Abstract
Background:
Chronic pulmonary diseases (CPDs) such as asthma and COPD are associated with particularly high rates of cost-related medication nonadherence (CRN), but the degree to which inhaler costs contribute to this is not known. Here, we examine the relationship between inhaler-specific out-of-pocket costs and CRN in CPD.
Methods:
Using data obtained in 2006 in a national stratified random sample (N = 16,072) of community-dwelling Medicare beneficiaries aged ≥ 65 years, we used logistic regression to examine the relationship between inhaled medications, various types of out-of-pocket costs, and CRN in persons with CPD.
Results:
The prevalence of CRN in Medicare recipients with CPD using inhalers was 31%. In multivariate models, the odds that respondents with CPD using inhalers would report CRN was 1.43 (95% CI, 1.21-1.69) compared with respondents without CPD who were not using inhalers. Adjustment for out-of-pocket inhaler costs—but not adjustment for total medication costs or non-inhaler costs—eliminated this excess risk of CRN (OR, 0.95; 95% CI, 0.71-1.28). Patients paying > $20 per month for inhalers were at significantly higher risk for CRN compared with those who had no out-of-pocket inhaler costs.
Conclusions:
Individuals with CPD and high out-of-pocket inhaler costs are at increased risk for CRN relative to individuals on other medications. Physicians should be aware that inhalers can pose a particularly high risk of medication nonadherence for some patients.
Medication nonadherence in the United States is common, resulting in unnecessary health-care costs that have been estimated at $100 billion annually.1,2 Given the high prevalence of prescription medication use among the elderly, cost-related medication nonadherence (CRN) in this population is of particular concern and has been shown to adversely impact health outcomes.3-5 The implementation of Medicare Part D in 2006 expanded prescription drug coverage and improved access to medications; however, many seniors continue to incur high out-of-pocket costs for prescription medications.6
Although Medicare Part D has decreased the overall CRN rate,7 little is known about disease-specific nonadherence patterns after the implementation of Medicare Part D. Previous work has demonstrated that chronic pulmonary diseases (CPDs), such as asthma and COPD, are associated with relatively high rates of overall nonadherence and cost-related nonadherence,1,6,8 and a wealth of anecdotal data suggests that some patients find the out-of-pocket cost of inhaled medications to be burdensome.9,10 Inhaled medications are the cornerstone of management of asthma and COPD, and long-term adherence to inhaled medications has been shown to improve outcomes in both asthma and COPD.11-13 However, there are few data regarding the potential link between inhaler costs and medication nonadherence.
We hypothesized that high out-of-pocket costs of inhaled medications are associated with particularly high rates of CRN and that this may partially explain the high rates of CRN observed in patients with CPD. Using a nationwide probability sample of > 16,000 seniors obtained in 2006 after implementation of the Medicare Part D benefit, we tested this hypothesis by examining the impact of inhaler use and various types of out-of-pocket medication costs on CRN.
Materials and Methods
Data Source and Sample
The survey and sampling methods have been reported previously.6,14 A 1% probability sample of noninstitutionalized Medicare beneficiaries aged ≥ 65 years was provided by the Centers for Medicare and Medicaid Services for sampling in 2003. For each beneficiary, the file included a Medicaid buy-in code indicating whether the individual was receiving full or partial Medicaid coverage. As detailed elsewhere,6,15 a random starting sample (N = 36,901) was drawn across the following three strata according to the following percentages: (1) beneficiaries with full Medicaid benefits (25%), (2) beneficiaries without Medicaid living in high-poverty neighborhoods (50%), and (3) beneficiaries without Medicaid residing outside of high-poverty neighborhoods (25%).
In 2006, Centers for Medicare and Medicaid Services provided updated information for all 2003 respondents, including vital status, 2006 Medicaid buy-in codes and history, and Medicare Advantage enrollment status and dates. We approached all surviving respondents (n = 15,274), along with 15,726 others in an augmented longitudinal design. After accounting for beneficiaries excluded because of death, institutionalization, relocation, non-English/Spanish language, or severe cognitive or physical impairment, the response rate was 56%, and the resulting sample size was 16,072. Nonresponse was moderately related to sociodemographic factors (eg, race, age). Out of the sample of 16,072 respondents, we excluded respondents who did not take any prescription medications, leaving a sample of 13,891 respondents. The Tufts Medical Center Institutional Review Board approved all protocols.
