Abstract
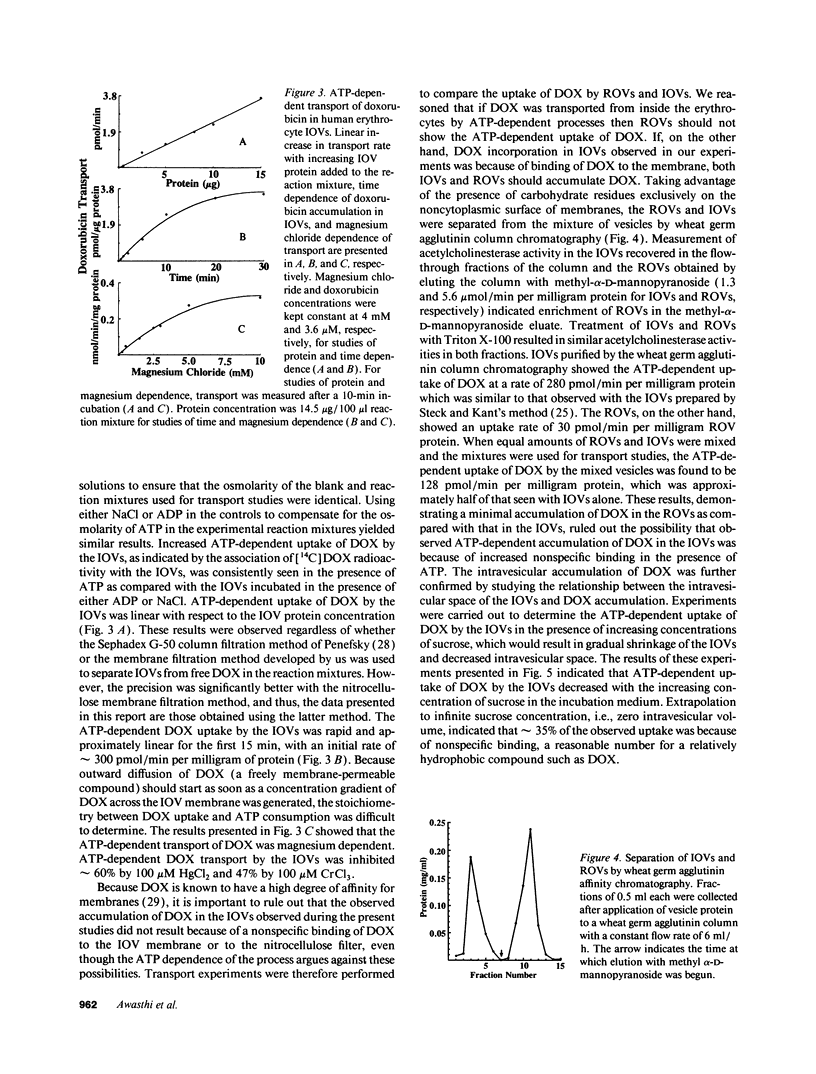
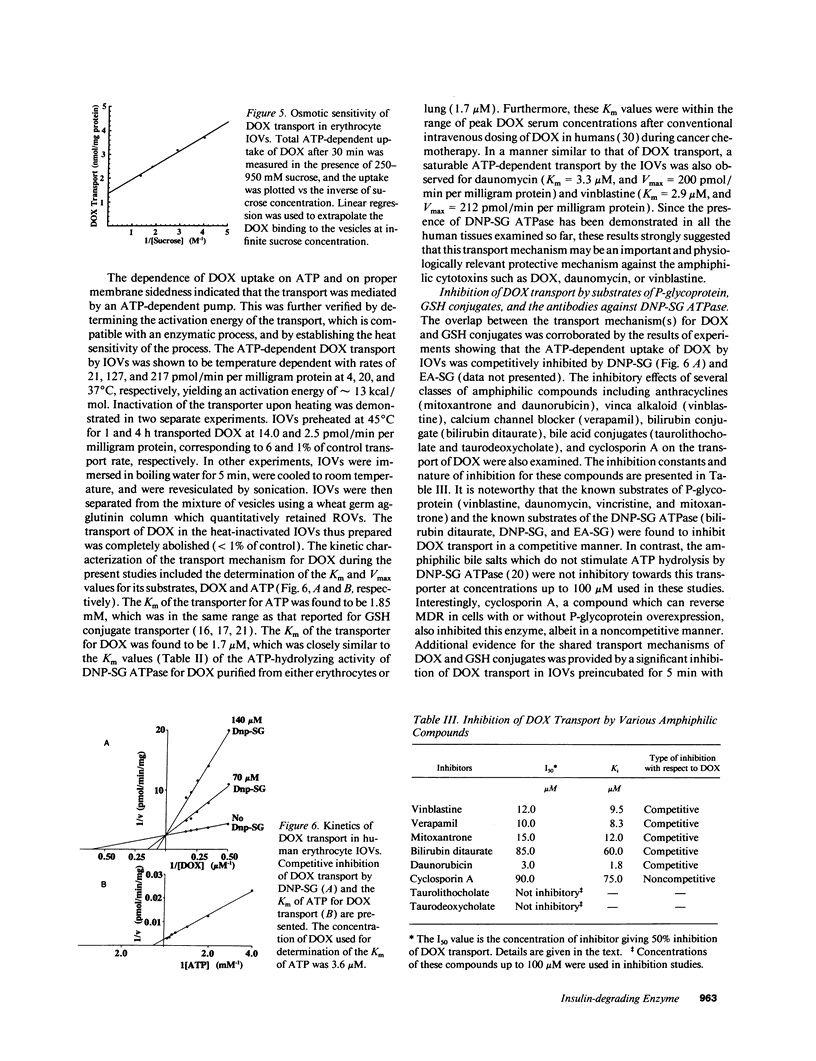
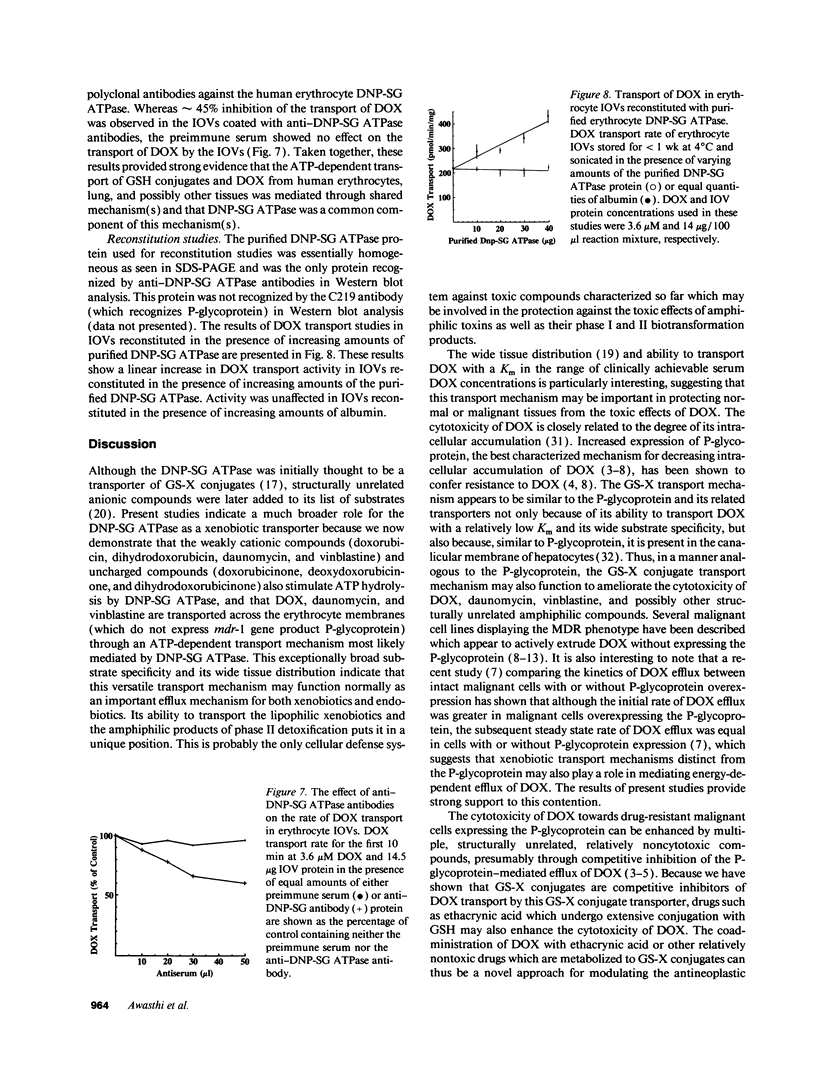
Previous studies have demonstrated that a human glutathione conjugate transporter, designated as dinitrophenyl-S-glutathione ATPase (DNP-SG ATPase), catalyzed ATP hydrolysis in the presence of several amphiphilic compounds other than glutathione conjugates (Singhal, S. S., R. Sharma, S. Gupta, H. Ahmad, P. Zimniak, A. Radominska, R. Lester, and Y. C. Awasthi. 1991. FEBS [Fed. Eur. Biochem. Soc.] Lett. 281:255-257). We now demonstrate that DNP-SG ATPase purified from human lung and erythrocyte membranes catalyzed the hydrolysis of ATP in the presence of doxorubicin and its metabolites. Doxorubicin-stimulated ATP hydrolysis by DNP-SG ATPase was saturable with respect to doxorubicin (Km 1.2 and 2.8 microM for the lung and erythrocyte enzymes, respectively). Antibodies against DNP-SG ATPase immunoprecipitated the ATP hydrolyzing activity stimulated by doxorubicin, its metabolites, and glutathione conjugates. Inside our vesicles prepared from erythrocyte membranes took up doxorubicin, daunomycin, and vinblastine in an ATP-dependent manner. The uptake was linear with respect to time and vesicle protein, was dependent on ATP and magnesium, was inhibited by heavy metal salts or by heating the vesicles, and was sensitive to both osmolarity and orientation of the vesicles. The transport had an activation energy of 13 kcal/mol, was saturable with respect to both doxorubicin and ATP (Km values of 1.8 microM and 1.9 mM, respectively), and was competitively inhibited by glutathione conjugates as well as by a number of amphiphiles such as daunomycin or vinblastine. Transport was diminished upon coating the vesicles with antibodies against DNP-SG ATPase. Incorporation of increasing amounts of purified DNP-SG ATPase into the vesicles resulted in a linear increase in transport of doxorubicin. These studies demonstrated for the first time that a membrane protein that catalyzed the transport of anionic amphiphilic molecules such as glutathione conjugates could also mediate the transport of weakly cationic antitumor antibiotic, doxorubicin. Notably, the Km of transport was in the range of doxorubicin concentration achievable in human serum after intravenous dosing of doxorubicin.
