Abstract
Objectives We evaluated whether the phenotypic pattern of higher verbal than nonverbal IQ in children with spina bifida meningomyelocele (SBM) is consistent across subgroups differing in ethnicity and SES. We also explored the relation of cognitive and academic performance. Methods Non-Hispanic White (n = 153) and Hispanic (n = 80) children with SBM received the Stanford Binet Test of Intelligence-IV and achievement subtests of the Woodcock–Johnson. Parents completed questionnaires assessing the family environment [socioeconomic status (SES), resources, and educational opportunities]. Results Multivariate analysis revealed that Hispanic children with lower SES had lower verbal than nonverbal scores. Hispanic children with higher SES and non-Hispanic White children demonstrated the reverse pattern. Verbal and nonverbal IQ interacted to predict reading and math performance. Conclusions Lower SES is associated with lower verbal IQ in economically disadvantaged Hispanic children with SBM. Academic achievement is largely correlated with verbal IQ, but children with lower verbal IQ may partially compensate with higher nonverbal ability.
Keywords: academic functioning, family functioning, race/ethnicity, spina bifida
Spina bifida, a defect in the closure of the neural tube, is the second most common congenital birth defect in North America after heart disease (Detrait et al., 2005). Since federal legislation required fortification of grains with folate, the rate of spina bifida has decreased, but it remains one of the most common birth defects in North America, affecting 0.3–0.5 per 1,000 live births per year (Williams, Rasmussen, Flores, Kirby, & Edmonds, 2005). Identified by the defining spinal lesion observed prenatally or at birth, the most common type of spina bifida, meningomyelocele (SBM), is associated with brain malformations affecting the cerebellum and hindbrain (Chiari II malformation), corpus callosum, and midbrain. In addition, the Chiari II malformation usually causes hydrocephalus that may require treatment with a diversionary shunt.
Cognitive Outcomes in SBM
Despite this cascade of central nervous system (CNS) insults that begin early in gestation, cognitive outcomes in SBM are not globally poor, but rather are characterized by principled variability around a modal pattern of strengths and weaknesses. Dennis, Landry, Barnes, and Fletcher (2006) reviewed the neuropsychological profile and presented a model of SBM that outlined the relation of primary and secondary CNS factors and the environment in explaining not only the modal cognitive outcomes but also variations in this profile.
The modal profile of SBM is characterized by strength in associative processing and weakness in assembled processing. In associative processing, meaning is stipulated and serves the formation of associations, categorization, or adaptive changes in response to repetition; in assembled processing, meaning is constructed through the integration of information, which allows the individual to go beyond given information to infer and construct new cognitive material. Processing differences are manifested within context domains. For example, children with SBM commonly show better development of verbal IQ, vocabulary, word recognition, exact calculation, face recognition, and motor learning versus poorer development of performance or nonverbal IQ, reading comprehension, pragmatic language, math estimation, spatial relations, motor control, and a variety of memory and executive functions requiring organization and the construction of information (Dennis et al., 2006).
Influence of Socioeconomic Factors on Outcomes
Dennis et al. (2006) suggested that the primary effect of the CNS factors associated with SBM was on assembled processing and that associative processes were preserved unless the child developed in a less advantageous environment characterized, for example, by poverty and lower socioeconomic status (SES). The issue of environmental factors such as SES is highly relevant for SBM because many children with this condition in North America are born in economically disadvantaged circumstances (Wasserman, Shaw, Selvin, Gould, & Syme, 1998). The impact of lower SES, including reduced economic status and lower parental education, on achievement (Gerber & Durgunoglu, 2004) and language (Hart & Risley, 1995) in typically developing children is well established. Similarly, lower SES is associated with poorer psychosocial outcomes across a number of conditions (Frank, Blount, & Brown, 1997; Wallander, Varni, Babani, Banis, & Wilcox, 1989). The mechanisms underlying these associations involve family access to economic resources and schooling, parenting practices, and maternal education (Bornstein, Hahn, Suwalsky, & Haynes, 2003; Bradley & Corwyn, 2002).
In studies of SBM, SES has emerged as a clear risk factor for poorer cognitive and psychosocial outcomes. In the cognitive area, Lomax-Bream, Barnes, Copeland, Taylor, and Landry (2007) evaluated the development of infants with SBM and controls from six to 36 months of age. An application of growth curve modeling showed that lower SES predicted lower scores and less growth of cognitive and language skills in both the group with SBM and the controls (i.e., no interaction). However, children with SBM and lower SES had the slowest rates of growth in the cognitive and language domains. In addition, there was an interaction showing poorest motor outcomes for children with SBM and lower SES.
Similarly, in the psychosocial domain, Holmbeck et al. (2003) found that low SES was a significant risk factor for many adjustment outcomes, including scholastic competence and behavioral autonomy, in both children with SBM and typically developing controls. Holmbeck et al. highlighted the potential additive effects of SBM and SES since children with SBM and lower SES had the poorest outcomes across multiple domains.
