Abstract
Background
Alpha-fetoprotein (AFP) is a well known widely used biomarker for the detection of hepatocellular carcinoma (HCC); however, it suffers from a low sensitivity and specificity. Protein or prothrombin induced by vitamin K absence or antagonist II (PIVKA-II) is another tumor marker elevated in HCC but not extensively used.
Aim
Evaluation of PIVKA-II and AFP in diagnosing HCC in India.
Patients and methods
The study group consisted of 70 consecutive HCC patients, 38 patients with cirrhosis, 30 patients with chronic hepatitis, and 30 normal healthy subjects. All patients were evaluated for PIVKA-II and AFP levels by ELISA.
Result
The mean plasma concentration of PIVKA-II in HCC, cirrhotic, chronic hepatitis patients and healthy controls was 101.07 ± 78.30 ng/ml, 2.45 ± 4.25 ng/ml, 1.50 ± 0.98 ng/ml and 0.79 ± 0.75 ng/ml, respectively. Receiver operating characteristic (ROC) curve was plotted for PIVKA-II and AFP. At a cutoff level of 9.2 ng/ml for PIVKA-II a sensitivity of 80% and a specificity of 92.1% was found, whereas AFP at a cutoff level of 13.02 ng/ml showed 72.9% sensitivity and 65.8% specificity. No significant relationship of plasma levels of PIVKA-II was observed in HCC with HBsAg/antiHCV positivity and associated portal vein thrombosis, but a positive correlation was seen with the tumor size (P = 0.001). However, no such significant association was found with AFP.
Conclusion
PIVKA-II was more sensitive and specific than AFP for diagnosing HCC in the Indian population.
Keywords: Protein induced by vitamin K absence or antagonist II, Alpha-fetoprotein, Hepatocellular carcinoma, Receiver operating characteristic curve, Area under curve
Introduction
Hepatocellular carcinoma (HCC) is a major public health problem worldwide. It is the fifth most prevalent cancer globally and the third leading cause of cancer related deaths [1]. According to recent trends a rise in the incidence of mortality from HCC has been observed in different countries [2]. The global distribution of HCC is highly variable due to the prevalence of different etiological factors in different geographic areas. According to the age-adjusted HCC incidence per 100,000 population per annum, different geographic regions can be divided into three incidence zones: low (<5), intermediate (between 5 and 15), and high (>15) [3]. Overall, most of the Asian countries come under the intermediate or high incidence zones of HCC [3]. In four population based registries, the mean incidence of HCC in India is 2.77% for males and 1.38% for females [4]. The prevalence of HCC in India varies from 0.2 to 1.6%. [4, 5].
HCC is one of the most difficult-to-treat cancers because most of the patients have either underlying cirrhosis or already have an advanced cancer at the time of diagnosis or both. As far as therapeutic strategies are concerned, surgical resection is the main curative treatment for HCC, but prognosis after resection remains poor due to high incidence of postoperative recurrence [6]. Besides this, liver transplant is another potential option for early HCC but its application is limited by the shortage of graft [7]. For unresectable HCC, various locoregional therapies like transcatheter arterial chemoembolization (TACE), percutaneous intratumoral ethanol injection (PEI) and radiofrequency ablation (RFA) are available, but these modalities are mainly for palliation and are applicable only to small group of patients with tumors localized in the liver [8].
Screening among the high risk population would benefit in detecting HCC at an early curable stage and yield a long-term survival [9, 10]. The principal diagnostic methods for HCC include imaging studies such as ultrasonography (US), computed tomography (CT), and magnetic resonance imaging (MRI) [11–16]. Tumor markers are also helpful in diagnosing HCC. Alpha-fetoprotein (AFP) is the most commonly used marker for the diagnosis of HCC. An elevated serum level of AFP is known to be associated with HCC but it is also elevated in some cases of chronic hepatitis and cirrhosis [17]. Thus, it has poor sensitivity and specificity. However, PIVKA-II is another useful marker for the diagnosis of HCC [18–21]. Till date there is no report available regarding the sensitivity and specificity of PIVKA-II in the Indian ethnic population. Thus, aim of this study was to evaluate the sensitivity and specificity of PIVKA-II in the Indian HCC patients.
Materials and methods
Patients
A total of 138 consecutive patients with liver disease (70 HCC; 38 cirrhosis; 30 chronic hepatitis) who attended the Liver Clinic from June 2006 to March 2009 at Post Graduate Institute of Medical Education and Research, Chandigarh, India and 30 healthy volunteers were included in the study. All patients were naive to treatment and did not receive any antiviral therapy for hepatitis B or C or HCC-directed therapy like TACE, RFA, PEI or resection prior to inclusion.
Inclusion criteria
HCC was diagnosed on the basis of either:
Histological confirmation or two concordant imaging studies with typical findings of HCC, which includes a high-density mass in the arterial phase and a low-density mass in the portal phase on dynamic computed tomography or magnetic resonance imaging (MRI).
Cirrhosis Liver was diagnosed on the basis of clinical, biochemical and radiological evidence suggestive of cirrhosis with or without endoscopy showing esophageal varices [22].
