Abstract
Background:
ARDS may occur after either septic or nonseptic injuries. Sepsis is the major cause of ARDS, but little is known about the differences between sepsis-related and non-sepsis-related ARDS.
Methods:
A total of 2,786 patients with ARDS-predisposing conditions were enrolled consecutively into a prospective cohort, of which 736 patients developed ARDS. We defined sepsis-related ARDS as ARDS developing in patients with sepsis and non-sepsis-related ARDS as ARDS developing after nonseptic injuries, such as trauma, aspiration, and multiple transfusions. Patients with both septic and nonseptic risks were excluded from analysis.
Results:
Compared with patients with non-sepsis-related ARDS (n = 62), patients with sepsis-related ARDS (n = 524) were more likely to be women and to have diabetes, less likely to have preceding surgery, and had longer pre-ICU hospital stays and higher APACHE III (Acute Physiology and Chronic Health Evaluation III) scores (median, 78 vs 65, P < .0001). There were no differences in lung injury score, blood pH, Pao2/Fio2 ratio, and Paco2 on ARDS diagnosis. However, patients with sepsis-related ARDS had significantly lower Pao2/Fio2 ratios than patients with non-sepsis-related ARDS patients on ARDS day 3 (P = .018), day 7 (P = .004), and day 14 (P = .004) (repeated-measures analysis, P = .011). Compared with patients with non-sepsis-related ARDS, those with sepsis-related had a higher 60-day mortality (38.2% vs 22.6%; P = .016), a lower successful extubation rate (53.6% vs 72.6%; P = .005), and fewer ICU-free days (P = .0001) and ventilator-free days (P = .003). In multivariate analysis, age, APACHE III score, liver cirrhosis, metastatic cancer, admission serum bilirubin and glucose levels, and treatment with activated protein C were independently associated with 60-day ARDS mortality. After adjustment, sepsis-related ARDS was no longer associated with higher 60-day mortality (hazard ratio, 1.26; 95% CI, 0.71-2.22).
Conclusion:
Sepsis-related ARDS has a higher overall disease severity, poorer recovery from lung injury, lower successful extubation rate, and higher mortality than non-sepsis-related ARDS. Worse clinical outcomes in sepsis-related ARDS appear to be driven by disease severity and comorbidities.
ARDS, the more severe form of acute lung injury (ALI), is a common and lethal disease in ICUs worldwide. Clinically, ARDS is characterized by acute respiratory failure with severe hypoxemia and diffuse pulmonary infiltrates. Despite recent advances in critical care and significant efforts invested in the basic research and clinical trials of ARDS, its mortality rate (35%-45%) has remained relatively unchanged since 1994.1
ARDS usually develops in patients with predisposing conditions that induce systemic inflammatory response, such as sepsis, pneumonia, major trauma, multiple transfusions, aspiration, and acute pancreatitis, among which sepsis is the most common cause of ARDS.2-5 In a large prospective cohort study, severe sepsis with a suspected pulmonary source (46%) or a nonpulmonary source (33%) was the most common risk factor for ALI.6 On the other hand, only a fraction of patients with sepsis (18%-38%) will develop ARDS.7
There is significant heterogeneity among patients with ARDS. Many efforts have been made to define pathogenetic or molecular phenotypes that might have significant clinical implications. Currently, ARDS usually is classified into subgroups based on predisposing clinical conditions, such as sepsis related, transfusion related, and trauma associated (or posttraumatic). Although prior studies have found patients with trauma-associated ALI to be less acutely and chronically ill with lower mortality than those with nontraumatic ALI,8-10 little is known about the differences between sepsis-related and non-sepsis-related ARDS. Characterization of these two subgroups hopefully will lead to improvements in future research and management of this syndrome.4
In this study, we hypothesized that sepsis-related ARDS is clinically different from non-sepsis-related ARDS. Therefore, we compared the clinical features and outcomes between these two subtypes in a large prospective cohort.
Materials and Methods
Study Design and Patient Inclusion
Study patients were recruited for the ongoing Molecular Epidemiology of ARDS Study at Massachusetts General Hospital (Boston, MA) from September 1999 to February 2009 and at Beth Israel Deaconess Medical Center (Boston, MA) from January 2007 to February 2009. Details of the study design have been described previously.11 Briefly, consecutive admissions to the ICUs were screened. Patients with predisposing conditions for ARDS, including bacteremia, sepsis, pneumonia, trauma, aspiration, or multiple transfusions as defined previously,12 were eligible for inclusion. Exclusion criteria were aged < 18 years, diffuse alveolar hemorrhage, chronic lung diseases other than COPD or asthma, directive to withhold intubation, immunosuppression (other than immunosuppression secondary to corticosteroid therapy), and treatment with granulocyte colony-stimulating factor. Enrolled patients were followed daily for ARDS development on the basis of the American-European Consensus Committee criteria for ARDS.13 The study was approved by the human subjects committees of Massachusetts General Hospital, Beth Israel Deaconess Medical Center, and the Harvard School of Public Health. Written informed consent was obtained from all participants or their surrogates.
Data Collection and Definitions
Patient demographic and baseline clinical characteristics were recorded on study enrollment. Vital signs and laboratory values in the first 24 h of ICU admission were collected, and Acute Physiology and Chronic Health Evaluation (APACHE) III scores were calculated. BMI was calculated based on admission height and weight. Patients were followed for specific treatments, including activated protein C (APC) and vasopressors use. Lung injury score was calculated as per Murray et al.14 To consider the influences of pre-ICU hospital stay on sepsis and ARDS, we created a variable indicating whether the pre-ICU hospital stay was ≥ 48 h. Patients transferred from other hospitals were considered to have a pre-ICU hospital stay of ≥ 48 h.
Sepsis was defined as a known or suspected source of systemic infection plus at least two of the following: temperature > 38°C or < 36°C; heart rate > 90 beats/min; respiratory rate > 20 breaths/min or Paco2 < 32 mm Hg; or WBC count > 12,000/mm3, < 4,000/mm3, or > 10% bandemia. Infections were determined based on the treating physician clinical judgment, imaging studies, microbiologic tests collected within 48 h before or after ICU admission, or a combination of these. We defined sepsis-related ARDS as that developing in patients with sepsis, and non-sepsis-related ARDS as that developing after nonseptic injuries such as trauma, aspiration, and multiple transfusions. Causes of ARDS were determined by the treating physicians on diagnosis. The classification of ARDS into sepsis-related or non-sepsis related were retrospectively made by two investigators according to causes of ARDS. Patients with both septic and nonseptic risks for ARDS were excluded from analysis. All patients with ARDS were followed until death or 60 days after diagnosis. We used all-cause 60-day mortality as the major clinical outcome. Other short-term outcomes were based on 28 days after ARDS diagnosis, including all-cause 28-day mortality, ICU length of stay (LOS), ICU-free days, total ventilator days, ventilator-free days, and successful extubation.