Ethnicity and SES
Tests of the relation of SES and cognitive outcomes in SBM are limited to the infancy studies of Lomax-Bream, Barnes et al. (2007). The study by Holmbeck et al. (2003) did not include cognitive measures. In contrast, Fletcher et al. (2005) evaluated cognitive outcomes in relation to spinal lesion level in Hispanic and non-Hispanic children with SBM, but did not model effects of SES. This study showed poorer outcomes in children with SBM of Hispanic ethnicity. Only non-Hispanic families with lower level spinal lesions (lumbar and sacral) showed the historically prominent pattern of lower verbal IQ than nonverbal IQ observed from the earliest studies of outcomes in SBM (Dennis et al., 1981). While most Hispanic children came from families with lower SES, Fletcher et al. argued that the lesion level findings could not be simply attributed to SES because the effects were observed within ethnicity groups, which were comparable in SES. However, specific tests of this hypothesis were not conducted.
Hispanic cohorts present additional challenges because of their language minority status. Most standardized tests of intelligence are available in English and standardized on English- speaking samples; it is well known than Hispanic children score lower on tests administered in their nonnative language even if nonlinguistic items are involved (Anastasi & Cordova, 1953). In one of few studies investigating IQ test performance in Hispanics, Bergan and Parra (1979) found that children who were administered the same IQ tests in English and Spanish scored higher than Hispanic children who received the test in English only, with a group that took the test in Spanish scoring in between. In the achievement domain, Kieffer (2008) found that language minority children not proficient in English had achievement growth trajectories well below those of native English speakers and language minority children proficient in English, but controlling for both SES and the poverty level of the school greatly reduced this gap. A recent consensus report on literacy in language minority children (August & Shanahan, 2006) concluded that while language minority status and limited English proficiency were risk factors, SES and schooling had significant impact on achievement outcomes.
The Present Study
Research attempting to disentangle effects of ethnicity, lesion level, and SES in SBM has not been attempted. As Holmbeck, Greenley, Coakley, Greco, and Hagstrom (2006) stated, “[I]t is recommended that future studies include samples with more ethnic and SES diversity. Most importantly, Hispanic/Latino families are understudied in this literature. This is surprising given the high prevalence rates of spina bifida in this population” (p. 255). The SES construct is quite broad and includes family resources and opportunities; however, the variable typically used to operationalize SES is an index of parental education and occupation, which is narrow. Multiple measures of the construct, including parental attitudes toward education and access to resources, may enhance the assessment of SES.
Building upon Fletcher et al. (2005), our primary objective was to examine how interactions of ethnicity, lesion level, and family environmental factors (including measures of SES, resources, and opportunities) were related to the commonly observed discrepancy in verbal and nonverbal IQ. We hypothesized that children of non-Hispanic White ethnicity would demonstrate the prototypical higher verbal than nonverbal cognitive processing pattern and that children of Hispanic ethnicity would demonstrate the reverse pattern (Hypothesis 1a). We also predicted a main effect of family environment factors on cognitive scores (i.e., that the association of family environment and cognitive scores would not differ by ethnicity; Hypothesis 1b). In returning to the model developed by Dennis et al. (2006), we hypothesized that environmental factors would be more strongly associated with verbal scores, and lesion level with nonverbal scores (Hypothesis 1c). Finally, we examined the impact of environmental factors on the well-established relation of verbal cognitive processing and achievement (Sattler, 2001). We hypothesized that verbal processing would better predict academic scores than would nonverbal processing (Hypothesis 2a), and that academic performance would be higher in children who were more economically advantaged and with lower lesion level (Hypothesis 2b). We examined the influence of ethnicity and SES on academic achievement, but did not have specific predictions regarding their relation, representing an exploration of possible ethnicity by SES interactions.
Methods
Participants
The present sample is a subset of a larger sample of children and adolescents with SBM and shunted hydrocephalus recruited from clinics in Houston and Toronto. The sample included many of the children in the Fletcher et al. (2005) study; however, there are children in each sample that were not in the other, due to composition of groups and additional recruitment since publication of the previous study. It is estimated that just over 90% of the children in the current study were also included in the sample in Fletcher et al. (2005). Children ranged in age from 5 to 18 years at the time of testing. Any child in the age range born with a meningomyelocele and shunted for hydrocephalus was included in the potential sample. Exclusion criteria included neurological disorders unrelated to SBM, severe psychiatric disorder, uncontrolled seizure disorder, uncorrected sensory disorder, or inability to control the upper limbs. Additional inclusion criteria for the present study included the availability of at least one of the three family environment variables that were of primary interest (97% of the sample had data on all three), presence of both cognitive variables, and presence of at least one of the academic achievement measures (97% had both). As part of the genetic component of this study, parents were asked to categorize their children in one of the following race/ethnicity categories: African, Asian, Hispanic, Caucasian (non-Hispanic White), or other. Children identified as Hispanic (n = 80) and non-Hispanic White (n = 153) comprised the largest proportion of children in this sample (and among children with spina bifida in the general population; Detrait et al., 2005), so these two groups were selected for the present study.
