Abstract
Purpose
Standard dose (13–15 mg/kg) ursodeoxycholic acid (UCDA) is ineffective in the treatment of nonalcoholic steatohepatitis (NASH), however, its immunomodulatory and hepatoprotective effects are dose related. Therefore, we examined the impact of high-dose (28–32 mg/kg) UCDA on aminotransaminase levels in a pilot study of patients with NASH.
Methods
Twelve patients with biopsy-proven NASH and elevated aminotransaminases were prescribed high-dose UCDA for 6 months. Liver function tests were monitored during and after treatment with the study endpoint defined as normalization of aminotransaminase levels.
Results
Normalization of aspartate aminotransaminase (AST) levels was observed in two (17%) patients, however, no patient normalized their alanine aminotransaminase (ALT) levels. A trend towards a minor reduction in median (range) ALT values from baseline to end of treatment was noted [124 (66–229) vs. 101 (53–188) IU/l, p = 0.07], whereas AST levels remained unchanged [85 (40–132) vs. 98 (28–147) IU/l, p = 0.83]. One patient discontinued treatment prematurely due to diarrhea. No significant change in fasting glucose, triglyceride or HDL cholesterol was observed with treatment. No significant change in ALT or AST levels was observed in the 6-month period after cessation of treatment.
Conclusion
High-dose UCDA does not normalize aminotransaminase levels in patients with NASH. Other inexpensive well-tolerated agents for the treatment of NASH need to be investigated.
Keywords: Ursodeoxycholic acid, Nonalcoholic steatohepatitis, Aminotransaminase
Introduction
Nonalcoholic fatty liver disease (NAFLD) is remarkably common in the general population affecting up to 30% of adults [1]; however, only a minority of individuals with NAFLD develop liver-related morbidity and even less die as a consequence of their liver disease [2]. Due to the predominately asymptomatic and chronic nature of NAFLD, treatment should be inexpensive with acceptable tolerability and a low risk of side effects. Weight loss and exercise are cheap and devoid of significant side effects and first line therapy for NAFLD, however, long-term efficacy is limited by a low level of compliance [3]. A number of insulin-sensitizing agents have been tested in NAFLD including PPARγ agonists rosiglitazone and pioglitazone. These have shown promise in improving liver histology in patients with nonalcoholic steatohepatitis (NASH), however, they are relatively expensive and have side effects of weight gain [4], and possibly increased cardiovascular risk [5].
Ursodeoxycholic acid (UDCA) has hepatoprotective and anti-apoptotic effects which may be beneficial in patients with NASH [6–8]. Despite preliminary evidence suggesting a possible beneficial effect of UDCA on liver injury in patients with NASH, a large randomized controlled trial of 166 patients using standard dose (13–15 mg/kg) UDCA, found no difference in biochemistry or liver histology with UDCA or placebo after 2 years of treatment [9]. However, biliary enrichment of UDCA is greater with high-dose UDCA than the standard doses used in this trial [10]. UDCA has exhibited a dose–response relationship for several diseases including primary sclerosing cholangitis, intrahepatic cholestasis of pregnancy and cystic fibrosis-associated liver disease [11–14]. Thus, the aim of this open-label pilot study was to assess the efficacy of 6 months of high-dose UCDA (28–32 mg/kg per day) on serum liver aminotransferases among patients with NASH.
Methods
Twelve patients were enrolled from general hepatology clinics at the Department of Gastroenterology, Mayo Clinic (Rochester, Minnesota). Inclusion criteria consisted of: age greater than 18 years, alanine aminotransferase (ALT) or aspartate aminotransferase (AST) level 1.5 times the upper limit of normal, liver biopsy prior to enrollment demonstrating NASH with greater than 10% steatosis, lobular inflammation and hepatocyte ballooning (with or without Mallory bodies or fibrosis) [15]. Exclusion criteria consisted of: pregnancy or lactation, advanced liver cirrhosis with decompensation, such as ascites, variceal hemorrhage, encephalopathy or hepatocellular carcinoma; secondary causes of NAFLD (medications, jejuno-ileal surgery, human immunodeficiency virus); clinical, serological or histological evidence of other liver disease such as viral hepatitis, autoimmune hepatitis, primary biliary cirrhosis, primary sclerosing hepatitis, hemochromatosis, Wilson’s disease or alpha-1 antitrypsin deficiency or alcohol intake of more than 40 g/week.
