within 6 months prior to enrollment, showed chronic hepatitis of different severity. Patients with a concurrent hepatitis B (seropositive for hepatitis B surface antigen), human immunodeficiency virus infection, autoimmune hepatitis, primary biliary cirrhosis, sclerosing cholangitis, Wilson’s disease, α1-antitrypsin deficiency, decompensated cirrhosis (Child-Pugh class B or C), overt hepatic failure, a current or past history of alcohol abuse (>40 g ethanol per day), psychiatric condition, or with evidence of hepatocellular carcinoma were excluded.
Community-based controls
A total of 95 age-, sex-matched healthy adults aged 30 years or more who were seronegative for antibody to hepatitis C virus (anti-HCV) were invited for hs-CRP examination during their multi-purpose health surveillance in The Clinic of Department of Preventive Medicine, Kaohsiung Medical University Hospital. The controls were recruited with the same exclusion criteria of CHC patients.
Laboratory measurements
Anti-HCV was detected by using a third-generation commercially available enzyme-linked immunosorbent assay kit (AxSYM 3.0, Abbott Laboratories, Chicago, IL, USA). HCV RNA levels were detected by a qualitative polymerase chain reaction (PCR) assay (Cobas Amplicor Hepatitis C Virus Test, version 2.0; Roche Diagnostics, Branchburg, NJ, USA; detection limit: 50 IU/ml). HCV genotypes were determined by the method described by Okamoto et al. [22].
Serum levels of aspartate and alanine aminotransferase (AST and ALT), gamma-glutamyl transpeptidase (rGT), fasting plasma glucose (FPG), total cholesterol, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and triglycerides (TG) were determined using a multichannel autoanalyzer (Hitachi Inc., Tokyo, Japan). Hs-CRP levels were analyzed before and 6 months after antiviral therapy with a latex turbidimetric immunoassay (Wako Chemicals, Neuss, Germany). The analytical detection limit for this method is 0.06 mg/L. When samples containing a known level of CRP were tested ten times, the coefficient of variation of the test was under 10%. Individuals with serum hs-CRP > 10 mg/L were excluded in the current study because of the possibility of concurrent inflammatory or infectious diseases [23].
Histological analyses
For each CHC patient, a liver biopsy specimen of at least 2 cm in length was taken and fixed in 10% formalin buffer. Biopsy samples were stained with hematoxylin-eosin and the results were then reported by one pathologist who was blinded to the treatment of each patient. Histological grading of chronic hepatitis was made based on histological activity index (HAI) of Knodell et al. Liver fibrosis was staged into F0-4 according to the Metavir scoring systems.
Regimens and treatment responses
CHC patients were treated with peginterferon-α-2a (PEGASYS, Roche, Basel, Switzerland), 180 μg/week subcutaneously, plus oral ribavirin at a dose of 1000–1200 mg/day for 48 weeks if they were infected with HCV genotype 1 (HCV-1) or peginterferon-α-2a, 180 μg/week, plus oral ribavirin at a dose of 800 mg/day for 24 weeks if they were infected with HCV genotype 2 (HCV-2), with a 24-week follow-up period.
Evaluation of efficacy of antiviral treatment was based on sustained virological response (SVR), defined as PCR-negativity by the end-of-treatment and throughout the follow-up period. Non-responders were defined as persistence of HCV RNA levels throughout the treatment course.
Statistical analysis
Hs-CRP levels were expressed as mean (SD) or median (interquartile range, IQR). Comparisons between groups were made using the Student’s test or the Mann–Whitney U test for continuous variables and the χ2 or Fisher exact probability test for categorical data if suitable. HCV RNA levels were analyzed with values log10 transformed before t test analysis. Statistical correlations between serum hs-CRP and other parameters were determined by the non-parametric Spearman test. Paired t test or Wilcoxon signed rank test was used to compare the changes of serum hs-CRP levels before and after antiviral therapy in CHC patients. A multivariate analysis based on a logistic regression study is used to establish associations as appropriate. The procedures were performed using the SPSS 12.0 statistical package (SPSS, Chicago, IL, USA). All statistical analyses were based on two-sided hypothesis tests with a significance level of P < 0.05.