Statistical Analyses
Categorical variables are presented as frequencies and percentages. All continuous variables were not normally distributed and, thus, are presented as median values and interquartile ranges (IQRs). In univariate analyses, categorical variables were compared by χ2 test or Fisher exact test, and continuous variables were compared by nonparametric Wilcoxon test. Repeated measurements were analyzed by the generalized estimating equation (GEE) model.
Mortality between patients with sepsis-related and non-sepsis-related ARDS was compared by χ2 test in univariate analysis. Kaplan-Meier estimates and Cox proportional hazards models were used to analyze time to death. In Cox regression models, candidate variables with P < .20 in univariate analyses were entered into the model. We replaced missing values (3.2% of BMI, 18.6% of serum bilirubin, and 19.5% of serum albumin) by the corresponding overall median values. Other covariates had < 1% missing values. We used a backward selection algorithm with criteria of P > .05 for eliminating variables. Considering advances in medical care over time, the analyses were performed with stratification by calendar year. For the final Cox regression models, we used Kolmogorov-type supremum test to check the proportional hazards assumption (all P > .90).
All data analyses were performed with statistical software SAS, version 9.1 (SAS Institute; Cary, NC). A two-sided P ≤ .05 was considered statistically significant.
Results
Enrollment, Follow-up, and Baseline Characteristics of Study Patients
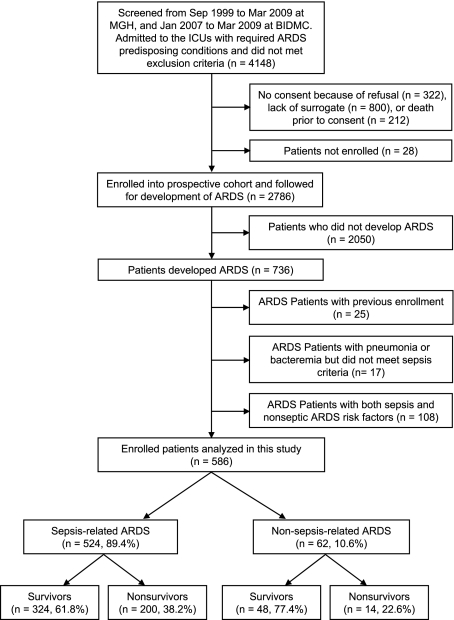
Figure 1 illustrates the enrollment and follow-up of study patients. A total of 2,786 patients with predisposing conditions for ARDS were enrolled into the prospective cohort, of which 736 (26.4%) developed ARDS. We excluded 25 patients with previous enrollment, 17 infected patients who did not fulfill sepsis criteria, and 108 patients who had both septic and nonseptic ARDS risk factors, leaving 586 patients for analysis.
Figure 1.
Enrollment and follow-up of study patients. BIDMC = Beth Israel Deaconess Medical Center; MGH = Massachusetts General Hospital.
Table 1 shows the baseline characteristics of study patients. Most patients were men (60.8%), white (90.4%), recruited at Massachusetts General Hospital (86.9%), and from the medical ICU (50.8%). Regarding predisposing conditions, 524 (89.4%) patients had sepsis, and 62 (10.6%) had nonseptic injuries. Among patients with sepsis, 373 (71.2%) had septic shock, 109 (20.8%) had bacteremia, and 408 (77.9%) had pneumonia. Seventy-eight patients had both pneumonia and bacteremia. Among patients with nonseptic injuries, 27 (43.6%) had trauma, 35 (56.5%) had multiple transfusions, and 8 (12.9%) had aspiration. Seven patients had both trauma and multiple transfusions, and one patient had both trauma and aspiration.
Table 1.
—Baseline Characteristics of Study Population
| Characteristic | ARDS (n = 586) |
| Age, y | 60 (45-73) |
| Male sex | 356 (60.8) |
| White | 530 (90.4) |
| APACHE III score | 77 (61-92) |
| BMI, kg/m2 | 26.9 (23.3-31.6) |
| Hospitals | |
| MGH | 509 (86.9) |
| BIDMC | 77 (13.1) |
| ICU | |
| Medical | 297 (50.8) |
| Surgical | 170 (29.1) |
| Coronary care unit | 88 (15.0) |
| Neurologic | 18 (3.1) |
| Others | 12 (2.0) |
| Predisposing conditions | |
| Sepsis related | 524 (89.4) |
| Sepsis without shock | 151 (28.8) |
| Septic shock | 373 (71.2) |
| Bacteremia | 109 (20.8) |
| Pneumonia | 408 (77.9) |
| Non-sepsis related | 62 (10.6) |
| Trauma | 27 (43.5) |
| Multiple transfusions | 35 (56.5) |
| Aspiration | 8 (12.9) |
| Postoperation | 26 (4.4) |
| Diabetes | 118 (20.5) |
| End-stage renal disease | 46 (8.0) |
| Liver cirrhosis | 46 (8.0) |
| Metastatic cancers | 13 (2.3) |
| History of alcohol abuse | 90 (15.4) |
Data are presented as median (25th-75th percentile) or No. (%). APACHE = Acute Physiology and Chronic Health Evaluation; BIDMC = Beth Israel Deaconess Medical Center; MGH = Massachusetts General Hospital.
Table 2 shows the demographics and clinical characteristics between ARDS survivors and nonsurvivors. Nonsurvivors were older, had higher APACHE III scores, and had lower BMI than survivors. Sepsis was more common in nonsurvivors, whereas trauma was more common in survivors. Compared with the survivors, nonsurvivors had higher percentages of liver cirrhosis and metastatic cancers and were more likely to have stayed in the hospital for > 48 h before ICU admission. The platelet counts were lower, whereas the serum glucose, bilirubin, and creatinine levels were higher in nonsurvivors than in survivors. There were no significant differences between survivors and nonsurvivors in lung injury scores, blood pH value, Pao2/Fio2 ratio, Paco2, tidal volume, and positive end-expiratory pressure (PEEP). Regarding treatments, more nonsurvivors had vasopressors used within 24 h after ARDS diagnosis.
