Abstract
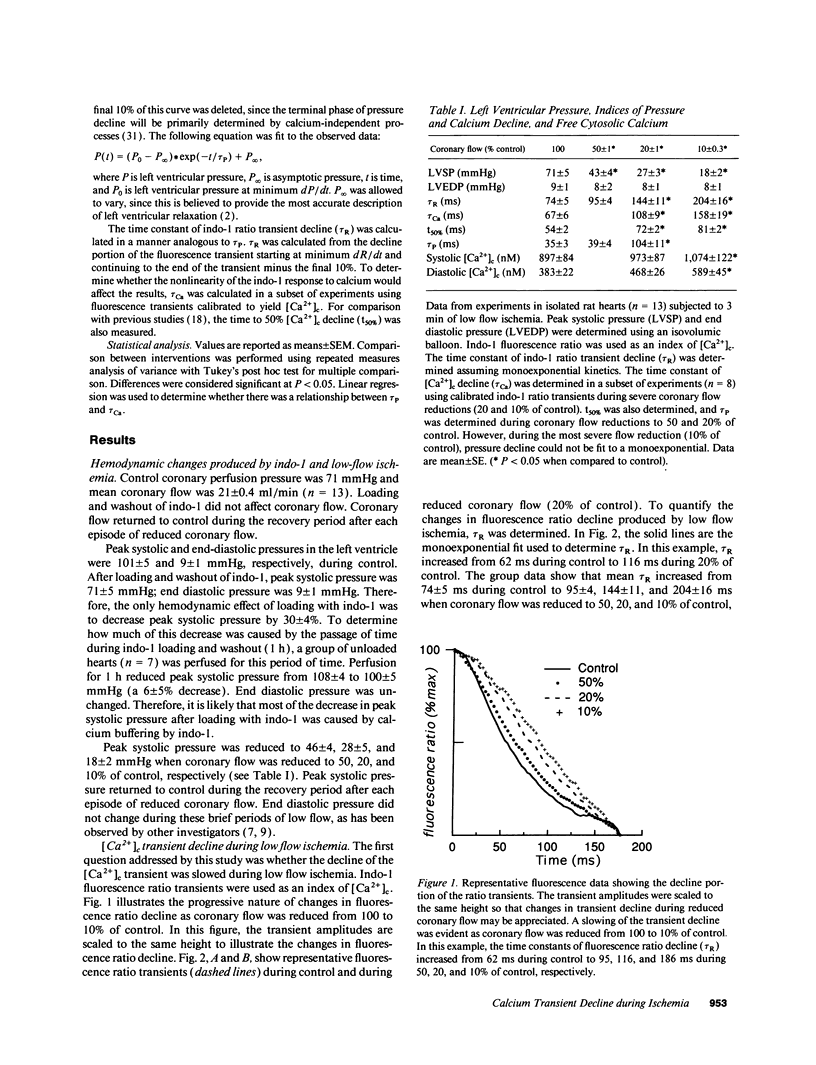
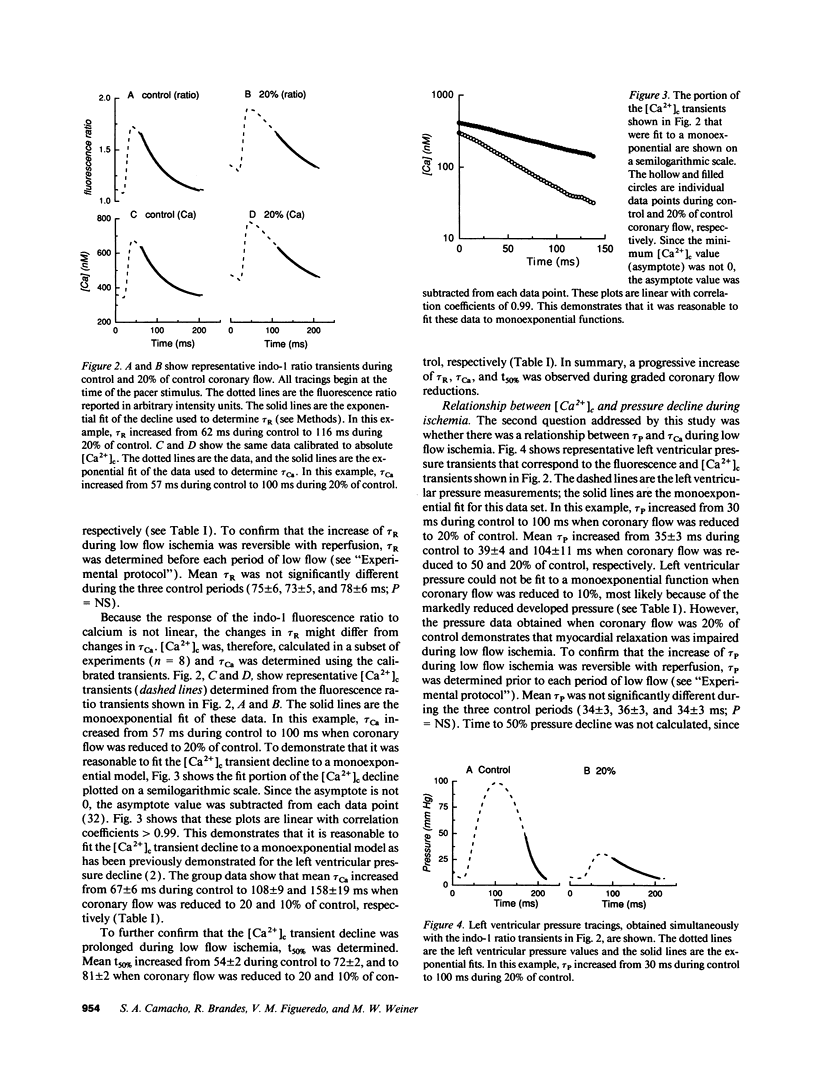
The mechanisms that impair myocardial relaxation during ischemia are believed to involve abnormalities of calcium handling. However, there is little direct evidence to support this hypothesis. Therefore, we sought to determine whether the time constant of cytosolic calcium ([Ca2+]c) decline (tau Ca) was increased during low flow ischemia, and if there was a relationship between the time constant of left ventricular pressure decline (tau P) and tau Ca. Isolated perfused hearts were studied using indo-1 fluorescence ratio as an index of [Ca2+]c.tau P was used as an index of myocardial relaxation. The time constant of decline of the indo-1 ratio increased from 74 +/- 5 ms to 95 +/- 4, 144 +/- 10, and to 204 +/- 16 ms when coronary flow was reduced was reduced to 50, 20, and 10% of control, respectively. Indo-1 transients were calibrated to calculate tau Ca. tau Ca increased from 67 +/- 6 ms to 108 +/- 9 and 158 +/- 19 ms when coronary flow was reduced to 20 and 10% of control, respectively. There was a linear relationship between tau Ca and tau P (r = 0.82). These data support the hypothesis that during low flow ischemia, impaired myocardial relaxation may be caused by slowing of [Ca2+]c decline.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. G., Orchard C. H. Intracellular calcium concentration during hypoxia and metabolic inhibition in mammalian ventricular muscle. J Physiol. 1983 Jun;339:107–122. doi: 10.1113/jphysiol.1983.sp014706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Applegate R. J., Walsh R. A., O'Rourke R. A. Effects of nifedipine on diastolic function during brief periods of flow-limiting ischemia in the conscious dog. Circulation. 1987 Dec;76(6):1409–1421. doi: 10.1161/01.cir.76.6.1409. [DOI] [PubMed] [Google Scholar]
- Arkhammar P., Nilsson T., Berggren P. O. Glucose-stimulated efflux of indo-1 from pancreatic beta-cells is reduced by probenecid. FEBS Lett. 1990 Oct 29;273(1-2):182–184. doi: 10.1016/0014-5793(90)81079-4. [DOI] [PubMed] [Google Scholar]
- Brandes R., Figueredo V. M., Camacho S. A., Massie B. M., Weiner M. W. Suppression of motion artifacts in fluorescence spectroscopy of perfused hearts. Am J Physiol. 1992 Sep;263(3 Pt 2):H972–H980. doi: 10.1152/ajpheart.1992.263.3.H972. [DOI] [PubMed] [Google Scholar]
- Brenner B. A new concept for the mechanism of Ca+(+)-regulation of muscle contraction. Implications for physiological and pharmacological approaches to modulate contractile function of myocardium. Basic Res Cardiol. 1991;86 (Suppl 3):83–92. doi: 10.1007/978-3-662-30769-4_8. [DOI] [PubMed] [Google Scholar]