Results
Comparison of baseline characteristics between CHC patients and controls
The basic demographical and clinical features of the 95 CHC patients and the 95 healthy controls were shown in Table 1. The mean viral load of the CHC patients was 5.50 ± 0.96 log IU/mL. Forty (42.1%) patients were of HCV-1 infection. The CHC patients had a significantly higher level of AST, ALT, and rGT and a significantly lower level of TG, total cholesterols, HDL-C and LDL-C than healthy controls (all P < 0.001). The mean and median hs-CRP of CHC patients were 0.97 ± 0.11 mg/L, and 0.62 (IQR = 0.30, 1.09) mg/L, respectively, which was significantly higher than that (mean = 0.24 ± 0.07 mg/L; median = 0.10, IQR = 0.05, 0.20 mg/L) of healthy controls (P < 0.001). When subjects were stratified into three groups dependent of severity of cardiovascular risks [14], 65 (68.4%) CHC patients had hs-CRP levels less than 1 mg/L, 25 (26.3%) patients of 1–3 mg/L, and 5 (5.3%) patients of >3 mg/L. CHC patients had a significantly higher proportion of the groups carrying hs-CRP > 1 mg/L than healthy controls (P < 0.001).
Table 1.
Basic demographical and clinical features of CHC patients and controls
| CHC patients (N = 95) | Healthy controls (N = 95) | P value | |
|---|---|---|---|
| Age (years)a | 52.8 ± 10.3 | 53.1 ± 10.3 | 0.87 |
| Sex (male), n (%) | 45 (47.4) | 45 (47.4) | 1 |
| BMI (kg/m2) | 24.5 ± 3.4 | 24.2 ± 3.3 | 0.59 |
| Blood sugar (mg/dL)a | 98.6 ± 23.6 | 103.0 ± 31.6 | 0.28 |
| Triglycerides (mg/dL)a | 94.5 ± 46.1 | 125.1 ± 63.5 | <0.001 |
| Total cholesterol (mg/dL)a | 168.0 ± 34.3 | 206.6 ± 42.3 | <0.001 |
| HDL-C (mg/dL)a | 42.6 ± 14.6 | 56.3 ± 14.6 | <0.001 |
| LDL-C (mg/dL)a | 102.5 + 30.0 | 123.3 ± 36.7 | <0.001 |
| AST (IU/L)a | 123.2 ± 5 1.5 | 23.6 ± 8.6 | <0.001 |
| ALT (IU/L)a | 157.7 ± 82.1 | 25.1 ± 13.1 | <0.001 |
| r-GT (U/L)a | 70.6 ± 67.2 | 29.5 ± 19.5 | <0.001 |
| hs-CRP, median (IQR) | 0.62 (0.30, 1.09) | 0.10 (0.05, 0.20) | <0.001 |
| < 1 mg/L, n (%) | 65 (68.4) | 92 (96.8) | <0.001 |
| 1–3 mg/L, n (%) | 25 (26.3) | 2 (2.1) | |
| >3 mg/L, n (%) | 5 (5.3) | 1 (1.1) |
CHC chronic hepatitis C, BMI body mass index, AST alanine aminotransferase, ALT aspartate aminotransferase, rGTr-Glutamyl transferase, HDL-C high-density lipoprotein cholesterol, LDL-C low-density lipoprotein cholesterol, hs-CRP high sensitivity C-reactive protein, IQR interquartile range
aExpressed as mean ± SD
Clinical profiles correlated with hs-CRP level in CHC patients
Lipid profiles including TG (r2 = 0.055, P = 0.023), total cholesterol (r2 = 0.072, P = 0.009) and LDL-C levels (r2 = 0.048, P = 0.033), were positively correlated with hs-CRP level in CHC patients. Further analysis demonstrated that 46 patients with LDL-C level > 100 mg/dL had significantly higher mean hs-CRP (1.38 ± 0.20 mg/L) than that of other 49 patients with LDL-C level < 100 mg/dL (0.59 ± 0.06 mg/L) (P < 0.001) (Fig. 1a). For those patients with high TG level (>150 mg/dL), the mean hs-CRP level (2.01 ± 0.55 mg/L) was substantially higher than that of patients without high TG level (0.85 ± 0.10 mg/L) (P = 0.06) (Fig. 1b).
Fig. 1.