Table 2.
—Characteristics Between ARDS Survivors and Nonsurvivors
| Characteristic | Survivors (n = 372) | Nonsurvivors (n = 214) | P Value |
| Age, y | 54 (39-67) | 68 (57-78) | < .0001 |
| Male sex | 230 (61.8) | 126 (58.9) | .481 |
| White | 333 (89.5) | 197 (92.1) | .314 |
| APACHE III score | 70 (54-85) | 88 (74-105) | < .0001 |
| BMI, kg/m2 | 27.5 (23.7-33.0) | 25.8 (22.7-29.6) | .002 |
| Predisposing conditions | |||
| Bacteremia | 64 (17.2) | 45 (21.0) | .252 |
| Sepsis | 324 (87.1) | 200 (93.5) | .017 |
| Pneumonia | 250 (67.2) | 158 (73.8) | .093 |
| Trauma | 26 (7.0) | 1 (0.5) | .0001 |
| Multiple transfusions | 23 (6.2) | 12 (5.6) | .778 |
| Aspiration | 7 (1.9) | 1 (0.5) | .269 |
| Comorbidities | |||
| Postoperation | 16 (4.3) | 10 (4.7) | .833 |
| Diabetes | 70 (19.2) | 48 (22.5) | .342 |
| End-stage renal disease | 26 (7.1) | 20 (9.4) | .336 |
| Liver cirrhosis | 16 (4.4) | 30 (14.1) | < .0001 |
| Metastatic cancers | 5 (1.4) | 8 (3.8) | .081 |
| History of alcohol abuse | 56 (15.1) | 34 (15.9) | .787 |
| Pre-ICU hospital stay ≥ 48 h | 142 (38.2) | 108 (50.5) | .004 |
| Laboratory values on ICU admission | |||
| WBC count, × 103/mm3 | 16.2 (11.6-21.6) | 16.6 (11.5-24.1) | .254 |
| Hematocrit, % | 29.5 (26.6-33.6) | 29.4 (25.9-33.0) | .505 |
| Platelet count, × 103/mm3 | 189 (116-282) | 161 (79-253) | .002 |
| Serum glucose, mg/dL | 172 (141-230) | 192 (149-258) | .009 |
| Serum bilirubin, mg/dL | 0.8 (0.5-1.5) | 1.1 (0.6-3.3) | .0002 |
| Serum creatinine, mg/dL | 1.2 (0.9-2.1) | 1.6 (0.9-2.8) | .0007 |
| Serum albumin, g/dL | 2.3 (1.8-2.7) | 2.2 (1.7-2.7) | .767 |
| ARDS diagnosis | .010 | ||
| Upon ICU admission | 158 (42.5) | 76 (35.5) | |
| After ICU admission | 214 (57.5) | 138 (64.5) | |
| On diagnosis of ARDS | |||
| Lung injury score | 2.7 (2.0-3.0) | 2.3 (2.0-3.0) | .947 |
| pH | 7.34 (7.26-7.40) | 7.33 (7.26-7.43) | .342 |
| Pao2/Fio2 ratio | 131 (84-195) | 122 (84-210) | .858 |
| Paco2, mm Hg | 42 (37-49) | 42 (34-50) | .359 |
| PEEP, cm H2O | 8.5 (5-10) | 8 (5-10) | .204 |
| Tidal volume, mL | 500 (420-600) | 500 (400-600) | .518 |
| Vasopressors used | |||
| < 24 h after ICU admission | 250 (67.2) | 157 (73.4) | .119 |
| < 24 h after ARDS diagnosis | 235 (63.7) | 165 (77.1) | .0005 |
| Treatment with activated protein C | 29 (7.8) | 8 (3.7) | .051 |
Data are presented as median (25th-75th percentile) or No. (%), unless otherwise indicated. H2O = water; PEEP = positive end-expiratory pressure. See Table 1 legend for expansion of other abbreviations.
Clinical Characteristics Between Sepsis-Related and Non-Sepsis-Related ARDS
The characteristics between patients with sepsis-related ARDS and non-sepsis-related ARDS are shown in Table 3. Compared to patients with non-sepsis-related ARDS, those with sepsis-related were more likely to be women (P = .010) and to have diabetes (P = .006), had longer pre-ICU hospital stays (P < .0001), and were less likely to have preceding surgery (P < .0001). Patients with sepsis-related ARDS also had significantly higher APACHE III scores (P < .0001); WBC counts (P < .0001), hematocrit levels (P = .006), and platelet counts (P < .0001); and lower serum albumin levels (P = .027) than those with non-sepsis-related ARDS.
Table 3.
