Abstract
Purpose
Concanavalin A (Con A)-induced hepatitis is an extensively used animal model of T cell-mediated acute hepatitis. A variety of cytokines, including interleukin 4 (IL-4), interferon gamma (IFN-γ), and tumor necrosis factor alpha (TNF-α), have been shown to play important roles in Con A-induced liver injury. However, the role of IL-2, a critical cytokine in the development and function of T cells and a clinical therapeutics for virus infection and tumor, has not been carefully examined in this model.
Methods
In this study, we investigated the function of IL-2 in Con A-induced hepatitis by using various strategies of rhIL-2 pretreatment. We treated mice with two rhIL-2 administration strategies: a single injection of high dose of rhIL-2 (IL-2hi, 50 × 103 U/mouse) and four injections of low dose of rhIL-2 (IL-24lo, 5 × 103 U/mouse).
Results
IL-2hi pretreatment ameliorated Con A-induced liver injury, while IL-24lo aggravated Con A-induced liver injury. IL-2hi pretreatment reduced Con A-induced elevation of serum TNF-α while IL-24lo pretreatment did not. Serum IL-4 and TNF-α were high 6 h after Con A injection in IL-24lo mice, while it was undetectable in IL-2hi and non-pretreated mice. IL-2hi pretreatment reduced Con A-induced accumulation of T cells in liver while IL-24lo pretreatment increased accumulation of NK cells.
Conclusion
Various strategies of rhIL-2 administration play different roles in Con A-induced hepatitis, suggesting the importance of IL-2 administrative regime in clinical liver diseases.
Keywords: Concanavalin A, Hepatitis, Interleukin 2
Introduction
Interleukin-2 (IL-2) is a typical four-α-helix cytokine produced primarily by activated CD4+ T cells, and also produced by naïve CD8+ T cells and dendritic cells [1]. A recent study found that IL-2 is critical for the development and peripheral expansion of CD4+CD25+ regulatory T cells, which promote self-tolerance by suppressing T cell responses in vivo [2]. In T cells, IL-2 synthesis is tightly regulated at the mRNA level by signals from the TCR and CD28 [3]. IL-2 binds to and signals through a receptor complex consisting of IL-2Rα (CD25), IL-2Rβ (CD122), and common γ-chain (CD132). All three subunits are required for high-affinity binding of IL-2 [4]. IL-2 also has been used to enhance T cell immunity in patients with AIDS or cancer in clinical therapy [5, 6], but the side effects of IL-2 treatment in liver disease, especially in hepatitis virus infectious diseases, is not explored.
Administration of concanavalin A (Con A) to mice leads to T cell-mediated liver injury, which is a well-established experimental murine model characterized by rapidly increased serum levels of aminotransferase and cytokines, leukocyte infiltration of liver, and hepatocyte necrosis [7]. The hepatic natural killer T (NKT) cells play essential roles in the Con A-induced liver injury by releasing a variety of cytokines, including interleukin 4 (IL-4), IL-5, interferon gamma (IFN-γ), and tumor necrosis factor alpha (TNF-α) [8–12]. Many other cytokines are also involved in Con A-induced liver injury; for example, IL-6, IL-10, IL-15, and IL-22 show hepatoprotective effects [13–16], whereas IL-12 and IL-18 aggravate Con A-induced hepatitis [17, 18]. T lymphocytes were proved as effector cells of Con A-induced liver injury, accompanying apoptotic cell death of hepatocytes and abounding cytokines, such as TNF-α, IFN-γ, granulocyte macrophage-colony stimulating factor, and IL-2, in the serum of the mice [19]. However, the role of IL-2 in this T cell-mediated liver injury remains unclear.
In this study, we investigated the role of IL-2 by two rhIL-2 administration strategies: a single injection of high dose of rhIL-2 (IL-2hi, 50 × 103 U/mouse) and multiple injections of low dose of rhIL-2 (IL-24lo, 5 × 103 U/mouse). We examined Con A-induced liver injury in the two strategies. We investigated the effects of IL-2 by detection of cytokines, and analysis of cell types and activation markers on lymphocytes in Con A model. We found that different strategies of rhIL-2 administration results in opposite effect in Con A-induced liver injury. Our findings may highlight the importance of IL-2 administrative regime in clinical liver diseases.
Materials and methods
Animals
Six- to eight-week-old male C57BL/6 (B6) mice were purchased from the Shanghai Laboratory Animal Center, Chinese Academy of Science and maintained in our animal facilities under specific pathogen-free conditions. Animal-handling protocols and experimental procedures conformed to the guidelines outlined in the Guide for the Care and Use of Laboratory Animals.