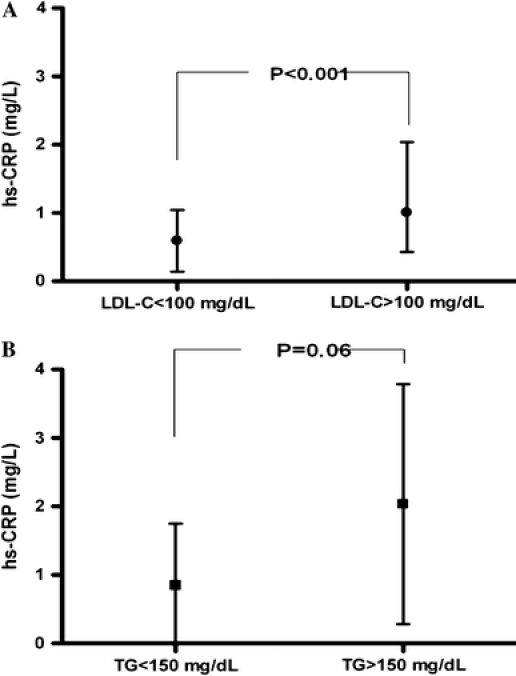
a The mean hs-CRP level between CHC patients with LDL-C level < 100 mg/dL and >100 mg/dL. b The mean hs-CRP level between CHC patients with TG level < 150 mg/dL and >150 mg/dL
Eighty-four (88.4%) CHC patients were regarded as high hs-CRP group, defined as 75 percentile of the healthy controls (0.197 mg/L). As shown in Table 2, univariate regression analysis demonstrated that the factors associated with high hs-CRP included fibrosis score, genotype non-1, ALT and total cholesterol levels.
Table 2.
Factors associated with high hs-CRP level in CHC patients
| High hs-CRP level | P value | ||
|---|---|---|---|
| Yes, n = 84 | No, n = 11 | ||
| Sex (male), n (%) | 38 (45.2) | 7 (63.6) | 0.25 |
| Age (years)a | 52.4 ± 10.4 | 56.0 ± 8.6 | 0.28 |
| Metabolic syndrome, n (%) | 23 (27.4) | 4 (36.4) | 0.72 |
| Hypertension, n (%) | 22 (26.2) | 3 (27.3) | 1 |
| BMI (kg/m2)a | 24.6 ± 3.2 | 23.0 ± 4.3 | 0.13 |
| HCV RNA > 400 kIU/mL, n (%) | 34 (40.5) | 4 (36.4) | 1 |
| HCV genotype 1, n (%) | 32 (38.1) | 8 (72.7) | 0.05 |
| FPG (mg/dL)a | 99 ± 25 | 96 ± 12 | 0.66 |
| Triglycerides (mg/dL)a | 96 ± 48 | 81 ± 26 | 0.29 |
| Cholesterol (mg/dL)a | 172 ± 34 | 141 ± 26 | 0.01 |
| HDL-C (mg/dL)a | 43 ± 15 | 38 ± 10 | 0.29 |
| LDL-C (mg/dL)a | 105 ± 29 | 87 ± 33 | 0.07 |
| AST (IU/L)a | 105 ± 53 | 93 ± 33 | 0.49 |
| ALT (IU/L)a | 161 ± 86 | 130 ± 40 | 0.05 |
| Fibrosis scorea | 1.82 ± 1.17 | 2.64 ± 0.92 | 0.03 |
| HAI scorea | 5.54 ± 2.77 | 6.45 ± 1.81 | 0.16 |
High hs-CRP level was defined as 75 percentile level of the healthy control group, 0.197 mg/L
BMI body mass index, CHC chronic hepatitis C, HCV hepatitis C virus, FPG fasting plasma glucose, AST alanine aminotransferase, ALT aspartate aminotransferase, HDL-C high-density lipoprotein cholesterol, LDL-C low-density lipoprotein cholesterol, hs-CRP high sensitivity C-reactive protein
aExpressed as mean ± SD
The independent predictive value of age, sex, BMI, AST, ALT, baseline HCV RNA levels, grade and stage of liver histopathology, blood sugar, total cholesterol, TG, HDL-C and LDL-C was determined by using stepwise logistic regression analysis.
Total cholesterol level was the only independent factor associated with high hs-CRP in CHC patients (odds ratio = 1.04, 95% CI = 1.01–1.08, P = 0.004).
Sequential changes of hs-CRP levels related to treatment response
A total 85 of the 95 CHC patients received peginterferon plus ribavirin combination therapy, including 36 HCV-1 and 49 HCV-non 1 patients, respectively. Two patients were excluded for comparison because of serum hs-CRP levels >10 mg/L after treatment. Sixty-eight (81.9%) patients had a SVR and 15 (18.1%) were non-SVR patients.