Data Collection
The questionnaire was administered between October and December 2006 using a standard, five-stage, mail and telephone survey protocol.16
Variables
The questionnaire was composed of previously validated questions to collect data on sociodemographic characteristics, comorbidities, insurance status, income level, physical functioning, out-of-pocket expenditures for medications, and medication nonadherence.17,18 Comorbidity data were obtained by asking individuals if they had ever been told by a physician that they had certain common illnesses, such as high blood pressure, heart disease, diabetes, arthritis, cancer, depression, osteoporosis, asthma, or COPD. For some analyses, we used disease-specific comorbidity information, and for other analyses we combined this information into a single variable representing the total number of comorbidities (adjusting the count appropriately for conditions already included in a specific regression model). Presence or absence of prescription drug insurance coverage was represented by a binary variable. Monthly income level was represented by a seven-level categorical variable with the lowest category representing < $800 per month and the highest category representing > $2,500 per month. To quantify out-of-pocket expenditures for inhalers we asked respondents how much money they had spent on inhalers over the previous 30 days. Data on physical functioning was obtained using the physical component score (PCS6), a six-item subset of the Medical Outcomes Study Short Form 36-Item Survey. PCS6 score represents level of physical function, with lower values representing lower levels of physical functioning. The PCS6 score is normalized to general, noninstitutionalized adult US population values such that the mean score is 50 with a standard deviation of 10.19
We assessed medication nonadherence using seven previously validated survey questions based on a conceptual model of three types of medication nonadherence: CRN (three items), nonadherence due to experiences with medications such as side-effects (two items), and nonadherence because of a perceived lack of need for medication (two items).20 All analyses described in this paper relate to CRN. For the purposes of our analysis, CRN was defined as the presence of any of three behaviors: not filling a prescription because of cost, skipping doses to make a prescription last longer, or taking smaller-than-prescribed doses to make a prescription last longer. Adherence questions addressed behavior occurring over the past 12 months.
Group Definitions
Respondents were determined to have CPD if they reported a physician diagnosis of COPD or asthma. Inhaler use was determined by the question, “Do you take any prescription medications that are inhalers?” Because it is possible that patients might not have made a distinction between pulmonary and nasal inhalers, we classified individuals into four categories: those with CPD using inhalers (Inhaler+/CPD+ group), those with CPD not using inhalers (Inhaler−/CPD+), those without a diagnosis of CPD on inhalers (Inhaler+/CPD−), and those without either CPD or inhaler use (Inhaler−/CPD−). These four categories are collectively referred to as Inhaler/CPD status, and the principal contrast of interest was between the Inhaler+/CPD+ and the Inhaler−/CPD− groups.
Analyses
Baseline characteristics by Inhaler/CPD status were compared using the Rao-Scott χ2 test for categorical variables and median regression for continuous variables, and we calculated unadjusted rates of CRN by Inhaler/CPD status. In the Inhaler+/CPD+ group, we calculated the rates of CRN stratified by the monthly amount of out-of-pocket inhaler costs, and we tested for a linear trend using the Cochran-Armitage trend test. We also tested the association between the out-of-pocket costs of inhalers and CRN in this group using logistic regression.
To test our hypothesis that inhaler-specific costs are responsible for most of the CRN attributable to CPD, we examined the impact of various types of out-of-pocket costs on CRN by constructing multivariate regression models with CRN as the dependent variable, Inhaler/CPD status as the independent variable, and the following covariates: age, sex, race, income level, prescription drug insurance status, comorbidities, and level of physical functioning. Building from this base model (model 1), we generated three additional models that examined the impact of out-of-pocket costs on the association between CRN and Inhaler/CPD status by adding various types of out-of-pocket medication costs to the base model. The resulting models included the following types of out-of-pocket costs: overall medication costs (model 2), noninhaler costs (model 3), and both noninhaler costs and inhaler-specific costs (model 4).