Reagents
Con A was purchased from Sigma Chemical Co. (St. Louis, MO) and dissolved in sterile phosphate-buffered saline (PBS) at a concentration of 1 mg/ml. Human recombinant IL-2 (Changshengan, Changchun, China) was dissolved in sterile PBS. The monoclonal antibodies (mAb) used in this study include FITC-conjugated anti-NK1.1 and -anti-CD4, PE-conjugated-anti-CD8, -anti-CD25, and -anti-CD69 (eBioscience, San Diego, CA), PE-Cy5-anti-CD3 (BD PharMigen, San Diego, CA).
Animal treatments
In a single injection of high dose of rhIL-2 group (IL-2hi), mice were subcutaneous injected with rhIL-2 (50 × 103 U/mouse) 6 h before Con A injection. In multiple injection of low dose of rhIL-2 group (IL-24lo), mice were subcutaneous injected with rhIL-2 (5 × 103 U/mouse) for four times in 24 h (8-h interval), the last administration finished 6 h later, mice received Con A injection. (Peripheral administration was used in rhIL-2 pretreatment.) Mice were i.v. injected with Con A at a dose of 15 μg/g of body weight to induced liver injury. Control mice were injected with the same volume of sterile PBS.
Cell preparation
Livers were harvested at indicated time points and pressed through a 200-gauge stainless steel mesh and suspended in PBS. After washing once with PBS, the cells were resuspended in 40% Percoll. The cell suspension was overlaid gently on the top of 70% Percoll and centrifuged at 2,400 rpm for 30 min at room temperature. Hepatic mononuclear cells (MNCs) were obtained from the interphase and washed twice with PBS.
Assay for serum aminotransferase activity
Serum samples from mice were obtained at different times. Serum alanine aminotransferase (ALT) activities were determined by using the serum aminotransferase test kit (Jian Cheng, Nanjing) according to manufactures’ instructions.
Measurement of serum cytokine levels
Blood samples were obtained from retro-orbital puncture and immediately centrifuged at 4,000 rpm for 15 min. Serum samples were stored at −20°C until ready to be used. Serum levels of IL-4, IFN-γ, and TNF-α were determined using ELISA kits from R&D systems (Minneapolis, MN, USA).
Histopathology
Livers were fixed in 10% buffered formalin, embedded in paraffin and tissue sections were then cut into 5 μm slices for routine hematoxylin (DakoCytomation, Carpintera, CA)–eosin (American Master Tech Scientific, Lodi, CA) staining and examined under light microscopy.
Flow cytometry
MNCs were stained with antibodies diluted in PBS containing 2% fetal calf serum and 0.1% NaN3. Cells were analyzed by FACSCalibur flow cytometer with CellQuest software (Becton–Dickinson, Franklin Lakes, NJ).
Statistical analysis
The results were analyzed using the Student’s t test or ANOVA where appropriate. P < 0.05 was considered significant.
Results
Pretreatment of mice with high or low doses of rhIL-2 in Con A-induced hepatitis
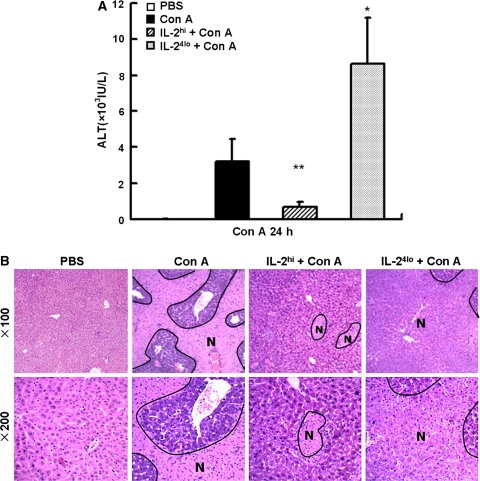
To investigate the role of rhIL-2 in Con A-induced liver injury, we measured serum ALT levels 24 h after Con A injection. As shown in Fig. 1a, when compared to the Con A alone group, IL-2hi pretreatment reduced Con A-induced elevation of ALT significantly, and IL-24lo pretreatment increased Con A-induced ALT significantly. We also detected Con A-induced liver injury by histopathological examination. As shown in the Fig. 1b, compared to the mice only with Con A injection, massive necrosis in the livers of IL-24lo pretreatment mice was observed, whereas mice pretreated with IL-2hi showed minor injury. The mice only with PBS injection did not show any necrotic hepatocyte.