Sequential changes of serum hs-CRP levels related to treatment response were shown in Table 3. There was statistically significant decrease of mean hs-CRP levels among those 83 CHC patients after treatment compared with their pretreatment levels (0.24 vs. 0.62 mg/L, P < 0.001). The changes were observed either in SVR group (0.25 vs. 0.64 mg/L, P < 0.001) or in non-SVR group (0.18 vs. 0.61 mg/L, P = 0.02). Nonetheless, the significant decrease of hs-CRP levels after treatment existed only in seven relapsers (0.13 vs. 0.85 mg/L, P = 0.03), but not in eight non-responders (0.18 vs. 0.54 mg/L, P = 0.33).
Table 3.
Sequential changes of hs-CRP levels according to treatment response in 83 CHC patients
| Before treatment [median (IQR)], mg/L | After treatment [median (IQR)], mg/L | P value | |
|---|---|---|---|
| All patients (n = 83) | 0.62 (0.33, 1.08) | 0.24 (0.10, 0.50) | <0.001 |
| SVR patients (n = 68) | 0.64 (0.30, 1.08) | 0.25 (0.10, 0.49) | <0.001 |
| Non-SVR patients (n = 15) | 0.61 (0.41, 1.57) | 0.18 (0.10, 0.52) | 0.02 |
| Relapsers (n = 7) | 0.85 (0.41, 2.16) | 0.13 (0.10, 0.24) | 0.03 |
| Non-responders (n = 8) | 0.54 (0.36, 0.93) | 0.31 (0.03, 0.88) | 0.33 |
hs-CRP high sensitivity C-reactive protein, IQR interquartile range, SVR sustained virological response
Discussion
Hs-CRP has been shown to be a competent predictor for future risks of cardiovascular diseases and metabolic abnormalities in apparently healthy persons independently of established major risk factors [24, 25]. It plays a major role in the scenario of an activated systemic inflammatory response, and is also a common feature of various chronic liver diseases, such as nonalcoholic fatty liver disease and nonalcoholic steatohepatitis [26, 27]. The current study demonstrated that the hs-CRP level of CHC patients was significantly higher than that of healthy controls. In addition, hs-CRP level was significantly ameliorated after antiviral therapy. It, therefore, implied that a more profound systemic inflammatory response existed in patients with HCV infection. It also provided evidence that systemic inflammatory response could effectively be relieved after antiviral therapy, particularly in patients achieving a SVR.
Elevated serum hs-CRP level has been consistently demonstrated in the emergence of atherosclerosis [11, 28]. The proinflammatory, proatherogenic effects of hs-CRP that have been documented in endothelial cells include the following features: atherosclerotic streaks and plaques, the emergence of inflammatory processes over intima layer, rupture of plaques and platelet aggregation, activation of glycoprotein IIb/IIIa receptor, cascading coagulating system, and thus lead to thrombus and acute coronary syndrome. HCV infection may lead to insulin resistance and/or metabolic syndrome, and which were clinical manifestations of systemic inflammatory response [4, 29–31]. Therefore, the association between HCV infection and elevated serum hs-CRP level is plausible. The current proof-of-concept study demonstrated that CHC patients had a higher hs-CRP level than healthy controls. This finding echoed our previous study showing that HCV infection carried a higher prevalence of metabolic syndrome and insulin resistance [4, 32]. In addition, the proportion of CHC patients with hs-CRP level > 3 mg/L, a condition indicative of a high cardiovascular risk, was also higher than healthy controls [14]. The relationships between HCV infection and vascular atherosclerosis remain an argument of debate [33–35]. The current study might provide insight for further studies to clarify subsequent cardiovascular risks in patient with HCV infection.
An association between HCV infection and lipid metabolism has been consistently reported [8, 36]. It has been reported that the LDL-receptor is one of the HCV receptors, and experiments in vitro showed competitive inhibition of binding between HCV and LDL-receptor by LDL-C [36]. In addition, lipoproteins play an important role in the process of HCV infection since complexing of the virus to VLDL-C or LDL-C could promote endocytosis of HCV via the LDL receptor [37]. Taken together, HCV infection is associated with clinically significant lower cholesterol (total cholesterol, HDL-C and LDL-C) and TG levels than those of normal subjects. The current study demonstrated that hs-CRP level was correlated with TG, total cholesterol, and LDL-C levels in CHC patients. For patients with high LDL-C or TG levels, their mean hs-CRP level was significantly or substantially higher than those without. However, advanced liver diseases may be associated with decreasing cholesterol level and the extent of liver injury should be taken into account when addressing this issue. Moreover, the lipid profiles are reflected by multiple factors, such as race, age, gender, life style and meal habits. Therefore, it might be too conclusive to imply that lipid profiles were the major factors correlated with hs-CRP level in CHC patients. Nevertheless, our data may suggest that hs-CRP may be used as a complementary surrogate marker for cardiovascular risks in this special population. Further study addressing the sequential changes and the correlation between lipid profiles and hs-CRP in a long-term follow-up basis will be needed to elucidate this intriguing observation.
