Abstract
Hepatocellular carcinoma (HCC) is naturally resistant to radiotherapy and cytotoxic chemotherapy, leaving surgery as the mainstream therapeutic approach. However, the 5-year recurrence rate after curative resection is as high as 61.5%. The background hepatitis B- or C-induced cirrhosis and the presence of micrometastases at the time of surgery have been regarded as two main causes of recurrence. Recently, accumulating evidence suggests that growth factors and cytokines released during the physiological process of post-surgical liver regeneration could induce the activation of dormant micrometastatic lesions. The establishment of neovasculature to support either liver regeneration or HCC growth involves multiple cell types including liver sinusoidal endothelial cells, Kupffer cells, hepatic stellate cells, and circulating endothelial progenitors. The crosstalks among these cells are driven by multiple molecules and signaling pathways, including vascular endothelial growth factors and their receptors, platelet-derived growth factor, the angiopoietin/Tie family, hepatocyte growth factor/c-Met signaling, and others. Anti-angiogenic agent targeting liver cancer vasculature has been reported to be able to generate limited survival benefit of the patients. In this review, discussions are focused on various angiogenic mechanisms of HCC and liver regeneration, as well as the prevailing anti-angiogenic strategies.
Keywords: Hepatocellular carcinoma, Angiogenesis, Liver regeneration, Metastasis
Introduction
Hepatocellular carcinoma (HCC) constitutes the majority of live malignancies. It is the sixth most common malignancy and the third most common cause of cancer death worldwide [1]. Potential curative therapies include surgical resection, liver transplantation, and local ablation of the tumor. Local ablation is mainly suitable for small HCC. Hence, surgical resection has been the mainstream therapy for decades. However, the 5-year recurrence rate after curative resection is as high as 61.5%; even after small HCC resection, it is up to 43.5% [2]. The background hepatitis B- or C-induced cirrhosis and the presence of intrahepatic micrometastases at the time of surgery are believed to be the two main causes of recurrence after partial hepatectomy (PH) for decades. Our previous study reveals that micrometastases are present in 50.4% of the HCC cases and that the distance of micrometastases from the primary tumor can be as far as 6.1 cm distant to the primary tumor margin [3]. The recurrence rate of the anatomical resection group is not different from that of the non-anatomical resection group [3, 4], implying the existence of other causative factors of recurrence in addition to anatomical blood supply carrying hypothesized cancer emboli.
Many clinical and animal studies suggest that liver regeneration after hepatectomy can stimulate remnant tumor growth and metastases [5–10], drawing more attentions on this physiological process.
Liver regeneration is a complicated process involving the secretions of numerous cytokines and growth factors, and the functioning of metabolic networks [11]. Many specific factors involved in liver regeneration are believed to be able to influence the growth of residual or dormant micrometastases after PH, and also modulating tumor angiogenesis [12]. These factors include hepatocyte growth factor (HGF), epidermal growth factor (EGF), transforming growth factor (TGF)-α, TGF-β, hypoxia-inducible factor 1 (HIF-1), vascular endothelial growth factor (VEGF), and matrix metalloproteinases (MMPs).
The mechanisms of cancer dormancy include angiogenic dormancy, cellular dormancy and immunosurveillance [13, 14]. Only a short-term of angiogenesis burst can awaken a dormant tumor [15]. In fact, during the late phase of regeneration after PH, which mainly involves re-establishment of liver structure with angiogenesis, accelerations of tumor growth, and metastasis have been observed [12, 16].
Notably, gene expression profiles of physiological and pathological angiogenesis are different [17], supporting the hypothesis that some unique hallmarks of HCC angiogenesis could be existing. In animal models, the endogenous angiogenic inhibitor angiostatin inhibits liver regeneration [18]; in contrast, the semi-synthetic angiogenic inhibitor TNP-470 suppresses HCC growth without retarding regeneration after PH [10], suggesting that different anti-angiogenic agents might target different part of vasculature in the liver and in the tumor.
In this review, the different angiogenic mechanisms of liver tumors and liver regeneration are summarized, and potential selective therapeutic strategies against HCC are discussed.
The process of liver regeneration
Regeneration of the liver after PH is a complicated process, and the mechanisms are not fully understood. Regeneration is carried out by proliferation of all of the mature cellular populations of the organ, which include hepatocytes (Fig. 1), biliary epithelial cells, liver sinusoidal endothelial cells (LSECs), Kupffer cells, and hepatic stellate cells (HSCs, which are pericytes in the liver) [19]. Liver regeneration undergoes three stages [12]. The first is the priming stage and occurs during the first few hours after PH. Hepatocytes become responsive to growth factors and the extracellular matrix (ECM) is broken down. Proliferation is the hallmark of the second stage. After PH, several signals are initiated simultaneously in the liver for proliferation [20]. Gut-derived factors, such as lipopolysaccharide are up-regulated and reach the liver through the portal blood supply. They activate hepatic non-parenchymal cells (including Kupffer cells and HSCs) and increase the production of tumor necrosis factor-α and interleukin-6. Other factors are released from the pancreas (insulin), duodenum or salivary gland (EGF), adrenal gland (norepinephrine), thyroid gland (triiodothyronine), and HSCs (HGF). Cooperative signals from these factors lead to hepatocyte proliferation. The proliferative hepatocytes provide mitogenic stimuli leading to the proliferation of the other cells [19]. The last stage of liver regeneration is the termination stage, in which the cells proliferation is terminated, new vessels are developed, and the ECM is remodeled, leading to new, fully functioning liver tissue.
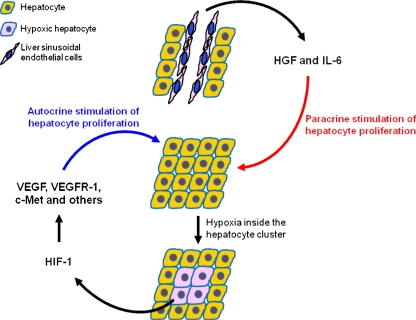
Fig. 1.
Mechanism of hepatocyte proliferation in liver regeneration. After partial hepatectomy, HGF and IL-6 released by liver sinusoidal endothelial cells can trigger a paracrine proliferation of hepatocytes. The initial proliferation leads to the formation of avascular clusters of hepatocytes, with hypoxia occurs in the center. HIF-1 then responds to hypoxic condition and triggers the expression of numerous growth factors and their receptors in the hypoxic hepatocytes, activating an autocrine proliferation of the hepatocytes
Angiogenesis in liver regeneration
Hepatic sinusoids are highly specialized capillary vessels. Like all blood vessels, they consist of two main cell types, endothelial and mural. The endothelial cells of the hepatic sinusoids are LSECs. Relative to the endothelial cells in other organs, LSECs have a unique phenotype characterized by forming a discontinuous, fenestrated endothelium without an organized basement membrane [21]. As the mural cells of the hepatic sinusoidal, HSCs are liver-specific pericytes [22, 23]. Cellular cross-talk among LSECs, HSCs and hepatocytes is believed to play an important role in physiologic angiogenesis during liver regeneration.
The initial proliferation of hepatocytes leads to the formation of avascular clusters of hepatocytes, in which the central cells reside outside the oxygen diffusion distance of capillaries [24, 25]. Hypoxic conditions activate the transcription factor HIF-1, which in turn induces the expression of downstream target genes including c-Met, erythropoietin (EPO), VEGF, and VEGF receptor 1 (VEGFR-1) [26–28].