—Clinical Characteristics Between Patients With Sepsis-Related and Non-Sepsis-Related ARDS
| Characteristics | Sepsis-Related ARDS (n = 524) | Non-Sepsis-Related ARDS (n = 62) | P Value |
| Age, y | 60 (45-73) | 58 (37-75) | .403 |
| Male sex | 309 (59.0) | 47 (75.8) | .010 |
| APACHE III score | 78 (62-94) | 65 (46-78) | < .0001 |
| BMI, kg/m2 | 27.0 (23.2-31.8) | 26.2 (24.0-30.6) | .634 |
| Predisposing conditions | |||
| Bacteremia | 109 (20.8) | 0 | < .0001 |
| Septic shock | 373 (71.2) | 0 | < .0001 |
| Pneumonia | 408 (77.9) | 0 | < .0001 |
| Trauma | 0 | 27 (43.5) | < .0001 |
| Multiple transfusions | 0 | 35 (56.5) | < .0001 |
| Aspiration | 0 | 8 (12.9) | < .0001 |
| Comorbidities | |||
| Postoperation | 9 (1.7) | 17 (27.4) | < .0001 |
| Diabetes | 114 (22.0) | 4 (6.8) | .006 |
| End-stage renal disease | 45 (8.7) | 1 (1.7) | .073 |
| Liver cirrhosis | 44 (8.5) | 2 (3.4) | .211 |
| Metastatic cancers | 12 (2.3) | 1 (1.7) | .761 |
| History of alcohol abuse | 85 (16.2) | 5 (8.1) | .092 |
| Pre-ICU hospital stay ≥ 48 h | 244 (46.6) | 6 (9.7) | < .0001 |
| Laboratory values on ICU admission | |||
| WBC count, × 103/mm3 | 16.8 (12.1-23.5) | 13.3 (9.7-16.8) | .0003 |
| Hematocrit, % | 29.7 (26.6-33.7) | 27.9 (25.0-31.2) | .006 |
| Platelet count, × 103/mm3 | 189 (114-279) | 97 (57-154) | < .0001 |
| Serum glucose, mg/dL | 177 (140-245) | 182 (162-288) | .351 |
| Serum bilirubin, mg/dL | 0.9 (0.5-1.9) | 0.7 (0.5-1.2) | .081 |
| Serum creatinine, mg/dL | 1.3 (0.9-2.5) | 1.2 (0.9-1.7) | .087 |
| Serum albumin, g/dL | 2.2 (1.8-2.7) | 2.4 (2.0-2.8) | .027 |
| ARDS development | .087 | ||
| On ICU admission | 203 (38.7) | 31 (50) | |
| After ICU admission | 321 (61.3) | 31 (50) | |
| On diagnosis of ARDS | |||
| Lung injury score | 2.3 (2.0-3.0) | 2.7 (2.0-3.0) | .592 |
| pH | 7.34 (7.26-7.41) | 7.32 (7.25-7.37) | .131 |
| Pao2/Fio2 ratio | 127 (84-191) | 140 (88-256) | .120 |
| Paco2, mm Hg | 41 (35-49) | 43.5 (38-52) | .182 |
| PEEP, cm H2O | 8 (5-10) | 9.3 (5-10) | .912 |
| Vasopressors | |||
| On ICU admission | 370 (70.6) | 37 (59.7) | .077 |
| On ARDS diagnosis | 372 (71.0) | 28 (45.2) | < .001 |
| Serial Pao2/Fio2 ratio after ARDS | |||
| ARDS day 1 | 127 (84-191) | 140 (88-256) | .120 |
| ARDS day 3 | 143 (87-216) | 181 (103-264) | .018 |
| ARDS day 7 | 145 (100-207) | 168 (135-260) | .004 |
| ARDS day 14 | 145 (101-202) | 168 (140-238) | .004 |
A slightly higher percentage of sepsis-related ARDS developed after ICU admission than of non-sepsis-related ARDS (61.3% vs 50%; P = .087). With regard to severity of lung injury on ARDS diagnosis, there were no differences between these two subtypes of ARDS in lung injury score, pH value, Pao2/Fio2 ratio, Paco2, and PEEP level. Although the Pao2/Fio2 ratios were not different on ARDS diagnosis, patients with sepsis-related ARDS had significantly lower Pao2/Fio2 ratios than those with non-sepsis-related ARDS on day 3 (P = .018), day 7 (P = .004), and day 14 (P = .004) after ARDS diagnosis. In repeated-measures analysis using a GEE model and adjusted for baseline values, the serial Pao2/Fio2 ratios (log-transformed before analysis) on day 3, day 7, and day 14 were significantly lower in patients with sepsis-related ARDS than in those with non-sepsis-related ARDS (P = .011).
Clinical Outcomes Between Sepsis-Related and Non-Sepsis-Related ARDS
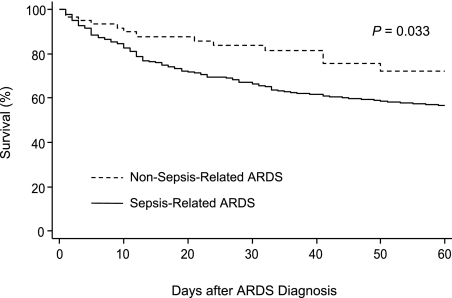
Patients with sepsis-related ARDS had worse clinical outcomes than those with non-sepsis-related ARDS, with significantly higher 28-day (31.1% vs 16.3%; P = .015) and 60-day (38.2% vs 22.6%; P = .016) mortality rates, fewer ICU-free days (P = .0001) and ventilator-free days (P = .003), and lower successful extubation rates (53.6% vs 72.6%; P = .005) in the first 28 days after ARDS diagnosis (Table 4). Among survivors, patients with sepsis-related ARDS had longer ICU LOS than those with non-sepsis-related ARDS (P = .010). The Kaplan-Meier estimates also showed a significant difference in 60-day survival between patients with sepsis-related and non-sepsis-related ARDS (P = .033 by log-rank test) (Fig 2).
Table 4.
—Clinical Outcomes Between Patients With Sepsis-Related and Non-Sepsis-Related ARDS
| Clinical Outcome | Sepsis-Related ARDS (n = 524) | Non-Sepsis-Related ARDS (n = 62) | P Value |
| 28-d mortality | 163 (31.1) | 10 (16.3) | .015 |
| 60-d mortality | 200 (38.2) | 14 (22.6) | .016 |
| ICU LOS, survivors, d | 16 (9-28) | 12 (8-18) | .010 |
| ICU-free days in 28 d | 1 (0-16) | 14 (0-20) | .0001 |
| Ventilator-free days in 28 d | 3 (0-18) | 13 (0-21) | .003 |
| Total ventilator days, survivors | 14 (8-26) | 12 (6-20) | .097 |
| Successful extubation in 28 d | 281 (53.6) | 45 (72.6) | .005 |
Data are presented as median (25th-75th percentile) or No. (%), unless otherwise indicated. LOS = length of stay.
Figure 2.
Kaplan-Meier curves for 60-day survival between patients with sepsis-related ARDS and patients with non-sepsis-related ARDS. P value was obtained by log-rank test.
Multivariate Analysis
In multivariate Cox regression analysis, we identified age (hazard ratio [HR], 1.03; 95% CI, 1.02-1.04), APACHE III score (HR, 1.02; 95% CI, 1.01-1.02), liver cirrhosis (HR, 1.85; 95% CI, 1.09-3.15), metastatic cancers (HR, 2.89; 95% CI, 1.32-6.37), serum bilirubin level (HR, 1.04; 95% CI, 1.02-1.06), serum glucose level (HR, 1.02; 95% CI, 1.00-1.03), and treatment with APC (HR, 0.47; 95% CI, 0.23-0.99) as independent predictors of ARDS mortality (Table 5). Sepsis-related ARDS was not independently associated with increased ARDS mortality compared with non-sepsis-related ARDS (HR, 1.26; 95% CI, 0.71-2.22).