Acute phase reactions with elicited acute phase proteins directly or indirectly from liver are common features in patients with different extent of liver insults ranging from acute liver injury to advanced liver cirrhosis [38]. The features of acute phase proteins include increased C1-inhibitor, C9, C4, and orosomucoid, while decreased transferrin and fetuin/a2HS-glycoprotein in CHC patients who responded to interferon-based antiviral therapy [39]. CRP is the most widely used acute phase protein in clinical setting. However, to our knowledge, the change of hs-CRP subsequent to peginterferon/ribavirin combination therapy has never been studied. Our data demonstrated that serum hs-CRP significantly decreased after pegylated interferon and ribavirin combination therapy. It may suggest that systemic inflammatory response in HCV infection could effectively be relieved after antiviral therapy, particularly in patients achieving a SVR. Intriguingly, among those non-SVR patients, decreases of serum hs-CRP levels were significantly observed only in relapsers but not in non-responders. It has been shown that those relapsers have more favorable outcomes after re-treatment programs compared with non-responders [40]. The decreased systemic inflammatory response in relapsers, which was reflected by significantly decreased hs-CRP level, may in a part contribute to the favorable outcomes. It is noteworthy whether the non-responders carry a higher risk of developing cardiovascular events, awaits further intervention.
In conclusion, the current study demonstrated that CHC patients had a higher serum hs-CRP level than healthy controls. CHC patients with elevated hs-CRP had substantially higher levels of lipid profiles. Furthermore, hs-CRP level was significantly decreased after pegylated interferon and ribavirin combination therapy, particularly for those achieving a SVR. Further studies are warranted to elucidate the causal relationship between HCV infection and cardiovascular risk in terms of hs-CRP level.
Acknowledgements
The authors thank secretary and serum processing helps from Taiwan Liver Research Foundation (TLRF). The foundation did not influence how the study was conducted or the approval of the manuscript. The authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. This study was partly supported by grant (NSC-96-2314-B-037-038) from National Science Council, The Executive Yuan, Taiwan.
Conflicts of interest None.
References
- 1.Mazzaro C, Panarello G, Tesio F, Santini G, Crovatto M, Mazzi G, Zorat F, Tulissi P, Pussini E, Baracetti S, Campanacci L, Pozzato G. Hepatitis C virus risk: a hepatitis C virus related syndrome. J Intern Med. 2000;247:535–545. doi: 10.1046/j.1365-2796.2000.00627.x. [DOI] [PubMed] [Google Scholar]
- 2.Huang JF, Chuang WL, Dai CY, Ho CK, Hwang SJ, Chen SC, Lin ZY, Wang LY, Chang WY, Yu ML. Viral hepatitis and proteinuria in an area endemic for hepatitis B and C infections: another chain of link? J Intern Med. 2006;260:255–262. doi: 10.1111/j.1365-2796.2006.01686.x. [DOI] [PubMed] [Google Scholar]
- 3.Huang JF, Dai CY, Hwang SJ, Ho CK, Hsiao PJ, Hsieh MY, Lee LP, Lin ZY, Chen SC, Wang LY, Shin SJ, Chang WY, Chuang WL, Yu ML. Hepatitis C viremia increases the association with type 2 diabetes mellitus in a hepatitis B and C endemic area: an epidemiological link with virological implication. Am J Gastroenterol. 2007;102:1237–1243. doi: 10.1111/j.1572-0241.2007.01181.x. [DOI] [PubMed] [Google Scholar]