- Brutsaert D. L., Sys S. U. Relaxation and diastole of the heart. Physiol Rev. 1989 Oct;69(4):1228–1315. doi: 10.1152/physrev.1989.69.4.1228. [DOI] [PubMed] [Google Scholar]
- Camacho S. A., Figueredo V. M., Brandes R., Weiner M. W. Ca(2+)-dependent fluorescence transients and phosphate metabolism during low-flow ischemia in rat hearts. Am J Physiol. 1993 Jul;265(1 Pt 2):H114–H122. doi: 10.1152/ajpheart.1993.265.1.H114. [DOI] [PubMed] [Google Scholar]
- Carroll J. D., Hess O. M., Hirzel H. O., Krayenbuehl H. P. Exercise-induced ischemia: the influence of altered relaxation on early diastolic pressures. Circulation. 1983 Mar;67(3):521–528. doi: 10.1161/01.cir.67.3.521. [DOI] [PubMed] [Google Scholar]
- Figueredo V. M., Brandes R., Weiner M. W., Massie B. M., Camacho S. A. Cardiac contractile dysfunction during mild coronary flow reductions is due to an altered calcium-pressure relationship in rat hearts. J Clin Invest. 1992 Nov;90(5):1794–1802. doi: 10.1172/JCI116054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueredo V. M., Brandes R., Weiner M. W., Massie B. M., Camacho S. A. Endocardial versus epicardial differences of intracellular free calcium under normal and ischemic conditions in perfused rat hearts. Circ Res. 1993 May;72(5):1082–1090. doi: 10.1161/01.res.72.5.1082. [DOI] [PubMed] [Google Scholar]
- Fry C. A numerical method for the calculation of the time constant of exponential functions. Cardiovasc Res. 1993 Aug;27(8):1552–1553. doi: 10.1093/cvr/27.8.1552. [DOI] [PubMed] [Google Scholar]
- Gilbert J. C., Glantz S. A. Determinants of left ventricular filling and of the diastolic pressure-volume relation. Circ Res. 1989 May;64(5):827–852. doi: 10.1161/01.res.64.5.827. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hove-Madsen L., Bers D. M. Indo-1 binding to protein in permeabilized ventricular myocytes alters its spectral and Ca binding properties. Biophys J. 1992 Jul;63(1):89–97. doi: 10.1016/S0006-3495(92)81597-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isoyama S., Apstein C. S., Wexler L. F., Grice W. N., Lorell B. H. Acute decrease in left ventricular diastolic chamber distensibility during simulated angina in isolated hearts. Circ Res. 1987 Dec;61(6):925–933. doi: 10.1161/01.res.61.6.925. [DOI] [PubMed] [Google Scholar]
- Jackson A. P., Timmerman M. P., Bagshaw C. R., Ashley C. C. The kinetics of calcium binding to fura-2 and indo-1. FEBS Lett. 1987 May 25;216(1):35–39. doi: 10.1016/0014-5793(87)80752-4. [DOI] [PubMed] [Google Scholar]
- Kaplan P., Hendrikx M., Mattheussen M., Mubagwa K., Flameng W. Effect of ischemia and reperfusion on sarcoplasmic reticulum calcium uptake. Circ Res. 1992 Nov;71(5):1123–1130. doi: 10.1161/01.res.71.5.1123. [DOI] [PubMed] [Google Scholar]
