Abstract
Background
Although chronic liver disease is associated with gastroesophageal reflux disease (GERD), the impact of chronic hepatitis B virus (HBV) infection on this association remains unclear. We thus aimed to evaluate the relationship between chronic HBV infection and GERD.
Methods
In this prospective population-based study, 1,001 adult subjects who underwent an upper gastrointestinal endoscopic examination in a health check-up and completed a gastroesophageal reflux questionnaire were consecutively enrolled. Endoscopic findings were classified according to the Los Angeles classification. Hepatitis B surface antigen was used as a marker of HBV infection. Univariate and multivariate approaches were used to evaluate the effects of chronic HBV infection on GERD.
Results
Chronic HBV infection was associated with heartburn sensation [odds ratio (OR) 1.27, 95% confidence interval 1.01–1.61, P = 0.037], and erosive esophagitis (adjusted OR 1.75, 1.03–2.97, P = 0.037). Although male gender is a risk factor of erosive esophagitis, further analyses stratified by gender and aspartate aminotransferase to platelet ratio index (APRI) showed that chronic HBV infection was associated with erosive esophagitis in female subjects (adjusted OR 2.70, 1.14–6.39, P = 0.024) and those with APRI of more than 0.3 (adjusted OR 3.94, 1.73–8.96, P = 0.001). Moreover, higher serum aspartate aminotransferase (AST) and triglyceride (TG) levels were risk factors of erosive esophagitis in patients with chronic HBV infection.
Conclusions
Our findings indicate a close association between chronic HBV infection and GERD, especially in female subjects and those with higher APRI levels. Moreover, HBV carriers with higher AST or TG levels have higher incidence of erosive esophagitis. The interactions between chronic HBV infection and GERD need further studies.
Electronic supplementary material
The online version of this article (doi:10.1007/s12072-010-9184-4) contains supplementary material, which is available to authorized users.
Keywords: Hepatitis B virus, Gastroesophageal reflux disease, Erosive esophagitis, Los Angeles classification, Endoscope, Questionnaire
Introduction
Hepatitis B virus (HBV) infection can cause acute and chronic liver disease, including chronic hepatitis, cirrhosis, and hepatocellular carcinoma (HCC) [1]. Worldwide over 2 billion people have been infected with HBV, and nearly 400 million people are chronic carriers of the virus. Although effective vaccines against HBV infection have been available for more than two decades, chronic HBV infection remains a global public health threat.
Gastroesophageal reflux disease (GERD) affects the quality of patients’ life, increases the risk for esophageal adenocarcinoma, impacts public health, increases the economic burden gradually, and is becoming increasingly prevalent in recent years [2]. Identification of risk factors or interaction with other diseases that could serve as the basis for the treatment or prevention of GERD is therefore highly warranted [3]. Several extra-esophageal manifestations have been linked with GERD, such as chronic bronchitis, interstitial pneumonia, bronchial asthma, laryngitis, and coronary artery disease [4]. Although several studies mentioned a link between chronic liver disease and GERD [5, 6], the major limitations included small sample size, specific focus on patients with cirrhosis or quality of life rather than liver disease per se.
Because both chronic HBV infection and GERD are prevalent globally, and have huge impacts on human health, it is thus important to elucidate the relationship and possible interactions between these two disorders. Taiwan is a country endemic for chronic HBV infection and this advantage prompted us to investigate the relationship between chronic HBV infection and GERD, including GERD symptoms and erosive esophagitis.
Methods
Study design
Cross-sectional design
A prospectively and population-based survey followed by endoscopy and ultrasonography.
Study population
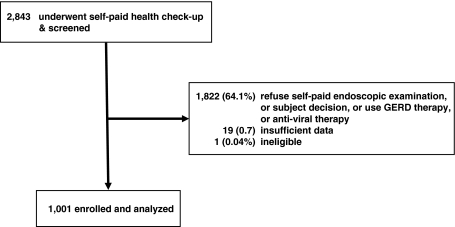
A total of 2,843 general healthy and potentially eligible subjects who underwent health examinations at the Health Management Center of Buddhist Tzu Chi General Hospital, Taipei branch, Taiwan between July and December 2007 were screened. Among them, subjects with obvious epigastric discomforts or GERD or anti-viral therapy were excluded, and followed or treated at the outpatient clinics. 1,021 (35.9%) subjects were willing to respond to the survey, and agreed to receive the upper gastrointestinal endoscopic examination. After excluding 20 subjects with ineligible or insufficient data, 1,001 were finally enrolled (Fig. 1). Chronic HBV infection was defined as the positivity of hepatitis B surface antigen (HBsAg) and a past history of HBV carriage. All of the subjects were negative for anti-HCV or HIV antibody, and none had a known history or serological evidence suggestive of autoimmune liver disease, inheritable disorders such as hemochromatosis or Wilson’s disease, renal insufficiency, or a history of drug abuse.
Fig. 1.
The flow diagram of this study
Demographic, serological and biochemical data
The data of age, gender, body weight, body height, body mass index (BMI), waist circumference, serum fasting blood glucose, hemoglobin A1c (HbA1c), triglyceride (TG), total cholesterol (TC), low density lipoprotein (LDL), high density lipoprotein (HDL), alkaline phosphatase (ALP), aspartate aminotransferase (AST), alanine aminotransferase (ALT) levels, and platelet counts were collected.