Table I presents sociodemographic information on children in each ethnic group, showing that the ethnicity groups were comparable in gender and handedness. The Hispanic group with SBM was younger than the non-Hispanic White group, t (231) = 3.77, p < .05, Cohen’s d = 0.49. However, because the cognitive and achievement outcomes were age-adjusted scores, we did not covary for this difference.
Table I.
Sociodemographic Information and Scores on Family Environment Variables, Cognitive Tests, and Academic Outcomes by Ethnicity
| Ethnicity |
Effect size | ||
|---|---|---|---|
| Hispanic | Caucasian | ||
| N | 80 | 153 | |
| Age in years: M (SD)* | 9.9 (2.57) | 11.5 (3.13) | 0.49 |
| Gender—n (%) | 0.07 | ||
| Male | 45 (56) | 75 (49) | |
| Female | 35 (54) | 78 (51) | |
| Handedness—n (%) | 0.02 | ||
| Right | 59 (76) | 95 (74) | |
| Left or Ambidextrous | 19 (24) | 33 (26) | |
| Hollingshead* | 24.6 (10.02) | 43.6 (12.04) | 1.58 |
| HELPS* | 88.6 (7.62) | 94.0 (6.23) | 0.87 |
| FRS* | 120.7 (19.83) | 135.5 (19.84) | 0.75 |
| SB-IV Vocabulary* | 73.7 (20.82) | 92.4 (15.96) | 1.17 |
| SB-IV Pattern Analysis | 83.7 (15.61) | 87.2 (18.93) | 0.19 |
| WJ-R Reading Skills* | 79.4 (28.66) | 95.9 (21.18) | 0.78 |
| WJ-R Math Skills* | 66.8 (31.22) | 81.5 (22.81) | 0.64 |
Note. All scores are presented as M (SD).
Effect sizes are Cohen’s d for continuous variables and Cramer’s phi for categorical variables.
*p < .05.
In this sample, 96% had a Chiari II malformation. Twenty-three percent of the sample had no shunt revisions, 50% had 1–2 revisions, and the remaining 27% had three or more. Consistent with recent conventions (Fletcher et al., 2005), spinal lesions at or above level T12 were designated High; lesions at or below L1 were designated Low. There were no statistically significant differences between ethnicity groups in shunt revisions or infections, oculomotor anomalies, tectal dysmorphology, ambulatory status, and other variables representing areas of impairment (p’s > .05). As expected, children of Hispanic ethnicity were slightly more likely to have upper level spinal lesions (35%) than children of non-Hispanic White ethnicity (25%), although this difference was not statistically significant.
Within each ethnic group (Hispanic, non-Hispanic White), children with SBM in this study are relatively homogeneous in terms of ethnicity and culture. A recent admixture study (Au et al., 2008) showed that the non-Hispanic Whites, who were born in the US (43%) or Canada (57%), were primarily of European ancestry (about 90%). Children in the Hispanic sample of the current study were born in the US (78%) or Mexico (21%), with <2% born in Central America. As a group, this sample is representative of the relatively large population of first and second generation of immigrants to the US that resides in Texas, California, and other southwestern states. Admixture studies show that individuals in this group of immigrants have similar ancestry to one another, with about 50% European, 45% Native American, and 5% African ancestries (Price et al., 2007). This population is economically disadvantaged (Table I), poorly educated, and the parents are either predominantly Spanish speaking or bilingual.
Thirty-two percent of the Hispanic sample received English as a Second Language (ESL) or bilingual services at the time of the assessment, and many of the older Hispanic children received ESL services when they were younger. The group receiving ESL services at the time of assessment was 2 years younger than the group not receiving the services (8 vs. 10 years, respectively). Relatedly, Hispanic children who were tested in Spanish were significantly younger than those tested in English, t(61) = 4.83, p < .0001, Cohen’s d = 0.63. This difference reflects Texas education laws, which mandate that children with limited English proficiency be initially taught in their native language and transitioned to English-speaking classrooms. There were no differences between Hispanic children who spoke English and those who spoke Spanish on other demographic variables, provision of special education, family environment factors, or test scores.
Measures
Adaptation Procedures
As part of the larger study, children were given the four subtest short form of the Stanford Binet Test of Intelligence-IV (SB-IV; Thorndike, Hagen, & Sattler, 1986) and the reading and math cluster skills of the Woodcock–Johnson – Revised (WJ-R; Woodcock & Johnson, 1989) in the child’s primary language of instruction. Where necessary, tests and questionnaires were adapted for Spanish language participants (27% of Hispanic children were tested in Spanish). English norms were used where Spanish-speaking norms were not available.