Initial evaluation
Each patient underwent a complete history and physical evaluation prior to enrollment. Alcohol consumption (type, amount, frequency, duration as well as alcohol abuse screening questions) was assessed by a patient history form filled out by the patient and reviewed by a nurse and physician. Fasting serum was taken to evaluate liver enzymes (bilirubin, aminotransferases, alkaline phosphatase, albumin), lipid profiles (triglycerides, cholesterol, HDL) and glucose level.
All liver biopsies were staged and graded by a dedicated liver pathologist, according to the scheme outlined by Brunt et al. [16]. Briefly, the degree of fat infiltration was assessed on a scale of 1–3 :1 = mild (10–33% of hepatocytes affected); 2 = moderate (33–66% of hepatocytes affected); and 3 = severe (>66% of hepatocytes affected). Severity of necroinflammatory activity was graded on a scale of 1–3: 1 = mild; 2 = moderate; and 3 = severe. The degree of fibrosis was staged using a 5-point scale: stage 0 = no fibrosis, normal connective tissue; stage 1 = perivenular/pericellular fibrosis in zone 3; stage 2 = perivenular or pericellular fibrosis confined to zone 3 plus periportal fibrosis; stage 3 = bridging or septal fibrosis; and stage 4 = cirrhosis.
Intervention
Patients were prescribed 500 mg tablets of UCDA (Axcan Pharma, Quebec, Canada) at a dose of 28–32 mg/kg, taken orally in two divided doses at breakfast and in the evening. Compliance was assessed by counting unused tablets at the end of each 3-month period. All patients were counseled regarding dietary modification and regular exercise at initial assessment. No patient had insulin-sensitizing agents, weight loss or anorectic agents commenced during the course of the trial. No patient underwent bariatric surgery.
Follow-up
Patients were monitored with three monthly blood tests (liver enzymes, glucose, lipid profile) as well as monthly phone contact by a research nurse to assess side effects. Patients were followed for further 6 months after cessation of medication with three monthly liver enzyme tests.
Consent
All patients supplied informed consent. The study was approved by the Mayo Clinic Institutional Review Board and was carried out according to the principals of the Declaration of Helsinki.
Statistical analysis
The primary endpoint of the trial was normalization of ALT or AST levels without an increase of more than 25% of the value of other liver tests. Descriptive data is presented as median and range or absolute number and proportion. The primary endpoint was assessed using Fishers exact test. Wilcoxon signed-rank test was used to assess change in liver enzymes, fasting glucose and lipid profiles from the beginning to the end of the trial and from the end of the trial to 6 months follow-up. A p value <0.05 was considered statistically significant. The statistical analysis was performed on SPSS for windows 15.0.
Results
Baseline clinical features
Twelve patients (three male, nine female) with a median (range) age of 58 (43–71) years were enrolled (Table 1). Features of the metabolic syndrome were common with the two-thirds being obese, half having impaired fasting glycemia or diabetes and three quarters being hypertensive. Liver enzymes were characteristic of patients with NASH with aminotransaminases being modestly elevated and all patients having an AST/ALT ratio of less than one. Liver biopsies performed prior to enrollment demonstrated predominately low-grade inflammation and minor or no fibrosis. Two patients had bridging fibrosis and one patient had cirrhosis without evidence of synthetic impairment or decompensation.
Table 1.
Baseline clinical, biochemical and histological features of cohort (n = 12)
| Gender (male/female) | 3/9 |
| Age (median, range) | 58 (43–71) years |
| Obese (BMI ≥30 kg/m2) | 8 (67%) |
| Impaired fasting glycemia (n, %) | 3 (25%) |
| Diabetes (n, %) | 3 (25%) |
| Hypertension (n, %) | 9 (75%) |
| Hypertriglyceridemia (n, %) | 8 (67%) |
| Fasting glucose (mg/dl) | 116 (108–188) |
| Triglyceride (mg/dl) | 148 (73–3,510 |
| HDL cholesterol (mg/dl) | 44 (33–126) |
| Bilirubin (mg/dl) | 0.6 (0.3–1.2) |
| ALT (IU/L) | 124 (66–229) |
| AST (IU/L) | 85 (40–132) |
| AST/ALT ratio | 0.62 (0.47–0.81) |
| Alkaline phosphatase (IU/L) | 82 (62–123) |
| Albumin (g/dl) | 4.2 (3.6–4.8) |
| Steatosis grade | |
| 1/2/3 (n, %) | 2 (17%)/7 (58%)/3 (25%) |
| Necroinflammatory grade | |
| 0/1/2/3 (n, %) | 0/5 (42%)/7 (58%)/0 |
| Fibrosis stage | |
| 0/1/2/3/4 (n, %) | 2 (17%)/5 (41%)/2 (17%)/2 (17%)/1 (8%) |
Biochemical values presented as median (range). Impaired fasting glycemia defined as fasting glucose >100 mg/dl. Histology scored according to the criteria of Brunt and colleagues [16]
Effect of high-dose UDCA on aminotransaminases
The primary endpoint of normalization of ALT or AST levels was reached by only two (17%) patients. Both of these patients had a normalization of their AST levels without normalization of ALT. No patient had normalization of their ALT level. Median (range) values of ALT tended to improve from baseline to end of treatment [124 (66–229) vs. 101 (53–188) IU/l, p = 0.07], whereas AST levels remained unchanged [85 (40–132) vs. 98 (28–147) IU/l, p = 0.83].