Chronic hepatitis B and C was diagnosed if there was an elevation of serum ALT levels at least two times upper limit of normal and HBsAg/antiHCV (HCV RNA positive) was present in the serum for at least 6 months [23].
The study was conducted only after approval from the ethical committee of the institute and an informed written consent was taken from all the patients before enrolling them in the study.
A detailed clinical examination was done in all patients. HBsAg and antiHCV were done in all the patient groups. Those found negative for the above markers; total antiHBc was done by ELISA. Patients negative for HBsAg, antiHBc and antiHCV were labeled as Non-B Non-C. Ultrasound and CT/MRI were done in all the HCC patients and on the basis of these imaging techniques, the tumor characteristics were studied including the diameter, single or multiplicity, and associated portal vein thrombosis, etc. All the HCC patients and other chronic liver disease patients were treated depending on the stage of the disease.
Measurement of PIVKA-II and AFP
PIVKA-II and AFP levels were measured in all the patients and healthy volunteers using commercially available kits according to the manufacturer’s instructions. Kits for the plasma PIVKA-II and serum AFP levels were purchased from Diagnostica Stago, France, and Smart Diagnostics, Israel, respectively.
Statistical analysis
Normality of all quantitative data variables was checked by means of skewness and Kolmogorov–Smirnov test. For normally distributed data, mean and standard deviation were calculated and for skewed data median and interquartile range (IQR) were given. For comparison of four groups analysis of variance (ANOVA) was applied with post hoc multiple comparison in case of normal distributed data. In case of skewed data with more than two groups, Kruskal–Wallis test was applied and for only two groups, Mann–Whitney test was used. Receiver operating characteristics (ROC) curves were constructed to compare the performance and also to set the optimal cutoff value of AFP and PIVKA-II. Sensitivity, specificity, positive predictive value, negative predictive value, and diagnostic accuracy of both markers alone as well as in different combination for the diagnosis of HCC were calculated. The combination of AFP and PIVKA-II was considered in two ways: if both AFP and PIVKA-II have values more than the cutoff and if either of the two (APF or PIVKA-II) has value greater than the cutoff. The relationship between different variables like AFP, PIVKA-II, and tumor size was analyzed by Spearman Rho correlation method. Variables that were significantly different between groups at univariate analysis were further analyzed by multivariate analysis. In addition, multivariate regression analysis was used to assess the association between AST, tumor size, and PIVKA-II. All statistical analysis was performed by using the SPSS 15.0 version. Differences and correlations were considered statistically significant at P < 0.05. VassarStats: Web Site for Statistical Computation was used for calculating the 95% confidence interval of sensitivity and specificity of AFP and PIVKA-II.
Results
Of the 70 HCC patients [mean age of 58.84 ± 11.23 (SD) years], 62 were males and 8 females. Twenty-four (34.2%) were HBsAg positive and 19 (27.1%) antiHCV positive. However, 6 (non HBsAg, non antiHCV) patients were found to be positive for antiHBc taking the total positivity for HBV associated HCC to 30 (42.8%) patients. Remaining 21 (30%) HCC patients were negative for all the viral markers. Cirrhosis was present in 70% of our HCC patients.
Tumor was single in 21 (30%) and multiple in 49 (70%) patients. The size of the tumor was found to be <3 cm in 9 (12.86%), 3–5 cm in 11 (15.71%), 5–6.5 cm in 8 (11.43%) and as many as 42 (60%) had a tumor size of >6.5 cm. Portal vein was thrombosed in 55.7% of HCC cases (Table 1).
Table 1.
Radiological profile (based on ultrasound/CT-scan/MRI findings) of HCC patients
| n (%) | |
|---|---|
| Cirrhosis | |
| Present | 49 (70) |
| Absent | 21 (30) |
| Portal vein thrombosis | 39 (55.7) |
| Localization (n = 70) | |
| Right lobe | 25 (35.71) |
| Left lobe | 7 (10) |
| Both lobes | 38 (54.24) |
| Tumor number | |
| Single | 21 (30) |
| Multiple (≥2) | 49 (70) |
| Tumor size (n = 70) | |
| <3 cm | 9 (12.86) |
| 3–5 cm | 11 (15.71) |
| 5–6.5 cm | 8 (11.43) |
| >6.5 | 42 (60) |
Out of 38 patients with cirrhosis (mean age: 47.92 ± 11.34; M:F—26:12), 16 were HBsAg positive, 15 antiHCV positive and 3 antiHBc positive whereas remaining 3 patients were negative for HBsAg, antiHCV and antiHBc. CT-Scan was done in all these patients to rule out HCC.
Chronic hepatitis (mean age: 35.93 ± 10.24; M:F—27:3) patients included 15 positive for HBsAg and 15 positive for antiHCV antibody). All our controls (mean age: 46.70 ± 10.24; M:F—21:9) had a normal biochemistry and were negative for HBsAg, AntiHCV and antiHBc.