The SB-IV was adapted by translating items into Spanish and then modifying the language so that it was appropriate for our sample, including substitution of vocabulary words and modification of grammar. The translation was performed by a team of bilingual individuals who were native Spanish speakers. After the adaptation by one person, another member of the team reviewed the adaptation to make sure it was linguistically and culturally appropriate and that it assessed items similar in difficulty level to the original item. Disagreements were reconciled by the team. A similar process was used for questionnaires.
Intellectual and Academic Ability
From the 4-subtest short form of the SB-IV, the Vocabulary subtest was used to indicate verbal processing and the Pattern Analysis subtest to indicate nonverbal processing. Vocabulary is a reliable subtest (r = .87) and correlates highly with the composite (r = .81); Pattern Analysis also has good reliability (r = .92) and a moderately high correlation (r = .74) with the composite (Sattler, 2001). We chose these subtests because they represent the best indicators of the basis for the discrepancy in verbal and nonverbal IQ in SBM, as well as strong indicators of more general constructs often referred to as “verbal comprehension” (vocabulary) and “perceptual organization” (pattern analysis; Sattler, 2001). The reading and math clusters from the English or Spanish versions of the WJ-R have reliability coefficients in the mid-.90s (Woodcock & Johnson, 1989; Woodcock & Muñoz-Sandoval, 1996).
Socioeconomic Variables
The Hollingshead 4-Factor Scale (Hollingshead, 1975), a composite of parent education and occupational status, was used to determine SES. Self-reported scores were weighted to obtain a single score for each caretaker (range 8–66, with higher values indicting higher SES). For families with multiple caretakers, we averaged scores to produce a single SES index.
The Henderson Environmental Learning Process Scale (HELPS; Henderson, Bergan, & Hurt, 1972) is a self-report questionnaire that assesses parental attitudes toward education and the degree to which the child is exposed to learning opportunities outside of school. Fifty-one items are answered on a 5-point Likert scale, and the dependent variable is the total score. The scale has adequate reliability (.80), and validity has been demonstrated across ethnic and clinical populations (Valencia, Henderson, & Rankin, 1985).
The Family Resource Scale (FRS; Dunst & Leet, 1987) is a self-report 30-item questionnaire that assesses the extent to which the respondent believes resources are adequate for their family, such as money for monthly bills, shelter, food, health care, and transportation; time to get enough sleep; and time to be with family and friends. Thirty items are answered on a 5-point Likert scale, and the dependent variable is the total score. Internal consistency is .92.
Procedure
Institutional review boards at all sites approved this study. Parents provided written consent, and children gave written assent for participation. Children were administered intellectual and achievement tests as part of a longer assessment battery in a quiet room in the researcher’s lab. All parent measures were provided in the informant’s preferred language (English or Spanish), with a native speaker of that language available if assistance was required. Medical variables (hydrocephalus, shunt status, lesion level, etc.) were obtained via parent interview and confirmed through examination of medical records.
Data Analysis
Hypothesis 1, involving verbal–nonverbal skill discrepancies, was evaluated with a series of multivariate analyses of variance (MANOVAs). Dependent variables were verbal and nonverbal scores, with ethnicity, lesion level (High, Low), and family environment variables entered as predictors. A separate analysis was run for each of the family environment variables.
Hypothesis 2, assessing the prediction of academic achievement, was evaluated with two univariate hierarchical regressions, with WJ-R Reading and Math Skills Composites as the respective dependent variables. In the first step, each child’s verbal and nonverbal scores (and their interaction) were entered to determine their respective influences on achievement. In the second step, ethnicity (non-Hispanic White, Hispanic), lesion level (High, Low), and family environment variables were entered. Only environmental measures (if any) that significantly contributed to the prediction of cognitive processing were used.
All interactions were tested, and nonsignificant interactions were trimmed in a hierarchical fashion. Alpha level for statistical significance was set at .05, corrected for the number of analyses within each hypothesis (p < .017 for each main analysis within hypothesis 1, and p < .025 for the main analyses of hypothesis 2).
Results
Table I presents scores on family environment variables and cognitive and academic outcomes by ethnic group. Notably, Hispanic children were more disadvantaged on all three types of environmental measures, underscoring the importance of including these variables. The three environmental measures were only moderately correlated with one another (r’s = .10–.40), indicating that the measures are related but distinct indices of a child’s family environment.
Hypothesis 1a: Verbal–Nonverbal Patterns
Preliminary analyses investigated the role of ethnicity and lesion level in predicting verbal and nonverbal cognitive scores. There was a significant main effect of ethnicity, F(1, 232) = 40.67, p < .0001, accounting for 15% of the variance. Univariate follow-up revealed that there was a significant impact of ethnicity on verbal (p < .0001), but not nonverbal (p = .15), scores. The direction of differences paralleled Fletcher et al. (2005) in a sample with greater ethnic diversity; non-Hispanic White children had higher mean verbal than nonverbal scores and Hispanic children showed the reverse pattern.