- 4.Huang JF, Chuang WL, Yu ML, Yu SH, Huang CF, Huang CI, Yeh ML, Hsieh MH, Yang JF, Lin ZY, Chen SC, Dai CY, Chang WY. Hepatitis C virus infection and metabolic syndrome—a community-based study in an endemic area of Taiwan. Kaohsiung J Med Sci. 2009;25:299–305. doi: 10.1016/S1607-551X(09)70520-0. [DOI] [PubMed] [Google Scholar]
- 5.Yu ML, Chuang WL. Treatment of chronic hepatitis C in Asia: when East meets West. J Gastroenterol Hepatol. 2009;24:336–345. doi: 10.1111/j.1440-1746.2009.05789.x. [DOI] [PubMed] [Google Scholar]
- 6.Hsieh MY, Dai CY, Lee LP, Huang JF, Tsai WC, Hou NJ, Lin ZY, Chen SC, Wang LY, Chang WY, Chuang WL, Yu ML. Antinuclear antibody is associated with a more advanced fibrosis and lower RNA levels of hepatitis C virus in patients with chronic hepatitis C. J Clin Pathol. 2008;61:333–337. doi: 10.1136/jcp.2006.046276. [DOI] [PubMed] [Google Scholar]
- 7.Kawaguchi T, Yoshida T, Harada M, Hisamoto T, Nagao Y, Ide T, Taniguchi E, Kumemura H, Hanada S, Maeyama M, Baba S, Koga H, Kumashiro R, Ueno T, Ogata H, Yoshimura A, Sata M. Hepatitis C virus down-regulates insulin receptor substrates 1 and 2 through up-regulation of suppressor of cytokine signaling 3. Am J Pathol. 2004;165:1499–1508. doi: 10.1016/S0002-9440(10)63408-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dai CY, Chuang WL, Ho CK, Hsieh MY, Huang JF, Lee LP, Hou NJ, Lin ZY, Chen SC, Hsieh MY, Wang LY, Tsai JF, Chang WY, Yu ML. Associations between hepatitis C viremia and low serum triglyceride and cholesterol levels: a community-based study. J Hepatol. 2008;49:9–16. doi: 10.1016/j.jhep.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 9.Kilic T, Jneid H, Ural E, Oner G, Sahin T, Kozdag G, Kahraman G, Ural D. Impact of the metabolic syndrome on high-sensitivity C reactive protein levels in patients with acute coronary syndrome. Atherosclerosis 2009;207:591–596 [DOI] [PubMed]
- 10.Koenig W, Khuseyinova N, Baumert J, Meisinger C. Prospective study of high-sensitivity C-reactive protein as a determinant of mortality: results from the MONICA/KORA Augsburg Cohort Study, 1984–1998. Clin Chem. 2008;54:335–342. doi: 10.1373/clinchem.2007.100271. [DOI] [PubMed] [Google Scholar]
- 11.Ridker PM, Buring JE, Cook NR, Rifai N. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: an 8-year follow-up of 14 719 initially healthy American women. Circulation. 2003;107:391–397. doi: 10.1161/01.CIR.0000055014.62083.05. [DOI] [PubMed] [Google Scholar]
- 12.Yudkin JS, Stehouwer CD, Emeis JJ, Coppack SW. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol. 1999;19:972–978. doi: 10.1161/01.atv.19.4.972. [DOI] [PubMed] [Google Scholar]
- 13.Zethelius B, Berglund L, Sundstrom J, Ingelsson E, Basu S, Larsson A, Venge P, Arnlov J. Use of multiple biomarkers to improve the prediction of death from cardiovascular causes. N Engl J Med. 2008;358:2107–2116. doi: 10.1056/NEJMoa0707064. [DOI] [PubMed] [Google Scholar]
- 14.Zacho J, Tybjaerg-Hansen A, Jensen JS, Grande P, Sillesen H, Nordestgaard BG. Genetically elevated C-reactive protein and ischemic vascular disease. N Engl J Med. 2008;359:1897–1908. doi: 10.1056/NEJMoa0707402. [DOI] [PubMed] [Google Scholar]
- 15.Kushner I. The phenomenon of the acute phase response. Ann NY Acad Sci. 1982;389:39–48. doi: 10.1111/j.1749-6632.1982.tb22124.x. [DOI] [PubMed] [Google Scholar]
- 16.Kalabay L, Nemesanszky E, Csepregi A, Pusztay M, David K, Horvath G, Ibranyi E, Telegdy L, Par A, Biro A, Fekete B, Gervain J, Horanyi M, Ribiczey P, Csondes M, Kleiber M, Walentin S, Prohaszka Z, Fust G. Paradoxical alteration of acute-phase protein levels in patients with chronic hepatitis C treated with IFN-alpha2b. Int Immunol. 2004;16:51–54. doi: 10.1093/intimm/dxh024. [DOI] [PubMed] [Google Scholar]