Bilirubin levels fell marginally on treatment [0.6 (0.3–1.2) vs. 0.5 (0.3–0.8), p = 0.046]. There was no change in levels of alkaline phosphatase [82 (62–123) vs. 85 (66–104) IU/L, p = 0.8]. There was no significant change in bilirubin or alkaline phosphatase levels from end of treatment when re-assessed at 3 and 6 months after end of treatment. No significant change in levels of fasting glucose, triglyceride or HDL cholesterol was observed with treatment.
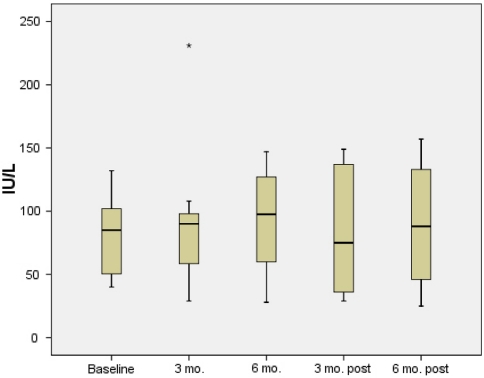
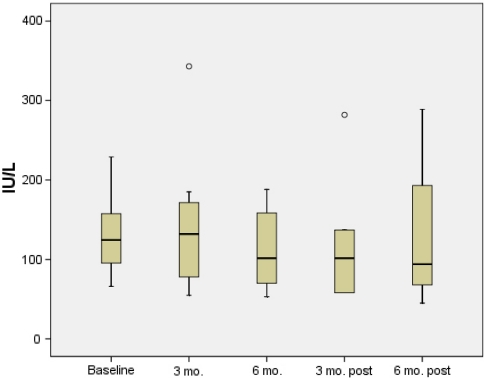
Six patients had aminotransaminase levels measured 6 months after stopping high-dose UCDA and four patients had aminotransaminase levels measured 6 months after stopping treatment (Figs. 1, 2). No significant worsening of ALT or AST levels was observed from end of treatment levels to 6 months after stopping high-dose UDCA [102 (58–282) vs. 101 (53–188) IU/l, p = 0.3 and 98 (28–147) vs. 75 (29–149) IU/L, p = 0.7, respectively]. Similarly, no change in ALT or AST levels was observed from end of treatment to 6 months after end of treatment [102 (58–282) vs. 94 (45–289) IU/l, p = 0.7 and 98 (28–147) vs. 88 (25–157) IU/L, p = 0.7, respectively].
Fig. 1.
AST levels during 6 months high-dose ursodeoxycholic acid and 6 months follow-up
Fig. 2.
ALT levels during 6 months high-dose ursodeoxycholic acid and 6 months follow-up
Side effects
The medication was generally well tolerated. Two patients suffered diarrhea, one of whom withdrew from the study. One patient complained of increased fatigue and mild nocturnal pruritus but was able to continue her medication.
Discussion
Morbidity and mortality related to NAFLD are likely to increase in the future as the prevalence of associated insulin resistant states of obesity and diabetes also increase [17]. Thus there is a critical need to identify effective therapeutic strategies for NAFLD. Due to the large proportion of individuals in the community with NAFLD and the chronic and largely asymptomatic course, treatment should ideally be inexpensive and non-toxic.