ELISA for PIVKA-II and AFP
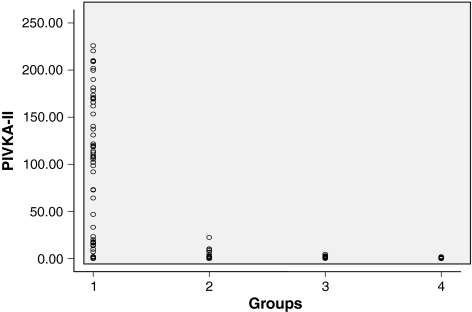
The mean plasma concentration of PIVKA-II in HCC, cirrhosis, chronic hepatitis and healthy volunteers was 101.07 ± 78.30 ng/ml, 2.45 ± 4.25 ng/ml, 1.50 ± 0.98 ng/ml and 0.79 ± 0.758 ng/ml, respectively (Table 2 and Fig. 1). The mean serum levels of AFP in these groups were 2347.97 ± 7185.56 ng/ml, 28.25 ± 4.58 ng/ml, 7.84 ± 1.43 ng/ml and 2.45 ± 0.448 ng/ml, respectively (Table 2).
Table 2.
Serum markers of the study population
| Parameter | HCC (n = 70) mean ± SD median (IQR) | Cirrhosis (n = 38) mean ± SD median (IQR) | CHC or CHB (n = 30) mean ± SD median (IQR) | Control (n = 30) mean ± SD median (IQR) | P value (ANOVA) |
|---|---|---|---|---|---|
| Bilirubin (0.2–0.8 mg/dl) | 1.89 ± 2.66 0.9 (0.6–2.0) | 1.751 ± 1.71 1 (0.7–2.7) | 1.078 ± 1.65 0.7 (0.6–0.8) | 0.64 ± 0.25 0.6 (0.5–0.7) | 0.023 |
| AST (2–40 IU/l) | 117.45 ± 82.09 93 (65–139) | 98.98 ± 93.63 82 (51–108) | 103.22 ± 87.89 78 (53–118) | 33.17 ± 8.69 32 (26–40) | 0.0001 |
| ALT (2–40 IU/l) | 74.93 ± 57.46 61 (40–88) | 77.72 ± 83.7 1 53 (40–75) | 116.23 ± 96.29 100 (48–143) | 31.17 ± 9.47 33 (23–39) | 0.0001 |
| ALP (98–306 IU/l) | 315.49 ± 201.33 275 (160–481) | 291.12 ± 177.88 259 (155–369) | 210.60 ± 96.50 195 (158–279) | 141.7 ± 57.56 170 (135–228) | 0.0001 |
| Albumin (3.5–5.0 g/dl) | 3.252 ± 0.6342 3.1 (2.8–3.8) | 3.123 ± 0.6876 3.0 (2.6–3.5) | 3.783 ± 0.7120 3.8 (3.3–4.4) | 4.043 ± 0.4739 4.1 (3.7–4.4) | 0.0001 |
| PIVKA-II (ng/ml) | 101.07 ± 78.30 108.03 (15.85–170.24) | 2.45 ± 4.25 1.21 (00–2.23) | 1.50 ± 0.98 1.41 (0.78–2.06) | 0.79 ± 0.758 0.50 (00–1.50) | 0.0001 |
| AFP (ng/ml) | 2347.97 ± 7185.56 81 (12–791) | 28.25 ± 4.58 9 (4–21) | 7.84 ± 1.43 5 (3–9) | 4.45 ± 2.45 4 (2–6) | 0.019 |
AST aspartate aminotransferase, ALT alanine aminotransferase, ALP alkaline phosphatase, HCC hepatocellular carcinoma, CHC chronic hepatitis C, CHB chronic hepatitis B, ANOVA analysis of variance
Fig. 1.
Scatter plot showing the mean plasma levels of PIVKA-II in different groups
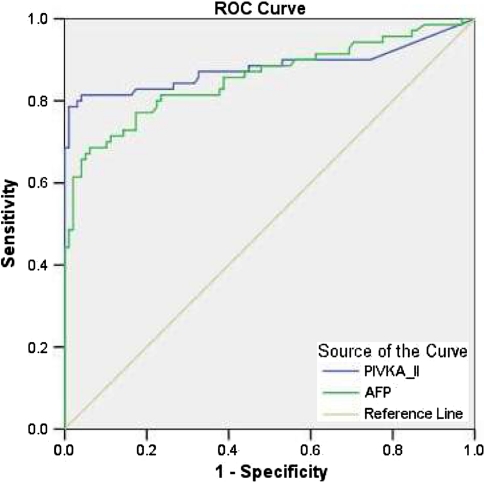
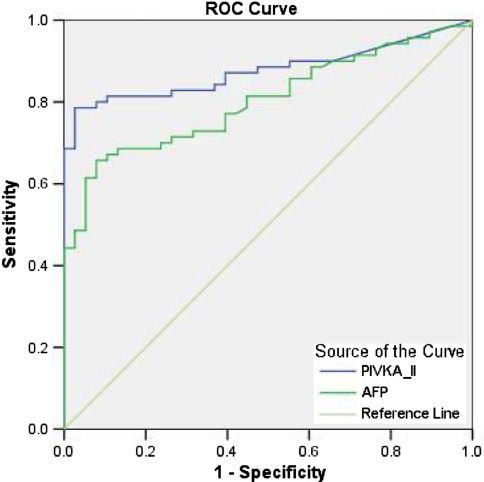
The ROC curve was plotted to identify cutoff values that would best distinguish HCC from other chronic liver diseases (Fig. 2). The optimal cutoff values for PIVKA-II and AFP were 6.98 and 11.88 ng/ml, respectively. These values yielded a sensitivity and specificity for PIVKA-II of 81.4 and 95.9% and for AFP of 77.1 and 82.7%, respectively. The AUROC indicated a better sensitivity and specificity for PIVKA-II than AFP for differentiating HCC from other groups (0.881 vs. 0.856, P = 0.0001). The optimal values for PIVKA-II and AFP in differentiating patients with HCC from those with cirrhosis were 9.20 and 13.02 ng/ml, respectively (Fig. 3). These values gave a sensitivity of 80% (95% CI; 0.68–0.88) and specificity of 92.1% (95% CI; 0.77–0.97) for PIVKA-II while for AFP it was 72.9% (95% CI; 0.60–0.82) and 65.8% (95% CI; 0.48–0.79), respectively. The AUROC indicated a better sensitivity and specificity for PIVKA-II than AFP for differentiating HCC from cirrhosis (0.87 vs. 0.803, P = 0.0001). Table 3 shows the comparison of sensitivity, specificity, positive predictive value, negative predictive value for both of these markers and their combination.