Hypothesis 1b: Family Environmental Factors as Predictors of Verbal–Nonverbal Patterns
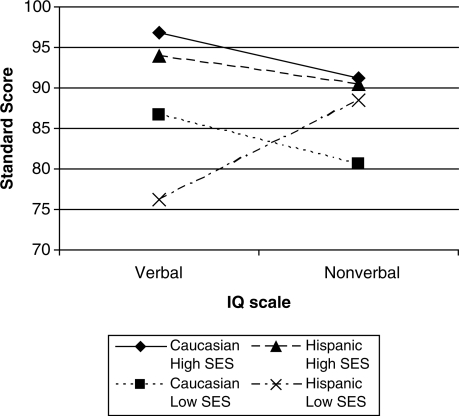
The measures of family environmental factors were used in three separate ANOVAs to investigate the role of environment in the relations between ethnicity, lesion level, and intelligence. Using the Hollingshead SES, there was a significant interaction between ethnicity and SES as predictors of verbal and nonverbal score patterns, F (1, 221) = 5.82, p = .016. This interaction accounted for 2% of the variance. Follow-up tests indicated that children of Hispanic ethnicity and lower SES had significantly lower verbal scores than children of non-Hispanic White ethnicity as well as children of Hispanic ethnicity and higher SES (p < .001). This difference resulted in different patterns of verbal–nonverbal scores. The results qualify the results reported in Fletcher et al. (2005); children with SBM of Hispanic ethnicity with lower SES showed higher nonverbal than verbal cognitive scores (Fig. 1). Children of Hispanic ethnicity and higher SES demonstrated the typically reported higher verbal than nonverbal pattern, as did children of non-Hispanic White ethnicity at all levels of SES. To evaluate language of assessment, the analyses were also run with separate Hispanic subgroups, and the findings were the same for both the English- and Spanish-speaking subgroups.
Figure 1.
Interaction of ethnicity and SES predicting cognitive score patterns
Using the FRS as a measure of family environment, results indicated no significant interactions of FRS with ethnicity in predicting patterns of scores. In the trimmed model, there was a significant main effect of ethnicity predicting patterns of scores, F(1, 225) = 35.98, p < .0001, η2 = .15. For both verbal and nonverbal scores, children of Caucasian ethnicity had higher scores. Similarly, in analyses using the HELPS as a predictor, there was a main effect of ethnicity predicting cognitive score patterns, F(1, 224) = 29.59, p < .0001, η2 = .15, but no significant interaction of the HELPS with ethnicity. These findings mirror the initial analyses and indicate that neither parent-reported family resources nor parent reports of the child’s exposure to educational opportunities were significant predictors of verbal–nonverbal score differences.
Hypothesis 1c: Family Environmental Variables, Lesion Level, and Cognitive Performance
Results from univariate portions of the multivariate ANOVAs were used to test the hypothesis that family environment variables would be related to verbal scores and lesion level would be related to nonverbal scores. SES was a significant predictor of verbal, but not nonverbal, cognitive scores, F(1, 221) = 11.85, p = .0007 (η2 = .06 for verbal, η2 = .02 for nonverbal), such that children at higher levels of SES had higher verbal processing scores. Neither the FRS nor the HELPS were significant predictors of either cognitive scale, p’s > .20, η2 < .01. As predicted, lesion level was a significant predictor of nonverbal cognitive scores, p’s < .006; however, lesion level was also a significant predictor of verbal cognitive scores, p = .0004 (both η2 = .03). Children with upper level spinal lesions had lower verbal and nonverbal cognitive performance.
Hypothesis 2: Intelligence, SES, Ethnicity, and Academic Achievement
Initial analyses of academic achievement showed a significant interaction between ethnicity and lesion level in predicting achievement scores, F(1, 226) = 3.92, p = .049, η2 < .01. These findings indicated that Hispanic children with upper level lesions (at or above spinal level T12) performed significantly worse than other children on both reading and math composites.
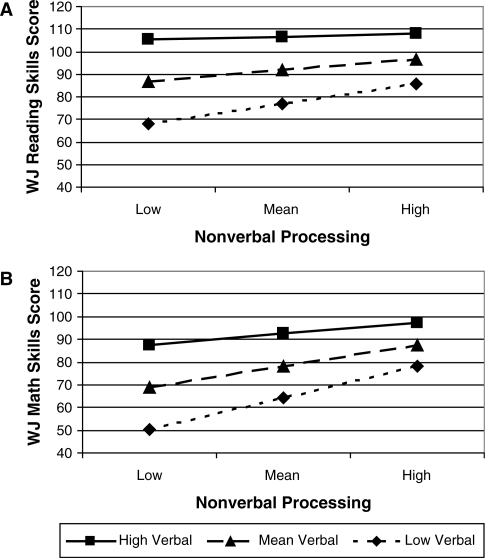
Analyses of the association of intelligence with academic skills were conducted separately for reading and math. Results for reading skills indicated that, in step one, there was a significant interaction of verbal and nonverbal scores, omnibus F(3, 222) = 93.93, p < .0001, R2 = .55. Children with higher verbal performance tended to perform relatively well on reading achievement regardless of nonverbal performance, whereas children with lower verbal performance only performed well on reading achievement when they also had higher nonverbal performance (Fig. 2A). In step two, the addition of the remaining variables (ethnicity, lesion level, SES) did not explain additional variance; R2 change = .003, F(3, 219) < 1, and none of the variables was significant, p’s > .43.