- Kihara Y., Grossman W., Morgan J. P. Direct measurement of changes in intracellular calcium transients during hypoxia, ischemia, and reperfusion of the intact mammalian heart. Circ Res. 1989 Oct;65(4):1029–1044. doi: 10.1161/01.res.65.4.1029. [DOI] [PubMed] [Google Scholar]
- Krause S., Hess M. L. Characterization of cardiac sarcoplasmic reticulum dysfunction during short-term, normothermic, global ischemia. Circ Res. 1984 Aug;55(2):176–184. doi: 10.1161/01.res.55.2.176. [DOI] [PubMed] [Google Scholar]
- Lee H. C., Mohabir R., Smith N., Franz M. R., Clusin W. T. Effect of ischemia on calcium-dependent fluorescence transients in rabbit hearts containing indo 1. Correlation with monophasic action potentials and contraction. Circulation. 1988 Oct;78(4):1047–1059. doi: 10.1161/01.cir.78.4.1047. [DOI] [PubMed] [Google Scholar]
- Lorell B. H., Apstein C. S., Cunningham M. J., Schoen F. J., Weinberg E. O., Peeters G. A., Barry W. H. Contribution of endothelial cells to calcium-dependent fluorescence transients in rabbit hearts loaded with indo 1. Circ Res. 1990 Aug;67(2):415–425. doi: 10.1161/01.res.67.2.415. [DOI] [PubMed] [Google Scholar]
- Lorell B. H. Significance of diastolic dysfunction of the heart. Annu Rev Med. 1991;42:411–436. doi: 10.1146/annurev.me.42.020191.002211. [DOI] [PubMed] [Google Scholar]
- MacLeod K. T. Regulation and interaction of intracellular calcium, sodium and hydrogen ions in cardiac muscle. Cardioscience. 1991 Jun;2(2):71–85. [PubMed] [Google Scholar]
- Mann T., Goldberg S., Mudge G. H., Jr, Grossman W. Factors contributing to altered left ventricular diastolic properties during angina pectoris. Circulation. 1979 Jan;59(1):14–20. doi: 10.1161/01.cir.59.1.14. [DOI] [PubMed] [Google Scholar]
- Marban E., Kusuoka H. Maximal Ca2+-activated force and myofilament Ca2+ sensitivity in intact mammalian hearts. Differential effects of inorganic phosphate and hydrogen ions. J Gen Physiol. 1987 Nov;90(5):609–623. doi: 10.1085/jgp.90.5.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohabir R., Lee H. C., Kurz R. W., Clusin W. T. Effects of ischemia and hypercarbic acidosis on myocyte calcium transients, contraction, and pHi in perfused rabbit hearts. Circ Res. 1991 Dec;69(6):1525–1537. doi: 10.1161/01.res.69.6.1525. [DOI] [PubMed] [Google Scholar]
- Momomura S., Iizuka M., Serizawa T., Sugimoto T. Separation of rate of left ventricular relaxation from chamber stiffness in rats. Am J Physiol. 1988 Dec;255(6 Pt 2):H1468–H1475. doi: 10.1152/ajpheart.1988.255.6.H1468. [DOI] [PubMed] [Google Scholar]
- Momomura S., Serizawa T., Ikenouchi H., Sugimoto T., Iizuka M. Effects of nifedipine on diastolic abnormalities in low-flow and pacing-induced ischemia in isolated rat hearts. Jpn Circ J. 1991 Jun;55(6):623–633. doi: 10.1253/jcj.55.623. [DOI] [PubMed] [Google Scholar]