Fig. 2.
ROC curves comparing PIVKA-II and AFP in patients with HCC versus those with non-HCC patients. The curves show the optimal cutoff value for PIVKA-II of 6.98 ng/ml and for AFP of 11.88 ng/ml
Fig. 3.
ROC curves comparing PIVKA-II and AFP in patients with HCC versus those with cirrhosis. The curves show the optimal cutoff value for PIVKA-II of 9.20 ng/ml and for AFP of 13.02 ng/ml
Table 3.
PIVKA-II versus AFP in differentiation of patients with HCC from cirrhosis
| Variables | TP | FP | TN | FN | Sensitivity [%(95% CI)] | Specificity [%(95% CI)] | PPV (%) | NPV (%) | Diagnostic accuracy (%) |
|---|---|---|---|---|---|---|---|---|---|
| PIVKA-II | 56 | 14 | 35 | 3 | 80.0 (0.68–0.88) | 92.1 (0.77–0.97) | 94.9 | 71.4 | 84.2 |
| AFP | 51 | 19 | 25 | 13 | 72.8 (0.60–0.82) | 65.8 (0.48–0.79) | 79.7 | 56.8 | 70.4 |
| AFP/PIVKA-II | 64 | 6 | 24 | 14 | 91.4 (0.81–0.96) | 63.1 (0.45–0.77) | 82.0 | 63.1 | 81.1 |
| PIVKA-II + AFP | 47 | 23 | 36 | 2 | 67.1 (0.54–0.77) | 94.7 (0.80–0.99) | 95.9 | 61.0 | 76.8 |
TP true positive, FP false positive, TN true negative, FN false negative, PPV positive predictive value, NPV negative predictive value
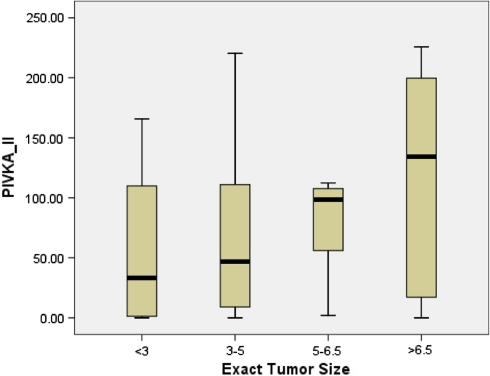
The combination showed a sensitivity of 67.1% (95% CI; 0.54–0.77) and specificity of 94.7% (95% CI; 0.80–0.99). The combination of these two markers resulted in an improvement in specificity compared to PIVKA-II alone, but the sensitivity decreased. A PIVKA-II value of 9.20 ng/ml had a PPV of 94.9% and NPV of 71.4%, respectively, for differentiating HCC from the cirrhosis, whereas the corresponding values for AFP at the level of 13.02 ng/ml were 79.7 and 56.8%, respectively. Univariate analysis identified albumin (P = 0.039), AST (P = 0.001), and tumor size (P = 0.0001) (Fig. 4) as variables that had a significant correlation with PIVKA-II. In multivariate analysis, AST and tumor size were the variables that significantly showed correlation with PIVKA-II values (P = 0.014; 0.031). Locoregional therapies like RFA and TACE were done only in 32 (44.3%) of HCC patients, whereas resection was done in 4 (5.7%) HCC patients. Other patients with advanced liver disease were either given systematic therapy or anti-angiogenic drugs.
Fig. 4.