Fig. 1.
Pretreatment with high or low doses of rhIL-2 in Con A-induced hepatitis. Mice were treated with PBS, Con A or IL-2/Con A. A single injection of high dose of IL-2 (IL-2hi) was injected to mice 6 h before Con A injection (15 μg/g body weight). Multiple injections of low dose of IL-2 (IL-24lo) were injected to mice in 24 h for four times (every 8 h) before Con A injection. Sera and liver samples were collected 24 h after Con A injection. a Serum ALT levels were measured. *P < 0.05; **P < 0.001 versus Con A. b representative results of histopathological examination of liver in PBS-, Con A- and IL-2/Con A-treated mice (original magnification ×100 and ×200). N indicated necrosis area. Similar results were obtained from 3 mice in three independent experiments
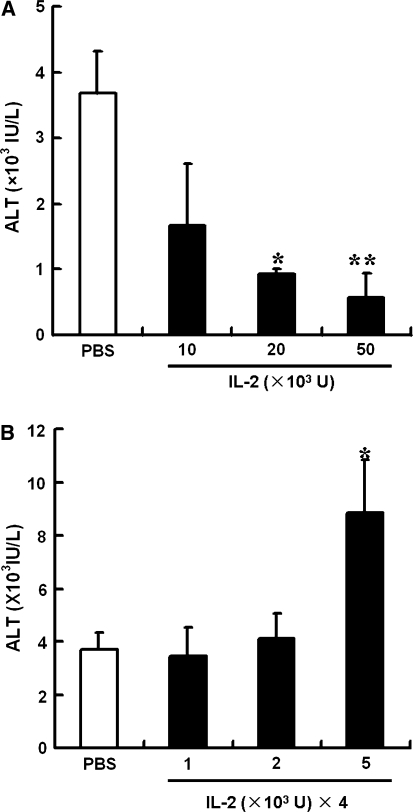
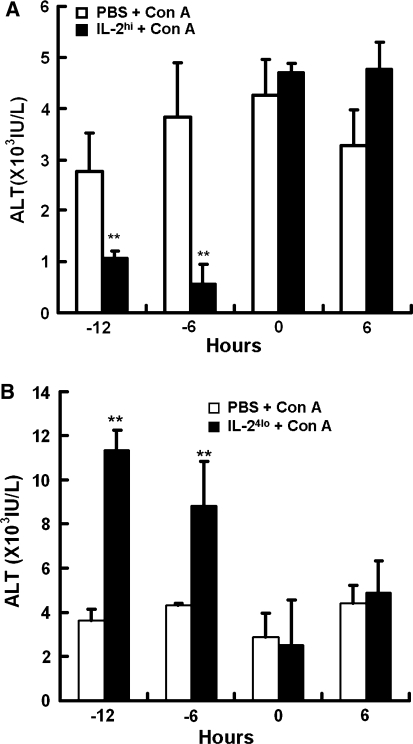
We evaluated the different doses of rhIL-2 in Con A-induced liver injury. Figure 2a illustrates that different doses of IL-2hi pretreatment exerted a dose-dependent protection against liver injury. Pretreatment of 50 × 103 and 20 × 103 U/mouse of rhIL-2 markedly inhibited Con A-induced elevation of serum ALT levels, whereas 10 × 103 U/mouse of rhIL-2 only partly alleviated ALT elevation. As shown in Fig. 2b, IL-24lo pretreatment aggravated Con A-induced liver injury in a dose-dependent manner. Pretreatment of 5 × 103 U/mouse of rhIL-2 markedly increased Con A-induced serum ALT elevation, whereas 1 × 103 and 2 × 103 U/mouse of rhIL-2 had no effect on ALT levels. Time points of rhIL-2 injection according to Con A administration were also examined. As shown in Fig. 3a, in the IL-2hi group, injection of rhIL-2, 12 or 6 h before Con A injection reduced the serum ALT significantly, whereas injection of rhIL-2 and Con A at the same time did not alleviate ALT levels, and injection of rhIL-2 after Con A injection increased ALT elevation mildly. The results of IL-24lo group were shown in Fig. 3b. When rhIL-2 were injected 12 or 6 h before Con A injection, the elevation of serum ALT was increased more significantly, whereas injection of rhIL-2 at the same time of or after Con A injection had no effect on ALT levels.
Fig. 2.
