Abstract
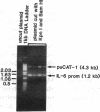
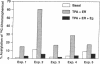
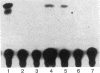
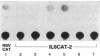
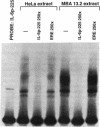
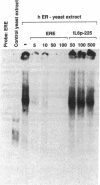
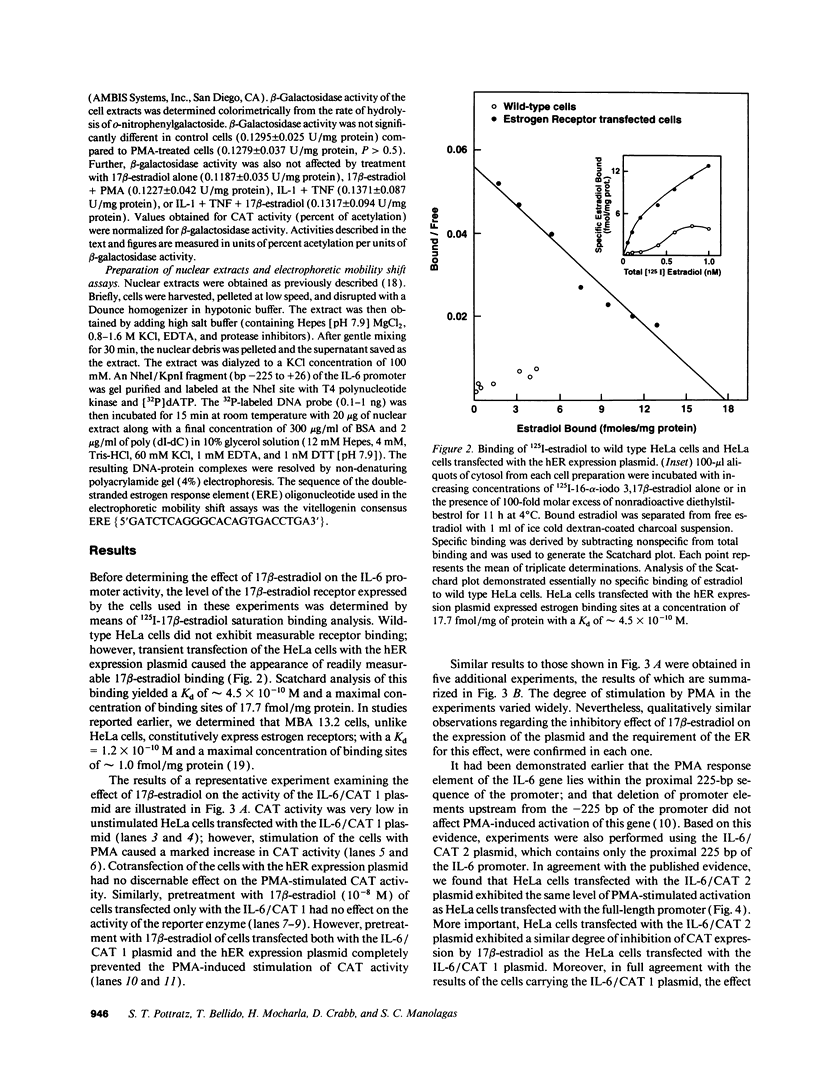
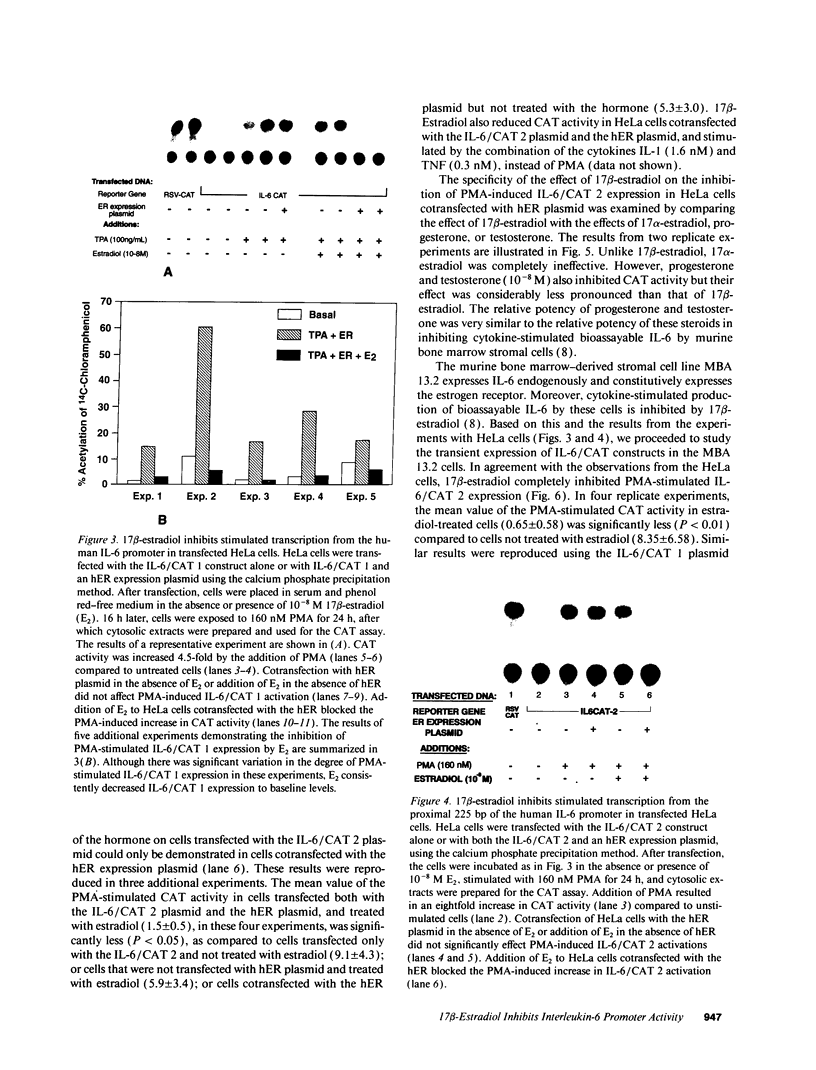
We previously reported that 17 beta-estradiol inhibits cytokine-stimulated bioassayable IL-6 and the steady-state level of IL-6 mRNA. To determine the molecular basis of this effect, the transient expression of chloramphenicol acetyltransferase (CAT) reporter plasmid driven by the human IL-6 promoter was studied here in HeLa or murine bone marrow stromal cells (MBA 13.2). 17 beta-estradiol (10(-8) M) completely suppressed stimulated CAT expression in HeLa cells cotransfected with IL-6/CAT constructs and a human estrogen receptor (hER) expression plasmid; but had no effect on reporter expression in HeLa cells not transfected with hER. 17 beta-estradiol also inhibited stimulated expression in MBA 13.2 cells (which express the estrogen receptor constitutively) without the requirement of cotransfection of the hER plasmid. The hormonal effects were indistinguishable between constructs containing a 1.2-kb fragment of the 5' flanking region of the IL-6 gene or only the proximal 225-bp fragment. However, yeast-derived recombinant hER did not bind to the 225-bp segment in DNA band shift assays, nor did the 225-bp fragment compete for binding of an estrogen response element oligonucleotide to yeast-derived estrogen receptor. These data suggest that 17 beta-estradiol inhibits the stimulated expression of the human IL-6 gene through an estrogen receptor mediated indirect effect on the transcriptional activity of the proximal 225-bp sequence of the promoter.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellido T., Girasole G., Passeri G., Yu X. P., Mocharla H., Jilka R. L., Notides A., Manolagas S. C. Demonstration of estrogen and vitamin D receptors in bone marrow-derived stromal cells: up-regulation of the estrogen receptor by 1,25-dihydroxyvitamin-D3. Endocrinology. 1993 Aug;133(2):553–562. doi: 10.1210/endo.133.2.8393768. [DOI] [PubMed] [Google Scholar]
- Benz D. J., Haussler M. R., Komm B. S. Estrogen binding and estrogenic responses in normal human osteoblast-like cells. J Bone Miner Res. 1991 Jun;6(6):531–541. doi: 10.1002/jbmr.5650060603. [DOI] [PubMed] [Google Scholar]