Figure 2.
Interaction of verbal and nonverbal process predicting reading (A) and math (B) skills
Results for analyses of math skills also showed a significant interaction of verbal and nonverbal scores in step one, omnibus F(3, 220) = 134.84, p < .0001, R2 = 0.65. The relation was the same as that found with reading skills (Fig. 2B). In step two, the addition of the remaining variables did not significantly explain additional variance; R2 change = .01, F(3, 217) = 2.22, p = .09, and none of the variables was significant, p’s > .05. These analyses were repeated using individual reading and math subtests, and comparable findings were obtained.
Discussion
This study assessed the relations of ethnicity and family environment variables in predicting verbal–nonverbal cognitive score patterns and utilized individual performance patterns to predict academic functioning. The results indicated that children with SBM and non-Hispanic White ethnicity tended to have higher verbal than nonverbal cognitive performance. However, compared to measures of resources and opportunities, parent education and occupation (SES) was a more important factor in predicting which children with SBM of Hispanic ethnicity would show a similar pattern. Children with higher levels of SES also showed higher verbal scores, while those with lower SES showed the reverse pattern. We note that our measurement of cognitive ability was based on a short form of the SB-IV; more complex patterns of performance might emerge if a full intelligence test were used. Furthermore, the absence of Spanish-speaking US norms for all the measures employed is a limitation of the study. Further research is needed on the revised SB-IV that was used with Spanish speakers, as well as on measures for diverse populations in general.
This difference in patterns may reflect the well-established relation of lower SES and poverty with language development (Hart & Risley, 1995), which is also related to ethnicity in this study. Limited English proficiency is less likely a factor since most Hispanic children in this sample are language minority students and come from homes where the predominant language is Spanish or both Spanish and English. In addition, a recent study recruited Hispanic children who were typically developing and low in SES (Carr, 2009). There were no interactions of language of assessment, etiology, or task, but there were main effects of these factors across multiple cognitive and achievement tests. In the current study, we did not find different patterns of results when the Hispanic subgroups tested in English and Spanish were examined separately, nor was there evidence that indices of SES varied across these subgroups.
Contrary to expectations, there was no relation of HELPS and FRS scores and intellectual outcomes, suggesting that variability in outcomes is not influenced by parent-reported access to educational opportunities, parents’ educational aspirations for their children, or adequacy of physical and economic resources. Future studies should incorporate broader assessments of familial environmental factors and parenting that do not rely only on maternal report. For example, the Home Observation for Measurement of the Environment (Caldwell & Bradley, 1984) is an observer-rated measure that assesses available educational and support resources. The present study provides only a limited sampling of family and environmental factors related to SBM (Holmbeck et al., 2006) and language minority status (August & Shanahan, 2006). Although we argued that the Hispanic sample is relatively homogeneous, we did not obtain measures of acculturation, language proficiency, or more in-depth assessments of family and school environments. There may also be cultural factors linked to parental (particularly maternal) education that are important to consider. Assessment of these aspects of culture would help further disentangle the myriad influences on cognitive development.
In finding that SES is more predictive than assessments of familial economic and physical resources (FRS), and aspirations and achievement-related efforts (HELPS), these results imply that parental education may be more important than economic resources. Research attempting to unpack SES indices like the Hollingshead has found that most of the variance is attributable to parental education (Bradley & Corwyn, 2002). SES and parenting have been linked to cognitive and social outcomes in typically developing children (Bornstein et al., 2003) as well as children with SBM (Holmbeck et al., 2006; Lomax-Bream, Taylor et al., 2007). It is possible that the more robust relation of SES in this study reflects the influence of parental education as it impacts parenting, issues that should be assessed in future research.
We also examined the relation between intelligence and academic achievement in children with SBM and shunted hydrocephalus, and whether this relation was influenced by SES and ethnicity. A significant interaction of verbal and nonverbal cognitive performance was consistent across predictions of various academic tasks. Higher nonverbal performance may be a protective factor, predicting academic performance that was close to age expectations in children with lower verbal performance. In contrast, children with higher verbal performance performed well on academic tasks tested here regardless of the level of nonverbal performance. Our study did not evaluate higher level academic skills, such as text-based inferential reading comprehension and written expression. It is possible that academic strengths would not be identified in these more complex skills in children with SBM and lower verbal abilities.