Pretreatment with different doses of rhIL-2 in Con A-induced hepatitis. Mice were treated with PBS or IL-2 before Con A administration. a A single injection of indicated high doses of IL-2 were injected to mice 6 h before Con A injection. b Multiple injections of indicated low doses of IL-2 were injected to mice in 24 h for four times (every 8 h) before Con A injection. Sera were collected 24 h after Con A injection and serum ALT levels were measured. Values in a and b are shown as means ± SEM (n = 3) from 3 mice in three independent experiments. *P < 0.05 versus PBS. **P < 0.001 versus PBS
Fig. 3.
Treatment with high or low doses of rhIL-2 at different time courses in Con A-induced hepatitis. Mice were treated with PBS or IL-2 before or after Con A. a A single injection of high dose of IL-2 (IL-2hi, 50,000 U) were injected to mice at indicated time points. The time of Con A injection was identified as 0 h. Sera were collected 24 h after Con A injection. b Multiple injections of low dose of IL-2 (IL-24lo, 5,000 U) were injected to mice in 24 h for four times (every 8 h) at indicated time points. Sera were collected 24 h after Con A injection. Values in a and b are shown as means ± SEM (n = 3) from 3 mice in three independent experiments. **P < 0.001 versus PBS
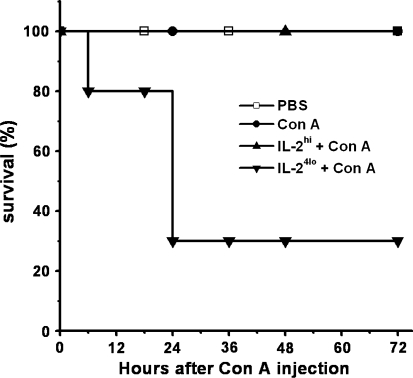
Survival of mice was also examined. As shown in the Fig. 4, all of PBS-, Con A- and IL-2hi + Con A-treated mice survived, whereas 20% of IL-24lo + Con A-treated mice began to die within 6 h after Con A injection and only 30% mice could live more than 24 h.
Fig. 4.
Effect of pretreatment with high or low doses of rhIL-2 on survival of Con A-treated mice. Analysis of the effect of different IL-2 administration on survival in mice treated with Con A (5 mice/group) was shown. Mice were injected with PBS or IL-2 (IL-2hi, 50,000 U; IL-24lo, 5,000 U) 6 h before administration with Con A
Serum cytokines profile of rhIL-2-pretreated Con A-induced hepatitic mice
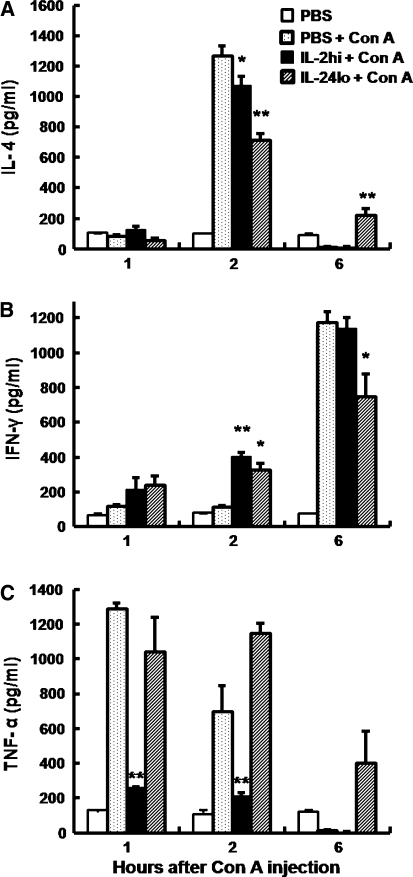
NKT cells rapidly secrete a large amount of cytokines, including IL-4, IFN-γ and TNF-α, which have been shown to play essential roles in Con A-induced liver injury. We examined whether IL-2hi and IL-24lo influenced the production of these cytokines. As shown in the Fig. 5, as compared to Con A-treated mice, IL-2hi pretreatment reduced serum levels of IL-4 at 2 h and reduced TNF-α at 1 and 2 h after Con A injection, whereas serum IFN-γ production increased at 2 h after Con A injection. IL-24lo pretreatment also reduced serum levels of IL-4 at 2 h and increased serum levels of IFN-γ at 2 h after Con A injection. Interestingly, IL-24lo pretreatment reduced production of IFN-γ at 6 h, but increased production of IL-4 at 6 h and TNF-α at 2 and 6 h after Con A injection.
Fig. 5.