In sensitivity analyses, we considered the possibility that the observed association between inhaler costs and CRN might be due to the fact that inhaler costs act as a surrogate for disease severity or for overall insurance generosity. To explore these scenarios, we first examined the association between inhaler cost and CRN before and after adjusting for markers of disease severity, namely PCS6 score and comorbidities. To explore the impact of insurance generosity, we used per-pill out-of-pocket costs as a measure of insurance generosity (ie, out-of-pocket pill costs/the number of pills an individual reported taking, defined to be zero when a patient reported taking no pills). Because this variable was based only on pill costs, it could be used to adjust for insurance generosity in models that included inhaler cost as a covariate. We entered this variable into the CRN models (models 1-4) described above to see if this substantially affected our results. In addition, we dichotomized this variable using the median value as a cutpoint to define “more generous” and “less generous” prescription drug plans, and we calculated estimated CRN rates adjusted for generosity of prescription drug coverage.
To place CPD in context with other common comorbidities, we examined the relationship between CRN and seven common medical conditions (CPD, hypertension, diabetes, osteoporosis, arthritis, heart disease, and depression) by constructing a logistic regression model with CRN as the dependent variable and the seven medical conditions as the independent variables. For each medical condition, the condition was coded as 1 if the patient reported taking medication for the specific condition and 0 otherwise. The β-coefficients obtained for each medical condition were compared using the Wald test. All analyses were appropriately weighted to account for the stratified sampling design.
Results
The total sample consisted of 13,891 individuals. After weighting for sampling design scheme, the median age was 75 years, 60% of the sample was women, and 86% were white. Ninety-five percent of the sample had prescription medication insurance, and the median monthly amount spent on out-of-pocket medication costs was $63. The baseline characteristics by Inhaler/CPD status are presented in Table 1. Compared with the Inhaler−/CPD− group, those in the Inhaler+/CPD+ group spent significantly more per month on prescription medications and had lower levels of physical health and higher rates of depression. The median monthly out-of-pocket expenditure for prescriptions drugs was $45 in the Inhaler−/CPD− group, $63 in the Inhaler−/CPD+ group, $73 in the Inhaler+/CPD− group, and $80 in the Inhaler+/CPD+ group.
Table 1.
—Participant Characteristics by Inhaler/Chronic Pulmonary Disease Status
| Characteristics | Inhaler-/CPD- (n = 10,275) | Inhaler-/CPD+(n = 632) | Inhaler+/CPD- (n = 811) | Inhaler+/CPD+ (n = 1,726) | P Valuea |
| Age, y, median (IQR) | 74 (69-80) | 74 (69-80) | 74 (70-80) | 74 (69-79) | NS |
| Women, % | 60 | 61 | 60 | 63 | NS |
| Race, % | |||||
| White | 86 | 88 | 83 | 88 | .04 |
| Nonwhite | 14 | 12 | 17 | 12 | |
| Chronic conditions, median (IQR)b | 2 (1-3) | 2 (2-3) | 2 (1-3) | 2 (1-3) | NS |
| Prescription drug insurance, % | 94 | 94 | 96 | 96 | < .005 |
| Monthly prescription medication cost, dollars, median (IQR) | 45 (20-98) | 63 (10-123) | 73 (35-186) | 80 (35-210) | < .001 |
| Depression, % | 16 | 29 | 24 | 25 | < .001 |
| PCS6 score, median (IQR) | 43 (32-52) | 35 (25-45) | 38 (29-47) | 32 (24-43) | < .001 |
CPD = chronic pulmonary disease; IQR = interquartile range; NS = not significant (ie,P value > 0.05); PCS6 = physical component score.
P values are obtained using quantile regression for continuous variables and the Rao Scott χ2 test for categorical variables.
The chronic condition number excludes CPD and depression.