Ursodeoxycholic acid is inexpensive and well tolerated and has a number of hepatoprotective and immunomodulatory effects in animal models of chronic liver disease. After ingestion, UDCA becomes the principal bile acid in the liver, replacing more toxic hydrophobic bile acids and stimulating secretion of bile acids [18, 19]. As steatotic hepatocytes are more susceptible to necrosis and generate increased quantities of hydroperoxides resulting in increased oxidative stress when exposed to hydrophobic bile acids, UDCA may potentially ameliorate this type of liver injury [20]. In addition to these effects, UDCA stabilizes mitochondrial membranes and inhibits caspase activation, having the net effect of inhibiting apoptosis [6, 7], which has been noted to be increased in patients with NASH [21]. UDCA also has immunomodulatory effects including the correction of defective NK cell activity [8], which has been postulated as mechanism of progression of NAFLD [22]. A mechanistic link between insulin resistance and tumor necrosis factor (TNF)-α has been identified in animal models of fatty liver [23]. TNF-α seems to play a key role in the development of insulin resistance and liver fibrosis, contributing to the pathogenesis of NASH [24]. At least among patients with chronic cholestasis, long-term therapy with UDCA diminishes production of TNF-α which is associated with biochemical and histological improvement [25].
Despite these observations in animal studies, high-dose UDCA failed to demonstrate efficacy to normalize aminotransaminase levels in this pilot study of patients with NASH. Although the sample size in this pilot study was relatively small, none of the 12 patients normalized their ALT levels, suggesting a consistent lack of benefit. There was a non-significant trend towards reduced ALT levels over the 6-month treatment course, however, the degree of improvement was marginal and is unlikely to be clinically significant. Furthermore, a recent study in primary sclerosing cholangitis with a similar dose of UDCA found more adverse events in the UDCA treated group than in the placebo group [26].
Assessment of TNF-α levels, insulin resistance and markers of oxidative stress would have been valuable to investigate possible mechanisms of action of UCDA. Unfortunately sera were not available to assess these endpoints in our study. Our pilot trial was primarily to assess the possibility of therapeutic effect rather than to investigate possible mechanisms of effect, thus our endpoint was normalization of aminotransaminases. We have previously demonstrated change in aminotransaminases to be a valid surrogate measure of histological improvement for use in screening therapies for NASH in clinical trials [27].
Knowledge of the pathogenesis of NASH is critical for developing effective treatment strategies. It is established that insulin resistance is the major pathogenic force leading to the development of hepatic steatosis in patients with NAFLD [26]. It is becoming increasingly recognized that insulin resistance is also an important pathogenic factor involved in progression to hepatocellular inflammation and fibrosis. For example, patients with diabetes are more likely to develop NASH cirrhosis and die of their liver disease [2, 28]. This aggressive clinical course may be due to hyperglycemia and hyperinsulinemia upregulating connective tissue growth factors and stimulating the production of collagen by hepatic stellate cells [29, 30]. Insulin resistance is also associated with reduced levels of adiponectin which is protective of liver injury in animal models [31–34]. Therefore pharmaceutical agents aimed at improving insulin resistance have been investigated as a means of improving liver histology in NASH. Thioglitazones such as pioglitazone has been demonstrated to improve levels of hepatic steatosis and necroinflammation [4]. However, larger studies are required to demonstrate a beneficial effect on liver fibrosis prior to routine recommendation. In addition, side effects such as weight gain may limit long-term compliance and concern has been raised regarding a potential link to increased cardiovascular events [35]. Metformin is a relatively inexpensive medication commonly utilized in the treatment of insulin resistance associated with type 2 diabetes. Preliminary studies have yielded encouraging results suggesting a beneficial effect on liver inflammation and fibrosis, however, these early findings need to be replicated in a controlled trial with comparative histological assessment before the routine use of metformin can be recommended [36].
In summary, patients with NASH are at risk of progressive fibrosis and liver-related morbidity and mortality. Apoptosis and oxidative stress are thought to be important mediators of ongoing hepatic inflammation and damage leading to elevated aminotransaminases. Although high-dose UCDA has anti-apoptotic and anti-oxidative properties, it does not appear to normalize aminotransaminase levels in patients with NASH.
Acknowledgements
This study was funded in part by Axcan Pharma Inc. The authors have no financial disclosures. The writing, data analysis and preparation of this paper was not funded by any organization.