Serum cytokines profile of mice with high or low doses of IL-2 pretreatment in Con A-induced Hepatitis. Mice were treated with PBS or different IL-2 administration (IL-2hi/50,000 U, IL-24lo/5,000 U) for 6 h, followed by administration with Con A. Serum samples were collected at various times post Con A injection (1, 2, 6 h), and serum levels of IL-4 (a), IFN-r (b), and TNF-α (c) were measured by ELISA. Data are expressed as means ± SEM (n = 3) from 3 mice in three independent experiments. *P < 0.05; **P < 0.001 versus PBS + Con A
Hepatic lymphocytes of rhIL-2-pretreated Con A-induced hepatitic mice
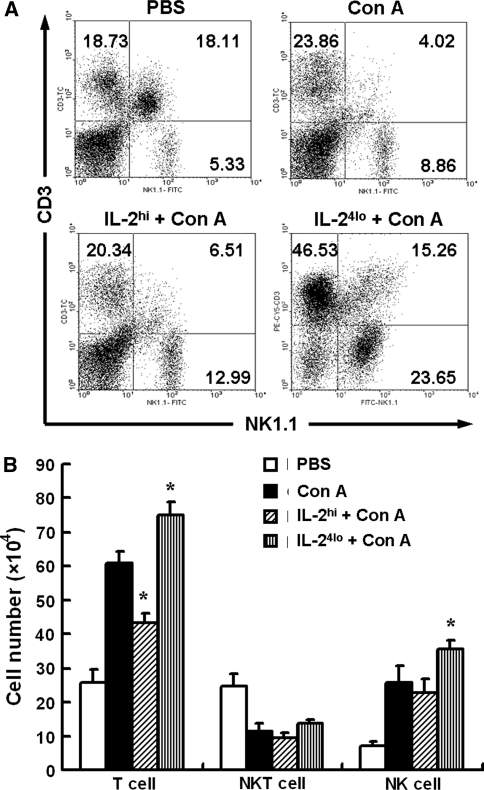
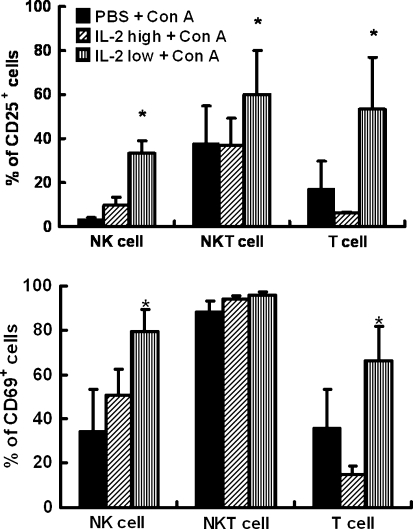
Con A injection often induces extensive T cell accumulation in liver. Therefore, we examined the change of hepatic lymphocytes. As shown in Fig. 6, when mice were treated with Con A, frequency and numbers of hepatic T cells and NK cells increased while NKT cells decreased. The increase of hepatic T cells decreased in IL-2hi-pretreated mice, whereas the increase of hepatic NK cells and T cells both pronounced more significantly in IL-24lo-pretreated mice. To explore the possible functional variation of lymphocyte populations, we observed the activation of hepatic lymphocytes in Con A-treated mice with and without rhIL-2 pretreatment. As shown in Fig. 7, the frequency of both CD25 and CD69 on NK and T cells markedly increased in IL-24lo-pretreated mice, whereas the frequency of CD25 and CD69 on T cells decreased significantly after IL-2hi-pretreated mice. The frequency of CD25+ NKT cells increased in IL-24lo-pretreated mice, and the frequency of CD69+ NKT cells had no change.
Fig. 6.
Hepatic lymphocytes content of mice with high or low doses of IL-2 pretreatment in Con A-induced Hepatitis. Mice were treated with PBS, Con A or different IL-2 administration (IL-2hi/50,000 U, IL-24lo/5,000 U) for 6 h, followed by administration with Con A. Liver samples were collected 24 h after Con A injection and hepatic MNCs were isolated. Hepatic MNCs were stained with anti-NK1.1 and anti-CD3 antibodies and analyzed by FACS. a Representative flow cytometry analysis of the percentage of NK (CD3−NK1.1+), NKT (CD3+NK1.1+) and T (CD3+NK1.1−) cells. b The absolute numbers of hepatic T, NKT and NK cells were calculated by multiplying the frequency of cells by the absolute numbers of hepatic MNCs, respectively. Values in b are expressed as means ± SEM (n = 3) from 3 mice in three independent experiments. *P < 0.05 versus Con A
Fig. 7.