Hepatocyte production of VEGF peaks 48–72 h after PH and is detected mainly in periportal hepatocytes [29]. As a potent survival factor of endothelial cells, VEGF promotes the proliferation of endothelial cells and regulates vascular permeability of LSECs [30, 31]. VEGF production is accompanied by increased expression of VEGFR-1 on hepatocytes and HSCs and of VEGFR-1 and VEGFR-2 on LSECs [25, 27, 32, 33]. After binding to the VEGFR-1 of hepatocytes, VEGF can induce autocrine proliferation of hepatocytes [29]. However, the proliferation of hepatocytes can also result from paracrine expression of HGF and IL-6 by LSECs [34]. Activation of VEGFR-2 stimulates LSEC proliferation.
Neuropilin-1 and neuropilin-2 are recognized to be VEGF co-receptors unrelated to VEGFR-1 and VEGFR-2; they have no intrinsic signaling, but enhance the binding of VEGF to VEGFR-2 [35]. Neuropilin-1 has been shown to be up-regulated in liver regeneration [36]. VEGF not only induces LSEC proliferation but also induces the expression of proteases-like collagenase [37], MMPs [38], and urokinase- and tissue-type plasminogen activators [39], which enable L1SECs to break down the surrounding extracellular matrix to migrate and form new blood vessels.
The angiopoietin/Tie family, including angiopoietin 1 (Ang-1), angiopoietin 2 (Ang-2), and receptor tyrosine kinases Tie-1, and Tie-2 are other important growth factors regulating angiogenesis in regeneration. Ang-1 regulates vessel stability by activating Tie-2, while Ang-2 acts as a natural antagonist of Ang-1 [22]. Tie-1 has an important role in endothelial cell differentiation and the maintenance of blood integrity [40, 41]. Tie-1 expression, which is up-regulated in regeneration, may stabilize nascent sinusoids.
Within the first 72 h following PH, resting HSCs start to proliferate (peaking at 48–72 h) and become activated, mediated by platelet-derived growth factor (PDGF) produced by hepatocytes and LSECs [42–45]. HSCs expresses angiopoietins [22]. It has been suggested that Ang-1 binds to Tie-2 on the surface of endothelial cells and promotes interaction between endothelial cells and pericytes to stabilize the mature vascular system [46]. Ang-2 initiates the angiogenesis process in the presence of VEGF by inhibiting the Ang-1 activity and disrupting existing blood vessels. However, in the absence of VEGF, Ang-2 leads to vessels regression [47, 48]. In the regenerating liver, VEGF expression peaked at 72 h. Angiopoietin/Tie factors peaked at 96 h except for Ang-2, which gradually increased and peaked at 168 h. It is possible that in the presence of VEGF, Ang-2 augments angiogenesis in the early phase of regeneration and inhibits angiogenesis in the absence of VEGF when the regeneration is completed [25, 32, 49, 50].
When the regenerating liver is approaching its preoperative mass at day 6 after PH, a wave of LSEC apoptosis can be detected with a maximum at day 8 in the mouse [49, 51, 52]. This is in contrast to hepatocytes, which do not show increased apoptosis during the regeneration process [51]. The end of angiogenesis may play a vital role in the termination of liver regeneration.
Sprouting angiogenesis in HCC
It is well known that most HCC emerges in a liver with extensive fibrosis due to HBV or HCV infection [53]. During the process of fibrogenesis, many growth factors, cytokines, and metalloproteinases with an inherent pro-angiogenic action are overexpressed [54].
Sakamoto et al. [55] divided the early development stage of HCC into ordinary adenomatous hyperplasia (OAH), atypical adenomatous hyperplasia, and well-differentiated HCC (early HCC), depending on the cellular morphology in nodule lesions. Arterialization (which means presence of new unpaired arteries not accompanied by bile duct [56]) and sinusoidal capillarization (involving transformation of fenestrated hepatic sinusoidal endothelial cells into continuous capillaries, coupled with collagenization of the extravascular spaces of Disse and deposition of laminin and basement membranes near the endothelial cells and hepatocytes [57]) are highest in HCC, develop from OAH and gradually increase [58]. Accordingly, the intranodular portal supply relative to the surrounding liver parenchyma is decreased, whereas the intranodular arterial supply is increased in accordance with elevation of the grade of malignancy of the nodules [59]. Arterialization can induce a partial transition of LSECs to capillary-type endothelial cells (sinusoidal capillarization) [60]. Sinusoidal capillarization is stimulated by Ang-1 due to hypoxia [22]. Subsequently, the progressing sinusoidal capillarization leads to an impairment of oxygen diffusion from the sinusoidal to hepatocytes [61, 62]. In addition, rapid proliferation of HCC cells continuously induces local hypoxia. Hence, angiogenesis is stimulated by the progressing increase in tissue hypoxia [63].
The mechanisms of hypoxia that induce angiogenesis in HCC are similar to those found in regeneration after PH. However, some special conditions are present in HCC. The X protein of hepatitis B virus has been shown to increase the transcriptional activity and protein level of HIF-1 [64]. Hypoxia stimulates angiogenesis through up-regulation of VEGF gene expression by at least two distinct molecular mechanisms: activation of VEGF gene transcription and stabilization of VEGF mRNA [65]. Whether the VEGFR1 or VEGFR2 plays a more important role in hypoxia-induced HCC angiogenesis is controversial. Most report that VEGFR2 were more important than VEGFR1 [66–70], but some show their reverse results [71, 72], while some other believe that both VEGFR1 and VEGFR2 played important roles, and lie in the different signaling cascades by which VEGF augments HCC development and angiogenesis [73]. The higher levels of VEGF expression during the development of HCC have been shown to be associated with an increase in arterialization and sinusoidal capillarization [58].
Angiopoietin/Tie-2 is also an important pathway in regulating angiogenesis of HCC, although it is not up-regulated by hypoxia [74]. Using immunohistochemistry, angiopoietin 1 (Ang-1) and Ang-2 can be detected in HCC cells, HSCs, and smooth muscle cells, whereas their receptor Tie-2 is detected in LSECs, HSCs, and smooth muscle cells, suggesting that multiple cell types are involved in the angiopoietin/Tie-2 signaling pathways to mediate tumor angiogenesis [75]. Ang-1 and Ang-2 expressions are positively correlated with tumor de-differentiation [75]. Ang-1 is more frequently expressed in normal liver, and Ang-2 is more frequently expressed in HCC [74]. The level of Ang-2 is found to be associated with the clinicopathological parameters of HCC patients, and the ratio between Ang-2 and Ang-1 indicated the status of angiogenesis [76]. Furthermore, Ang-2 displays a VEGF-dependent synergistic effect on angiogenesis in a mouse HCC model, similar to that in regeneration after PH [77].
It has been a general conception that VEGF/Ang-driven sprouting angiogenesis is the main mechanism of neovascularization in HCC (Fig. 2). However, a recent paper challenges this conception [78]. Zeng et al. investigated gene and protein expression levels of VEGF-A, VEGFR-1, VEGFR-2, Ang-1, Ang-2, and Tie-2 using quantitative (real-time) reverse transcription polymerase chain reaction and Western blot analysis in tumors, adjacent liver tissues, and normal liver tissues. HCC in non-cirrhotic and cirrhotic livers expresses VEGF and its receptors to a similar extent as normal liver, although in a cirrhotic background, VEGFR-2 levels in both tumor and adjacent tissue are decreased. Tumor Ang-1 expression is slightly increased when compared with normal liver, whereas Tie-2 is strongly down-regulated in the tumor vasculature. The Ang-2 mRNA level is also low in HCC with both non-cirrhotic or cirrhotic liver. These results indicate that HCC vascularization may not be driven by VEGF or angiopoietin. Obviously, further investigation is demanded to clarify the molecular mechanism of sprouting angiogenesis in HCC.
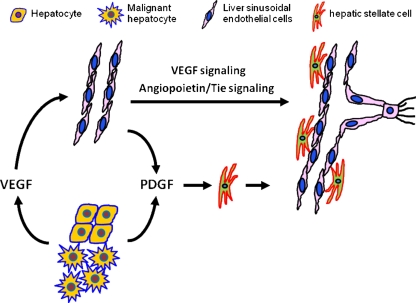
Fig. 2.