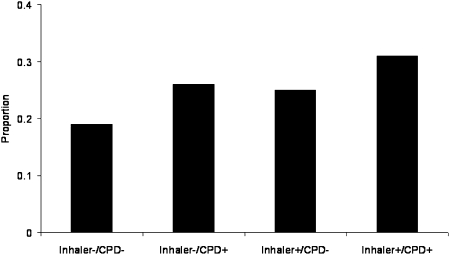
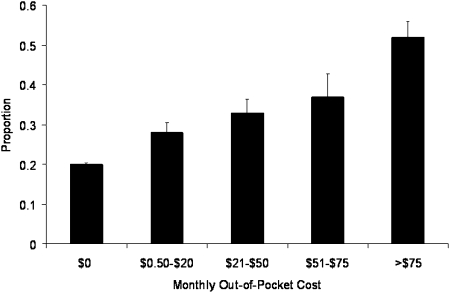
The unadjusted rates of CRN by Inhaler/CPD status are presented in Figure 1. Rates of CRN in the Inhaler−/CPD− and Inhaler+/CPD+ groups were 19% and 31%, respectively. The unadjusted relationship between inhaler costs and CRN in the Inhaler+/CPD+ group is presented in Figure 2. The risk of CRN increases linearly with the out-of-pocket cost of inhalers (P < .0001). Even at monthly out-of-pocket costs ≤ $20, this trend is evident. As shown in Table 2, there is a strong association between cost and CRN in a regression model relating CRN to out-of-pocket inhaler costs in the Inhaler+/CPD+ group. This association remains significant after adjustment for surrogate markers of disease severity.
Figure 1.
Proportion of cost-related nonadherence presented in the four study groups. Only adjustment is a sample weight correction. CPD = chronic pulmonary disease.
Figure 2.
Rates of cost-related nonadherence (black bars) by monthly out-of-pocket inhaler costs in the Inhaler+/CPD+ group. There is a roughly linear increase in cost-related nonadherence as the out-of-pocket costs of inhalers increases (test for trend, P <.0001). Error bars represent SEM. See Figure 1 for expansion of abbreviation.
Table 2.
—Association Between Inhaler Cost and Cost-Related Nonadherence in Inhaler+/CPD+ Individuals
| Variable | Unadjusted | Adjusted for Indicators of Disease Severity |
| Inhaler cost (No. of subjects) | … | … |
| $0 (431) | Reference | Reference |
| $0.50-$20 (507) | 1.31 (0.89-1.91) | 1.28 (0.87-1.87) |
| $21-$50 (305) | 1.72 (1.14-2.62) | 1.86 (1.23-2.82) |
| $51-$75 (116) | 2.14 (1.20-3.81) | 2.34 (1.29-4.23) |
| >$75 (221) | 2.98 (1.94-4.59) | 3.15 (2.02-4.90) |
| PCS6 | … | 0.97 (0.96-0.99) |
| Number of comorbidities | … | 0.99 (0.89-1.11) |
Results are OR (CI) from logistic regression models. The only covariates are those specified in the table. CRN = cost-related nonadherence; OR = odds ratio. See Table 1 for expansion of other abbreviation.
In an analysis of the entire sample, multivariable regression models relating total and inhaler-specific out-of-pocket costs to CRN are shown in Table 3. In the base model, (model 1) membership in the Inhaler+/CPD+ group was strongly associated with CRN (OR, 1.43; 95% CI, 1.21-1.69). This effect was larger than that seen for the Inhaler−/CPD+ group, suggesting that the increased risk of CRN observed in the Inhaler+/CPD+ group is at least partly due to inhaler-specific, rather than pulmonary disease-specific factors. After adjusting for total out-of-pocket costs (model 2), this association remained statistically significant (OR, 1.25; 95% CI, 1.06-1.47). Similar results were obtained after adjusting for noninhaler out-of-pocket costs (model 3). However, as is seen in model 4, adjustment for out-of-pocket inhaler costs completely attenuates the association between Inhaler+/CPD+ status and CRN (OR, 0.95; 95% CI, 0.71-1.28), suggesting that inhaler cost is an important link between CPD and risk of CRN. Out-of-pocket inhaler cost categories of $21 to $50, $51 to $75, and > $75 had ORs for CRN of 1.82 (95% CI, 1.20-2.76), 2.06 (95% CI, 1.82-2.76), and 2.33 (95% CI, 1.46-3.71), respectively, compared with the reference group of $0. Not surprisingly, noninhaler out-of-pocket costs are also significantly associated with CRN (OR, 1.29; 95% CI, 1.23-1.35).