BMI was calculated as weight in kilograms divided by height in square meters. Blood samples were collected in the morning after 12 h fasting and measured by standard laboratory techniques. Platelet counts were performed by an automated Sysmex XE-2100 hematology analyzer (Sysmex, Kobe, Japan). Serum fasting blood glucose, TG, TC, LDL, HDL, ALP, AST, and ALT levels were measured by an auto analyzer (ROCHE ANALYTICS; Roche Professional Diagnostics, Penzberg, Germany).
Metabolic syndrome was defined according to the latest International Diabetes Federation consensus worldwide definition[7, 8] as the presence of central obesity (a waist circumference ≧90 cm for Taiwanese men and ≧80 cm for Taiwanese women) plus any two of the following four risk factors: TG concentration ≧150 mg/dL, or specific treatment for this lipid abnormality; HDL-cholesterol concentration <40 mg/dL in men and <50 mg/dL in women, or specific treatment for this lipid abnormality; blood pressure ≧130/85 mmHg, or treatment of previously diagnosed hypertension; or fasting plasma glucose ≧100 mg/dL, or previously diagnosed type 2 diabetes.
Aspartate aminotransferase to platelet ratio index (APRI) was used to assess liver fibrosis severity by the following formula and upper limit of normal (ULN) of serum AST level was set at 40 IU/L as previously described [9]:
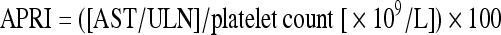 |
HBsAg and anti-HCV were assayed with commercial kits (Abbott Laboratories, North Chicago, IL, USA).
Questionnaire and additional medical information
The Reflux Disease Diagnostic Questionnaire (RDQ) is a reliable and valid instrument for the diagnosis of GERD that could be easily administered by primary care physicians in community settings. It examines symptoms of heartburn, acid regurgitation and dyspepsia, and contains 12 questions on the frequency, severity, and duration of burning and pain behind breastbone, acid taste in mouth, movement of materials upward from the stomach, and burning and pain in the upper stomach [10]. Most response options are scaled with categories ranging from one to five points for frequency, severity, and duration of symptoms.
Before receiving upper gastrointestinal endoscopic examination, all patients and controls underwent personal interviews conducted by trained professional interviewers who completed questionnaires and recorded additional medical information, including smoking, alcohol consumption, history of diabetes mellitus, hypertension, and use of the following medications: aspirin or non-steroid anti-inflammatory drugs, histamine 2 receptor antagonist, proton pump inhibitor, over the counter GERD therapy, and anti-viral therapy.
Smoking status was categorized into ever smokers (including past and current smokers at the time of filling in the questionnaires) and never smokers. Alcohol consumption was evaluated by summing the total amount of ethanol (beer, wine and spirits) consumed per week in grams, and categorized into no excessive alcohol intake (<140 g/week) and excessive alcohol consumer (≧140 g/week).
Endoscopic examination
Upper gastrointestinal endoscopy was offered to all participants who responded to the survey. Standardized sedated endoscopic examination of the esophagus, stomach, and duodenum was performed. Eight experienced endoscopists who were blinded to the results of the questionnaire performed all procedures, and one of them (P. C. Wang) reviewed all the endoscopic pictures. The severity of erosive esophagitis was graded from A to D according to the Los Angeles (LA) classification [11]. However, only 20 subjects were graded as B and 1 as C, so we collapsed the grading and the presence of erosive esophagitis defined as yes/or no was used. Any discordance in diagnosis was discussed by at least three experienced endoscopists, and the final diagnosis was made by consensus. We used the aforementioned methods to avoid interobserver and intraobserver variability.
Ultrasound examination
A GE LOGIO 5 Pro Ultrasound system (GE Medical Systems, Seoul, Korea) with a 4-MHz electronic probe was used to examine the livers. The parenchyma of liver was assessed on the basis of ultrasonographic findings of coarsened or heterogeneous echo pattern, increased parenchymal echogenicity, and nodularity of liver surface as previously described [12], and was classified into the following three categories: normal (N), none of the above findings; parenchymal liver disease (PLD), one of the findings; liver cirrhosis (LC), two or more of the findings.
Ethical considerations
The study was performed in accordance with the principles of the Declaration of Helsinki, and was approved by the Ethical Committee of the Buddhist Tzu Chi General Hospital in Taipei, Taiwan. Written informed consent was obtained from each participant.
Statistical analysis
The data were presented as percentages for categorical variables and mean with standard deviations for continuous variables unless mentioned otherwise. Chi-square tests, t tests, and Wilcoxon’s tests were used to analyze categorical, parametric continuous and non-parametric variables, respectively. Unadjusted and adjusted odds ratio (OR) and 95% confidence interval (CI) as well as P values were estimated for each variable by logistic regression.
To elucidate the associations between chronic HBV infection and GERD, multivariate analyses using a general logistic regression model with serostatus of HBsAg as a dependent variable were conducted. Risk factors of GERD or HBV infection or a P value of <0.05 in univariate analysis were candidates to be added into the model. Several factors were known to increase the risk of GERD, such as age, sex, BMI, and metabolic factors, so we finally included erosive esophagitis, age at enrollment, gender, BMI, fasting blood glucose, TC, AST, ALP levels and platelet counts into this model.
To further examine aforementioned relationships in terms of different genders and APRI levels, the 75 percentile of APRI was used as a cutoff point and stepwise linear regression analysis (backward) was performed to examine the association between chronic HBV infection and GERD. The presence of erosive esophagitis was used as dependent variable, and age, sex, BMI, waist circumference, systolic blood pressure (SBP), diastolic blood pressure (DBP), HBsAg, fasting blood glucose, HbA1c, TG, TC, LDL, HDL level, AST, ALT, history of DM, hypertension, smoking, alcohol consumption, and APRI as independent variables. A P value of ≥0.2 is the significant level for removal from the model, and a P value of <0.1 is the significant level for addition to the model. All analyses were performed with Stata statistical software (version 8.0, Stata Corp., College Station, TX, USA). All tests were two-sided and a P value of <0.05 was considered statistically significant.
