Abstract
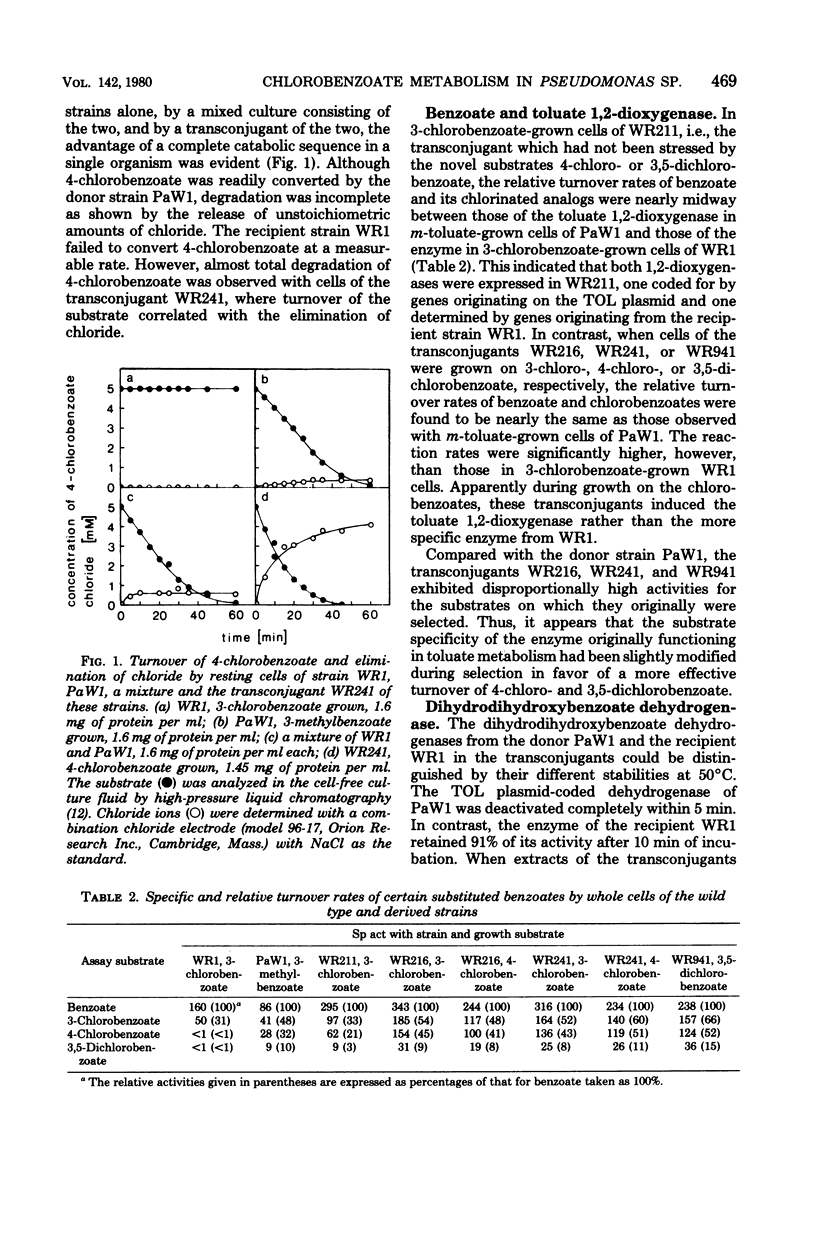
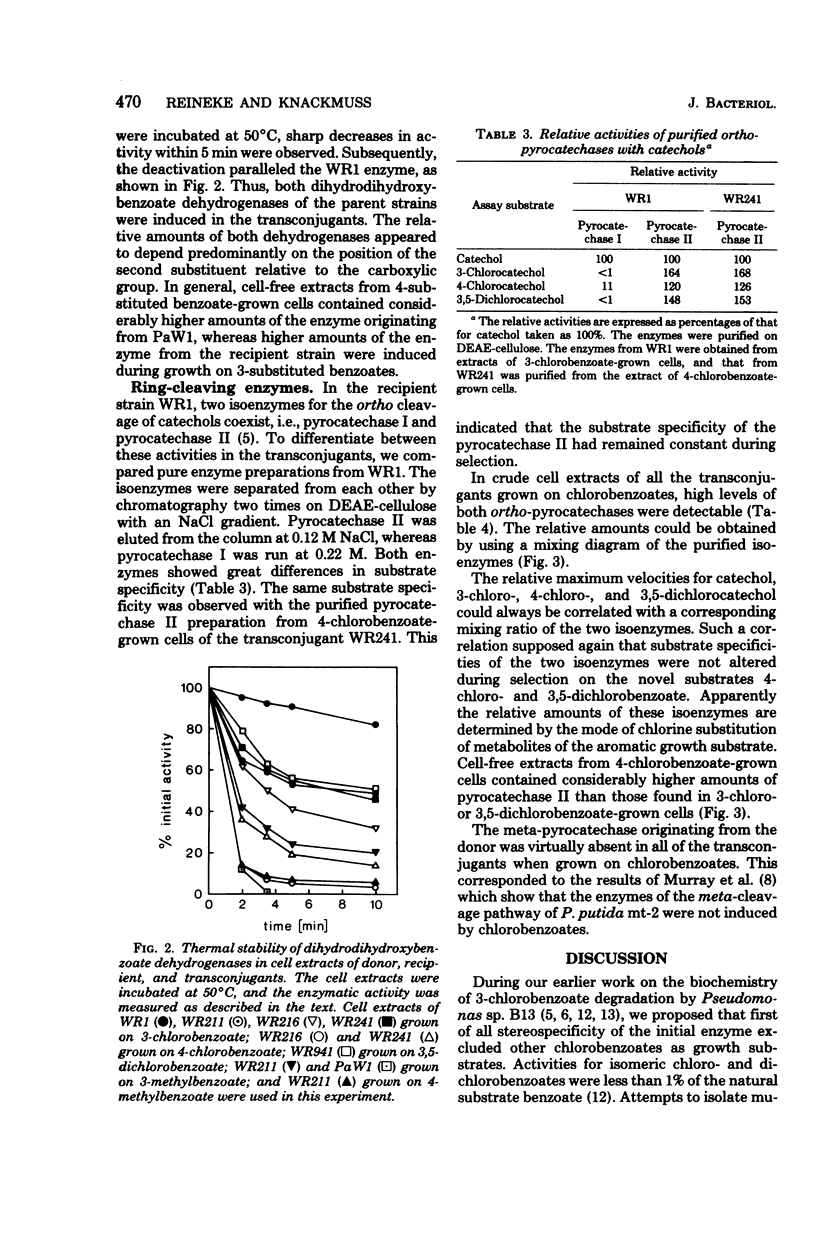
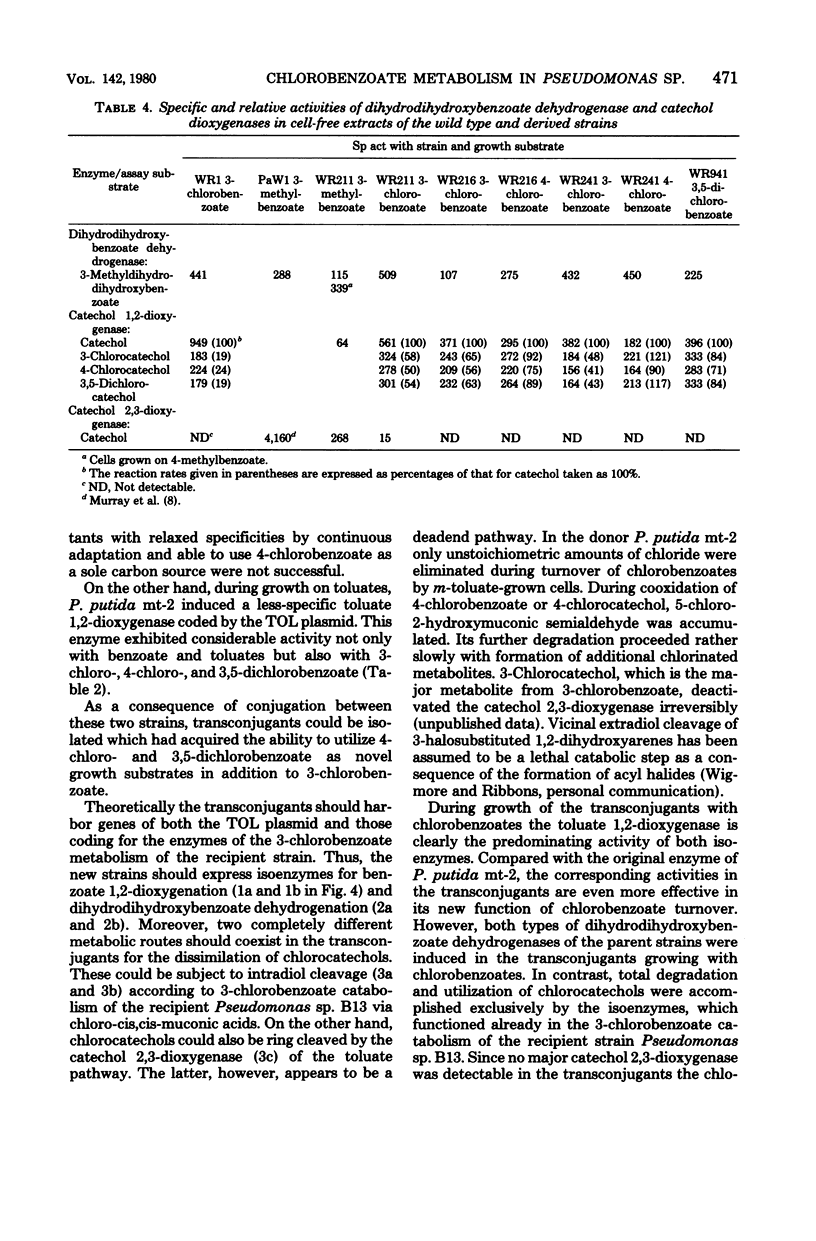
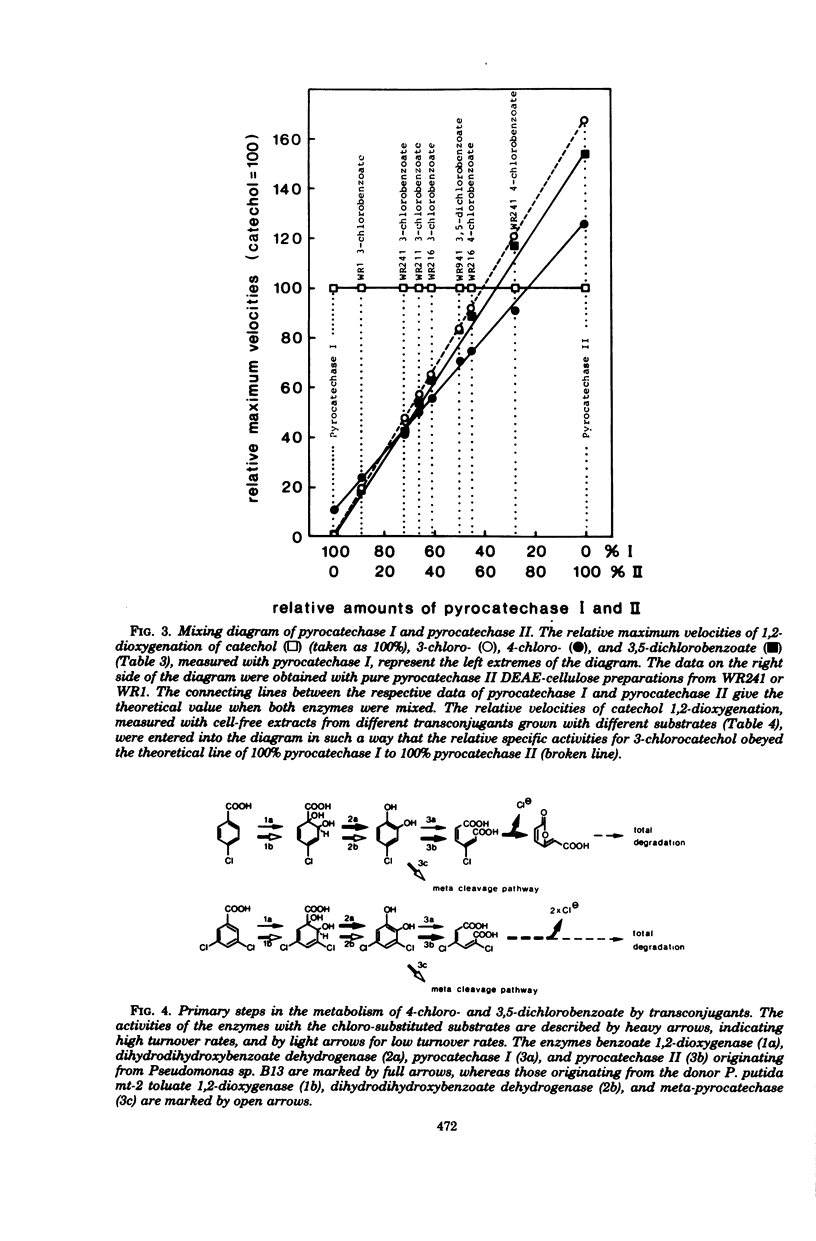
Derivatives of Pseudomonas sp. B13 which had acquired the capability to utilize 4-chloro- and 3,5-dichlorobenzoate as a consequence of the introduction of genes of the TOL plasmid of Pseudomonas putida mt-2 were studied. The utilization of these substrates, a property not shared by the parent strains, was shown to depend upon the combined activities of enzymes from the donor and from the recipient. During growth on 3-chloro-, 4-chloro-, and 3,5-dichlorobenzoate, predominantly the toluate 1,2-deoxygenase and both dihydrodihydroxybenzoate dehydrogenases of the parent strains were induced. On the other hand, no catechol 2,3-dioxygenase from P. putida mt-2 was detectable, so that degradation of chlorocatechols by the nonproductive meta-cleavage pathway was avoided. Instead of that, chlorocatechols were subject to ortho cleavage and totally degraded by the preexisting enzymes of Pseudomonas sp. B13.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AZOUZ W. M., PARKE D. V., WILLIAMS R. T. Studies in detoxication. 