Table 3.
—Association of Inhaler/CPD Status and Out-of-Pocket Inhaler Costs With Cost-Related Nonadherence After Multivariate Adjustment
| Variable | Base Model (Model 1) (n = 12,216) | Model With Total OOP Costs (Model 2) (n = 12,088) | Model With Noninhaler OOP Costs (Model 3) (n = 12,088) | Model With Inhaler and Noninhaler OOP Costs (Model 4) (n = 12,088) |
| Inhaler/CPD status | ||||
| Inhaler-/CPD- | Reference | Reference | Reference | Reference |
| Inhaler-/CPD+ | 1.22 (0.92-1.61) | 1.19 (0.90-1.57) | 1.22 (0.92-1.61) | 1.14 (0.86-1.52) |
| Inhaler+/CPD- | 1.21 (0.96-1.52) | 1.12 (0.89-1.34) | 1.12 (0.89-1.40) | 1.12 (0.89-1.40) |
| Inhaler+/CPD+ | 1.43 (1.21-1.69) | 1.25 (1.06-1.47) | 1.40 (1.19-1.65) | 0.95 (0.71-1.28) |
| Total OOP medication costsa | … | 1.29 (1.23-1.34) | … | … |
| All noninhaler OOP costsa | … | … | 1.29 (1.23-1.35) | 1.27 (1.21-1.33) |
| Inhaler cost | … | … | … | |
| $0 | Reference | |||
| $0.50-$20 | … | … | 1.16 (0.79-1.70) | |
| $21-$50 | … | … | … | 1.82 (1.20-2.76) |
| $51-$75 | … | … | … | 2.06 (1.82-2.76) |
| > $75 | … | … | … | 2.33 (1.46-3.71) |
All models adjusted for age, sex, race, income level, depression, other comorbidities, prescription drug coverage status, and PCS score. Results are given as OR (CI). OOP = out-of-pocket. See Tables 1 and 2 for expansion of other abbreviations.
Units for total OOP medication cost and noninhaler OOP cost, 1 unit = $100/mo.
We considered the possibility that the association of inhaler costs with CRN might be explained by an underlying association between inhaler costs and overall insurance generosity. In order to examine this further, we created an insurance generosity variable that represented the out-of-pocket amount spent per prescription pill. We entered this variable into the analysis above, and, although this variable was significantly associated with CRN, the association between inhaler costs and CRN remained significant. We estimated the impact of insurance generosity on CRN rates by using a logistic regression model to relate CRN to Inhaler/CPD status and dichotomized insurance generosity (more generous/less generous). If insurance was more generous in covering medication copayments, predicted CRN rates were 21% for the Inhaler−/CPD− group and 26% for the Inhaler+/CPD+ group. If insurance was less generous, predicted CRN rates increased to 30% and 36%, respectively.
The multivariable relationship between selected chronic conditions and CRN is shown in Table 4. The odds of CRN conferred by CPD are significantly higher than those conferred by hypertension (P < .001) and diabetes (P < .01). Although the point estimate for association between depression and CRN is higher than that of CPD, this difference is not statistically significant (P = .44).
Table 4.
—Comparison of Strength of Association With Cost-Related Nonadherence for Selected Diseases
| Diseasea | CRN, OR (CI) | Comparison With CPD OR,b P Value |
| CPD | 1.37 (1.15-1.63) | … |
| Hypertension | 0.85 (0.74-0.98) | < .0001 |
| Heart diseasec | 1.19 (1.04-1.37) | .22 |
| Osteoporosis | 1.22 (1.03-1.44) | .34 |
| Depression | 1.51 (1.28-1.78) | .44 |
| Arthritis | 1.19 (1.03-1.38) | .23 |
| Diabetes | 1.00 (0.86-1.15) | .005 |
Logistic regression model adjusted for age, sex, race, income level, other comorbidities, prescription drug insurance status, and PCS6 score. See Tables 1 and 2 for expansion of abbreviations.