- Crabb D. W., Minth C. D., Dixon J. E. Assaying the reporter gene chloramphenicol acetyltransferase. Methods Enzymol. 1989;168:690–701. doi: 10.1016/0076-6879(89)68050-0. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorshkind K. Regulation of hemopoiesis by bone marrow stromal cells and their products. Annu Rev Immunol. 1990;8:111–137. doi: 10.1146/annurev.iy.08.040190.000551. [DOI] [PubMed] [Google Scholar]
- Girasole G., Jilka R. L., Passeri G., Boswell S., Boder G., Williams D. C., Manolagas S. C. 17 beta-estradiol inhibits interleukin-6 production by bone marrow-derived stromal cells and osteoblasts in vitro: a potential mechanism for the antiosteoporotic effect of estrogens. J Clin Invest. 1992 Mar;89(3):883–891. doi: 10.1172/JCI115668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. Transformation of rat cells by DNA of human adenovirus 5. Virology. 1973 Aug;54(2):536–539. doi: 10.1016/0042-6822(73)90163-3. [DOI] [PubMed] [Google Scholar]
- Green S., Walter P., Kumar V., Krust A., Bornert J. M., Argos P., Chambon P. Human oestrogen receptor cDNA: sequence, expression and homology to v-erb-A. Nature. 1986 Mar 13;320(6058):134–139. doi: 10.1038/320134a0. [DOI] [PubMed] [Google Scholar]
- Hattersley G., Kerby J. A., Chambers T. J. Identification of osteoclast precursors in multilineage hemopoietic colonies. Endocrinology. 1991 Jan;128(1):259–262. doi: 10.1210/endo-128-1-259. [DOI] [PubMed] [Google Scholar]
- Jilka R. L., Hangoc G., Girasole G., Passeri G., Williams D. C., Abrams J. S., Boyce B., Broxmeyer H., Manolagas S. C. Increased osteoclast development after estrogen loss: mediation by interleukin-6. Science. 1992 Jul 3;257(5066):88–91. doi: 10.1126/science.1621100. [DOI] [PubMed] [Google Scholar]
- Jonat C., Rahmsdorf H. J., Park K. K., Cato A. C., Gebel S., Ponta H., Herrlich P. Antitumor promotion and antiinflammation: down-modulation of AP-1 (Fos/Jun) activity by glucocorticoid hormone. Cell. 1990 Sep 21;62(6):1189–1204. doi: 10.1016/0092-8674(90)90395-u. [DOI] [PubMed] [Google Scholar]
- Kishimoto T., Akira S., Taga T. Interleukin-6 and its receptor: a paradigm for cytokines. Science. 1992 Oct 23;258(5082):593–597. doi: 10.1126/science.1411569. [DOI] [PubMed] [Google Scholar]
- Klein B., Wijdenes J., Zhang X. G., Jourdan M., Boiron J. M., Brochier J., Liautard J., Merlin M., Clement C., Morel-Fournier B. Murine anti-interleukin-6 monoclonal antibody therapy for a patient with plasma cell leukemia. Blood. 1991 Sep 1;78(5):1198–1204. [PubMed] [Google Scholar]
- Kumar V., Green S., Staub A., Chambon P. Localisation of the oestradiol-binding and putative DNA-binding domains of the human oestrogen receptor. EMBO J. 1986 Sep;5(9):2231–2236. doi: 10.1002/j.1460-2075.1986.tb04489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara N., Chenu C., Miller M., Civin C., Roodman G. D. Identification of committed mononuclear precursors for osteoclast-like cells formed in long term human marrow cultures. Endocrinology. 1990 May;126(5):2733–2741. doi: 10.1210/endo-126-5-2733. [DOI] [PubMed] [Google Scholar]