62. The metabolism of halogenobenzenes; ortho- and para-dichlorobenzenes. Biochem J. 1955 Mar;59(3):410–415. doi: 10.1042/bj0590410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Dorn E., Hellwig M., Reineke W., Knackmuss H. J. Isolation and characterization of a 3-chlorobenzoate degrading pseudomonad. Arch Microbiol. 1974;99(1):61–70. doi: 10.1007/BF00696222. [DOI] [PubMed] [Google Scholar]
- Dorn E., Knackmuss H. J. Chemical structure and biodegradability of halogenated aromatic compounds. Substituent effects on 1,2-dioxygenation of catechol. Biochem J. 1978 Jul 15;174(1):85–94. doi: 10.1042/bj1740085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn E., Knackmuss H. J. Chemical structure and biodegradability of halogenated aromatic compounds. Two catechol 1,2-dioxygenases from a 3-chlorobenzoate-grown pseudomonad. Biochem J. 1978 Jul 15;174(1):73–84. doi: 10.1042/bj1740073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann J., Reineke W., Knackmuss H. J. Metabolism of 3-chloro-, 4-chloro-, and 3,5-dichlorobenzoate by a pseudomonad. Appl Environ Microbiol. 1979 Mar;37(3):421–428. doi: 10.1128/aem.37.3.421-428.