Sprouting angiogenesis in liver regeneration and HCC. Sprouting angiogenesis is believed to be a major type of vasculature development in both liver regeneration and HCC. VEGF released by hepatocytes and cancer cells is the main driver for the liver sinusoidal endothelial cells to undergo sprouting angiogenesis. In addition to VEGF signaling, angiopoietin/Tie signaling is also involved in this process. PDGF released by hepatocytes, malignant hepatocytes, and endothelial cells can stimulate the proliferation of hepatic stellate cells, which participate in the stabilization of the newly formed vessels during sprouting angiogenesis
Intussusceptive angiogenesis in HCC
In a rat model of HCC treated by mTOR inhibitor sirolimus, the HCC of control animals primarily present sprouting angiogenesis, which is nearly absent and replaced by intussusceptive angiogenesis in that of treated animals. The results indicate that inhibition of sprouting angiogenesis may stimulate the process of intussusceptive angiogenesis [79].
Intussusceptive angiogenesis is an alternative mode of angiogenesis that consists of microvascular remodeling by transcapillary pillar formation; it relies much less on endothelial cell proliferation. Growth of these endothelial pillars leads to sinusoidal multiplication by successive fusion and partitioning of the existing vascular lumens [80]. A recent study with human endothelial cells shows that chronic hypoxia attenuates VEGF signaling and angiogenic responses by down-regulation of VEGFR-2 [81]. As stated above, most of HCCs originate from fibrosis and cirrhosis, which undergo chronic hypoxia and VEGFR-2 levels were down-regulated in both tumor and in adjacent tissue [78], preferring intussusceptive angiogenesis instead of sprouting angiogenesis. Besides, the rate of endothelial cell proliferation is low in a cirrhotic background [82], further suggesting that another mechanism different from sprouting angiogenesis might exist. Hence, intussusception may play an important role in the angiogenesis of HCC. However, the direct evidence is absent.
Vasculogenesis in HCC
Recently, vasculogenesis in HCC has been shown to involve vessel changes in which the formation of blood vessels is due to the arrival and differentiation of endothelial progenitor cells (EPCs) generated from bone marrow. Ho et al. reported that the level of circulating EPCs is significantly higher in HCC patients than that in patients with cirrhosis or in healthy controls, and higher circulating levels of EPCs are detectable in the patients with advanced unresectable HCC relative to patients with resectable HCC [83]. Others report that EPCs can incorporate into vessel walls of different sizes, mostly in the microvessels in cirrhotic and tumor tissues of the patients with HCC [84]. Although the exact mechanisms for recruitment and homing of EPCs to liver cirrhosis or liver cancer are unclear, it is evident that the mobilized EPCs participate in the vasculogenesis of HCC.
Vasculogenic mimicry in HCC
Vasculogenic mimicry has increasingly been recognized as an important form of vasculogenic structure in solid tumors. This process of cell plasticity occurs mainly in aggressive tumors; the tumor cells de-differentiate to an endothelial phenotype and form tube-like structures. This provides tumor cells with a secondary circulation system of vasculogenic structures lined by tumor cells, independent of angiogenesis [85]. Vasculogenic mimicry has been shown in HCC [86–88]. It has been identified by the presence of red blood cells in vessels lined by HCC cells, which are CD31- and CD105-negative and HGF- and VEGF-positive. The presence of vasculogenic mimicry is associated with a high tumor grade, invasion and metastasis, and shorter survival. The exact mechanisms underlying mimicry still needs to be clarified, but the related molecules, mainly involving extracellular matrix and hypoxia have been summarized in a recent review [89].
Vessel co-option in HCC
Tumor cells can grow along existing vessels without evoking an angiogenic response, which is defined as vessel co-option. The phenomenon has been described in glioblastoma multiforme and non-small cell lung cancer [90, 91]. Holash et al. reported that a subset of tumors rapidly co-opt existing host vessels to form an initially well-vascularized tumor mass in which the co-opted vessels will undergo a widespread regression, leading to a secondarily avascular tumor and massive tumor cell apoptosis, but the remaining tumor is ultimately rescued by robust angiogenesis at the tumor margin [92]. The expression patterns of VEGF and the natural Tie-2 receptor antagonist Ang-2 strongly implicate them in these processes [46, 92]. Ang-1 expression does not change significantly throughout tumor development. The co-opted vessels display striking and specific up-regulation of Ang-2. In the early stage, there is minimal up-regulation of VEGF, and the elevated Ang-2 may induce vessel regression in the absence of VEGF. Subsequently, VEGF up-regulation coincident with Ang-2 expression at the tumor periphery is associated with robust angiogenesis.
The hypothesis that vessel co-option exists in HCC is put forward by Zeng et al. without further testing due to the lack of a vascular marker for endothelial co-option [78]. However, accumulating evidence supports the hypothesis that co-option does exist in HCC. First, liver is a well-vascularized organ providing efficient vasculature for vessel co-option. Second, the two main characteristics of arterialization and sinusoidal capillarization in HCC developed from OAH gradually increase following lesions progression [58], suggesting a continuous remodeling of tumor vasculature from the pre-existing vessels. CD4, CD14, and CD32, the specific phenotypes of LSECs expressed in early and well-differentiated HCC cases are similar to those of the LSECs in normal liver, but they are not expressed in poorly differentiated HCC [93] suggesting a regression or differentiation of pre-existing vasculature after being integrated into tumor vasculature. Third, the rate of LSEC proliferation is low—from 0.02 to 0.03—in HCC [82] suggesting that other source(s) of endothelial cells including vessel co-option should exist in addition to conventional angiogenesis for the rapid establishment of tumor vasculature. Fourth, vessel co-option is present in liver metastases [94]. Last and most important, early HCC does not destroy the preexisting architecture of liver lobule and pseudolobule [95]. Taken together, vessel co-option might be an important component of tumor vasculature development in HCC worthy of further investigation.
Lymphangiogenesis in HCC
Lymphatic vessels are also part of the vascular circulatory system. The lymphatic vascular endothelial hyaluronan receptor-1 (LYVE-1), podoplanin, and the transcription factor Prox1 are three lymphatic-specific markers for lymphatic endothelial cells [96]. By immunohistochemistry, LYVE-1 is present not only in lymph vessels, but also in LSECs; it is absent from angiogenic blood vessels of HCC and only weakly present in the microcirculation of regeneration hepatic nodules in cirrhosis [97]. Prox1 is abundant in cirrhosis; it is restricted to the tumor margin and surrounding liver in HCC [97]. Podoplanin is present in the stroma weakly, but not present in the parenchyma of healthy liver tissue or cirrhosis; it is present within the tumor parenchyma as well as within the intratumor septa in HCC [98].
This limited evidence suggests that lymphatic endothelial cells may be special LSECs whose phenotype alters following the development of HCC. Tumor-associated lymphangiogenesis is involved in the neovascularization of HCC. The lymphatic microvessel density showed a trend toward association with reduced survival and represents an independent prognostic factor for disease-free survival, indicating that the role of lymphangiogenesis for tumor progression in HCC is related to the risk of recurrence rather than to local tumor growth [98]. Lymphangiogenesis is mainly regulated by the VEGF-C/VEGF-D/VEGFR-3 system [99–102], however, not much is known about the role of this signaling system in the lymphangiogenesis of HCC.
Strategies of anti-angiogenic therapy against HCC
Preliminary results from clinical trials of single-agent anti-angiogenic therapy in advanced solid cancers have shown poor efficacy [103]. Many molecular-targeted drugs have been tested for HCC [104]. The multi-tyrosine kinase inhibitor sorafenib is the first (and so far the only) drug that has shown an overall survival benefit to the patients with HCC in two multi-centre, double-blind, placebo-controlled randomized phase III trials (SHARP trial and Asia-Pacific trial) [105, 106].