Table 5.
—Multivariate Analysis for Predictors of ARDS Mortality
| Predictor | HR (95% CI)a | P Valueb |
| Sepsis-related vs non-sepsis-related ARDS | 1.26 (0.71-2.22) | .434 |
| Age, y | 1.03 (1.02-1.04) | < .0001 |
| APACHE III score | 1.02 (1.01-1.02) | < .0001 |
| Liver cirrhosis | 1.85 (1.09-3.15) | .023 |
| Metastatic cancers | 2.89 (1.32-6.37) | .008 |
| Serum bilirubin, mg/dL | 1.04 (1.02-1.06) | .0007 |
| Serum glucose, 10 mg/dL | 1.02 (1.00-1.03) | .029 |
| Treatment with activated protein C | 0.47 (0.23-0.99) | .046 |
HR = hazard ratio. See Table 1 legend for expansion of other abbreviation.
The HR and 95% CI were calculated in a multivariate Cox proportional hazard model, with stratification by calendar year.
Candidate variables with P < .20 in Table 2 were entered into the model. The variable representing sepsis-related vs non-sepsis-related ARDS was forced to be retained in the final model, whereas the covariates were selected using backward selection algorithm with criteria of P > .05 for eliminating variables.
Patients With ARDS Excluded From Comparisons
There were 17 infected patients who did not meet sepsis criteria, including 15 with pneumonia, one with bacteremia, and one with both pneumonia and bacteremia. The median APACHE III score (69; IQR, 62-80) and 28-day (23.5%) and 60-day (29.4%) mortality rates of these patients were between the data in patients with sepsis-related and non-sepsis-related ARDS. Another 108 patients with both sepsic and nonseptic ARDS risk factors also were excluded from comparisons. Their median APACHE III score (72; IQR, 55-89) and 28-day (31.5%) and 60-day (38.9%) mortality rates were close to the data in the patients with sepsis-related ARDS.
Discussion
Although sepsis as a cause of ARDS generally is associated with higher mortality than other risk factors,15,16 to our knowledge, no study has comprehensively examined the clinical difference between sepsis-related and non-sepsis-related ARDS. Unlike previous studies, we considered ARDS developing in patients with pneumonia who also fulfilled sepsis criteria as sepsis-related ARDS, grouped all non-sepsis-related ARDS together, and took into account the reality that a fraction of ARDS may be caused by both septic and nonseptic injuries. Our study demonstrates significant differences between sepsis-related and non-sepsis-related ARDS in clinical features and outcomes. In general, patients with sepsis-related ARDS had a higher disease severity and worse clinical outcomes than those with non-sepsis-related ARDS.
ARDS is a heterogeneous syndrome associated with complex interactions among the predisposing conditions, comorbidities, and genetic determinants. This heterogeneity leads to complexity and uncertainty in the study of this syndrome.4 It is possible that clinical trials have not found a treatment effect that truly exists because a therapy that benefits one subgroup may not benefit another subgroup.17 The genetic susceptibility to ARDS also shows differences among subgroups.18 A better classification of ARDS subgroups, therefore, is crucial in the future research and management of ARDS. In 1998, Gattinoni and colleagues19 first described the differences of underlying pathology, respiratory mechanics, and response to mechanical ventilation between pulmonary and extrapulmonary ARDS. However, later studies showed that there are no differences in mortality or ICU LOS between these two groups.16,20-22 In the present study, we found significant differences in characteristics and outcomes between sepsis-related and non-sepsis-related ARDS. Our findings warrant further studies to understand whether these two ARDS subtypes may represent different syndromes.
How sepsis-related ARDS differs pathophysiologically from non-sepsis-related ARDS remains largely unknown. Studies measuring circulating biomarkers in patients with ARDS showed that protein C level was lower in patients with sepsis-related ARDS than in those with non-sepsis-related ARDS, whereas procalcitonin, neopterin, von Willebrand factor antigen, soluble intercellular adhesion molecule-1, and soluble E-selectin levels were higher.23-25 Plasma cytokines also vary among clinical risk factors because interleukin-6, -8, and -10 levels are known to be higher in patients with ARDS caused by sepsis and pneumonia.26 These factors together suggest a higher degree of acute inflammation, endothelial cell activity, and coagulation activation in sepsis-related ARDS than in non-sepsis-related ARDS. Hemodynamics, ventricular function, and oxygen delivery and consumption, however, are not different between sepsis-related and non-sepsis-related ARDS.23 Our study revealed no significant difference between sepsis-related and non-sepsis-related ARDS in baseline Pao2/Fio2 ratio and severity of lung injury. However, patients with sepsis-related ARDS had lower serial Pao2/Fio2 ratios after ARDS diagnosis, indicating a poorer recovery from lung injury than patients with non-sepsis-related ARDS.
We found diabetes to be more common in sepsis-related ARDS than in non-sepsis-related ARDS. Diabetes is associated with lower risk of developing ARDS, but how it may protect against ARDS remains unclear.27,28 Studies have shown defects of neutrophil chemotactic, phagocytic, and microbicidal function in patients with diabetes.29 Our finding is consistent with the premise that deficient neutrophil function may predispose these patients to severe infections but, conversely, may protect the lung from profound inflammation during severe infections.
We identified seven independent predictors of ARDS mortality. From prior studies, age, APACHE III score, liver cirrhosis, and metastatic cancers are known predictors of ARDS mortality.5,20,30-32 In a recent study, we found that higher admission serum bilirubin levels were associated with subsequent ARDS development and mortality.33 The independent associations of admission serum glucose level and APC therapy with ARDS mortality have not been previously reported.
Stress hyperglycemia is common in acute critical illnesses. Although admission hyperglycemia has been associated with increased mortality in critically ill patients, this association is not uniformly observed in all ICU populations,34,35 with more evidence in surgical ICU patients but less in medical ICU patients.34,36-38 Many studies demonstrating admission blood glucose level as an independent outcome predictor were carried out in patients with acute vascular problems like stroke, myocardial infarction, and coronary artery bypass grafting.39-42 Interestingly, ARDS, with diffuse pulmonary microvascular damage as the pathologic hallmark, is also a syndrome of acute vascular illness. A randomized controlled trial of early blood glucose control in at-risk subjects is ongoing and will further clarify to role of hyperglycemia (or insulin therapy) on ARDS (trial registration: clinicaltrials.gov; Identifier: NCT00605696).