Results
The characteristics and biochemical profiles of study populations
A total of 1,001 persons (93 HBV carriers and 908 non-HBV subjects) were enrolled. Demographic, biochemical, status of HBsAg, endoscopic and ultrasonographic characteristics of the study population were compared between HBV carriers and non-HBV subjects (Table 1). The mean value of BMI in enrollers fell in the normal range for the Southeast Asian population as a whole. The distribution of BMI was comparable between HBV carriers and non-HBV subjects: <24 kg/m2, 54/550 (58.1 vs. 60.6%); 24–27 kg/m2, 25/239 (26.9 vs. 26.3%); 27–30 kg/m2, 12/86 (12.9 vs. 9.5%); >30 kg/m2, 2/33 (2.2 vs. 3.6%). Although the mean value of fasting blood glucose levels tended to be higher in HBV carriers than in non-HBV subjects, the distribution of fasting blood glucose levels was comparable between HBV carriers and non-HBV subjects (P = 0.089): <100 mg/dL, 58/615 (62.4 vs. 67.7%); 100–126 mg/dL, 23/230 (24.7 vs. 25.3%); >126 mg/dL, 12/63 (12.9 vs. 6.9%). In contrast, the mean level of APRI was comparable between HBV carriers and non-HBV subjects, but the distribution of APRI levels was different (HBV vs. non-HBV, P = 0.005): <25 percentile of APRI, 13/237 (14.0 vs. 26.1%); 25 percentile to 50 percentile, 21/229 (22.6 vs. 25.2%); 50–75 percentile, 23/228 (24.7 vs. 25.1%); >75 percentile, 36/214 (38.7 vs. 23.6%).
Table 1.
Demographic, biochemical features, and components of metabolic syndrome of enrolled subjects
| Characteristics | Mean ± SD or n (%) | OR (95% CI) | P value | |
|---|---|---|---|---|
| Non-HBV (n = 908) | HBV (n = 93) | |||
| Age (years) | 52.0 ± 11.7 | 52.2 ± 9.1 | 1.00 (0.98–1.02) | 0.866 |
| Male [n (%)] | 419 (46.1) | 54 (58.1) | 1.62 (1.05–2.49) | 0.028 |
| BMI (kg/m2) | 23.4 ± 3.4 | 23.6 ± 3.1 | 1.02 (0.96–1.08) | 0.549 |
| Waist circumference (cm)a | 80.4 ± 9.5 | 81.5 ± 9.3 | 1.01 (0.99–1.03) | 0.325 |
| SBP (mmHg) | 121.8 ± 17.0 | 122.1 ± 15.7 | 1.00 (0.99–1.01) | 0.882 |
| DBP (mmHg) | 75.4 ± 12.0 | 77.3 ± 12.2 | 1.01 (1.00–1.03) | 0.131 |
| Fasting blood glucose (mg/dL) | 99.3 ± 23.8 | 107.7 ± 38.3 | 1.01 (1.00–1.01) | 0.002 |
| HbA1c (%)a | 5.5 ± 0.7 | 5.7 ± 1.2 | 1.22 (1.00–1.49) | 0.037 |
| Diabetes mellitus, yes = 1 [n (%)] | 39 (4.3) | 6 (6.5) | 1.54 (0.63–3.73) | 0.339 |
| Hypertension, yes = 1 [n (%)] | 157 (17.3) | 11 (11.8) | 0.64 (0.33–1.23) | 0.179 |
| Alcohol, yes = 1 [n (%)] | 157 (17.3) | 19 (20.4) | 1.23 (0.72–2.09) | 0.449 |
| Smoking, yes = 1 [n (%)] | 73 (8.0) | 12 (12.9) | 1.69 (0.88–3.25) | 0.109 |
| NSAID user, yes = 1 [n (%)] | 26 (2.9) | 2 (2.2) | 0.75 (0.17–3.19) | 0.691 |
| Aspirin user, yes = 1 [n (%)] | 16 (1.8) | 1 (1.1) | 0.61 (0.08–4.62) | 0.625 |
| AST (IU/L) | 24.3 ± 10.7 | 26.8 ± 11.1 | 1.02 (1.00–1.03) | 0.034 |
| ALT (IU/L) | 26.9 ± 21.0 | 30.2 ± 20.3 | 1.01 (1.00–1.01) | 0.155 |
| Alkaline phosphatase (AP, IU/L) | 68.4 ± 19.4 | 72.7 ± 22.7 | 1.01 (1.00–1.02) | 0.046 |
| Platelet (K/μL) | 246.1 ± 59.1 | 227.7 ± 68.4 | 0.99 (0.99–1.00) | 0.005 |
| Triglyceride (mg/dL) | 132.6 ± 95.3 | 121.3 ± 62.4 | 1.00 (1.00–1.00) | 0.262 |
| Total cholesterol (mg/dL) | 196.3 ± 37.4 | 188.8 ± 33.3 | 0.99 (0.99–1.00) | 0.063 |
| LDL (mg/dL) | 124.9 ± 32.6 | 121.7 ± 29.9 | 1.00 (0.99–1.00) | 0.357 |
| HDL (mg/dL) | 53.3 ± 14.8 | 51.3 ± 14.4 | 0.99 (0.98–1.01) | 0.225 |
| Metabolic syndrome | 173 (19.1) | 16 (17.2) | 0.88 (0.50–1.55) | 0.664 |
| Liver sonography (N/PLD/LC) | 888/20/0 | 88/3/2 | 2.96 (1.30–6.72) | <0.001 |
| APRI | 0.28 ± 0.41 | 0.32 ± 0.18 | 1.17 (0.83–1.67) | 0.371 |
| Erosive esophagitis (EE)b | ||||
| LA classification A/B/C | 125/17/1 | 20/3/0 | ||
| Presence of EE, yes = 1 [n (%)] | 143 (15.7) | 23 (24.7) | 1.76 (1.06–2.91) | 0.027 |
Data are shown by mean ± standard deviation or proportion of the character
N normal liver, PLD parenchymal liver disease, LC liver cirrhosis, NSAID non-steroid anti-inflammatory drug, APRI aspartate aminotransferase to platelet ratio index, EE erosive esophagitis
aThree non-HBV enrollers did not have waist circumference data, and two without HbA1c
bThe severity of erosive esophagitis was graded from A to D according to the LA classification. However, only 20 subjects were graded as B and one as C, so we collapsed the grading and the presence of erosive esophagitis defined as yes/or no was used
Hepatitis B e antigen (HBeAg), a marker representing active replication of HBV, is not routinely screened during the healthy examination in Taiwan. However, we collected available medical records of our HBV carriers and found those who had available HBeAg data were all negative for HBeAg [54/93 (58.1%)].