References
- 1.Browning JD, Szczepaniak LS, Dobbins R, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 2.Adams LA, Lymp JF, St Sauver J, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–121. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 3.Foster GD, Wyatt HR, Hill JO, et al. A randomized trial of a low-carbohydrate diet for obesity. N Engl J Med. 2003;348:2082–2090. doi: 10.1056/NEJMoa022207. [DOI] [PubMed] [Google Scholar]
- 4.Belfort R, Harrison SA, Brown K, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355:2297–2307. doi: 10.1056/NEJMoa060326. [DOI] [PubMed] [Google Scholar]
- 5.Lincoff AM, Wolski K, Nicholls SJ, Nissen SE. Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis of randomized trials. JAMA. 2007;298:1180–1188. doi: 10.1001/jama.298.10.1180. [DOI] [PubMed] [Google Scholar]
- 6.Rodrigues CM, Fan G, Wong PY, Kren BT, Steer CJ. Ursodeoxycholic acid may inhibit deoxycholic acid-induced apoptosis by modulating mitochondrial transmembrane potential and reactive oxygen species production. Mol Med. 1998;4:165–178. doi: 10.1007/s0089480040165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodrigues CM, Ma X, Linehan-Stieers C, Fan G, Kren BT, Steer CJ. Ursodeoxycholic acid prevents cytochrome c release in apoptosis by inhibiting mitochondrial membrane depolarization and channel formation. Cell Death Differ. 1999;6:842–854. doi: 10.1038/sj.cdd.4400560. [DOI] [PubMed] [Google Scholar]
- 8.Nishigaki Y, Ohnishi H, Moriwaki H, Muto Y. Ursodeoxycholic acid corrects defective natural killer activity by inhibiting prostaglandin E2 production in primary biliary cirrhosis. Dig Dis Sci. 1996;41:1487–1493. doi: 10.1007/BF02088577. [DOI] [PubMed] [Google Scholar]
- 9.Lindor KD, Kowdley KV, Heathcote EJ, et al. Ursodeoxycholic acid for treatment of nonalcoholic steatohepatitis: results of a randomized trial. Hepatology. 2004;39:770–778. doi: 10.1002/hep.20092. [DOI] [PubMed] [Google Scholar]
- 10.Rost D, Rudolph G, Kloeters-Plachky P, Stiehl A. Effect of high-dose ursodeoxycholic acid on its biliary enrichment in primary sclerosing cholangitis. Hepatology. 2004;40:693–698. doi: 10.1002/hep.20370. [DOI] [PubMed] [Google Scholar]
- 11.Mitchell SA, Bansi DS, Hunt N, Bergmann K, Fleming KA, Chapman RW. A preliminary trial of high-dose ursodeoxycholic acid in primary sclerosing cholangitis. Gastroenterology. 2001;121:900–907. doi: 10.1053/gast.2001.27965. [DOI] [PubMed] [Google Scholar]
- 12.Harnois DM, Angulo P, Jorgensen RA, Larusso NF, Lindor KD. High-dose ursodeoxycholic acid as a therapy for patients with primary sclerosing cholangitis. Am J Gastroenterol. 2001;96:1558–1562. doi: 10.1111/j.1572-0241.2001.03777.x. [DOI] [PubMed] [Google Scholar]
- 13.Mazzella G, Rizzo N, Azzaroli F, et al. Ursodeoxycholic acid administration in patients with cholestasis of pregnancy: effects on primary bile acids in babies and mothers. Hepatology. 2001;33:504–508. doi: 10.1053/jhep.2001.22647. [DOI] [PubMed] [Google Scholar]
- 14.Meeberg PC, Houwen RH, Sinaasappel M, Heijerman HG, Bijleveld CM, Vanberge-Henegouwen GP. Low-dose versus high-dose ursodeoxycholic acid in cystic fibrosis-related cholestatic liver disease. Results of a randomized study with 1-year follow-up. Scand J Gastroenterol. 1997;32:369–373. doi: 10.3109/00365529709007686. [DOI] [PubMed] [Google Scholar]
- 15.Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology. 2003;37:1202–1219. doi: 10.1053/jhep.2003.50193. [DOI] [PubMed] [Google Scholar]
- 16.Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467–2474. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 17.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 18.Angulo P. Use of ursodeoxycholic acid in patients with liver disease. Curr Gastroenterol Rep. 2002;4:37–44. doi: 10.1007/s11894-002-0036-9. [DOI] [PubMed] [Google Scholar]
- 19.Paumgartner G, Beuers U. Ursodeoxycholic acid in cholestatic liver disease: mechanisms of action and therapeutic use revisited. Hepatology. 2002;36:525–531. doi: 10.1053/jhep.2002.36088. [DOI] [PubMed] [Google Scholar]