Box plot showing the relation between the PIVKA-II levels and tumor size
Discussion
AFP and ultrasonography have most commonly been used for HCC screening and diagnosis [24]. However, many studies have already shown that AFP has a poor sensitivity and specificity for HCC and the likelihood of the disease being present is different at different concentrations of AFP (e.g., HCC is more likely if the AFP is 200 ng/ml than if it is 5 ng/ml). Therefore, the optimal diagnostic cutoff having a higher sensitivity, specificity and positive and negative predictive values has to be determined. The other most widely used tool for HCC diagnosis is ultrasonography. Although, many improvements in ultrasound techniques including contrast-enhanced ultrasound are available or under development, a major drawback of using ultrasound as a screening tool, is its high operator dependence [25, 26]. PIVKA-II is another serological test used to diagnose HCC.
For the first time in 1984 Leibman et al. [27] reported PIVKA-II levels to be significantly elevated in HCC patients in contrast to undetectable levels in normal healthy volunteers. The clinical usefulness of PIVKA-II in the detection of HCC has been demonstrated in many studies. Nevertheless, it is still controversial as to whether AFP is superior to PIVKA-II [28–30]. In a recent Japanese study, the performance of PIVKA-II was rather lower than AFP; the AUROC of each marker was 0.812 and 0.887, respectively (P < 0.0001) [31]. On the other hand, in a Western study PIVKA-II showed higher sensitivity and specificity compared to AFP and AFP-L3, irrespective of tumor size [31]. Based on our results, the ROC curves indicated a higher sensitivity and specificity for PIVKA-II than AFP in differentiating HCC from other chronic liver diseases in the Indian population. Such different results might be related to a difference in etiologies of underlying liver disease and the ethnicity of the population studies.
There are two different kits commonly used for estimation of PIVKA-II. One gives the PIVKA-II value in mAU/ml (Eisai, Japan) and other gives value in ng/ml (Diagnostica Stago, France). Different cutoff values have been proposed by different authors in different ethnic populations. A recent report by Tanaka et al. [18] estimated the serum PIVKA-II concentration (mAU/ml) in patients with HCC, cirrhosis, chronic hepatitis and normal controls and took the cutoff as 40 mAU/ml for PIVKA-II; they reported a significantly higher PIVKA-II concentration in HCC (5420.3 ± 3960.0 mAu/ml) when compared to patients with cirrhosis (26.3 ± 7.2 mAu/ml), chronic hepatitis (16.1 ± 2.0 mAU/ml) and normal controls (15.7 ± 1.1 mAu/ml). Another study by Marrero et al. [32] compared the PIVKA-II and AFP levels in an American cohort, and used 63 mAU/ml as a cutoff for PIVKA-II and found elevated levels in 3 (5%), 9 (17%), and 50 (90%) subjects with chronic hepatitis, cirrhosis and HCC, respectively. Lamerz et al. [33] in 1999 used the two commercially available PIVKA-II kits simultaneously (Stago and Eisai) for discriminating the benign group from malignant group and found significantly elevated levels of PIVKA-II in malignant group by both kits. A recent study by Beale et al. [20] has compared the PIVKA-II levels in HCC and cirrhotic patients, and claimed a higher median PIVKA-II levels in HCC (42.74 ng/ml) when compared with cirrhosis (7.8 ng/ml). Similar results have been shown by Grosley et al. [34]. Our results are no different from the above studies. Based on the ROC, we propose a cutoff of 9.20 ng/ml for the diagnosis of HCC and its distinction from cirrhosis and chronic hepatitis. At this level, the sensitivity is 80% and specificity is 92.1%. Although PIVKA-II is highly specific for HCC, it is not quite sensitive enough for the diagnosis of small HCC [35, 36]. Therefore, PIVKA-II is not currently used for surveillance of HCC. Similar results were found in our study. We categorized HCC patients on the basis of their tumor size and found 59.74 ± 67.36, 70.28 ± 74.87, 77.20 ± 45.95 and 120.45 ± 80.89 PIVKA-II levels (ng/ml) in patients with tumor size <3 cm, 3–5 cm, 5–6.5 cm and >6.5 cm, respectively.
Comparison of the clinicopathological variables with PIVKA-II levels by multivariate analysis revealed that AST and tumor size were two factors that independently affected the PIVKA-II levels. A higher AST-to-ALT ratio has been reported in patients with HCC compared with cirrhosis, likely due to an increase in cytosolic AST [37, 38]. A larger tumor size is also known to be associated with the elevated PIVKA-II levels [39, 40]. Our results also suggest that PIVKA-II was closely related to tumor size in comparison to AFP.
PIVKA-II also has been reported to predict the progression of HCC patients as those with higher PIVKA-II levels had a significantly higher frequency of intrahepatic metastasis, portal or hepatic vein tumor thrombosis and capsular infiltration [41, 42]. In our study, however, we did not find any relationship between the portal vein invasion and PIVKA-II levels.
Some studies have shown that the PIVKA-II and AFP levels are associated with the etiology of the liver disease [27, 43, 44]. Collier et al. [39] in a study has shown that the performance of AFP for HCC may be dependent on the cause of underlying liver disease. Moreover, Ohhira et al. [44] found higher PIVKA-II levels in alcohol-induced liver disease when compared with that of chronic viral hepatitis. In our study, we could not find any such relationship between the etiologies of HCC, supporting the results of Liebman et al. [27] who also did not show any correlation between the etiologies.