- Peterson J. N., Hunter W. C., Berman M. R. Estimated time course of Ca2+ bound to troponin C during relaxation in isolated cardiac muscle. Am J Physiol. 1991 Mar;260(3 Pt 2):H1013–H1024. doi: 10.1152/ajpheart.1991.260.3.H1013. [DOI] [PubMed] [Google Scholar]
- Serizawa T., Momomura S., Kohmoto O., Ohya T., Sato H., Takahashi T., Mochizuki T., Iizuka M., Sugimoto T. Mechanisms of abnormal myocardial relaxation induced by ischemia: comparison of low flow ischemia and hypoxia in isolated rabbit heart. Jpn Circ J. 1987 Jan;51(1):90–97. doi: 10.1253/jcj.51.90. [DOI] [PubMed] [Google Scholar]
- Serizawa T., Vogel W. M., Apstein C. S., Grossman W. Comparison of acute alterations in left ventricular relaxation and diastolic chamber stiffness induced by hypoxia and ischemia. Role of myocardial oxygen supply-demand imbalance. J Clin Invest. 1981 Jul;68(1):91–102. doi: 10.1172/JCI110258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman H. S., Ninomiya M., Blank P. S., Hano O., Miyata H., Spurgeon H. A., Lakatta E. G., Stern M. D. A cellular mechanism for impaired posthypoxic relaxation in isolated cardiac myocytes. Altered myofilament relaxation kinetics at reoxygenation. Circ Res. 1991 Jul;69(1):196–208. doi: 10.1161/01.res.69.1.196. [DOI] [PubMed] [Google Scholar]
- Smith G. L., Steele D. S., Crichton C. A. Effects of inorganic phosphate on calcium and tension oscillations in saponin-treated rat cardiac muscle. Adv Exp Med Biol. 1992;311:387–388. doi: 10.1007/978-1-4615-3362-7_47. [DOI] [PubMed] [Google Scholar]
- Spurgeon H. A., Stern M. D., Baartz G., Raffaeli S., Hansford R. G., Talo A., Lakatta E. G., Capogrossi M. C. Simultaneous measurement of Ca2+, contraction, and potential in cardiac myocytes. Am J Physiol. 1990 Feb;258(2 Pt 2):H574–H586. doi: 10.1152/ajpheart.1990.258.2.H574. [DOI] [PubMed] [Google Scholar]
- Spurgeon H. A., duBell W. H., Stern M. D., Sollott S. J., Ziman B. D., Silverman H. S., Capogrossi M. C., Talo A., Lakatta E. G. Cytosolic calcium and myofilaments in single rat cardiac myocytes achieve a dynamic equilibrium during twitch relaxation. J Physiol. 1992 Feb;447:83–102. doi: 10.1113/jphysiol.1992.sp018992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson D. S., Waldron C. B., Juul S. M., Naqvi N., Swanton R. H., Coltart D. J., Jenkins B. S., Webb-Peploe M. M. Analysis of left ventricular pressure during isovolumic relaxation in coronary artery disease. Circulation. 1982 Apr;65(4):690–697. doi: 10.1161/01.cir.65.4.690. [DOI] [PubMed] [Google Scholar]
- Weiss J. L., Frederiksen J. W., Weisfeldt M. L. Hemodynamic determinants of the time-course of fall in canine left ventricular pressure. J Clin Invest. 1976 Sep;58(3):751–760. doi: 10.1172/JCI108522. [DOI] [PMC free article] [PubMed] [Google Scholar]