Full text
PDF


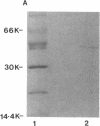
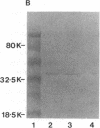
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Awasthi S., Sharma R., Awasthi Y. C., Belli J. A., Frenkel E. P. The relationship of doxorubicin binding to membrane lipids with drug resistance. Cancer Lett. 1992 Apr 15;63(2):109–116. doi: 10.1016/0304-3835(92)90060-9. [DOI] [PubMed] [Google Scholar]
- Awasthi S., Srivastava S. K., Ahmad F., Ahmad H., Ansari G. A. Interactions of glutathione S-transferase-pi with ethacrynic acid and its glutathione conjugate. Biochim Biophys Acta. 1993 Jul 10;1164(2):173–178. doi: 10.1016/0167-4838(93)90245-m. [DOI] [PubMed] [Google Scholar]
- Awasthi Y. C., Garg H. S., Dao D. D., Partridge C. A., Srivastava S. K. Enzymatic conjugation of erythrocyte glutathione with 1-chloro-2,4-dinitrobenzene: the fate of glutathione conjugate in erythrocytes and the effect of glutathione depletion on hemoglobin. Blood. 1981 Oct;58(4):733–738. [PubMed] [Google Scholar]
- Baas F., Jongsma A. P., Broxterman H. J., Arceci R. J., Housman D., Scheffer G. L., Riethorst A., van Groenigen M., Nieuwint A. W., Joenje H. Non-P-glycoprotein mediated mechanism for multidrug resistance precedes P-glycoprotein expression during in vitro selection for doxorubicin resistance in a human lung cancer cell line. Cancer Res. 1990 Sep 1;50(17):5392–5398. [PubMed] [Google Scholar]
- Benjamin R. S. Pharmacokinetics of adriamycin (NSC-123127) in patients with sarcomas. Cancer Chemother Rep. 1974 Mar-Apr;58(2):271–273. [PubMed] [Google Scholar]
- Biedler J. L. Genetic aspects of multidrug resistance. Cancer. 1992 Sep 15;70(6 Suppl):1799–1809. doi: 10.1002/1097-0142(19920915)70:4+<1799::aid-cncr2820701623>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Chen Y. N., Mickley L. A., Schwartz A. M., Acton E. M., Hwang J. L., Fojo A. T. Characterization of adriamycin-resistant human breast cancer cells which display overexpression of a novel resistance-related membrane protein. J Biol Chem. 1990 Jun 15;265(17):10073–10080. [PubMed] [Google Scholar]