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray K., Duggleby C. J., Sala-Trepat J. M., Williams P. A. The metabolism of benzoate and methylbenzoates via the meta-cleavage pathway by Pseudomonas arvilla mt-2. Eur J Biochem. 1972 Jul 24;28(3):301–310. doi: 10.1111/j.1432-1033.1972.tb01914.x. [DOI] [PubMed] [Google Scholar]
- Nakazawa T., Yokota T. Isolation of a mutant TOL plasmid with increased activity and transmissibility from Pseudomonas putida (arvilla) mt-2. J Bacteriol. 1977 Jan;129(1):39–46. doi: 10.1128/jb.129.1.39-46.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reineke W., Knackmuss H. J. Chemical structure and biodegradability of halogenate aromatic compounds. Substituent effects on 1,2-dioxygenation of benzoic acid. Biochim Biophys Acta. 1978 Sep 6;542(3):412–423. doi: 10.1016/0304-4165(78)90372-0. [DOI] [PubMed] [Google Scholar]
- Reineke W., Knackmuss H. J. Chemical structure and biodegradability of halogenated aromatic compounds. Substituent effects on dehydrogenation of 3,5-cyclohexadiene-1,2-diol-1-carboxylic acid. Biochim Biophys Acta. 1978 Sep 6;542(3):424–429. doi: 10.1016/0304-4165(78)90373-2. [DOI] [PubMed] [Google Scholar]