The following reasons are speculated to explain the limited efficacy of current anti-angiogenic therapy in HCC: first, most of anti-angiogenic agents, such as sorafenib, bevacizumab, sirolimus, everolimus, sunitinib are mainly targeting sprouting angiogenesis, leaving other angiogenic modalities unaffected. For example, the vascular remodeling can present as substitute [79]. Second, anti-angiogenic agents mainly interfere with newly formed blood vessels, but not with mature blood vessel supported by pericytes [107, 108], leaving the mature vessels fully functioning. Third, some anti-angiogenic agents can block the cell cycle of tumor cells, but cannot induce tumors apoptosis [109], therefore, weakening its antitumor effect.
For better efficacy, several strategies of anti-angiogenic agents against HCC must be considered: (1) combination of different angiogenic pathway inhibitors; (2) combination of anti-angiogenic agents with vascular disrupting agents, which mainly disrupt established blood vessel; and (3) combination of anti-angiogenic drugs with surgery, chemotherapy, radiotherapy, and biotherapy.
Conclusion
The establishment of vasculature is a complicated process regulated by pro- and anti-angiogenic factors [110]. During liver regeneration, the balance is broken mainly due to hypoxia, which activates VEGF/Ang-driven sprouting neovascularization, leading to the formation of a functionally normal blood system. In addition to sprouting angiogenesis, other mechanisms identified in tumor include intussusceptive angiogenesis, the recruitment of EPCs, vessel co-option, vasculogenic mimicry and lymphangiogenesis [89]. All of these angiogenesis models have been shown to be present in HCC, as summarized in Table 1, suggesting that neovasculogenesis of HCC is even more complicated than predicted. Anti-angiogenic therapy is becoming more and more important in HCC management. Its advantage and shortcoming should be recognized for proper selection of anti-angiogenic agents combined with standard therapeutic modalities. Understanding the exact mechanisms of neovasculogenesis in HCC remains fundamental for the development of more effective treatments and the prevention of HCC recurrence.
Table 1.
Characteristics of different patterns of vasculature establishment in liver regeneration and HCC
| Characteristics | Sprouting angiogenesis in liver regeneration | Sprouting angiogenesis in HCC | Intussusceptive angiogenesis in HCC | Vasculogenesis in HCC | Vasculogenic mimicry in HCC | Vessel cooption in HCC | Lymphangiogenesis in HCC |
|---|---|---|---|---|---|---|---|
| Main cell type | LSEC, HSC | LSEC | LSEC | EPC | Cancer cells | EC, LSEC, HSC | Lymphatic endothelial cells |
| VEGF signaling | Up-regulated | Up-regulated | Down-regulated | Unclear | Unclear | Up-regulated | Unclear |
| Angiopoietin/Tie signaling | Up-regulated | Up-regulated | Unclear | Unclear | Unclear | Up-regulated | Unclear |
| Proliferation of endothelial cells | High rate | High rate | Low rate | Unclear | Unclear | Unclear | Unclear |
| Apoptosis of endothelial cells | Occurs in late stage | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
HCC hepatocellular carcinoma, LSEC liver sinusoidal endothelial cell, HSC hepatic stellate cell, EPC endothelial progenitor cells, VEGF vascular endothelial growth factor
Acknowledgements
We thank David Nadziejka of Van Andel Research Institute, Grand Rapids, Michigan, for technical editing of the manuscript. This work is funded by National Key Sci-Tech Special Project of China (No. 2008ZX10002-019).
Conflict of interest None.
Abbreviations
- AAH
Atypical adenomatous hyperplasia
- ECM
Extracellular matrix
- EGF
Epidermal growth factor
- EPC
Endothelial progenitor cell
- HBV
Hepatitis B virus
- HCC
Hepatocellular carcinoma
- HCV
Hepatitis C virus
- HGF
Hepatocyte growth factor
- HIF
Hypoxia-inducible factor
- HSC
Hepatic stellate cell
- LPS
Lipopolysaccharide
- LSEC
Liver sinusoidal endothelial cell
- MMPs
Matrix metalloproteinases
- OAH
Ordinary adenomatous hyperplasia
- PDGF
Platelet-derived growth factor
- PH
Partial hepatectomy
- TGF
Transforming growth factor
- VEGF
Vascular endothelial growth factor
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55(2):74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Tang ZY, Ye SL, Liu YK, Qin LX, Sun HC, Ye QH, Wang L, Zhou J, Qiu SJ, Li Y, Ji XN, Liu H, Xia JL, Wu ZQ, Fan J, Ma ZC, Zhou XD, Lin ZY, Liu KD. A decade’s studies on metastasis of hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004;130(4):187–196. doi: 10.1007/s00432-003-0511-1. [DOI] [PubMed] [Google Scholar]
- 3.Shi M, Zhang CQ, Zhang YQ, Liang XM, Li JQ. Micrometastases of solitary hepatocellular carcinoma and appropriate resection margin. World J Surg. 2004;28(4):376–381. doi: 10.1007/s00268-003-7308-x. [DOI] [PubMed] [Google Scholar]
- 4.Tanaka K, Shimada H, Matsumoto C, Matsuo K, Nagano Y, Endo I, Togo S. Anatomic versus limited nonanatomic resection for solitary hepatocellular carcinoma. Surgery. 2008;143(5):607–615. doi: 10.1016/j.surg.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 5.Schweinitz D, Faundez A, Teichmann B, Birnbaum T, Koch A, Hecker H, Gluer S, Fuchs J, Pietsch T. Hepatocyte growth-factor-scatter factor can stimulate post-operative tumor-cell proliferation in childhood hepatoblastoma. Int J Cancer. 2000;85(2):151–159. doi: 10.1002/(SICI)1097-0215(20000115)85:2<151::AID-IJC1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 6.Ichihashi H, Mabuchi H, Suenaga M, Kondo T. Liver regeneration and tumor growth in the rat after partial hepatectomy. Jpn J Surg. 1984;14(6):510–514. doi: 10.1007/BF02469795. [DOI] [PubMed] [Google Scholar]
- 7.Mizutani J, Hiraoka T, Yamashita R, Miyauchi Y. Promotion of hepatic metastases by liver resection in the rat. Br J Cancer. 1992;65(6):794–797. doi: 10.1038/bjc.1992.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Picardo A, Karpoff HM, Ng B, Lee J, Brennan MF, Fong Y. Partial hepatectomy accelerates local tumor growth: potential roles of local cytokine activation. Surgery. 1998;124(1):57–64. [PubMed] [Google Scholar]
- 9.Yokoyama H, Goto S, Chen CL, Pan TL, Kawano K, Kitano S. Major hepatic resection may suppress the growth of tumours remaining in the residual liver. Br J Cancer. 2000;83(8):1096–1101. doi: 10.1054/bjoc.2000.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kinoshita S, Hirai R, Yamano T, Yuasa I, Tsukuda K, Shimizu N. Angiogenesis inhibitor TNP-470 can suppress hepatocellular carcinoma growth without retarding liver regeneration after partial hepatectomy. Surg Today. 2004;34(1):40–46. doi: 10.1007/s00595-003-2645-9. [DOI] [PubMed] [Google Scholar]