- 17.Tsui JI, Whooley MA, Monto A, Seal K, Tien PC, Shlipak M. Association of hepatitis C virus seropositivity with inflammatory markers and heart failure in persons with coronary heart disease: data from the Heart and Soul study. J Card Fail. 2009;15:451–456. doi: 10.1016/j.cardfail.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reingold J, Wanke C, Kotler D, Lewis C, Tracy R, Heymsfield S, Tien P, Bacchetti P, Scherzer R, Grunfeld C, Shlipak M. Association of HIV infection and HIV/HCV coinfection with C-reactive protein levels: the fat redistribution and metabolic change in HIV infection (FRAM) study. J Acquir Immune Defic Syndr. 2008;48:142–148. doi: 10.1097/QAI.0b013e3181685727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Floris-Moore M, Howard AA, Lo Y, Schoenbaum EE, Arnsten JH, Klein RS. Hepatitis C infection is associated with lower lipids and high-sensitivity C-reactive protein in HIV-infected men. AIDS Patient Care STDS. 2007;21:479–491. doi: 10.1089/apc.2006.0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yelken B, Gorgulu N, Caliskan Y, Elitok A, Cimen AO, Yazici H, Oflaz H, Turkmen A, Sever MS. Association between chronic hepatitis C infection and coronary flow reserve in dialysis patients with failed renal allografts. Transplant Proc. 2009;41:1519–1523. doi: 10.1016/j.transproceed.2009.03.069. [DOI] [PubMed] [Google Scholar]
- 21.Nascimento MM, Bruchfeld A, Suliman ME, Hayashi SY, Pecoits-Filho R, Manfro RC, Pachaly MA, Renner L, Stenvinkel P, Riella MC, Lindholm B. Effect of hepatitis C serology on C-reactive protein in a cohort of Brazilian hemodialysis patients. Braz J Med Biol Res. 2005;38:783–788. doi: 10.1590/S0100-879X2005000500017. [DOI] [PubMed] [Google Scholar]
- 22.Okamoto H, Tokita H, Sakamoto M, Horikita M, Kojima M, Iizuka H, Mishiro S. Characterization of the genomic sequence of type V (or 3a) hepatitis C virus isolates and PCR primers for specific detection. J Gen Virol. 1993;74:2385–2390. doi: 10.1099/0022-1317-74-11-2385. [DOI] [PubMed] [Google Scholar]
- 23.Lahiri DK, Nurnberger JI., Jr A rapid non-enzymatic method for the preparation of HMW DNA from blood for RFLP studies. Nucleic Acids Res. 1991;19:5444. doi: 10.1093/nar/19.19.5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Black S, Kushner I, Samols D. C-reactive Protein. J Biol Chem. 2004;279:48487–48490. doi: 10.1074/jbc.R400025200. [DOI] [PubMed] [Google Scholar]
- 25.Libby P, Ridker PM. Inflammation and atherosclerosis: role of C-reactive protein in risk assessment. Am J Med. 2004;116:9S–16S. doi: 10.1016/j.amjmed.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 26.Riquelme A, Arrese M, Soza A, Morales A, Baudrand R, Perez-Ayuso RM, Gonzalez R, Alvarez M, Hernandez V, Garcia-Zattera MJ, Otarola F, Medina B, Rigotti A, Miquel JF, Marshall G, Nervi F. Non-alcoholic fatty liver disease and its association with obesity, insulin resistance and increased serum levels of C-reactive protein in Hispanics. Liver Int. 2009;29:82–88. doi: 10.1111/j.1478-3231.2008.01823.x. [DOI] [PubMed] [Google Scholar]
- 27.Kogiso T, Moriyoshi Y, Shimizu S, Nagahara H, Shiratori K. High-sensitivity C-reactive protein as a serum predictor of nonalcoholic fatty liver disease based on the Akaike Information Criterion scoring system in the general Japanese population. J Gastroenterol. 2009;44:313–321. doi: 10.1007/s00535-009-0002-5. [DOI] [PubMed] [Google Scholar]