Recombinant human APC has both anticoagulant and antiinflammatory properties and is US Food and Drug Administration-approved for the treatment of high disease severity severe sepsis. Given that inflammation and coagulation both play important roles in the pathogenesis of ARDS43,44 and that lower levels of plasma protein C were independent predictors of ARDS mortality,45 APC may also be effective in treating ARDS. A recent phase II clinical trial showed that APC did not improve outcome in lower disease severity ALI but did improve dead space fraction.46 Our study included patients with ARDS with greater severity of illness, of which 89% had sepsis. We found that treatment with APC was independently associated with decreased mortality. However, the survival benefit of APC in patients with ARDS might come from the effective treatment of severe sepsis or septic shock, not from effective treatment of ARDS per se. Of note, the effectiveness of APC for septic shock recently has been called into question, and an international trial of APC in septic shock is under way.47 Hopefully, this trial will lead to a better understanding of the role of APC, if any, in sepsis-related ARDS.
A major strength of this study is that it was conducted within a large, well-defined, two-center, multiple-ICU cohort of ARDS. All data were collected prospectively, thus avoiding recall biases. In addition, excluding from analyses patients with both septic-related and non-septic-related ARDS reduced possible bias from misclassification. Nevertheless, we acknowledge several limitations to our study. First, the number of patients with non-sepsis-related ARDS was relatively small largely due to the exclusion of 108 patients with both sepsis-related and non-sepsis-related ARDS. Second, we did not collect data for antibiotic appropriateness, time delay to the diagnosis of sepsis, and time to meeting resuscitation goals, all of which might affect the outcomes in critically ill patients.48-50 Finally, patients with immunosuppression (other than secondary to steroids) were excluded in our study, thus generalization to populations including such patients should be made with caution.
In summary, sepsis-related ARDS is associated with a higher overall disease severity, poorer recovery from lung injury, lower successful extubation rate, longer ICU stay, and higher mortality than non-sepsis-related ARDS. Worse clinical outcomes in sepsis-related ARDS appear to be driven by disease severity and comorbidities. Our findings warrant further studies on potential pathophysiologic differences to understand whether sepsis-related and non-sepsis-related ARDS may represent different disease entities.
Acknowledgments
Author contributions: Dr Sheu: contributed to the planning of the study, study design, data analyses and interpretation, and manuscript preparation and review.
Dr Gong: contributed to the planning of the study, study design, assembly of the study patients, and manuscript preparation and review.
Dr Zhai: contributed to the planning of the study, study design, and manuscript preparation and review.
Dr Chen: contributed to the planning of the study, data analysis and interpretation, and manuscript preparation and review.
Dr Bajwa: contributed to the planning of the study, assembly of study patients, and manuscript preparation and review.
Dr Clardy: contributed to the planning of the study, assembly of study patients, and manuscript preparation and review.
Dr Gallagher: contributed to the planning of the study, assembly of the study patients, and manuscript preparation and review.
Dr Thompson: contributed to the planning of the study, assembly of the study patients, and manuscript preparation and review.
Dr Christiani: contributed to the planning of the study, study design, and manuscript preparation and review.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Thompson reports financial support from Eli Lilly for his role as the co-principal investigator of the PROWESS-SHOCK study, a randomized trial of activated protein C vs placebo for septic shock. Drs Sheu, Gong, Zhai, Chen, Bajwa, Clardy, Gallagher, and Christiani have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Other contributions: We thank Thomas McCabe, Julia Shin, Hanae Fujii-Rios, Ian Taggart, and Kezia Ellison for patient recruitment; Andrea Shafer and Starr Sumpter for research support; Janna Frelich, Marcia Chertok, and Julie DelPrato for data management; and the patients and staff of the ICUs at Massachusetts General Hospital and Beth Israel Deaconess Medical Center.
Abbreviations
- ALI
acute lung injury
- APACHE
Acute Physiology and Chronic Health Evaluation
- APC
activated protein C
- GEE
generalized estimating equation
- HR
hazard ratio
- IQR
interquartile range
- LOS
length of stay
- PEEP
positive end-expiratory pressure
Footnotes
Funding/Support: The work was supported by National Institutes of Health [Grants ES00002, HL60710, and HL087934].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (http://www.chestpubs.org/site/misc/reprints.xhtml).