- Reineke W., Knackmuss H. J. Construction of haloaromatics utilising bacteria. Nature. 1979 Feb 1;277(5695):385–386. doi: 10.1038/277385a0. [DOI] [PubMed] [Google Scholar]
- Reiner A. M. Metabolism of aromatic compounds in bacteria. Purification and properties of the catechol-forming enzyme, 3,5-cyclohexadiene-1,2-diol-1-carboxylic acid (NAD + ) oxidoreductase (decarboxylating). J Biol Chem. 1972 Aug 25;247(16):4960–4965. [PubMed] [Google Scholar]
- SCHMIDT K., LIAAENJENSEN S., SCHLEGEL H. G. DIE CAROTINOIDE DER THIORHODACEAE. I. OKENON ALS HAUPTEAROTINOID VON CHROMATIUM OKENII PERTY. Arch Mikrobiol. 1963 Aug 1;46:117–126. [PubMed] [Google Scholar]
- Williams P. A., Murray K. Metabolism of benzoate and the methylbenzoates by Pseudomonas putida (arvilla) mt-2: evidence for the existence of a TOL plasmid. J Bacteriol. 1974 Oct;120(1):416–423. doi: 10.1128/jb.120.1.416-423.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong C. L., Dunn N. W. Combined chromosomal and plasmid encoded control for the degradation of phenol in Pseudomonas putida. Genet Res. 1976 Jun;27(3):405–412. doi: 10.1017/s001667230001661x. [DOI] [PubMed] [Google Scholar]