- Coley H. M., Workman P., Twentyman P. R. Retention of activity by selected anthracyclines in a multidrug resistant human large cell lung carcinoma line without P-glycoprotein hyperexpression. Br J Cancer. 1991 Mar;63(3):351–357. doi: 10.1038/bjc.1991.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L., COURTNEY K. D., ANDRES V., Jr, FEATHER-STONE R. M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961 Jul;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Horio M., Gottesman M. M., Pastan I. ATP-dependent transport of vinblastine in vesicles from human multidrug-resistant cells. Proc Natl Acad Sci U S A. 1988 May;85(10):3580–3584. doi: 10.1073/pnas.85.10.3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano R. L., Ling V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim Biophys Acta. 1976 Nov 11;455(1):152–162. doi: 10.1016/0005-2736(76)90160-7. [DOI] [PubMed] [Google Scholar]
- Keizer H. G., Schuurhuis G. J., Broxterman H. J., Lankelma J., Schoonen W. G., van Rijn J., Pinedo H. M., Joenje H. Correlation of multidrug resistance with decreased drug accumulation, altered subcellular drug distribution, and increased P-glycoprotein expression in cultured SW-1573 human lung tumor cells. Cancer Res. 1989 Jun 1;49(11):2988–2993. [PubMed] [Google Scholar]
- Kondo T., Dale G. L., Beutler E. Studies on glutathione transport utilizing inside-out vesicles prepared from human erythrocytes. Biochim Biophys Acta. 1981 Jul 6;645(1):132–136. doi: 10.1016/0005-2736(81)90520-4. [DOI] [PubMed] [Google Scholar]
- Kondo T., Kawakami Y., Taniguchi N., Beutler E. Glutathione disulfide-stimulated Mg2+-ATPase of human erythrocyte membranes. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7373–7377. doi: 10.1073/pnas.84.21.7373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzmich S., Vanderveer L. A., Walsh E. S., LaCreta F. P., Tew K. D. Increased levels of glutathione S-transferase pi transcript as a mechanism of resistance to ethacrynic acid. Biochem J. 1992 Jan 1;281(Pt 1):219–224. doi: 10.1042/bj2810219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBelle E. F., Singh S. V., Ahmad H., Wronski L., Srivastava S. K., Awasthi Y. C. A novel dinitrophenylglutathione-stimulated ATPase is present in human erythrocyte membranes. FEBS Lett. 1988 Feb 8;228(1):53–56. doi: 10.1016/0014-5793(88)80583-0. [DOI] [PubMed] [Google Scholar]