References
- 1.Phua J, Badia JR, Adhikari NK, et al. Has mortality from acute respiratory distress syndrome decreased over time? A systematic review. Am J Respir Crit Care Med. 2009;179(3):220–227. doi: 10.1164/rccm.200805-722OC. [DOI] [PubMed] [Google Scholar]
- 2.Wheeler AP, Bernard GR. Acute lung injury and the acute respiratory distress syndrome: a clinical review. Lancet. 2007;369(9572):1553–1564. doi: 10.1016/S0140-6736(07)60604-7. [DOI] [PubMed] [Google Scholar]
- 3.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 4.Matthay MA, Zimmerman GA, Esmon C, et al. Future research directions in acute lung injury: summary of a National Heart, Lung, and Blood Institute working group. Am J Respir Crit Care Med. 2003;167(7):1027–1035. doi: 10.1164/rccm.200208-966WS. [DOI] [PubMed] [Google Scholar]
- 5.Hudson LD, Steinberg KP. Epidemiology of acute lung injury and ARDS. Chest. 1999;116(1) Suppl:74S–82S. doi: 10.1378/chest.116.suppl_1.74s-a. [DOI] [PubMed] [Google Scholar]
- 6.Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353(16):1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 7.Fein AM, Calalang-Colucci MG. Acute lung injury and acute respiratory distress syndrome in sepsis and septic shock. Crit Care Clin. 2000;16(2):289–317. doi: 10.1016/s0749-0704(05)70111-1. [DOI] [PubMed] [Google Scholar]
- 8.Ware LB, Eisner MD, Thompson BT, Parsons PE, Matthay MA. Significance of von Willebrand factor in septic and nonseptic patients with acute lung injury. Am J Respir Crit Care Med. 2004;170(7):766–772. doi: 10.1164/rccm.200310-1434OC. [DOI] [PubMed] [Google Scholar]
- 9.Estenssoro E, Dubin A, Laffaire E, et al. Incidence, clinical course, and outcome in 217 patients with acute respiratory distress syndrome. Crit Care Med. 2002;30(11):2450–2456. doi: 10.1097/00003246-200211000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Calfee CS, Eisner MD, Ware LB, et al. Acute Respiratory Distress Syndrome Network, National Heart, Lung, and Blood Institute Trauma-associated lung injury differs clinically and biologically from acute lung injury due to other clinical disorders. Crit Care Med. 2007;35(10):2243–2250. doi: 10.1097/01.ccm.0000280434.33451.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gong MN, Thompson BT, Williams P, Pothier L, Boyce PD, Christiani DC. Clinical predictors of and mortality in acute respiratory distress syndrome: potential role of red cell transfusion. Crit Care Med. 2005;33(6):1191–1198. doi: 10.1097/01.ccm.0000165566.82925.14. [DOI] [PubMed] [Google Scholar]
- 12.Sheu CC, Zhai R, Wang Z, et al. Heme oxygenase-1 microsatellite polymorphism and haplotypes are associated with the development of acute respiratory distress syndrome. Intensive Care Med. 2009;35(8):1343–1351. doi: 10.1007/s00134-009-1504-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149(3 Pt 1):818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 14.Murray JF, Matthay MA, Luce JM, Flick MR. An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis. 1988;138(3):720–723. doi: 10.1164/ajrccm/138.3.720. [DOI] [PubMed] [Google Scholar]
- 15.Hudson LD, Milberg JA, Anardi D, Maunder RJ. Clinical risks for development of the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1995;151(2 Pt 1):293–301. doi: 10.1164/ajrccm.151.2.7842182. [DOI] [PubMed] [Google Scholar]
- 16.Eisner MD, Thompson T, Hudson LD, et al. Acute Respiratory Distress Syndrome Network Efficacy of low tidal volume vetilation in patients with different clinical risk factors for acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;164(2):231–236. doi: 10.1164/ajrccm.164.2.2011093. [DOI] [PubMed] [Google Scholar]
- 17.Phua J, Stewart TE, Ferguson ND. Acute respiratory distress syndrome 40 years later: time to revisit its definition. Crit Care Med. 2008;36(10):2912–2921. doi: 10.1097/CCM.0b013e31817d20bd. [DOI] [PubMed] [Google Scholar]
- 18.Gong MN. Genetic epidemiology of acute respiratory distress syndrome: implications for future prevention and treatment. Clin Chest Med. 2006;27(4):705–724. doi: 10.1016/j.ccm.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gattinoni L, Pelosi P, Suter PM, Pedoto A, Vercesi P, Lissoni A. Acute respiratory distress syndrome caused by pulmonary and extrapulmonary disease. Different syndromes? Am J Respir Crit Care Med. 1998;158(1):3–11. doi: 10.1164/ajrccm.158.1.9708031. [DOI] [PubMed] [Google Scholar]
- 20.Rubenfeld GD, Herridge MS. Epidemiology and outcomes of acute lung injury. Chest. 2007;131(2):554–562. doi: 10.1378/chest.06-1976. [DOI] [PubMed] [Google Scholar]
- 21.Agarwal R, Srinivas R, Nath A, Jindal SK. Is the mortality higher in the pulmonary vs the extrapulmonary ARDS? A meta analysis. Chest. 2008;133(6):1463–1473. doi: 10.1378/chest.07-2182. [DOI] [PubMed] [Google Scholar]
- 22.Agarwal R, Aggarwal AN, Gupta D, Behera D, Jindal SK. Etiology and outcomes of pulmonary and extrapulmonary acute lung injury/ARDS in a respiratory ICU in North India. Chest. 2006;130(3):724–729. doi: 10.1378/chest.130.3.724. [DOI] [PubMed] [Google Scholar]
- 23.Moss M, Gillespie MK, Ackerson L, Moore FA, Moore EE, Parsons PE. Endothelial cell activity varies in patients at risk for the adult respiratory distress syndrome. Crit Care Med. 1996;24(11):1782–1786. doi: 10.1097/00003246-199611000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Matthay MA, Ware LB. Plasma protein C levels in patients with acute lung injury: prognostic significance. Crit Care Med. 2004;32(5) Suppl:S229–S232. doi: 10.1097/01.ccm.0000126121.56990.d3. [DOI] [PubMed] [Google Scholar]
- 25.Brunkhorst FM, Eberhard OK, Brunkhorst R. Discrimination of infectious and noninfectious causes of early acute respiratory distress syndrome by procalcitonin. Crit Care Med. 1999;27(10):2172–2176. doi: 10.1097/00003246-199910000-00016. [DOI] [PubMed] [Google Scholar]
- 26.Parsons PE, Eisner MD, Thompson BT, et al. NHLBI Acute Respiratory Distress Syndrome Clinical Trials Network Lower tidal volume ventilation and plasma cytokine markers of inflammation in patients with acute lung injury. Crit Care Med. 2005;33(1):1–6. doi: 10.1097/01.ccm.0000149854.61192.dc. [DOI] [PubMed] [Google Scholar]
- 27.Moss M, Guidot DM, Steinberg KP, et al. Diabetic patients have a decreased incidence of acute respiratory distress syndrome. Crit Care Med. 2000;28(7):2187–2192. doi: 10.1097/00003246-200007000-00001. [DOI] [PubMed] [Google Scholar]