Associations between chronic HBV infection and GERD
Table 2 shows the multivariate analyses between chronic HBV infection and erosive esophagitis. A P value of <0.05 in univariate analysis (Table 1) was the significance level for addition to the model (see “Statistical analysis”). Erosive esophagitis, higher fasting blood glucose, lower TC, higher ALP levels and lower platelet counts were associated with chronic HBV infection (P < 0.05).
Table 2.
Multivariate analyses identifying factors associated with chronic HBV infection (n = 1,001)
| OR (95% CI) | P value | |
|---|---|---|
| Erosive esophagitis | 1.75 (1.03–2.97) | 0.037 |
| Age (years) | 0.99 (0.97–1.01) | 0.348 |
| Sex (male = 1) | 1.11 (0.69–1.78) | 0.677 |
| BMI (kg/m2) | 0.99 (0.93–1.06) | 0.849 |
| Fasting blood glucose (mg/dL) | 1.01 (1.00–1.01) | 0.012 |
| Total cholesterol (mg/dL) | 0.99 (0.99–1.00) | 0.036 |
| AST (IU/L) | 1.01 (0.99–1.03) | 0.269 |
| ALP (IU/L) | 1.01 (1.00–1.02) | 0.027 |
| Platelet (K/μL) | 0.99 (0.99–1.00) | 0.012 |
Multivariate logistic regression model was used. Use HBsAg (+) as reference and dependent variable, and age, sex, BMI, fasting blood glucose, total cholesterol, AST, ALP, platelet and erosive esophagitis as independent variables
P < 0.05 is considered as statistical significance
Considering the possible confounding effects on the analyses of chronic HBV infection and GERD, we also conducted subgroup analyses with correlates of GERD. The association between erosive esophagitis and chronic HBV infection was unchanged in subjects with waist circumference ≦90 cm, LDL ≦130 mg/dL, fasting blood glucose level ≧100 mg/dL, or lower HDL levels (supplementary Tables 1, 2), and was similar in subjects without using aspirin or non-steroid anti-inflammatory drugs, or alcohol consumption (supplementary Table 3).
Further evaluation of the associations between chronic HBV infection and GERD symptoms, such as heartburn, acid regurgitation, and dyspepsia (scaled with RDQ points, see questionnaire for details) showed that the sum of RDQ points or the points of each RDQ category were similar between subjects with and without chronic HBV infection, except that HBV carriers had more severe heartburn than non-HBV subjects (OR 1.27, 95% CI 1.01–1.61, P = 0.037) (Table 3).
Table 3.
The differences of the Reflux Disease Questionnaire (RDQ) between HBV carriers and non-HBV subjects
| Characteristics | Mean ± SD or n (%) | OR (95% CI) | P value | |
|---|---|---|---|---|
| Non-HBV (n = 908) | HBV (n = 93) | |||
| Burning feeling behind breastbone, frequency | 0.13 ± 0.51 | 0.22 ± 0.67 | 1.27 (0.93–1.74) | 0.118 |
| Burning feeling behind breastbone, severity | 0.17 ± 0.67 | 0.33 ± 0.97 | 1.27 (1.01–1.61) | 0.037 |
| Pain behind breastbone, frequency | 0.17 ± 0.60 | 0.15 ± 0.51 | 0.94 (0.64–1.39) | 0.767 |
| Pain behind breastbone, severity | 0.21 ± 0.68 | 0.25 ± 0.86 | 1.08 (0.81–1.43) | 0.599 |
| Acid taste in mouth, frequency | 0.50 ± 0.98 | 0.52 ± 0.96 | 1.02 (0.82–1.26) | 0.887 |
| Acid taste in mouth, severity | 0.59 ± 1.05 | 0.67 ± 1.19 | 1.07 (0.89–1.29) | 0.485 |
| Unpleasant movement of material upwards from the stomach, frequency | 0.17 ± 0.61 | 0.18 ± 0.62 | 1.03 (0.73–1.44) | 0.882 |
| Unpleasant movement of material upwards from the stomach, severity | 0.19 ± 0.63 | 0.23 ± 0.68 | 1.07 (0.78–1.47) | 0.655 |
| Pain in the central of the stomach, frequency | 0.42 ± 0.99 | 0.54 ± 1.14 | 1.11 (0.92–1.34) | 0.292 |
| Pain in the central of the stomach, severity | 0.54 ± 1.13 | 0.72 ± 1.31 | 1.13 (0.95–1.33) | 0.156 |
| Burning feeling in the central of the stomach, frequency | 0.17 ± 0.65 | 0.18 ± 0.66 | 1.03 (0.76–1.41) | 0.841 |
| Burning feeling in the central of the stomach, severity | 0.21 ± 0.74 | 0.29 ± 0.92 | 1.13 (0.88–1.44) | 0.335 |
Data are shown by mean ± standard deviation or proportion of the character
P < 0.05 is considered as statistical significance
Factors affecting the associations between chronic HBV infection and GERD
Gender is known to affect the development of GERD and the disease progression in HBV carriers. To adjust the effects of gender and chronic HBV infection on GERD, further analyses stratified by gender revealed a sexual difference on the association of chronic HBV infection with erosive esophagitis. Male subjects with erosive esophagitis had more alcohol consumption, and higher LDL levels, but no increased frequency of chronic HBV infection. In sharp contrast, female subjects with erosive esophagitis were independently associated with chronic HBV infection (adjusted OR 2.70, 1.14–6.39, P = 0.024) (Table 4).