- 11.Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006;43(2 Suppl. 1):S45–S53. doi: 10.1002/hep.20969. [DOI] [PubMed] [Google Scholar]
- 12.Christophi C, Harun N, Fifis T. Liver regeneration and tumor stimulation: a review of cytokine and angiogenic factors. J Gastrointest Surg. 2008;12(5):966–980. doi: 10.1007/s11605-007-0459-6. [DOI] [PubMed] [Google Scholar]
- 13.Naumov GN, Akslen LA, Folkman J. Role of angiogenesis in human tumor dormancy: animal models of the angiogenic switch. Cell Cycle. 2006;5(16):1779–1787. doi: 10.4161/cc.5.16.3018. [DOI] [PubMed] [Google Scholar]
- 14.Aguirre-Ghiso JA. Models, mechanisms and clinical evidence for cancer dormancy. Nat Rev Cancer. 2007;7(11):834–846. doi: 10.1038/nrc2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Indraccolo S, Favaro E, Amadori A. Dormant tumors awaken by a short-term angiogenic burst: the spike hypothesis. Cell Cycle 2006;5(16):1751–1755 [DOI] [PubMed]
- 16.Harun N, Nikfarjam M, Muralidharan V, Christophi C. Liver regeneration stimulates tumor metastases. J Surg Res. 2007;138(2):284–290. doi: 10.1016/j.jss.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 17.Seaman S, Stevens J, Yang MY, Logsdon D, Graff-Cherry C, St Croix B. Genes that distinguish physiological and pathological angiogenesis. Cancer Cell. 2007;11(6):539–554. doi: 10.1016/j.ccr.2007.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drixler TA, Vogten MJ, Ritchie ED, Vroonhoven TJ, Gebbink MF, Voest EE, Borel Rinkes IH. Liver regeneration is an angiogenesis-associated phenomenon. Ann Surg. 2002;236(6):703–711. doi: 10.1097/00000658-200212000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276(5309):60–66. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- 20.Taub R. Liver regeneration: from myth to mechanism. Nat Rev Mol Cell Biol. 2004;5(10):836–847. doi: 10.1038/nrm1489. [DOI] [PubMed] [Google Scholar]
- 21.Wisse E, Zanger RB, Charels K, Smissen P, McCuskey RS. The liver sieve: considerations concerning the structure and function of endothelial fenestrae, the sinusoidal wall and the space of Disse. Hepatology. 1985;5(4):683–692. doi: 10.1002/hep.1840050427. [DOI] [PubMed] [Google Scholar]
- 22.Lee JS, Semela D, Iredale J, Shah VH. Sinusoidal remodeling and angiogenesis: a new function for the liver-specific pericyte? Hepatology. 2007;45(3):817–825. doi: 10.1002/hep.21564. [DOI] [PubMed] [Google Scholar]
- 23.Semela D, Das A, Langer D, Kang N, Leof E, Shah V. Platelet-derived growth factor signaling through ephrin-b2 regulates hepatic vascular structure and function. Gastroenterology. 2008;135(2):671–679. doi: 10.1053/j.gastro.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wack KE, Ross MA, Zegarra V, Sysko LR, Watkins SC, Stolz DB. Sinusoidal ultrastructure evaluated during the revascularization of regenerating rat liver. Hepatology. 2001;33(2):363–378. doi: 10.1053/jhep.2001.21998. [DOI] [PubMed] [Google Scholar]
- 25.Ross MA, Sander CM, Kleeb TB, Watkins SC, Stolz DB. Spatiotemporal expression of angiogenesis growth factor receptors during the revascularization of regenerating rat liver. Hepatology. 2001;34(6):1135–1148. doi: 10.1053/jhep.2001.29624. [DOI] [PubMed] [Google Scholar]
- 26.Maeno H, Ono T, Dhar DK, Sato T, Yamanoi A, Nagasue N. Expression of hypoxia inducible factor-1alpha during liver regeneration induced by partial hepatectomy in rats. Liver Int. 2005;25(5):1002–1009. doi: 10.1111/j.1478-3231.2005.01144.x. [DOI] [PubMed] [Google Scholar]
- 27.Redaelli CA, Semela D, Carrick FE, Ledermann M, Candinas D, Sauter B, Dufour JF. Effect of vascular endothelial growth factor on functional recovery after hepatectomy in lean and obese mice. J Hepatol. 2004;40(2):305–312. doi: 10.1016/j.jhep.2003.10.027. [DOI] [PubMed] [Google Scholar]
- 28.Yim SH, Shah Y, Tomita S, Morris HD, Gavrilova O, Lambert G, Ward JM, Gonzalez FJ. Disruption of the Arnt gene in endothelial cells causes hepatic vascular defects and partial embryonic lethality in mice. Hepatology. 2006;44(3):550–560. doi: 10.1002/hep.21284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taniguchi E, Sakisaka S, Matsuo K, Tanikawa K, Sata M. Expression and role of vascular endothelial growth factor in liver regeneration after partial hepatectomy in rats. J Histochem Cytochem. 2001;49(1):121–130. doi: 10.1177/002215540104900112. [DOI] [PubMed] [Google Scholar]
- 30.Shimizu H, Miyazaki M, Wakabayashi Y, Mitsuhashi N, Kato A, Ito H, Nakagawa K, Yoshidome H, Kataoka M, Nakajima N. Vascular endothelial growth factor secreted by replicating hepatocytes induces sinusoidal endothelial cell proliferation during regeneration after partial hepatectomy in rats. J Hepatol. 2001;34(5):683–689. doi: 10.1016/s0168-8278(00)00055-6. [DOI] [PubMed] [Google Scholar]
- 31.Yokomori H, Oda M, Yoshimura K, Nagai T, Ogi M, Nomura M, Ishii H. Vascular endothelial growth factor increases fenestral permeability in hepatic sinusoidal endothelial cells. Liver Int. 2003;23(6):467–475. doi: 10.1111/j.1478-3231.2003.00880.x. [DOI] [PubMed] [Google Scholar]
- 32.Sato T, El-Assal ON, Ono T, Yamanoi A, Dhar DK, Nagasue N. Sinusoidal endothelial cell proliferation and expression of angiopoietin/Tie family in regenerating rat liver. J Hepatol. 2001;34(5):690–698. doi: 10.1016/s0168-8278(00)00109-4. [DOI] [PubMed] [Google Scholar]
- 33.Ankoma-Sey V, Wang Y, Dai Z. Hypoxic stimulation of vascular endothelial growth factor expression in activated rat hepatic stellate cells. Hepatology. 2000;31(1):141–148. doi: 10.1002/hep.510310122. [DOI] [PubMed] [Google Scholar]
- 34.LeCouter J, Moritz DR, Li B, Phillips GL, Liang XH, Gerber HP, Hillan KJ, Ferrara N. Angiogenesis-independent endothelial protection of liver: role of VEGFR-1. Science. 2003;299(5608):890–893. doi: 10.1126/science.1079562. [DOI] [PubMed] [Google Scholar]
- 35.Neufeld G, Kessler O, Herzog Y. The interaction of Neuropilin-1 and Neuropilin-2 with tyrosine-kinase receptors for VEGF. Adv Exp Med Biol. 2002;515:81–90. doi: 10.1007/978-1-4615-0119-0_7. [DOI] [PubMed] [Google Scholar]
- 36.Braet F, Shleper M, Paizi M, Brodsky S, Kopeiko N, Resnick N, Spira G. Liver sinusoidal endothelial cell modulation upon resection and shear stress in vitro. Comp Hepatol. 2004;3(1):7. doi: 10.1186/1476-5926-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Unemori EN, Ferrara N, Bauer EA, Amento EP. Vascular endothelial growth factor induces interstitial collagenase expression in human endothelial cells. J Cell Physiol. 1992;153(3):557–562. doi: 10.1002/jcp.1041530317. [DOI] [PubMed] [Google Scholar]