Multicentric studies involving large number of subjects with chronic hepatitis and cirrhosis are indicated to induct PIVKA-II as a necessary investigation for the diagnosis of HCC.
Acknowledgments
This work was financially supported by Indian Council of Medical Research (ICMR), New Delhi, India.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153–156. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745–750. doi: 10.1056/NEJM199903113401001. [DOI] [PubMed] [Google Scholar]
- 3.Bosch FX. Global epidemiology of hepatocellular carcinoma. In: Okuda K, Tabor E, editors. Liver Cancer. New York: Churchill Livingstone; 1997. pp. 13–27. [Google Scholar]
- 4.Report Annual. National Cancer Registry Programme. New Delhi: Indian Council of Medical Research; 1987. p. 1990. [Google Scholar]
- 5.Jayant K, Rao RS, Nene BM, Dale PS. Rural Cancer Registry at Barshi—Report 1988–1992. Barshi: Rural Cancer Registry;1994 [PubMed]
- 6.Poon RT, Fan ST, Lo CM, Ng IO, Liu CL, Lam CM, Wong J. Improving survival results after resection of hepatocellular carcinoma: a prospective study of 377 patients over 10 years. Ann Surg. 2001;234:63–70. doi: 10.1097/00000658-200107000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poon RT, Fan ST. Resection prior to liver transplantation for hepatocellular carcinoma: a strategy of optimizing the role of resection and transplantation in cirrhotic patients with preserved liver function. Liver Transpl. 2004;10:813–815. doi: 10.1002/lt.20175. [DOI] [PubMed] [Google Scholar]
- 8.Poon RT, Fan ST, Tsang FH, Wong J. Locoregional therapies for hepatocellular carcinoma: a critical review from the surgeon’s perspective. Ann Surg. 2002;235:466–486. doi: 10.1097/00000658-200204000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liaw YF, Tai DI, Chu CM, Lin DY, Sheen IS, Chen TJ, Pao CC. Early detection of hepatocellular carcinoma in patients with chronic type B hepatitis. A prospective study. Gastroenterology. 1986;90:263–267. doi: 10.1016/0016-5085(86)90919-4. [DOI] [PubMed] [Google Scholar]
- 10.Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004;130:417–422. doi: 10.1007/s00432-004-0552-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shinagawa T, Ohto M, Kimura K, Tsunetomi S, Morita M, Saisho H, Tsuchiya Y, Saotome N, Karasawa E, Miki M, et al. Diagnosis and clinical features of small hepatocellular carcinoma with emphasis on the utility of real-time ultrasonography. A study in 51 patients. Gastroenterology. 1984;86:495–502. [PubMed] [Google Scholar]
- 12.Ikeda K, Saitoh S, Koida I, Tsubota A, Arase Y, Chayama K, Kumada H. Diagnosis and follow-up of small hepatocellular carcinoma with selective intraarterial digital subtraction angiography. Hepatology. 1993;17:1003–1007. doi: 10.1002/hep.1840170610. [DOI] [PubMed] [Google Scholar]
- 13.Oka H, Tamori A, Kuroki T, Kobayashi K, Yamamoto S. Prospective study of alpha-fetoprotein in cirrhotic patients monitored for development of hepatocellular carcinoma. Hepatology. 1994;19:61–66. doi: 10.1002/hep.1840190111. [DOI] [PubMed] [Google Scholar]
- 14.Takayasu K, Moriyama N, Muramatsu Y, Makuuchi M, Hasegawa H, Okazaki N, Hirohashi S. The diagnosis of small hepatocellular carcinomas: efficacy of various imaging procedures in 100 patients. Am J Roentgenol. 1990;155:49–54. doi: 10.2214/ajr.155.1.1693808. [DOI] [PubMed] [Google Scholar]
- 15.Takayasu K, Furukawa H, Wakao F, Muramatsu Y, Abe H, Terauchi T, Winter TC, III, Sakamoto M, Hirohashi S. CT diagnosis of early hepatocellular carcinoma: sensitivity, findings, and CT-pathologic correlation. Am J Roentgenol. 1995;164:885–890. doi: 10.2214/ajr.164.4.7726041. [DOI] [PubMed] [Google Scholar]
- 16.Ebara M, Ohto M, Watanabe Y, Kimura K, Saisho H, Tsuchiya Y, Okuda K, Arimizu N, Kondo F, Ikehira H, et al. Diagnosis of small hepatocellular carcinoma: correlation of MR imaging and tumor histologic studies. Radiology. 1986;159:371–377. doi: 10.1148/radiology.159.2.3008213. [DOI] [PubMed] [Google Scholar]
- 17.Hu KQ, Kyulo NL, Lim N, Elhazin B, Hillebrand DJ, Bock T. Clinical significance of elevated alpha-fetoprotein (AFP) in patients with chronic hepatitis C, but not hepatocellular carcinoma. Am J Gastroenterol. 2004;99:860–865. doi: 10.1111/j.1572-0241.2004.04152.x. [DOI] [PubMed] [Google Scholar]