Table 4.
Multivariate analyses identifying factors associated with erosive esophagitis stratified by gender
| Male (n = 471) | Female (n = 526) | |||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Alcohol drinking (yes = 1) | 1.74 (1.10–2.75) | 0.017 | ||
| HbA1c (%) | 1.21 (0.98–1.49) | 0.070 | 1.79 (1.11–2.89) | 0.018 |
| Triglyceride (mg/dL) | 1.00 (0.99–1.00) | 0.096 | ||
| HBsAg (+ve = 1) | 1.30 (0.67–2.51) | 0.435 | 2.70 (1.14–6.39) | 0.024 |
| HDL (mg/dL) | 1.01 (1.00–1.03) | 0.090 | ||
| LDL (mg/dL) | 1.01 (1.00–1.02) | 0.026 | ||
| ALP (IU/L) | 0.98 (0.97–1.00) | 0.089 | ||
| SBP (mmHg) | 1.01 (1.00–1.03) | 0.052 | 0.99 (0.97–1.01) | 0.241 |
| Waist circumference (cm)a | 1.06 (1.02–1.09) | 0.003 | ||
Stepwise estimation with backward-selection logistic regression model was used. Use the presence of erosive esophagitis (yes or no) as reference and dependent variable, and age, waist circumference, SBP, DBP, HBsAg, triglyceride, total cholesterol, HbA1c, LDL, HDL level, AST, ALT, ALP levels, history of DM, hypertension, smoking, alcohol consumption and APRI as independent variables
P ≥ 0.2 is the significant level for removal from the model and P < 0.1 is the significant level for addition to the model
aThree non-HBV enrollers did not have waist circumference data, and two without HbA1c
Liver cirrhosis is also linked to GERD and is an adverse outcome of chronic HBV infection. In univariate analysis, we consistently found that ultrasonographic liver fibrosis (PLD and cirrhosis) was a significant risk factor of erosive esophagitis [N vs. PLD/LC, 158 (16.2%) vs. 8 (32%), P = 0.036]. To examine the effect of liver fibrosis stage on the incidence of GERD in HBV infection, the 75 percentile of APRI (a non-invasive predictor of liver fibrosis and cirrhosis [13–15]) was used as a cutoff point. Multivariate analysis showed that HBV carriers with higher APRI levels had an increased risk of erosive esophagitis than those with lower APRI levels (adjusted OR 3.94, 1.73–8.96, P = 0.001) (Table 5).
Table 5.
Multivariate analyses identifying factors associated with erosive esophagitis stratified by APRI level
| APRI ≦ 0.3a (n = 737) | APRI > 0.3 (n = 260) | |||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Alcohol drinking (yes = 1) | 2.82 (1.34–5.91) | 0.006 | ||
| Sex (male = 1) | 2.71 (1.76–4.19) | <0.001 | ||
| BMI (kg/m2) | 0.85 (0.77–0.95) | 0.003 | 1.13 (1.04–1.23) | 0.005 |
| HBsAg (+ve = 1) | 0.89 (0.42–1.87) | 0.760 | 3.94 (1.73–8.96) | 0.001 |
| LDL (mg/dL) | 1.01 (1.00–1.02) | 0.004 | ||
| Waist circumference (cm)b | 1.05 (1.01–1.09) | 0.007 | ||
| AST (IU/L) | 1.04 (1.00–1.09) | 0.062 | ||
| HbA1c (%) | 1.19 (0.95–1.47) | 0.126 | ||
Stepwise estimation with backward-selection logistic regression model was used. Use the presence of erosive esophagitis (yes or no) as reference and dependent variable, and age, sex, waist circumference, SBP, DBP, HBsAg, triglyceride, total cholesterol, HbA1c, LDL, HDL, AST, ALT levels, history of DM, hypertension, smoking, and alcohol consumption as independent variables
P ≥ 0.2 is the significant level for removal from the model and P < 0.1 is the significant level for addition to the model
aA cut off level of 0.3 was used for APRI, because the 75 percentile of APPI is 0.3060254
bThree non-HBV enrollers did not have waist circumference data, and two without HbA1c
Finally, stepwise linear regression analysis (backward) was performed to identify factors associated with erosive esophagitis in HBV carriers, and the results showed that higher AST and TG levels were risk factors of erosive esophagitis in HBV carriers (Table 6).