- 38.Zucker S, Mirza H, Conner CE, Lorenz AF, Drews MH, Bahou WF, Jesty J. Vascular endothelial growth factor induces tissue factor and matrix metalloproteinase production in endothelial cells: conversion of prothrombin to thrombin results in progelatinase A activation and cell proliferation. Int J Cancer. 1998;75(5):780–786. doi: 10.1002/(sici)1097-0215(19980302)75:5<780::aid-ijc19>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 39.Pepper MS, Ferrara N, Orci L, Montesano R. Vascular endothelial growth factor (VEGF) induces plasminogen activators and plasminogen activator inhibitor-1 in microvascular endothelial cells. Biochem Biophys Res Commun. 1991;181(2):902–906. doi: 10.1016/0006-291x(91)91276-i. [DOI] [PubMed] [Google Scholar]
- 40.Sato TN, Tozawa Y, Deutsch U, Wolburg-Buchholz K, Fujiwara Y, Gendron-Maguire M, Gridley T, Wolburg H, Risau W, Qin Y. Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature. 1995;376(6535):70–74. doi: 10.1038/376070a0. [DOI] [PubMed] [Google Scholar]
- 41.Puri MC, Rossant J, Alitalo K, Bernstein A, Partanen J. The receptor tyrosine kinase TIE is required for integrity and survival of vascular endothelial cells. EMBO J. 1995;14(23):5884–5891. doi: 10.1002/j.1460-2075.1995.tb00276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Michalopoulos GK. Liver regeneration. J Cell Physiol. 2007;213(2):286–300. doi: 10.1002/jcp.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Balabaud C, Bioulac-Sage P, Desmouliere A. The role of hepatic stellate cells in liver regeneration. J Hepatol. 2004;40(6):1023–1026. doi: 10.1016/j.jhep.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 44.Budny T, Palmes D, Stratmann U, Minin E, Herbst H, Spiegel HU. Morphologic features in the regenerating liver—a comparative intravital, lightmicroscopical and ultrastructural analysis with focus on hepatic stellate cells. Virchows Arch. 2007;451(4):781–791. doi: 10.1007/s00428-007-0472-x. [DOI] [PubMed] [Google Scholar]
- 45.Mabuchi A, Mullaney I, Sheard PW, Hessian PA, Mallard BL, Tawadrous MN, Zimmermann A, Senoo H, Wheatley AM. Role of hepatic stellate cell/hepatocyte interaction and activation of hepatic stellate cells in the early phase of liver regeneration in the rat. J Hepatol. 2004;40(6):910–916. doi: 10.1016/j.jhep.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 46.Holash J, Wiegand SJ, Yancopoulos GD. New model of tumor angiogenesis: dynamic balance between vessel regression and growth mediated by angiopoietins and VEGF. Oncogene. 1999;18(38):5356–5362. doi: 10.1038/sj.onc.1203035. [DOI] [PubMed] [Google Scholar]
- 47.Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277(5322):55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- 48.Hanahan D. Signaling vascular morphogenesis and maintenance. Science. 1997;277(5322):48–50. doi: 10.1126/science.277.5322.48. [DOI] [PubMed] [Google Scholar]
- 49.Shimizu H, Mitsuhashi N, Ohtsuka M, Ito H, Kimura F, Ambiru S, Togawa A, Yoshidome H, Kato A, Miyazaki M. Vascular endothelial growth factor and angiopoietins regulate sinusoidal regeneration and remodeling after partial hepatectomy in rats. World J Gastroenterol. 2005;11(46):7254–7260. doi: 10.3748/wjg.v11.i46.7254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kraizer Y, Mawasi N, Seagal J, Paizi M, Assy N, Spira G. Vascular endothelial growth factor and angiopoietin in liver regeneration. Biochem Biophys Res Commun. 2001;287(1):209–215. doi: 10.1006/bbrc.2001.5548. [DOI] [PubMed] [Google Scholar]
- 51.Greene AK, Wiener S, Puder M, Yoshida A, Shi B, Perez-Atayde AR, Efstathiou JA, Holmgren L, Adamis AP, Rupnick M, Folkman J, O’Reilly MS. Endothelial-directed hepatic regeneration after partial hepatectomy. Ann Surg. 2003;237(4):530–535. doi: 10.1097/01.SLA.0000059986.96051.EA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov. 2007;6(4):273–286. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- 53.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42(5):1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 54.Pinzani M, Marra F. Cytokine receptors and signaling in hepatic stellate cells. Semin Liver Dis. 2001;21(3):397–416. doi: 10.1055/s-2001-17554. [DOI] [PubMed] [Google Scholar]
- 55.Sakamoto M, Hirohashi S, Shimosato Y. Early stages of multistep hepatocarcinogenesis: adenomatous hyperplasia and early hepatocellular carcinoma. Hum Pathol. 1991;22(2):172–178. doi: 10.1016/0046-8177(91)90039-r. [DOI] [PubMed] [Google Scholar]
- 56.Himeno H, Enzan H, Saibara T, Onishi S, Yamamoto Y. Hitherto unrecognized arterioles within hepatocellular carcinoma. J Pathol. 1994;174(3):217–222. doi: 10.1002/path.1711740311. [DOI] [PubMed] [Google Scholar]
- 57.Kin M, Torimura T, Ueno T, Inuzuka S, Tanikawa K. Sinusoidal capillarization in small hepatocellular carcinoma. Pathol Int. 1994;44(10–11):771–778. doi: 10.1111/j.1440-1827.1994.tb02925.x. [DOI] [PubMed] [Google Scholar]
- 58.Park YN, Kim YB, Yang KM, Park C. Increased expression of vascular endothelial growth factor and angiogenesis in the early stage of multistep hepatocarcinogenesis. Arch Pathol Lab Med. 2000;124(7):1061–1065. doi: 10.5858/2000-124-1061-IEOVEG. [DOI] [PubMed] [Google Scholar]
- 59.Hayashi M, Matsui O, Ueda K, Kawamori Y, Kadoya M, Yoshikawa J, Gabata T, Takashima T, Nonomura A, Nakanuma Y. Correlation between the blood supply and grade of malignancy of hepatocellular nodules associated with liver cirrhosis: evaluation by CT during intraarterial injection of contrast medium. AJR Am J Roentgenol. 1999;172(4):969–976. doi: 10.2214/ajr.172.4.10587130. [DOI] [PubMed] [Google Scholar]
- 60.Yamasaki M, Ikeda K, Nakatani K, Yamamoto T, Kawai Y, Hirohashi K, Kinoshita H, Kaneda K. Phenotypical and morphological alterations to rat sinusoidal endothelial cells in arterialized livers after portal branch ligation. Arch Histol Cytol. 1999;62(5):401–411. doi: 10.1679/aohc.62.401. [DOI] [PubMed] [Google Scholar]
- 61.Corpechot C, Barbu V, Wendum D, Kinnman N, Rey C, Poupon R, Housset C, Rosmorduc O. Hypoxia-induced VEGF and collagen I expressions are associated with angiogenesis and fibrogenesis in experimental cirrhosis. Hepatology. 2002;35(5):1010–1021. doi: 10.1053/jhep.2002.32524. [DOI] [PubMed] [Google Scholar]
- 62.Fernandez M, Semela D, Bruix J, Colle I, Pinzani M, Bosch J. Angiogenesis in liver disease. J Hepatol. 2009;50(3):604–620. doi: 10.1016/j.jhep.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 63.Kim KR, Moon HE, Kim KW. Hypoxia-induced angiogenesis in human hepatocellular carcinoma. J Mol Med. 2002;80(11):703–714. doi: 10.1007/s00109-002-0380-0. [DOI] [PubMed] [Google Scholar]