Activation of hepatic lymphocytes with high or low doses of IL-2 pretreatment in Con A-induced hepatitis. Mice were treated with Con A or IL-2/Con A (IL-2hi/50,000 U, IL-24lo/5,000 U). For flow cytometric analysis. Liver samples were collected 24 h after Con A injection and hepatic MNCs were isolated. Hepatic MNCs were stained with anti-CD3, anti-NK1.1, anti-CD25 or anti-CD69 mAb. T (CD3+NK1.1−), NKT (CD3+NK1.1+) and NK (CD3−NK1.1+) cells were then examined for CD25 (upper) and CD69 (lower) expression. Values in a and b are shown as means ± SEM (n = 3) from 3 mice in three independent experiments. *P < 0.05 versus PBS + Con A
Discussion
Interleukin-2 is a potent T cell growth factor, which can potently induce T cell expansion in vitro and in vivo [2]. This assumption led to the development of therapeutic strategies for clinical benefit, such as to enhance T cell numbers and function in patients with cancer or AIDS [5, 6]. Although these examples support that IL-2 serves as an important T cell growth factor in vivo, in some cases, mice lacking the IL-2 or IL-2R genes are not markedly immunocompromised but instead develop severe T cell-mediated autoimmune disease [20–22]. High-dose and continuous IL-2 therapy used in the clinical were proved to be associated with significant toxicities that influenced a variety of human organs. Alternative IL-2 therapy approaches have been evaluated in order to decrease the toxicities. Administration of lower doses of IL-2 showed the similar effect to those with high-dose IL-2 therapy in several clinical studies [5], but no study in hepatitic virus diseases. So, it needs to better understand the administration regime of IL-2 in liver immune injury, in order to provide a clinical manipulation of IL-2 to achieve the maximum therapeutic benefit in patients.
Con A-induced liver injury is characterized by markedly increasing of serum aminotransferase levels and massive infiltration of lymphocytes into the liver [23, 24]. Con A activates lymphocytes which residing in the liver, such as NKT cells and CD4+ T cells [7, 8], mainly cause liver injury. Con A can also activate peripheral T cells accumulating into liver to mediate liver injury [19]. These activated cells release inflammatory cytokines, which is also an important reason for liver injury. It has been shown that TNF-α, IFN-γ and IL-4 are involved in this liver injury model [7, 10, 11, 19, 23]. In our studies, we found that different strategies of IL-2 administration result in different effect in Con A-induced hepatitis. In this model, a single injection of high-dose IL-2 mainly decreased the number of activated cells accumulating in the liver, suppressing activation of T cells and reduced the level of TNF-α. Multiple injection of low-dose IL-2 aggravated liver injury, but inflammatory cytokines levels were not all found increased in serum.
IL-2hi pretreatment reduced Con A-mediated induction of IL-4 and TNF-α. TNF-α is one of the most important cytokines in Con A-induced liver injury that caused damage to liver tissues [12]. Our studies showed that IL-2hi pretreatment through regulating these cytokines level to alleviate the damage that caused by TNF-α. In IL-24lo pretreatment, we found that TNF-α increased after Con A injection, the damage of liver tissues was aggravated, indicating that TNF-α level is a major cause for this pretreatment effect. Regulatory T cells (Tregs) may play a crucial role in tolerance in the liver. Different IL-2 administrations may have some effects on the Tregs, and the frequency of other cell types may not have the same changes as usually in Con A-induced hepatitis. However, further studies need to be made to explain how IL-2 affects on the Tregs. In addition, we observed changes in liver lymphocytes and found that in IL-2hi mice the accumulation of T cells decreased in the liver after Con A injection, whereas the number of both T cells and NK cells increased more pronouncedly in the liver of IL-24lo mice. Our results demonstrated that NKT cells had no remarkable changes after these two IL-2 administrations. NKT cells play a critical role in Con A-induced hepatitis, and it might be eliminated quickly after they accomplished their effector functions in the liver. It has been reported that some members of the TNF family other than FasL, including TNF and TRAIL, are involved in activation-induced cell death (AICD) of pre-activated T cells. Therefore, these molecules and cytokines may contribute to apoptosis of NKT cells. We showed that IL-2hi pretreatment prevented liver tissues from injury induced by Con A injection and IL-24lo pretreatment aggravated liver tissues damage as detected by tissue histology. However, it remains uncertain whether IL-2hi inhibits lymphocyte proliferation directly or by promoting the survival advantage of hepatocytes, which in turn render them resistant to T cell-mediated injury. The hepatocytes in the liver exhibit remarkable regenerative potential [19, 25], it remains to be determined how IL-2hi would affect the proliferation of hepatocytes in this model. Whether IL-24lo pretreatment aggravated liver injury via an NKT cell-dependent manner or other mechanism needs to be further researched.