- 18.Kim HS, Park JW, Jang JS, Kim HJ, Shin WG, Kim KH, Lee JH, Kim HY, Jang MK. Prognostic values of alpha-fetoprotein and protein induced by vitamin K absence or antagonist-II in hepatitis B virus-related hepatocellular carcinoma: a prospective study. J Clin Gastroenterol. 2009;43:482–488. doi: 10.1097/MCG.0b013e318182015a. [DOI] [PubMed] [Google Scholar]
- 19.Sterling RK, Jeffers L, Gordon F, Venook AP, Reddy KR, Satomura S, Kanke F, Schwartz ME, Sherman M. Utility of Lens culinaris agglutinin-reactive fraction of alpha-fetoprotein and des-gamma-carboxy prothrombin, alone or in combination, as biomarkers for hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2009;7:104–113. doi: 10.1016/j.cgh.2008.08.041. [DOI] [PubMed] [Google Scholar]
- 20.Beale G, Chattopadhyay D, Gray J, Stewart S, Hudson M, Day C, Trerotoli P, Giannelli G, Manas D, Reeves H. AFP, PIVKAII, GP3, SCCA-1 and follisatin as surveillance biomarkers for hepatocellular cancer in non-alcoholic and alcoholic fatty liver disease. BMC Cancer. 2008;8:200. doi: 10.1186/1471-2407-8-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Durazo FA, Blatt LM, Corey WG, Lin JH, Han S, Saab S, Busuttil RW, Tong MJ. Des-gamma-carboxyprothrombin, alpha-fetoprotein and AFP-L3 in patients with chronic hepatitis, cirrhosis and hepatocellular carcinoma. J Gastroenterol Hepatol. 2008;23:1541–1548. doi: 10.1111/j.1440-1746.2008.05395.x. [DOI] [PubMed] [Google Scholar]
- 22.Gentilini P, Laffi G, Villa G, Romanelli RG, Buzzelli G, Casini-Raggi V, Melani L, Mazzanti R, Riccardi D, Pinzani M, Zignego AL. Long course and prognostic factors of virus-induced cirrhosis of the liver. Am J Gastroenterol. 1997;92:66–72. [PubMed] [Google Scholar]
- 23.Lok AS, Heathcote EJ, Hoofnagle JH. Management of hepatitis B: 2000—summary of a workshop. Gastroenterology. 2001;120:1828–1853. doi: 10.1053/gast.2001.24839. [DOI] [PubMed] [Google Scholar]
- 24.Okuda K. Early recognition of hepatocellular carcinoma. Hepatology. 1986;6:729–738. doi: 10.1002/hep.1840060432. [DOI] [PubMed] [Google Scholar]
- 25.Zoli M, Magalotti D, Bianchi G, Gueli C, Marchesini G, Pisi E. Efficacy of a surveillance program for early detection of hepatocellular carcinoma. Cancer. 1996;78:977–985. doi: 10.1002/(SICI)1097-0142(19960901)78:5<977::AID-CNCR6>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 26.Sherman M. Hepatocellular carcinoma: epidemiology, risk factors, and screening. Semin Liver Dis. 2005;25:143–154. doi: 10.1055/s-2005-871194. [DOI] [PubMed] [Google Scholar]
- 27.Liebman HA, Furie BC, Tong MJ, Blanchard RA, Lo KJ, Lee SD, Coleman MS, Furie B. Des-gamma-carboxy (abnormal) prothrombin as a serum marker of primary hepatocellular carcinoma. N Engl J Med. 1984;310:1427–1431. doi: 10.1056/NEJM198405313102204. [DOI] [PubMed] [Google Scholar]
- 28.Tsai SL, Huang GT, Yang PM, Sheu JC, Sung JL, Chen DS. Plasma des-gamma-carboxyprothrombin in the early stage of hepatocellular carcinoma. Hepatology. 1990;11:481–488. doi: 10.1002/hep.1840110321. [DOI] [PubMed] [Google Scholar]
- 29.Grazi GL, Mazziotti A, Legnani C, Jovine E, Miniero R, Gallucci A, Palareti G, Gozzetti G. The role of tumor markers in the diagnosis of hepatocellular carcinoma, with special reference to the des-gamma-carboxy prothrombin. Liver Transpl Surg. 1995;1:249–255. doi: 10.1002/lt.500010410. [DOI] [PubMed] [Google Scholar]
- 30.Ishii M, Gama H, Chida N, Ueno Y, Shinzawa H, Takagi T, Toyota T, Takahashi T, Kasukawa R. Simultaneous measurements of serum alpha-fetoprotein and protein induced by vitamin K absence for detecting hepatocellular carcinoma. South Tohoku District Study Group. Am J Gastroenterol. 2000;95:1036–1040. doi: 10.1111/j.1572-0241.2000.01978.x. [DOI] [PubMed] [Google Scholar]
- 31.Volk ML, Hernandez JC, Su GL, Lok AS, Marrero JA. Risk factors for hepatocellular carcinoma may impair the performance of biomarkers: a comparison of AFP, DCP, and AFP-L3. Cancer Biomark. 2007;3:79–87. doi: 10.3233/cbm-2007-3202. [DOI] [PubMed] [Google Scholar]