- 64.Moon EJ, Jeong CH, Jeong JW, Kim KR, Yu DY, Murakami S, Kim CW, Kim KW. Hepatitis B virus X protein induces angiogenesis by stabilizing hypoxia-inducible factor-1alpha. FASEB J. 2004;18(2):382–384. doi: 10.1096/fj.03-0153fje. [DOI] [PubMed] [Google Scholar]
- 65.Marschall Z, Cramer T, Hocker M, Finkenzeller G, Wiedenmann B, Rosewicz S. Dual mechanism of vascular endothelial growth factor upregulation by hypoxia in human hepatocellular carcinoma. Gut. 2001;48(1):87–96. doi: 10.1136/gut.48.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yoshiji H, Kuriyama S, Hicklin DJ, Huber J, Yoshii J, Miyamoto Y, Kawata M, Ikenaka Y, Nakatani T, Tsujinoue H, Fukui H. KDR/Flk-1 is a major regulator of vascular endothelial growth factor-induced tumor development and angiogenesis in murine hepatocellular carcinoma cells. Hepatology. 1999;30(5):1179–1186. doi: 10.1002/hep.510300509. [DOI] [PubMed] [Google Scholar]
- 67.Shimamura T, Saito S, Morita K, Kitamura T, Morimoto M, Kiba T, Numata K, Tanaka K, Sekihara H. Detection of vascular endothelial growth factor and its receptor expression in human hepatocellular carcinoma biopsy specimens. J Gastroenterol Hepatol. 2000;15(6):640–646. doi: 10.1046/j.1440-1746.2000.02201.x. [DOI] [PubMed] [Google Scholar]
- 68.Yamaguchi R, Yano H, Nakashima Y, Ogasawara S, Higaki K, Akiba J, Hicklin DJ, Kojiro M. Expression and localization of vascular endothelial growth factor receptors in human hepatocellular carcinoma and non-HCC tissues. Oncol Rep. 2000;7(4):725–729. doi: 10.3892/or.7.4.725. [DOI] [PubMed] [Google Scholar]
- 69.Raskopf E, Dzienisowicz C, Hilbert T, Rabe C, Leifeld L, Wernert N, Sauerbruch T, Prieto J, Qian C, Caselmann WH, Schmitz V. Effective angiostatic treatment in a murine metastatic and orthotopic hepatoma model. Hepatology. 2005;41(6):1233–1240. doi: 10.1002/hep.20724. [DOI] [PubMed] [Google Scholar]
- 70.Nakamura K, Zen Y, Sato Y, Kozaka K, Matsui O, Harada K, Nakanuma Y. Vascular endothelial growth factor, its receptor Flk-1, and hypoxia inducible factor-1alpha are involved in malignant transformation in dysplastic nodules of the liver. Hum Pathol. 2007;38(10):1532–1546. doi: 10.1016/j.humpath.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 71.Ng IO, Poon RT, Lee JM, Fan ST, Ng M, Tso WK. Microvessel density, vascular endothelial growth factor and its receptors Flt-1 and Flk-1/KDR in hepatocellular carcinoma. Am J Clin Pathol. 2001;116(6):838–845. doi: 10.1309/FXNL-QTN1-94FH-AB3A. [DOI] [PubMed] [Google Scholar]
- 72.Dhar DK, Naora H, Yamanoi A, Ono T, Kohno H, Otani H, Nagasue N. Requisite role of VEGF receptors in angiogenesis of hepatocellular carcinoma: a comparison with angiopoietin/Tie pathway. Anticancer Res. 2002;22(1A):379–386. [PubMed] [Google Scholar]
- 73.Yoshiji H, Noguchi R, Kuriyama S, Yoshii J, Ikenaka Y, Yanase K, Namisaki T, Kitade M, Yamazaki M, Uemura M, Fukui H. Different cascades in the signaling pathway of two vascular endothelial growth factor (VEGF) receptors for the VEGF-mediated murine hepatocellular carcinoma development. Oncol Rep. 2005;13(5):853–857. [PubMed] [Google Scholar]
- 74.Sugimachi K, Tanaka S, Taguchi K, Aishima S, Shimada M, Tsuneyoshi M. Angiopoietin switching regulates angiogenesis and progression of human hepatocellular carcinoma. J Clin Pathol. 2003;56(11):854–860. doi: 10.1136/jcp.56.11.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Torimura T, Ueno T, Kin M, Harada R, Taniguchi E, Nakamura T, Sakata R, Hashimoto O, Sakamoto M, Kumashiro R, Sata M, Nakashima O, Yano H, Kojiro M. Overexpression of angiopoietin-1 and angiopoietin-2 in hepatocellular carcinoma. J Hepatol. 2004;40(5):799–807. doi: 10.1016/j.jhep.2004.01.027. [DOI] [PubMed] [Google Scholar]
- 76.Zhang ZL, Liu ZS, Sun Q. Expression of angiopoietins, Tie2 and vascular endothelial growth factor in angiogenesis and progression of hepatocellular carcinoma. World J Gastroenterol. 2006;12(26):4241–4245. doi: 10.3748/wjg.v12.i26.4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yoshiji H, Kuriyama S, Noguchi R, Yoshii J, Ikenaka Y, Yanase K, Namisaki T, Kitade M, Uemura M, Masaki T, Fukui H. Angiopoietin 2 displays a vascular endothelial growth factor dependent synergistic effect in hepatocellular carcinoma development in mice. Gut. 2005;54(12):1768–1775. doi: 10.1136/gut.2005.067900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zeng W, Gouw AS, Heuvel MC, Zwiers PJ, Zondervan PE, Poppema S, Zhang N, Platteel I, Jong KP, Molema G. The angiogenic makeup of human hepatocellular carcinoma does not favor vascular endothelial growth factor/angiopoietin-driven sprouting neovascularization. Hepatology. 2008;48(5):1517–1527. doi: 10.1002/hep.22490. [DOI] [PubMed] [Google Scholar]
- 79.Semela D, Piguet AC, Kolev M, Schmitter K, Hlushchuk R, Djonov V, Stoupis C, Dufour JF. Vascular remodeling and antitumoral effects of mTOR inhibition in a rat model of hepatocellular carcinoma. J Hepatol. 2007;46(5):840–848. doi: 10.1016/j.jhep.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 80.Djonov V, Schmid M, Tschanz SA, Burri PH. Intussusceptive angiogenesis: its role in embryonic vascular network formation. Circ Res. 2000;86(3):286–292. doi: 10.1161/01.res.86.3.286. [DOI] [PubMed] [Google Scholar]
- 81.Olszewska-Pazdrak B, Hein TW, Olszewska P, Carney DH. Chronic hypoxia attenuates VEGF signaling and angiogenic responses by downregulation of KDR in human endothelial cells. Am J Physiol Cell Physiol. 2009;296(5):C1162–C1170. doi: 10.1152/ajpcell.00533.2008. [DOI] [PubMed] [Google Scholar]
- 82.Weng J, Gouw AS, Heuvel MC, Poppema S, Jong KP. Angiogenic characteristics in hepatocellular carcinomas: a comparison of HCCs in cirrhotic and non-cirrhotic livers and the influence of grading. Hepatology. 2006;44(Suppl 1):506A. [Google Scholar]
- 83.Ho JW, Pang RW, Lau C, Sun CK, Yu WC, Fan ST, Poon RT. Significance of circulating endothelial progenitor cells in hepatocellular carcinoma. Hepatology. 2006;44(4):836–843. doi: 10.1002/hep.21353. [DOI] [PubMed] [Google Scholar]
- 84.Yu D, Sun X, Qiu Y, Zhou J, Wu Y, Zhuang L, Chen J, Ding Y. Identification and clinical significance of mobilized endothelial progenitor cells in tumor vasculogenesis of hepatocellular carcinoma. Clin Cancer Res. 2007;13(13):3814–3824. doi: 10.1158/1078-0432.CCR-06-2594. [DOI] [PubMed] [Google Scholar]