Although our sample was restricted to children with SBM and shunted hydrocephalus, this profile represents the largest subgroup of children with spina bifida (over 90%; Menkes, 1995). The results would not be expected to generalize to subgroups with other spinal lesions because they are generally not associated with malformations of the brain. Some children with myelomeningocele have arrested hydrocephalus that is not shunted; this population is growing because of changes in neurosurgical practice in which children with myelomeningocele are not automatically shunted at birth (Bowman, Boshnjaku, & McLone, 2009). It will be important to re-evaluate these findings when sufficiently large samples are available.
Clinical and Public Policy Implications
Clinicians assessing children with SBM and shunted hydrocephalus should be aware of the differences that emerge in cognitive function in association with SES, ethnicity, and lesion level. Across these factors, a preliminary implication is that better-developed nonverbal skills can act as a protective factor in children with weaker verbal skills. The nature of this relation needs to be further investigated in more complex academic tasks and in real-world outcomes such as social competence and independent functioning.
More generally, SES and ethnicity are associated with variations in expected cognitive performance patterns in SBM, particularly in language development. Given that SES is uniquely associated with lower vocabulary and lexical processing (Hart & Risley, 1995) and parenting practices that are less optimal for cognitive development (Bornstein et al., 2003), there is a need for public policy to support delivery of interventions to maximize language development in those children most at risk for difficulty with vocabulary. Early intervention may be especially important for economically disadvantaged children with disabilities, with implementation of both language-based interventions and parenting interventions to enhance cognitive outcomes.
Funding
This research was supported in part by grant P01-HD35946 awarded from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NICHD or the National Institutes of Health.
Conflicts of interest: None declared.
References
- Anastasi A, Cordova FA. Some effects of bilingualism upon the intelligence test performance of Puerto Rican children in New York City. Journal of Educational Psychology. 1953;44:1–19. [Google Scholar]
- Au KS, Tran PX, Tsai CC, O'B;yrne MR, Lin J, Morrison AC, et al. Characteristics of a spina bifida population including North American Caucasian and Hispanic individuals. Birth Defects Research Part A. Clinical and Molecular Teratology. 2008;82:692–700. doi: 10.1002/bdra.20499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- August D, Shanahan T. Developing literacy in second-language learners: Report of the National Literacy Panel on Language-Minority Children and Youth. Mahwah, NJ: Lawrence Erlbaum; 2006. [Google Scholar]
- Bergan JR, Parra EB. Variations in IQ testing and instruction and the letter learning and achievement of Anglo and bilingual Mexican-American children. Journal of Educational Psychology. 1979;71:819–826. [Google Scholar]
- Bornstein MH, Hahn C, Suwalsky JTD, Haynes OM. Socioeconomic status, parenting, and child development: The Hollingshead Four-Factor Index of Social Status and The Socioeconomic Index of Occupations. In: Bornstein MH, Robert H, editors. Socioeconomic status, parenting, and child development. Mahwah, NJ: Lawrence Erlbaum; 2003. pp. 29–82. [Google Scholar]
- Bowman RM, Boshnjaku V, McLone DG. The changing incidence of myelomeningocele and its impact on pediatric neurosurgery: A review from the Children’s Memorial Hospital. Child’s Nervous System. 2009;25:801–806. doi: 10.1007/s00381-009-0865-z. [DOI] [PubMed] [Google Scholar]
- Bradley RH, Corwyn RF. Socioeconomic status and child development. Annual Review of Psychology. 2002;53:371–399. doi: 10.1146/annurev.psych.53.100901.135233. [DOI] [PubMed] [Google Scholar]
- Caldwell BM, Bradley RH. Home observation for measurement of the environment. Little Rock: University of Arkansas at Little Rock, Center for Child Development and Education; 1984. [Google Scholar]
- Carr L. Neuropsychological profiles of English- and Spanish- speaking Hispanic children with spina bifida. 2009. Unpublished doctoral dissertation, University of Houston. [Google Scholar]
- Dennis M, Fitz CR, Netley CT, Sugar J, Harwood-Nash DC, Hendrick EB, et al. The intelligence of hydrocephalic children. Archives of Neurology. 1981;38:607–615. doi: 10.1001/archneur.1981.00510100035004. [DOI] [PubMed] [Google Scholar]
- Dennis M, Landry SH, Barnes M, Fletcher JM. A model of neurocognitive function in spina bifida over the life span. Journal of the International Neuropsychological Society. 2006;12:285–296. doi: 10.1017/S1355617706060371. [DOI] [PubMed] [Google Scholar]