- 32.Marrero JA, Su GL, Wei W, Emick D, Conjeevaram HS, Fontana RJ, Lok AS. Des-gamma carboxyprothrombin can differentiate hepatocellular carcinoma from nonmalignant chronic liver disease in American patients. Hepatology. 2003;37:1114–1121. doi: 10.1053/jhep.2003.50195. [DOI] [PubMed] [Google Scholar]
- 33.Lamerz R, Runge M, Stieber P, Meissner E. Use of serum PIVKA-II (DCP) determination for differentiation between benign and malignant liver diseases. Anticancer Res. 1999;19:2489–2493. [PubMed] [Google Scholar]
- 34.Grosley BM, Hirschauer C, Chambrette B, Bezeaud A, Amiral J. Specific measurement of hypocarboxylated prothrombin in plasma or serum and application to the diagnosis of hepatocellular carcinoma. J Lab Clin Med. 1996;127:553–564. doi: 10.1016/S0022-2143(96)90146-8. [DOI] [PubMed] [Google Scholar]
- 35.Kasahara A, Hayashi N, Fusamoto H, Kawada Y, Imai Y, Yamamoto H, Hayashi E, Ogihara T, Kamada T. Clinical evaluation of plasma des-gamma-carboxy prothrombin as a marker protein of hepatocellular carcinoma in patients with tumors of various sizes. Dig Dis Sci. 1993;38:2170–2176. doi: 10.1007/BF01299891. [DOI] [PubMed] [Google Scholar]
- 36.Okuda H, Nakanishi T, Takatsu K, Saito A, Hayashi N, Takasaki K, Takenami K, Yamamoto M, Nakano M. Serum levels of des-gamma-carboxy prothrombin measured using the revised enzyme immunoassay kit with increased sensitivity in relation to clinicopathologic features of solitary hepatocellular carcinoma. Cancer. 2000;88:544–549. doi: 10.1002/(SICI)1097-0142(20000201)88:3<544::AID-CNCR8>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 37.Lai CL, Lam KC, Wong KP, Wu PC, Todd D. Clinical features of hepatocellular carcinoma: review of 211 patients in Hong Kong. Cancer. 1981;47:2746–2755. doi: 10.1002/1097-0142(19810601)47:11<2746::AID-CNCR2820471134>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 38.Shimokawa Y, Okuda K, Kubo Y, Kaneko A, Arishima T, Nagata E, Hashimoto M, Sawa Y, Nagasaki Y, Kojiro M, Sakamoto K, Nakashima T. Serum glutamic oxalacetic transaminase/glutamic pyruvic transaminase ratios in hepatocellular carcinoma. Cancer. 1977;40:319–324. doi: 10.1002/1097-0142(197707)40:1<319::AID-CNCR2820400145>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 39.Nakamura S, Nouso K, Sakaguchi K, Ito YM, Ohashi Y, Kobayashi Y, Toshikuni N, Tanaka H, Miyake Y, Matsumoto E, Shiratori Y. Sensitivity and specificity of des-gamma-carboxy prothrombin for diagnosis of patients with hepatocellular carcinomas varies according to tumor size. Am J Gastroenterol. 2006;101:2038–2043. doi: 10.1111/j.1572-0241.2006.00681.x. [DOI] [PubMed] [Google Scholar]
- 40.Suehiro T, Sugimachi K, Matsumata T, Itasaka H, Taketomi A, Maeda T. Protein induced by vitamin K absence or antagonist II as a prognostic marker in hepatocellular carcinoma. Comparison with alpha-fetoprotein. Cancer. 1994;73:2464–2471. doi: 10.1002/1097-0142(19940515)73:10<2464::AID-CNCR2820731004>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 41.Gotoh M, Nakatani T, Masuda T, Mizuguchi Y, Sakamoto M, Tsuchiya R, Kato H, Furuta K. Prediction of invasive activities in hepatocellular carcinomas with special reference to alpha-fetoprotein and des-gamma-carboxyprothrombin. Jpn J Clin Oncol. 2003;33:522–526. doi: 10.1093/jjco/hyg096. [DOI] [PubMed] [Google Scholar]
- 42.Koike Y, Shiratori Y, Sato S, Obi S, Teratani T, Imamura M, Yoshida H, Shiina S, Omata M. Des-gamma-carboxy prothrombin as a useful predisposing factor for the development of portal venous invasion in patients with hepatocellular carcinoma: a prospective analysis of 227 patients. Cancer. 2001;91:561–569. doi: 10.1002/1097-0142(20010201)91:3<561::AID-CNCR1035>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 43.Collier J, Sherman M. Screening for hepatocellular carcinoma. Hepatology. 1998;27:273–278. doi: 10.1002/hep.510270140. [DOI] [PubMed] [Google Scholar]
- 44.Ohhira M, Saito H, Suzuki Y, Naraki T, Sakurai S, Ohtake T, Suzuki M, Ohhira M, Fujimoto And Y, Kohgo Y. A variant of des-gamma-carboxy prothrombin was increased in alcoholic liver disease without hepatocellular carcinoma. Alcohol Clin Exp Res. 2001;25(suppl 6):46S–50S. doi: 10.1097/00000374-200106001-00011. [DOI] [PubMed] [Google Scholar]