Table 6.
Multivariate analyses identifying factors associated with erosive esophagitis in 93 subjects with chronic HBV infection
| Characteristics | OR (95% CI) | P value |
|---|---|---|
| Sex (male = 1) | 3.25 (0.87–12.1) | 0.080 |
| Total cholesterol (mg/dL) | 1.04 (0.99–1.09) | 0.085 |
| Triglyceride (mg/dL) | 1.01 (1.00–1.02) | 0.021 |
| LDL (mg/dL) | 0.96 (0.92–1.01) | 0.134 |
| AST (IU/L) | 1.11 (1.01–1.23) | 0.039 |
| ALT (IU/L) | 0.94 (0.88–1.01) | 0.097 |
Stepwise estimation with backward-selection logistic regression model was used. Use the presence of erosive esophagitis (yes or no) as reference and dependent variable, and age, sex, BMI, waist circumference, SBP, DBP, fasting blood glucose, triglyceride, total cholesterol, HbA1c, LDL, HDL, AST, ALT, ALP levels, and APRI as independent variables
P ≥ 0.2 is the significant level for removal from the model and P < 0.1 is the significant level for addition to the model
Discussion
In this study, we demonstrated that chronic HBV infection was strongly associated with an increased incidence of GERD, including GERD symptoms and erosive esophagitis. To the best of our knowledge, this is the first prospective population-based study to explore an association between chronic HBV infection and GERD. The increased incidence of GERD in HBV carriers was independent of other known risk factors [16–18]. Moreover, we found a differential association between genders and APRI levels, and chronic HBV infection was an independent risk factor of erosive esophagitis, especially in female subjects and those with higher APRI levels. Finally, HBV carriers with higher AST or TG levels had an increased incidence of erosive esophagitis.
Several studies indicated a higher frequency of GERD in patients with cirrhosis than those without liver disease [6], and an impaired quality of life in chronic liver disease patients with GERD symptoms [5]. However, their conclusions were undermined by small sample size, enrollment of only patients with cirrhosis, or specific focus on the aspect of life quality. In addition, several risk factors were not analyzed or controlled in previous studies. These factors included age, sex, BMI, metabolic factors, non-steroid anti-inflammatory drug use, and tobacco smoking [2, 19]. In this study, a large health check-up population was enrolled and all possible risk factors were analyzed at the same time. We not only evaluated the association of chronic HBV infection with endoscopic findings (erosive esophagitis), but also with GERD symptoms. All these measures made our findings more informative and convincible.
Our results demonstrated a gender-related association between chronic HBV infection and GERD. In this study, male gender was a risk factor of erosive esophagitis of all enrollers (OR 2.43, 95% CI 1.63–3.64, P < 0.001) (supplementary Table 4), but HBV infection was associated with erosive esophagitis only in females (OR 2.70, 95% CI 1.14–6.39, P = 0.024), not in males (OR 1.30, 95% CI 0.67–2.51, P = 0.435). The contrary association between HBV and non-HBV subjects suggests a close interaction between HBV and sex on the incidence of erosive esophagitis. It is generally believed that estrogen may play a favorable role in the clinical outcomes of chronic HBV infection. A more progressive clinical course and a predominance of cirrhosis as well as HCC in male and postmenopausal female HBV carriers have been observed [20]. In addition, several studies indicated a possible interaction between estrogen/its receptors and HBV, or HBx protein and androgen receptor-responsive gene expression [21]. Taking these lines of evidence together, it is reasonable to speculate that the effect of chronic HBV infection on GERD could be enhanced in female subjects, but attenuated in male subjects. In this study, a stronger association between erosive esophagitis and chronic HBV infection was shown in females than males. These findings not only confirmed an association between chronic HBV and GERD, but also suggested an enhanced association between HBV and GERD in females. Female sex hormones may increase the risk of GERD symptoms through a relaxing effect on the lower esophageal sphincter [22], whether HBV could strengthen this relaxing effect or aggravate GERD through the actions of sex hormone-related mechanisms need further examinations.
The associations of GERD and HBV infection between different stages of hepatic fibrosis are worthy of attention. Although LC has been shown to be associated with the occurrence of GERD, the underlying mechanisms which link both diseases together remained largely unknown. Metabolic syndrome, diabetes mellitus and obesity are known risk factors of LC development [23]. Of particular note is that diabetes mellitus, metabolic syndrome and obesity could also increase the risk of GERD [2]. Thus, metabolic factors may contribute, at least in part, to the linkage between GERD and chronic liver disease. This speculation was not only supported by our findings that the association between HBV infection and GERD was more prominent in subjects with higher fasting blood glucose or lower HDL levels (supplementary Table 2), but further supported by a recent study in which an association was demonstrated between lower adiponectin level, a key factor of metabolic syndrome [24], and GERD [25]. The emerging evidence seems to shed some light on the mechanisms related to both disorders. In addition, our data also showed a probable additive effect of TG on the occurrence of GERD in HBV carriers. Whether chronic HBV infection could affect the emergence of GERD through metabolic factors as well as related pathways deserve further in vitro and in vivo studies.