In summary, our data suggests that under some circumstances where liver injury is mediated by activation of immune system, treatment of patients with IL-2 may be highly beneficial in reducing morbidity and mortality associated with liver injury. However, it needs to be noticed that IL-2 administration may affect the therapy results. As similar immune-mediated mechanisms are important in autoimmune and viral hepatitis, these results are also relevant for the pathogenesis of liver injury in humans. Therefore, further investigations in this model may have implications for the development of new treatment options.
Acknowledgements
This work was funded by Natural Science Foundation of China (#30630059 and #30721002) and National 973 Basic Science Project (#2007CB512405 and #2007CB512807).
Footnotes
X. Zhang and H.-X. Wei contributed equally to this work.
References
- 1.Bassiri H, Carding SR. A requirement for IL-2/IL-2 receptor signaling in intrathymic negative selection. J Immunol. 2001;166:5945–5954. doi: 10.4049/jimmunol.166.10.5945. [DOI] [PubMed] [Google Scholar]
- 2.Nelson BH. IL-2, regulatory T cells, and tolerance. J Immunol. 2004;172:3983–3988. doi: 10.4049/jimmunol.172.7.3983. [DOI] [PubMed] [Google Scholar]
- 3.Powell JD, Ragheb JA, Kitagawa-Sakakida S, Schwartz RH. Molecular regulation of interleukin-2 expression by CD28 co-stimulation and anergy. Immunol Rev. 1998;165:287–300. doi: 10.1111/j.1600-065X.1998.tb01246.x. [DOI] [PubMed] [Google Scholar]
- 4.Nelson BH, Willerford DM. Biology of the interleukin-2 receptor. Adv Immunol 1998;70:1–81 [DOI] [PubMed]
- 5.Dutcher J. Current status of interleukin-2 therapy for metastatic renal cell carcinoma and metastatic melanoma. Oncology (New York) 2002;16:4–10. [PubMed] [Google Scholar]
- 6.David D, Bani L, Moreau JL, Treilhou MP, Nakarai T, Joussemet M, Ritz J, Dupont B, Pialoux G, Theze J. Regulatory dysfunction of the interleukin-2 receptor during HIV infection and the impact of triple combination therapy. Proc Natl Acad Sci USA. 1998;95:11348–11353. doi: 10.1073/pnas.95.19.11348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tiegs G, Hentschel J, Wendel A. A T-cell-dependent experimental liver-injury in mice inducible by concanavalin-A. J Clin Invest. 1992;90:196–203. doi: 10.1172/JCI115836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takeda K, Hayakawa Y, Kaer L, Matsuda H, Yagita H, Okumura K. Critical contribution of liver natural killer T cells to a murine model of hepatitis. Proc Natl Acad Sci USA. 2000;97:5498–5503. doi: 10.1073/pnas.040566697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kusters S, Gantner F, Kunstle G, Tiegs G. Interferon gamma plays a critical role in T cell-dependent liver injury in mice initiated by concanavalin A. Gastroenterology. 1996;111:462–471. doi: 10.1053/gast.1996.v111.pm8690213. [DOI] [PubMed] [Google Scholar]
- 10.Kunstle G, Hentze H, Germann PG, Tiegs G, Meergans T, Wendel A. Concanavalin A hepatotoxicity in mice: tumor necrosis factor-mediated organ failure independent of caspase-3-like protease activation. Hepatology. 1999;30:1241–1251. doi: 10.1002/hep.510300517. [DOI] [PubMed] [Google Scholar]
- 11.Trautwein C, Rakemann T, Brenner DA, Streetz K, Licato L, Manns MP, Tiegs G. Concanavalin A-induced liver cell damage: activation of intracellular pathways triggered by tumor necrosis factor in mice. Gastroenterology. 1998;114:1035–1045. doi: 10.1016/S0016-5085(98)70324-5. [DOI] [PubMed] [Google Scholar]
- 12.Wolf D, Hallmann R, Sass G, Sixt M, Kusters S, Fregien B, Trautwein C, Tiegs G. TNF-alpha-induced expression of adhesion molecules in the liver is under the control of TNFR1—relevance for concanavalin A-induced hepatitis. J Immunol. 2001;166:1300–1307. doi: 10.4049/jimmunol.166.2.1300. [DOI] [PubMed] [Google Scholar]