- 20.Kobak GE, Deutsch G, Dahl R, Devereaux MW, Gumpricht E, Sokol RJ. Fat laden hepatocytes are more prone to cellular necrosis than apoptosis when exposed to hydrophobic bile acids. Gastroenterology 2002;122:A642 (abstract)
- 21.Feldstein AE, Canbay A, Angulo P, et al. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology. 2003;125:437–443. doi: 10.1016/S0016-5085(03)00907-7. [DOI] [PubMed] [Google Scholar]
- 22.Li Z, Lin H, Yang S, Diehl AM. Murine leptin deficiency alters Kupffer cell production of cytokines that regulate the innate immune system. Gastroenterology. 2002;123:1304–1310. doi: 10.1053/gast.2002.35997. [DOI] [PubMed] [Google Scholar]
- 23.Yuan M, Konstantopoulos N, Lee J, et al. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science. 2001;293:1673–1677. doi: 10.1126/science.1061620. [DOI] [PubMed] [Google Scholar]
- 24.Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature. 1997;389:610–614. doi: 10.1038/39335. [DOI] [PubMed] [Google Scholar]
- 25.Neuman M, Angulo P, Malkiewicz I, et al. Tumor necrosis factor-alpha and transforming growth factor-beta reflect severity of liver damage in primary biliary cirrhosis. J Gastroenterol Hepatol. 2002;17:196–202. doi: 10.1046/j.1440-1746.2002.02672.x. [DOI] [PubMed] [Google Scholar]
- 26.Browning JD, Horton JD. Molecular mediators of hepatic steatosis and liver injury. J Clin Invest. 2004;114:147–152. doi: 10.1172/JCI22422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suzuki A, Lymp J, Sauver JS, Angulo P, Lindor K. Values and limitations of serum aminotransferases in clinical trials of nonalcoholic steatohepatitis. Liver Int. 2006;26:1209–1216. doi: 10.1111/j.1478-3231.2006.01362.x. [DOI] [PubMed] [Google Scholar]
- 28.Younossi ZM, Gramlich T, Matteoni CA, Boparai N, McCullough AJ. Nonalcoholic fatty liver disease in patients with type 2 diabetes. Clin Gastroenterol Hepatol. 2004;2:262–265. doi: 10.1016/S1542-3565(04)00014-X. [DOI] [PubMed] [Google Scholar]
- 29.Sugimoto R, Enjoji M, Kohjima M, et al. High glucose stimulates hepatic stellate cells to proliferate and to produce collagen through free radical production and activation of mitogen-activated protein kinase. Liver Int. 2005;25:1018–1026. doi: 10.1111/j.1478-3231.2005.01130.x. [DOI] [PubMed] [Google Scholar]
- 30.Paradis V, Perlemuter G, Bonvoust F, et al. High glucose and hyperinsulinemia stimulate connective tissue growth factor expression: a potential mechanism involved in progression to fibrosis in nonalcoholic steatohepatitis. Hepatology. 2001;34:738–744. doi: 10.1053/jhep.2001.28055. [DOI] [PubMed] [Google Scholar]
- 31.Musso G, Gambino R, Biroli G, et al. Hypoadiponectinemia predicts the severity of hepatic fibrosis and pancreatic beta-cell dysfunction in nondiabetic nonobese patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2005;100:2438–2446. doi: 10.1111/j.1572-0241.2005.00297.x. [DOI] [PubMed] [Google Scholar]
- 32.Masaki T, Chiba S, Tatsukawa H, et al. Adiponectin protects LPS-induced liver injury through modulation of TNF-alpha in KK-Ay obese mice. Hepatology. 2004;40:177–184. doi: 10.1002/hep.20282. [DOI] [PubMed] [Google Scholar]
- 33.Kamada Y, Tamura S, Kiso S, et al. Enhanced carbon tetrachloride-induced liver fibrosis in mice lacking adiponectin. Gastroenterology. 2003;125:1796–1807. doi: 10.1053/j.gastro.2003.08.029. [DOI] [PubMed] [Google Scholar]
- 34.Xu A, Wang Y, Keshaw H, Xu LY, Lam KS, Cooper GJ. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J Clin Invest. 2003;112:91–100. doi: 10.1172/JCI17797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 36.Bugianesi E, Gentilcore E, Manini R, et al. A randomized controlled trial of metformin versus vitamin E or prescriptive diet in nonalcoholic fatty liver disease. Am J Gastroenterol. 2005;100:1082–1090. doi: 10.1111/j.1572-0241.2005.41583.x. [DOI] [PubMed] [Google Scholar]