Several limitations existed in this study. First, this study was a cross-sectional design, and hence only associations between chronic HBV infection and GERD could be determined. Further studies with longitudinal design and paired controls, such as chronic HCV infection, autoimmune hepatitis, as well as non-alcoholic liver disease, are required to elucidate possible causal relationships. Second, most of the participants were generally healthy, indicating our results may only be applied to asymptomatic HBV carriers. Further studies are necessary to clarify the relationship between GERD and HBV-related chronic hepatitis, cirrhosis or HCC. Third, because few patients with overt liver fibrosis, by which the power to detect a difference may be decreased. We thus used APRI as a surrogate marker to evaluate the severity of liver fibrosis, aiming to increase the power to detect a possible difference between different stages of liver fibrosis. Fourth, because it is not possible to apply both pH-metry and manometric studies to the general population, we only focused on the GERD symptoms and erosive esophagitis. Further evaluation with both methods in HBV cohorts will be informative. Finally, it is difficult to explore all GERD-related factors in a single study. Many factors, such as dietary components, asthma, psychosocial factors, or Helicobacter pylori infection have been linked to GERD. Although not fully confirmatory, these associations are clinically important and deserve future studies.
In summary, our data demonstrate a significant association between chronic HBV infection and GERD. The association is differently affected by gender and APRI level. HBV carriers with higher AST or TG levels have an increased incidence of erosive esophagitis. Whether these associations imply an interaction between chronic HBV infection and GERD await further studies.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank colleagues in the Buddhist Tzu Chi General Hospital, Taipei Branch, Taiwan, who enrolled and followed the patients, and research assistants who assisted in laboratory analyses and collected clinical information. This work was supported by grants from the Buddhist Tzu Chi General Hospital, Taipei Branch, the National Taiwan University Hospital, Liver Disease Prevention and Treatment Research Foundation, the Department of Health, and the National Science Council, Executive Yuan, Taiwan. All authors declare the independence of researchers from funders.
Conflict of interest statement P.-J. Chen: consultant for Novartis and Roche. D.-S. Chen: consultant for Novartis and GlaxoSmithKline. J.-H. Kao: consultant for Bristol-Myers Squibb, GlaxoSmithKline, Novartis, Omrix, Roche, and Schering-Plough; on speaker’s bureau for Roche, Bristol-Myers Squibb, GlaxoSmithKline, Novartis, and Schering-Plough. All other authors declare that the answer to the questions on your competing interest form are all no and therefore have nothing to declare.
Abbreviations
- HBV
Hepatitis B virus
- HCV
Hepatitis C virus
- ALT
Alanine transaminase
- AST
Aspartate transaminase
- ALP
Alkaline phosphatase
- AFP
Alfa fetoprotein
- TG
Triglyceride
- TC
Total cholesterol
- HDL
High-density lipoprotein-cholesterol
- LDL
Low-density lipoprotein-cholesterol
- HbA1c
Hemoglobin A1c
- PLT
Platelet count
- BMI
Body mass index
- GERD
Gastroesophageal reflux disease
- EE
Erosive esophagitis
- APRI
Aspartate aminotransferase to platelet ratio index
- ULN
Upper limit of normal
- RDQ
Reflux Disease Diagnostic Questionnaire
- NSAID
Non-steroid anti-inflammatory drug
- SBP
Systolic blood pressure
- DBP
Diastolic blood pressure
References
- 1.Kao JH, Chen DS. Global control of hepatitis B virus infection. Lancet Infect Dis. 2002;2:395–403. doi: 10.1016/S1473-3099(02)00315-8. [DOI] [PubMed] [Google Scholar]
- 2.Lee YC, Yen AM, Tai JJ, Chang SH, Lin JT, Chiu HM, et al. The effect of metabolic risk factors on the natural course of gastro-oesophageal reflux disease. Gut. 2009;58:174–181. doi: 10.1136/gut.2008.162305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zheng Z, Nordenstedt H, Pedersen NL, Lagergren J, Ye W. Lifestyle factors and risk for symptomatic gastroesophageal reflux in monozygotic twins. Gastroenterology. 2007;132:87–95. doi: 10.1053/j.gastro.2006.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katz PO, Castell DO. Approach to the patient with unexplained chest pain. Am J Gastroenterol. 2000;95:S4–S8. doi: 10.1016/S0002-9270(00)01072-8. [DOI] [PubMed] [Google Scholar]
- 5.Suzuki K, Koizumi K, Takada H, Nishiki R, Ichimura H, Oka S, et al. Effect of symptomatic gastroesophageal reflux disease on quality of life of patients with chronic liver disease. Hepatol Res. 2008;38:335–339. doi: 10.1111/j.1872-034X.2007.00275.x. [DOI] [PubMed] [Google Scholar]
- 6.Ahmed AM, al Karawi MA, Shariq S, Mohamed AE. Frequency of gastroesophageal reflux in patients with liver cirrhosis. Hepatogastroenterology. 1993;40:478–480. [PubMed] [Google Scholar]
- 7.IDF consensus worldwide definition of the metabolic syndrome. 2006. http://www.idf.org/webdata/docs/IDF_Meta_def_final.pdf