- Löwik C. W., van der Pluijm G., Bloys H., Hoekman K., Bijvoet O. L., Aarden L. A., Papapoulos S. E. Parathyroid hormone (PTH) and PTH-like protein (PLP) stimulate interleukin-6 production by osteogenic cells: a possible role of interleukin-6 in osteoclastogenesis. Biochem Biophys Res Commun. 1989 Aug 15;162(3):1546–1552. doi: 10.1016/0006-291x(89)90851-6. [DOI] [PubMed] [Google Scholar]
- Metcalf D. The molecular control of cell division, differentiation commitment and maturation in haemopoietic cells. Nature. 1989 May 4;339(6219):27–30. doi: 10.1038/339027a0. [DOI] [PubMed] [Google Scholar]
- Ray A., Tatter S. B., May L. T., Sehgal P. B. Activation of the human "beta 2-interferon/hepatocyte-stimulating factor/interleukin 6" promoter by cytokines, viruses, and second messenger agonists. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6701–6705. doi: 10.1073/pnas.85.18.6701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roodman G. D., Kurihara N., Ohsaki Y., Kukita A., Hosking D., Demulder A., Smith J. F., Singer F. R. Interleukin 6. A potential autocrine/paracrine factor in Paget's disease of bone. J Clin Invest. 1992 Jan;89(1):46–52. doi: 10.1172/JCI115584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadduck R. K., Waheed A., Greenberger J. S., Dexter T. M. Production of colony stimulating factor in long-term bone marrow cultures. J Cell Physiol. 1983 Jan;114(1):88–92. doi: 10.1002/jcp.1041140115. [DOI] [PubMed] [Google Scholar]
- Yang-Yen H. F., Chambard J. C., Sun Y. L., Smeal T., Schmidt T. J., Drouin J., Karin M. Transcriptional interference between c-Jun and the glucocorticoid receptor: mutual inhibition of DNA binding due to direct protein-protein interaction. Cell. 1990 Sep 21;62(6):1205–1215. doi: 10.1016/0092-8674(90)90396-v. [DOI] [PubMed] [Google Scholar]
- Yasukawa K., Hirano T., Watanabe Y., Muratani K., Matsuda T., Nakai S., Kishimoto T. Structure and expression of human B cell stimulatory factor-2 (BSF-2/IL-6) gene. EMBO J. 1987 Oct;6(10):2939–2945. doi: 10.1002/j.1460-2075.1987.tb02598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipori D., Toledo J., von der Mark K. Phenotypic heterogeneity among stromal cell lines from mouse bone marrow disclosed in their extracellular matrix composition and interactions with normal and leukemic cells. Blood. 1985 Aug;66(2):447–455. [PubMed] [Google Scholar]