- LaBelle E. F., Singh S. V., Srivastava S. K., Awasthi Y. C. Dinitrophenyl glutathione efflux from human erythrocytes is primary active ATP-dependent transport. Biochem J. 1986 Sep 1;238(2):443–449. doi: 10.1042/bj2380443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minamide L. S., Bamburg J. R. A filter paper dye-binding assay for quantitative determination of protein without interference from reducing agents or detergents. Anal Biochem. 1990 Oct;190(1):66–70. doi: 10.1016/0003-2697(90)90134-u. [DOI] [PubMed] [Google Scholar]
- Nielsen D., Skovsgaard T. P-glycoprotein as multidrug transporter: a critical review of current multidrug resistant cell lines. Biochim Biophys Acta. 1992 Jul 7;1139(3):169–183. doi: 10.1016/0925-4439(92)90131-6. [DOI] [PubMed] [Google Scholar]
- Penefsky H. S. Reversible binding of Pi by beef heart mitochondrial adenosine triphosphatase. J Biol Chem. 1977 May 10;252(9):2891–2899. [PubMed] [Google Scholar]
- Roepe P. D. Analysis of the steady-state and initial rate of doxorubicin efflux from a series of multidrug-resistant cells expressing different levels of P-glycoprotein. Biochemistry. 1992 Dec 22;31(50):12555–12564. doi: 10.1021/bi00165a003. [DOI] [PubMed] [Google Scholar]
- Saxena M., Singhal S. S., Awasthi S., Singh S. V., Labelle E. F., Zimniak P., Awasthi Y. C. Dinitrophenyl S-glutathione ATPase purified from human muscle catalyzes ATP hydrolysis in the presence of leukotrienes. Arch Biochem Biophys. 1992 Oct;298(1):231–237. doi: 10.1016/0003-9861(92)90117-f. [DOI] [PubMed] [Google Scholar]
- Sharma R., Gupta S., Singh S. V., Medh R. D., Ahmad H., LaBelle E. F., Awasthi Y. C. Purification and characterization of dinitrophenylglutathione ATPase of human erythrocytes and its expression in other tissues. Biochem Biophys Res Commun. 1990 Aug 31;171(1):155–161. doi: 10.1016/0006-291x(90)91370-8. [DOI] [PubMed] [Google Scholar]