- 28.Honiden S, Gong MN. Diabetes, insulin, and development of acute lung injury. Crit Care Med. 2009;37(8):2455–2464. doi: 10.1097/CCM.0b013e3181a0fea5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alba-Loureiro TC, Munhoz CD, Martins JO, et al. Neutrophil function and metabolism in individuals with diabetes mellitus. Braz J Med Biol Res. 2007;40(8):1037–1044. doi: 10.1590/s0100-879x2006005000143. [DOI] [PubMed] [Google Scholar]
- 30.Seeley E, McAuley DF, Eisner M, Miletin M, Matthay MA, Kallet RH. Predictors of mortality in acute lung injury during the era of lung protective ventilation. Thorax. 2008;63(11):994–998. doi: 10.1136/thx.2007.093658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cooke CR, Shah CV, Gallop R, et al. National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome Network A simple clinical predictive index for objective estimates of mortality in acute lung injury. Crit Care Med. 2009;37(6):1913–1920. doi: 10.1097/CCM.0b013e3181a009b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cooke CR, Kahn JM, Caldwell E, et al. Predictors of hospital mortality in a population-based cohort of patients with acute lung injury. Crit Care Med. 2008;36(5):1412–1420. doi: 10.1097/CCM.0b013e318170a375. [DOI] [PubMed] [Google Scholar]
- 33.Zhai R, Sheu CC, Su L, et al. Serum bilirubin levels on ICU admission are associated with ARDS development and mortality in sepsis. Thorax. 2009;64(9):784–790. doi: 10.1136/thx.2009.113464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whitcomb BW, Pradhan EK, Pittas AG, Roghmann MC, Perencevich EN. Impact of admission hyperglycemia on hospital mortality in various intensive care unit populations. Crit Care Med. 2005;33(12):2772–2777. doi: 10.1097/01.ccm.0000189741.44071.25. [DOI] [PubMed] [Google Scholar]
- 35.Falciglia M, Freyberg RW, Almenoff PL, D’Alessio DA, Render ML. Hyperglycemia-related mortality in critically ill patients varies with admission diagnosis. Crit Care Med. 2009;37(12):3001–3009. doi: 10.1097/CCM.0b013e3181b083f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Freire AX, Bridges L, Umpierrez GE, Kuhl D, Kitabchi AE. Admission hyperglycemia and other risk factors as predictors of hospital mortality in a medical ICU population. Chest. 2005;128(5):3109–3116. doi: 10.1378/chest.128.5.3109. [DOI] [PubMed] [Google Scholar]
- 37.Christiansen C, Toft P, Jørgensen HS, Andersen SK, Tønnesen E. Hyperglycaemia and mortality in critically ill patients. A prospective study. Intensive Care Med. 2004;30(8):1685–1688. doi: 10.1007/s00134-004-2325-2. [DOI] [PubMed] [Google Scholar]
- 38.Bochicchio GV, Salzano L, Joshi M, Bochicchio K, Scalea TM. Admission preoperative glucose is predictive of morbidity and mortality in trauma patients who require immediate operative intervention. Am Surg. 2005;71(2):171–174. doi: 10.1177/000313480507100215. [DOI] [PubMed] [Google Scholar]
- 39.Zindrou D, Taylor KM, Bagger JP. Admission plasma glucose: an independent risk factor in nondiabetic women after coronary artery bypass grafting. Diabetes Care. 2001;24(9):1634–1639. doi: 10.2337/diacare.24.9.1634. [DOI] [PubMed] [Google Scholar]
- 40.Stranders I, Diamant M, van Gelder RE, et al. Admission blood glucose level as risk indicator of death after myocardial infarction in patients with and without diabetes mellitus. Arch Intern Med. 2004;164(9):982–988. doi: 10.1001/archinte.164.9.982. [DOI] [PubMed] [Google Scholar]
- 41.Norhammar AM, Rydén L, Malmberg K. Admission plasma glucose. Independent risk factor for long-term prognosis after myocardial infarction even in nondiabetic patients. Diabetes Care. 1999;22(11):1827–1831. doi: 10.2337/diacare.22.11.1827. [DOI] [PubMed] [Google Scholar]
- 42.Bruno A, Levine SR, Frankel MR, et al. NINDS rt-PA Stroke Study Group Admission glucose level and clinical outcomes in the NINDS rt-PA Stroke Trial. Neurology. 2002;59(5):669–674. doi: 10.1212/wnl.59.5.669. [DOI] [PubMed] [Google Scholar]
- 43.Welty-Wolf KE, Carraway MS, Ortel TL, Piantadosi CA. Coagulation and inflammation in acute lung injury. Thromb Haemost. 2002;88(1):17–25. [PubMed] [Google Scholar]
- 44.Bastarache JA, Ware LB, Bernard GR. The role of the coagulation cascade in the continuum of sepsis and acute lung injury and acute respiratory distress syndrome. Semin Respir Crit Care Med. 2006;27(4):365–376. doi: 10.1055/s-2006-948290. [DOI] [PubMed] [Google Scholar]
- 45.Ware LB, Matthay MA, Parsons PE, Thompson BT, Januzzi JL, Eisner MD. National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome Clinical Trials Network Pathogenetic and prognostic significance of altered coagulation and fibrinolysis in acute lung injury/acute respiratory distress syndrome. Crit Care Med. 2007;35(8):1821–1828. doi: 10.1097/01.CCM.0000221922.08878.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu KD, Levitt J, Zhuo H, et al. Randomized clinical trial of activated protein C for the treatment of acute lung injury. Am J Respir Crit Care Med. 2008;178(6):618–623. doi: 10.1164/rccm.200803-419OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Finfer S, Ranieri VM, Thompson BT, et al. Design, conduct, analysis and reporting of a multi-national placebo-controlled trial of activated protein C for persistent septic shock. Intensive Care Med. 2008;34(11):1935–1947. doi: 10.1007/s00134-008-1266-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kumar A, Ellis P, Arabi Y, et al. Cooperative Antimicrobial Therapy of Septic Shock Database Research Group Initiation of inappropriate antimicrobial therapy results in a fivefold reduction of survival in human septic shock. Chest. 2009;136(5):1237–1248. doi: 10.1378/chest.09-0087. [DOI] [PubMed] [Google Scholar]
- 49.Iscimen R, Cartin-Ceba R, Yilmaz M, et al. Risk factors for the development of acute lung injury in patients with septic shock: an observational cohort study. Crit Care Med. 2008;36(5):1518–1522. doi: 10.1097/CCM.0b013e31816fc2c0. [DOI] [PubMed] [Google Scholar]
- 50.Garnacho-Montero J, Garcia-Garmendia JL, Barrero-Almodovar A, Jimenez-Jimenez FJ, Perez-Paredes C, Ortiz-Leyba C. Impact of adequate empirical antibiotic therapy on the outcome of patients admitted to the intensive care unit with sepsis. Crit Care Med. 2003;31(12):2742–2751. doi: 10.1097/01.CCM.0000098031.24329.10. [DOI] [PubMed] [Google Scholar]