- 85.Maniotis AJ, Folberg R, Hess A, Seftor EA, Gardner LM, Pe’er J, Trent JM, Meltzer PS, Hendrix MJ. Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am J Pathol. 1999;155(3):739–752. doi: 10.1016/S0002-9440(10)65173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sun T, Zhao N, Zhao XL, Gu Q, Zhang SW, Che N, Wang XH, Du J, Liu YX, Sun BC. Expression and functional significance of Twist1 in hepatocellular carcinoma: its role in vasculogenic mimicry. Hepatology 2010;51(2):545–556 [DOI] [PubMed]
- 87.Sun B, Zhang S, Zhang D, Du J, Guo H, Zhao X, Zhang W, Hao X. Vasculogenic mimicry is associated with high tumor grade, invasion and metastasis, and short survival in patients with hepatocellular carcinoma. Oncol Rep. 2006;16(4):693–698. [PubMed] [Google Scholar]
- 88.Guzman G, Cotler SJ, Lin AY, Maniotis AJ, Folberg R. A pilot study of vasculogenic mimicry immunohistochemical expression in hepatocellular carcinoma. Arch Pathol Lab Med. 2007;131(12):1776–1781. doi: 10.5858/2007-131-1776-apsovm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hillen F, Griffioen AW. Tumour vascularization: sprouting angiogenesis and beyond. Cancer Metastasis Rev. 2007;26(3,4):489–502. doi: 10.1007/s10555-007-9094-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wesseling P, Laak JA, Leeuw H, Ruiter DJ, Burger PC. Quantitative immunohistological analysis of the microvasculature in untreated human glioblastoma multiforme. Computer-assisted image analysis of whole-tumor sections. J Neurosurg. 1994;81(6):902–909. doi: 10.3171/jns.1994.81.6.0902. [DOI] [PubMed] [Google Scholar]
- 91.Pezzella F, Pastorino U, Tagliabue E, Andreola S, Sozzi G, Gasparini G, Menard S, Gatter KC, Harris AL, Fox S, Buyse M, Pilotti S, Pierotti M, Rilke F. Non-small-cell lung carcinoma tumor growth without morphological evidence of neo-angiogenesis. Am J Pathol. 1997;151(5):1417–1423. [PMC free article] [PubMed] [Google Scholar]
- 92.Holash J, Maisonpierre PC, Compton D, Boland P, Alexander CR, Zagzag D, Yancopoulos GD, Wiegand SJ. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science. 1999;284(5422):1994–1998. doi: 10.1126/science.284.5422.1994. [DOI] [PubMed] [Google Scholar]
- 93.Nakamura S, Muro H, Suzuki S, Sakaguchi T, Konno H, Baba S, Syed AS. Immunohistochemical studies on endothelial cell phenotype in hepatocellular carcinoma. Hepatology. 1997;26(2):407–415. doi: 10.1002/hep.510260222. [DOI] [PubMed] [Google Scholar]
- 94.Vermeulen PB, Colpaert C, Salgado R, Royers R, Hellemans H, Heuvel E, Goovaerts G, Dirix LY, Marck E. Liver metastases from colorectal adenocarcinomas grow in three patterns with different angiogenesis and desmoplasia. J Pathol. 2001;195(3):336–342. doi: 10.1002/path.966. [DOI] [PubMed] [Google Scholar]
- 95.Kanai T, Hirohashi S, Upton MP, Noguchi M, Kishi K, Makuuchi M, Yamasaki S, Hasegawa H, Takayasu K, Moriyama N, et al. Pathology of small hepatocellular carcinoma. A proposal for a new gross classification. Cancer. 1987;60(4):810–819. doi: 10.1002/1097-0142(19870815)60:4<810::aid-cncr2820600417>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 96.Scavelli C, Weber E, Agliano M, Cirulli T, Nico B, Vacca A, Ribatti D. Lymphatics at the crossroads of angiogenesis and lymphangiogenesis. J Anat. 2004;204(6):433–449. doi: 10.1111/j.0021-8782.2004.00293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mouta Carreira C, Nasser SM, di Tomaso E, Padera TP, Boucher Y, Tomarev SI, Jain RK. LYVE-1 is not restricted to the lymph vessels: expression in normal liver blood sinusoids and down-regulation in human liver cancer and cirrhosis. Cancer Res. 2001;61(22):8079–8084. [PubMed] [Google Scholar]
- 98.Thelen A, Jonas S, Benckert C, Weichert W, Schott E, Botcher C, Dietz E, Wiedenmann B, Neuhaus P, Scholz A. Tumor-associated lymphangiogenesis correlates with prognosis after resection of human hepatocellular carcinoma. Ann Surg Oncol. 2009;16(5):1222–1230. doi: 10.1245/s10434-009-0380-1. [DOI] [PubMed] [Google Scholar]
- 99.Thelen A, Scholz A, Benckert C, Marschall Z, Schroder M, Wiedenmann B, Neuhaus P, Rosewicz S, Jonas S. VEGF-D promotes tumor growth and lymphatic spread in a mouse model of hepatocellular carcinoma. Int J Cancer. 2008;122(11):2471–2481. doi: 10.1002/ijc.23439. [DOI] [PubMed] [Google Scholar]
- 100.Ohtani O, Ohtani Y. Lymph circulation in the liver. Anat Rec (Hoboken) 2008;291(6):643–652. doi: 10.1002/ar.20681. [DOI] [PubMed] [Google Scholar]
- 101.Lian Z, Liu J, Wu M, Wang HY, Arbuthnot P, Kew M, Feitelson MA. Hepatitis B × antigen up-regulates vascular endothelial growth factor receptor 3 in hepatocarcinogenesis. Hepatology. 2007;45(6):1390–1399. doi: 10.1002/hep.21610. [DOI] [PubMed] [Google Scholar]
- 102.Yamaguchi R, Yano H, Nakashima O, Akiba J, Nishida N, Kurogi M, Kojiro M. Expression of vascular endothelial growth factor-C in human hepatocellular carcinoma. J Gastroenterol Hepatol. 2006;21(1 Pt 1):152–160. doi: 10.1111/j.1440-1746.2005.04217.x. [DOI] [PubMed] [Google Scholar]
- 103.Gasparini G, Longo R, Fanelli M, Teicher BA. Combination of antiangiogenic therapy with other anticancer therapies: results, challenges, and open questions. J Clin Oncol. 2005;23(6):1295–1311. doi: 10.1200/JCO.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 104.Greten TF, Korangy F, Manns MP, Malek NP. Molecular therapy for the treatment of hepatocellular carcinoma. Br J Cancer. 2009;100(1):19–23. doi: 10.1038/sj.bjc.6604784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Haussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J. Sorafenib in advanced hepatocellular carcinoma. New Engl J Med. 2008;359(4):378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 106.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, Xu J, Sun Y, Liang H, Liu J, Wang J, Tak WY, Pan H, Burock K, Zou J, Voliotis D, Guan Z. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 107.Tozer GM, Kanthou C, Baguley BC. Disrupting tumour blood vessels. Nat Rev Cancer. 2005;5(6):423–435. doi: 10.1038/nrc1628. [DOI] [PubMed] [Google Scholar]
- 108.Baguley BC. Antivascular therapy of cancer: DMXAA. Lancet Oncol. 2003;4(3):141–148. doi: 10.1016/s1470-2045(03)01018-0. [DOI] [PubMed] [Google Scholar]
- 109.Huynh H, Chow KP, Soo KC, Toh HC, Choo SP, Foo KF, Poon D, Ngo VC, Tran E. RAD001 (everolimus) inhibits tumor growth in xenograft models of human hepatocellular carcinoma. J Cell Mol Med. 2009;13(7):1371–1380. doi: 10.1111/j.1582-4934.2008.00364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Semela D, Dufour JF. Angiogenesis and hepatocellular carcinoma. J Hepatol. 2004;41(5):864–880. doi: 10.1016/j.jhep.2004.09.006. [DOI] [PubMed] [Google Scholar]