- Singhal S. S., Sharma R., Gupta S., Ahmad H., Zimniak P., Radominska A., Lester R., Awasthi Y. C. The anionic conjugates of bilirubin and bile acids stimulate ATP hydrolysis by S-(dinitrophenyl)glutathione ATPase of human erythrocyte. FEBS Lett. 1991 Apr 9;281(1-2):255–257. doi: 10.1016/0014-5793(91)80405-r. [DOI] [PubMed] [Google Scholar]
- Slovak M. L., Hoeltge G. A., Dalton W. S., Trent J. M. Pharmacological and biological evidence for differing mechanisms of doxorubicin resistance in two human tumor cell lines. Cancer Res. 1988 May 15;48(10):2793–2797. [PubMed] [Google Scholar]
- Spoelstra E. C., Westerhoff H. V., Dekker H., Lankelma J. Kinetics of daunorubicin transport by P-glycoprotein of intact cancer cells. Eur J Biochem. 1992 Jul 15;207(2):567–579. doi: 10.1111/j.1432-1033.1992.tb17083.x. [DOI] [PubMed] [Google Scholar]
- Srivastava S. K., Beutler E. The transport of oxidized glutathione from human erythrocytes. J Biol Chem. 1969 Jan 10;244(1):9–16. [PubMed] [Google Scholar]
- Steck T. L., Kant J. A. Preparation of impermeable ghosts and inside-out vesicles from human erythrocyte membranes. Methods Enzymol. 1974;31:172–180. doi: 10.1016/0076-6879(74)31019-1. [DOI] [PubMed] [Google Scholar]
- Tsuchida S., Sato K. Glutathione transferases and cancer. Crit Rev Biochem Mol Biol. 1992;27(4-5):337–384. doi: 10.3109/10409239209082566. [DOI] [PubMed] [Google Scholar]
- Versantvoort C. H., Broxterman H. J., Pinedo H. M., de Vries E. G., Feller N., Kuiper C. M., Lankelma J. Energy-dependent processes involved in reduced drug accumulation in multidrug-resistant human lung cancer cell lines without P-glycoprotein expression. Cancer Res. 1992 Jan 1;52(1):17–23. [PubMed] [Google Scholar]
- Zimniak P., Ziller S. A., 3rd, Panfil I., Radominska A., Wolters H., Kuipers F., Sharma R., Saxena M., Moslen M. T., Vore M. Identification of an anion-transport ATPase that catalyzes glutathione conjugate-dependent ATP hydrolysis in canalicular plasma membranes from normal rats and rats with conjugated hyperbilirubinemia (GY mutant). Arch Biochem Biophys. 1992 Feb 1;292(2):534–538. doi: 10.1016/0003-9861(92)90027-t. [DOI] [PubMed] [Google Scholar]