- 13.Mizuhara H, Oneill E, Seki N, Ogawa T, Kusunoki C, Otsuka K, Satoh S, Niwa M, Senoh H, Fujiwara H. T-Cell activation-associated hepatic-injury—mediation by tumor necrosis factors and protection by interleukin-6. J Exp Med. 1994;179:1529–1537. doi: 10.1084/jem.179.5.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun R, Tian ZG, Kulkarni S, Gao B. IL-6 prevents T cell-mediated hepatitis via inhibition of NKT cells in CD4(+) T cell- and STAT3-dependent manners. J Immunol. 2004;172:5648–5655. doi: 10.4049/jimmunol.172.9.5648. [DOI] [PubMed] [Google Scholar]
- 15.Bozza M, Bliss JL, Maylor R, Erickson J, Donnelly L, Bouchard P, Dorner AJ, Trepicchio WL. Interleukin-11 reduces T-cell-dependent experimental liver injury in mice. Hepatology. 1999;30:1441–1447. doi: 10.1002/hep.510300616. [DOI] [PubMed] [Google Scholar]
- 16.Radaeva S, Sun R, Pan HN, Hong F, Gao B. Interleukin 22 (IL-22) plays a protective role in T cell-mediated murine hepatitis: IL-22 is a survival factor for hepatocytes via STAT3 activation. Hepatology. 2004;39:1332–1342. doi: 10.1002/hep.20184. [DOI] [PubMed] [Google Scholar]
- 17.Nicoletti F, Di Marco R, Zaccone P, Salvaggio A, Magro G, Bendtzen K, Meroni P. Murine concanavalin A-induced hepatitis is prevented by interleukin 12 (IL-12) antibody and exacerbated by exogenous IL-12 through an interferon-gamma-dependent mechanism. Hepatology. 2000;32:728–733. doi: 10.1053/jhep.2000.17701. [DOI] [PubMed] [Google Scholar]
- 18.Faggioni R, Jones-Carson J, Reed DA, Dinarello CA, Feingold KR, Grunfeld C, Fantuzzi G. Leptin-deficient (ob/ob) mice are protected from T cell-mediated hepatotoxicity: role of tumor necrosis factor alpha and IL-18. Proc Natl Acad Sci USA. 2000;97:2367–2372. doi: 10.1073/pnas.040561297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gantner F, Leist M, Lohse AW, Germann PG, Tiegs G. Concanavalin A-induced T-cell-mediated hepatic-injury in mice—the role of tumor-necrosis-factor. Hepatology. 1995;21:190–198. doi: 10.1016/0270-9139(95)90428-x. [DOI] [PubMed] [Google Scholar]
- 20.Morris JC, Waldmann TA. Advances in interleukin 2 receptor targeted treatment. Ann Rheum Dis. 2000;59:109–114. doi: 10.1136/ard.59.suppl_1.i109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Willerford DM, Chen JZ, Ferry JA, Davidson L, Ma A, Alt FW. Interleukin-2 receptor-alpha chain regulates the size and content of the peripheral lymphoid compartment. Immunity. 1995;3:521–530. doi: 10.1016/1074-7613(95)90180-9. [DOI] [PubMed] [Google Scholar]
- 22.Schorle H, Holtschke T, Hunig T, Schimpl A, Horak I. Development and function of T-cells in mice rendered interleukin-2 deficient by gene targeting. Nature. 1991;352:621–624. doi: 10.1038/352621a0. [DOI] [PubMed] [Google Scholar]
- 23.Sakamoto T, Ezure T, Lunz J, Murase N, Tsuji H, Fung JJ, Demetris AJ. Concanavalin A simultaneously primes liver hematopoietic and epithelial progenitor cells for parallel expansion during liver regeneration after partial hepatectomy in mice. Hepatology. 2000;32:256–267. doi: 10.1053/jhep.2000.9406. [DOI] [PubMed] [Google Scholar]
- 24.Trautwein C, Rakemann T, Malek NP, Plumpe J, Tiegs G, Manns MP. Concanavalin A-induced liver injury triggers hepatocyte proliferation. J Clin Invest. 1998;101:1960–1969. doi: 10.1172/JCI504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fausto N. Liver regeneration and repair: hepatocytes, progenitor cells, and stem cells. Hepatology. 2004;39:1477–1487. doi: 10.1002/hep.20214. [DOI] [PubMed] [Google Scholar]