- Detrait ER, George TM, Etchevers HC, Gilbert JR, Vekemans M, Speer MC. Human neural tube defects: Developmental biology, epidemiology, and genetics. Neurotoxicology and Teratology. 2005;27:515–524. doi: 10.1016/j.ntt.2004.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunst CJ, Leet HE. Measuring the adequacy of resources in households with young children. Child: Care, Health, and Development. 1987;13:111–125. doi: 10.1111/j.1365-2214.1987.tb00528.x. [DOI] [PubMed] [Google Scholar]
- Fletcher JM, Copeland K, Frederick JA, Blaser SE, Kramer LA, Northrup H, et al. Spinal lesion level in spina bifida: A source of neural and cognitive heterogeneity. Journal of Neurosurgery: Pediatrics. 2005;102:268–279. doi: 10.3171/ped.2005.102.3.0268. [DOI] [PubMed] [Google Scholar]
- Frank NC, Blount RL, Brown RT. Attributions, coping, and adjustment in children with cancer. Journal of Pediatric Psychology. 1997;22:563–576. doi: 10.1093/jpepsy/22.4.563. [DOI] [PubMed] [Google Scholar]
- Gerber M, Durgunoglu AY. Reading risk and intervention for young English learners: Evidence from longitudinal intervention research. Introduction to special series. Learning Disabilities Research & Practice. 2004;19:199–201. [Google Scholar]
- Hart B, Risley TR. Meaningful differences in the everyday experience of young American children. Baltimore: Brookes; 1995. [Google Scholar]
- Henderson RW, Bergen JR, Hurt M., Jr. Development and validation of the Henderson Environmental Learning Process scale. The Journal of Social Psychology. 1972;88:185–196. [Google Scholar]
- Hollingshead A. Four factor index of social status. New Haven, CT: Yale University, Department of Sociology; 1975. [Google Scholar]
- Holmbeck GN, Greenley RN, Coakley RM, Greco J, Hagstrom J. Family functioning in children and adolescents with spina bifida: An evidence-based review of research and interventions. Journal of Developmental and Behavioral Pediatrics. 2006;27:249–277. doi: 10.1097/00004703-200606000-00012. [DOI] [PubMed] [Google Scholar]
- Holmbeck GN, Westhoven VC, Phillips WS, Bowers R, Gruse C, Nikolopoulos T, et al. A multi-method, multi-informant, and multidimensional perspective on psychosocial adjustment in preadolescents with spina bifida. Journal of Consulting and Clinical Psychology. 2003;71:782–796. doi: 10.1037/0022-006x.71.4.782. [DOI] [PubMed] [Google Scholar]
- Kieffer MJ. Catching up or falling behind? Initial English proficiency, concentrated poverty, and the reading growth of language minority learners in the United States. Journal of Educational Psychology. 2008;100:851–868. [Google Scholar]
- Lomax-Bream LE, Barnes M, Copeland K, Taylor HB, Landry SH. The impact of spina bifida on development across the first 3 years. Developmental Neuropsychology. 2007;31:1–20. doi: 10.1207/s15326942dn3101_1. [DOI] [PubMed] [Google Scholar]
- Lomax-Bream LE, Taylor HB, Landry SH, Barnes MA, Fletcher JM, Swank P. Role of early parenting and motor skills on development in children with spina bifida. Journal of Applied Developmental Psychology. 2007;28:250–263. [Google Scholar]
- Menkes JH. Textbook of Child Neurology (5th ed.) Baltimore: Williams & Wilkens; 1995. [Google Scholar]
- Price AL, Patterson N, Yu F, Cox DR, Waliszewska A, McDonald GJ, et al. A genomewide admixture map for Latino populations. The American Journal of Human Genetics. 2007;80:1024–1036. doi: 10.1086/518313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler JM. Assessment of children: Cognitive applications (4th ed.) San Diego: Author; 2001. [Google Scholar]
- Thorndike RL, Hagen EP, Sattler JM. Stanford-Binet Intelligence Scale: Fourth Edition. Chicago: Riverside; 1986. [Google Scholar]
- Valencia RR, Henderson RW, Rankin RJ. Family status, family constellation, and home environmental variables as predictors of cognitive performance of Mexican American children. Journal of Educational Psychology. 1985;77:323–331. [Google Scholar]
- Wallander JL, Varni JL, Babani L, Banis HT, Wilcox KT. Family resources as resistance factors for psychological maladjustment in chronically ill and handicapped children. Journal of Pediatric Psychology. 1989;14:157–173. doi: 10.1093/jpepsy/14.2.157. [DOI] [PubMed] [Google Scholar]
- Wasserman CR, Shaw GM, Selvin S, Gould JB, Syme SL. Socioeconomic status, neighborhood conditions, and neural tube defects. American Journal of Public Health. 1998;88:1674–1680. doi: 10.2105/ajph.88.11.1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LJ, Rasmussen SA, Flores A, Kirby RS, Edmonds LD. Decline in the prevalence of spina bifida and anencephaly by race/ethnicity: 1995-2002. Pediatrics. 2005;116:580–586. doi: 10.1542/peds.2005-0592. [DOI] [PubMed] [Google Scholar]
- Woodcock RW, Johnson MB. Woodcock-Johnson Psychoeducational Battery – Revised. Chicago: Riverside; 1989. [Google Scholar]
- Woodcock RW, Muñoz-Sandoval AF. Batería Woodcock-Muñoz Pruebas de Aprovechamiento-Revisada. Itasca, IL: Riverside; 1996. [Google Scholar]