- 8.He J, Neal B, Gu D, Suriyawongpaisal P, Xin X, Reynolds R, et al. International collaborative study of cardiovascular disease in Asia: design, rationale, and preliminary results. Ethn Dis. 2004;14:260–268. [PubMed] [Google Scholar]
- 9.Liu CH, Lin JW, Tsai FC, Yang PM, Lai MY, Chen JH, et al. Noninvasive tests for the prediction of significant hepatic fibrosis in hepatitis C virus carriers with persistently normal alanine aminotransferases. Liver Int. 2006;26:1087–1094. doi: 10.1111/j.1478-3231.2006.01355.x. [DOI] [PubMed] [Google Scholar]
- 10.Shaw MJ, Talley NJ, Beebe TJ, Rockwood T, Carlsson R, Adlis S, et al. Initial validation of a diagnostic questionnaire for gastroesophageal reflux disease. Am J Gastroenterol. 2001;96:52–57. doi: 10.1111/j.1572-0241.2001.03451.x. [DOI] [PubMed] [Google Scholar]
- 11.Armstrong D, Bennett JR, Blum AL, Dent J, Dombal FT, Galmiche JP, et al. The endoscopic assessment of esophagitis: a progress report on observer agreement. Gastroenterology. 1996;111:85–92. doi: 10.1053/gast.1996.v111.pm8698230. [DOI] [PubMed] [Google Scholar]
- 12.Brown JJ, Naylor MJ, Yagan N. Imaging of hepatic cirrhosis. Radiology. 1997;202:1–16. doi: 10.1148/radiology.202.1.8988182. [DOI] [PubMed] [Google Scholar]
- 13.Hongbo L, Xiaohui L, Hong K, Wei W, Yong Z. Assessing routine and serum markers of liver fibrosis in CHB patients using parallel and serial interpretation. Clin Biochem. 2007;40:562–566. doi: 10.1016/j.clinbiochem.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 14.Shin WG, Park SH, Jang MK, Hahn TH, Kim JB, Lee MS, et al. Aspartate aminotransferase to platelet ratio index (APRI) can predict liver fibrosis in chronic hepatitis B. Dig Liver Dis. 2008;40:267–274. doi: 10.1016/j.dld.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 15.Lebensztejn DM, Skiba E, Sobaniec-Lotowska M, Kaczmarski M. A simple noninvasive index (APRI) predicts advanced liver fibrosis in children with chronic hepatitis B. Hepatology. 2005;41:1434–1435. doi: 10.1002/hep.20736. [DOI] [PubMed] [Google Scholar]
- 16.Labenz J, Jaspersen D, Kulig M, Leodolter A, Lind T, Meyer-Sabellek W, et al. Risk factors for erosive esophagitis: a multivariate analysis based on the ProGERD study initiative. Am J Gastroenterol. 2004;99:1652–1656. doi: 10.1111/j.1572-0241.2004.30390.x. [DOI] [PubMed] [Google Scholar]
- 17.Dickman R, Mattek N, Holub J, Peters D, Fass R. Prevalence of upper gastrointestinal tract findings in patients with noncardiac chest pain versus those with gastroesophageal reflux disease (GERD)-related symptoms: results from a national endoscopic database. Am J Gastroenterol. 2007;102:1173–1179. doi: 10.1111/j.1572-0241.2007.01117.x. [DOI] [PubMed] [Google Scholar]
- 18.Fujiwara Y, Higuchi K, Shiba M, Yamamori K, Watanabe Y, Sasaki E, et al. Differences in clinical characteristics between patients with endoscopy-negative reflux disease and erosive esophagitis in Japan. Am J Gastroenterol. 2005;100:754–758. doi: 10.1111/j.1572-0241.2005.40966.x. [DOI] [PubMed] [Google Scholar]
- 19.Ruszniewski P, Soufflet C, Barthelemy P. Nonsteroidal anti-inflammatory drug use as a risk factor for gastro-oesophageal reflux disease: an observational study. Aliment Pharmacol Ther. 2008;28:1134–1139. doi: 10.1111/j.1365-2036.2008.03821.x. [DOI] [PubMed] [Google Scholar]
- 20.Yu MW, Yang YC, Yang SY, Cheng SW, Liaw YF, Lin SM, et al. Hormonal markers and hepatitis B virus-related hepatocellular carcinoma risk: a nested case-control study among men. J Natl Cancer Inst. 2001;93:1644–1651. doi: 10.1093/jnci/93.21.1644. [DOI] [PubMed] [Google Scholar]
- 21.Chiu CM, Yeh SH, Chen PJ, Kuo TJ, Chang CJ, Yang WJ, et al. Hepatitis B virus X protein enhances androgen receptor-responsive gene expression depending on androgen level. Proc Natl Acad Sci USA. 2007;104:2571–2578. doi: 10.1073/pnas.0609498104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nordenstedt H, Zheng Z, Cameron AJ, Ye W, Pedersen NL, Lagergren J. Postmenopausal hormone therapy as a risk factor for gastroesophageal reflux symptoms among female twins. Gastroenterology. 2008;134:921–928. doi: 10.1053/j.gastro.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong GL, Wong VW, Choi PC, Chan AW, Chim AM, Yiu KK, et al. Metabolic syndrome increases the risk of liver cirrhosis in chronic hepatitis B. Gut. 2009;58:111–117. doi: 10.1136/gut.2008.157735. [DOI] [PubMed] [Google Scholar]
- 24.Hui CK, Zhang HY, Lee NP, Chan W, Yueng YH, Leung KW, et al. Serum adiponectin is increased in advancing liver fibrosis and declines with reduction in fibrosis in chronic hepatitis B. J Hepatol. 2007;47:191–202. doi: 10.1016/j.jhep.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 25.Iwasaki E, Suzuki H, Sugino Y, Iida T, Nishizawa T, Masaoka T, et al. Decreased levels of adiponectin in obese patients with gastroesophageal reflux evaluated by videoesophagography: possible relationship between gastroesophageal reflux and metabolic syndrome. J Gastroenterol Hepatol. 2008;23(Suppl 2):S216–S221. doi: 10.1111/j.1440-1746.2008.05441.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Below is the link to the electronic supplementary material.