- 28.Duncan BB, Schmidt MI, Pankow JS, Ballantyne CM, Couper D, Vigo A, Hoogeveen R, Folsom AR, Heiss G. Low-grade systemic inflammation and the development of type 2 diabetes: the atherosclerosis risk in communities study. Diabetes. 2003;52:1799–1805. doi: 10.2337/diabetes.52.7.1799. [DOI] [PubMed] [Google Scholar]
- 29.Shintani Y, Fujie H, Miyoshi H, Tsutsumi T, Tsukamoto K, Kimura S, Moriya K, Koike K. Hepatitis C virus infection and diabetes: direct involvement of the virus in the development of insulin resistance. Gastroenterology. 2004;126:840–848. doi: 10.1053/j.gastro.2003.11.056. [DOI] [PubMed] [Google Scholar]
- 30.Yoneda M, Saito S, Ikeda T, Fujita K, Mawatari H, Kirikoshi H, Inamori M, Nozaki Y, Akiyama T, Takahashi H, Abe Y, Kubota K, Iwasaki T, Terauchi Y, Togo S, Nakajima A. Hepatitis C virus directly associates with insulin resistance independent of the visceral fat area in nonobese and nondiabetic patients. J Viral Hepat. 2007;14:600–607. doi: 10.1111/j.1365-2893.2006.00836.x. [DOI] [PubMed] [Google Scholar]
- 31.Huang JF, Yu ML, Dai CY, Hsieh MY, Hwang SJ, Hsiao PJ, Lee LP, Lin ZY, Chen SC, Hsieh MY, Wang LY, Shin SJ, Chang WY, Chuang WL. Reappraisal of the characteristics of glucose abnormalities in patients with chronic hepatitis C infection. Am J Gastroenterol. 2008;103:1933–1940. doi: 10.1111/j.1572-0241.2008.01996.x. [DOI] [PubMed] [Google Scholar]
- 32.Dai CY, Huang JF, Hsieh MY, Hou NJ, Lin ZY, Chen SC, Wang LY, Chang WY, Chuang WL, Yu ML. Insulin resistance predicts response to peginterferon-alpha/ribavirin combination therapy in chronic hepatitis C patients. J Hepatol. 2009;50:712–718. doi: 10.1016/j.jhep.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 33.Oyake N, Shimada T, Murakami Y, Ishibashi Y, Satoh H, Suzuki K, Matsumory A, Oda T. Hepatitis C virus infection as a risk factor for increased aortic stiffness and cardiovascular events in dialysis patients. J Nephrol. 2008;21:345–353. [PubMed] [Google Scholar]
- 34.Moritani M, Adachi K, Arima N, Takashima T, Miyaoka Y, Niigaki M, Furuta K, Sato S, Kinoshita Y. A study of arteriosclerosis in healthy subjects with HBV and HCV infection. J Gastroenterol. 2005;40:1049–1053. doi: 10.1007/s00535-005-1655-3. [DOI] [PubMed] [Google Scholar]
- 35.Ishizaka N, Ishizaka Y, Takahashi E, Tooda E, Hashimoto H, Nagai R, Yamakado M. Association between hepatitis C virus seropositivity, carotid-artery plaque, and intima-media thickening. Lancet. 2002;359:133–135. doi: 10.1016/S0140-6736(02)07339-7. [DOI] [PubMed] [Google Scholar]
- 36.Monazahian M, Bohme I, Bonk S, Koch A, Scholz C, Grethe S, Thomssen R. Low density lipoprotein receptor as a candidate receptor for hepatitis C virus. J Med Virol. 1999;57:223–229. doi: 10.1002/(SICI)1096-9071(199903)57:3<223::AID-JMV2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 37.Agnello V, Abel G, Elfahal M, Knight GB, Zhang QX. Hepatitis C virus and other flaviviridae viruses enter cells via low density lipoprotein receptor. Proc Natl Acad Sci USA. 1999;96:1276. doi: 10.1073/pnas.96.22.12766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramadori G, Armbrust T. Cytokines in the liver. Eur J Gastroenterol Hepatol 2001;13:777–784 [DOI] [PubMed]
- 39.Biro L, Varga L, Par A, Nemesanszky E, Csepregi A, Telegdy L, Ibranyi E, David K, Horvath G, Szentgyorgyi L, Nagy I, Dalmi L, Abonyi M, Fust G, Horanyi M. Changes in the acute phase complement component and IL-6 levels in patients with chronic hepatitis C receiving interferon alpha-2b. Immunol Lett 2000;72:69–74 [DOI] [PubMed]
- 40.Keeffe EB. Chronic hepatitis C: management of treatment failures. Clin Gastroenterol Hepatol 2005;3:S102–S105 [DOI] [PubMed]