Disease groups defined by diagnosis plus disease-specific medication use.
Wald test of coefficients for each medical condition in comparison with CPD.
Myocardial infarction or heart failure.
Discussion
Our study demonstrates that Medicare beneficiaries with CPD who use inhalers are at increased risk of CRN. This increased risk is apparent even at relatively modest levels of out-of-pocket cost. In regression models, the strong association between CRN and inhaler use in the setting of CPD persists despite adjustment for factors such as income level, presence of prescription drug coverage, and level of physical function. However, the inclusion of out-of-pocket inhaler costs attenuates this association, suggesting that inhaler cost may be a key link between CPD and CRN.
It is clear from previous research that out-of-pocket costs are a major factor in medication nonadherence,3,4,21,22 and there are similarly strong data demonstrating that nonadherence rates vary by disease condition.1,8,22 In the 2004 study of condition-specific rates of CRN by Piette et al,8 asthma and COPD were among the conditions associated with the highest rates of condition-specific and general CRN. Our work extends these findings by demonstrating a strong association between the out-of-pocket costs of inhalers and CRN in patients with CPD. These findings are clinically important since inhaled medications (bronchodilators and corticosteroids) are a mainstay of outpatient management for asthma and COPD. Bronchodilators provide immediate symptomatic relief, and inhaled corticosteroids contribute to better long-term outcomes in asthma (FEV1 level, steroid dependence)11,12 and COPD (exacerbations, mortality).13
From a policy perspective, the data from this study are of particular interest because they reflect patient experiences in 2006 after the implementation of Medicare Part D. Overall, Part D has been successful in reducing CRN rates.7 However, although Part D has extended prescription drug coverage to a number of previously uninsured seniors, it has been less beneficial in terms of reducing out-of-pocket costs, and it is not clear that the reduction in CRN extends to the sickest and most vulnerable segments of the Medicare population.6,7 As a consequence, CRN remains a significant problem among Medicare seniors, and our study demonstrates that for inhaled medications, which are of clear benefit, out-of-pocket costs remain an important cause of medication nonadherence after the implementation of Part D.
Many health-care providers and patients are well aware that inhalers are more expensive than many other commonly prescribed medications. This is due, in part, to unique patent and approval considerations arising from the method of delivery of inhaled medications. The federally mandated switch from chlorofluorocarbon inhalers to hydrofluoroalkane inhalers in 2008 served as an opportunity for pharmaceutical companies to reapply for patent protections for inhaled medications based on use of a new delivery system. Although a single albuterol inhaler typically costs approximately $40, long-acting β-agonists and steroid inhalers, which are crucial for long-term symptom control, typically cost between $100 and $200 per inhaler.23 In the tiered copayment systems currently used by most major insurers, long-acting β-agonists and inhaled steroids typically incur large copayments. In our sample, patients with CPD using inhalers spent on average $35 more in out-of-pocket monthly medication expenditures than their counterparts without CPD who did not use inhalers. Our study, in line with previous work in diabetes, demonstrates that out-of-pocket expenditures of $20 per month or more significantly increase the risk of CRN.24 Point estimates suggest that this risk may be present at levels < $20.
An important strength of our study is that our survey was specifically designed to evaluate the impact of Medicare Part D on medication nonadherence and, as a result, we had access to information regarding different types of medication nonadherence, insurance status, income level, comorbidities, level of physical functioning, and inhaler-specific out-of pocket costs. In addition, our data sample is large and reasonably representative of the population of home-dwelling senior citizens on Medicare. Although the survey response rate was 56%, nonresponse was moderately related to sociodemographic factors (eg, race, age) but unrelated to substantive variables of interest (eg, prescription coverage, use, spending). Nonresponse effects were thus controlled in multivariate analyses by inclusion of the relevant sociodemographic variables.
Our study has several limitations. First, there were a number of individuals who reported inhaler use without reporting a diagnosis of CPD, raising questions of why these individuals were using inhalers. There are a number of possible explanations for membership in this group, including a failure to distinguish between inhalers and nasal sprays. We addressed this potential misclassification by analyzing individuals by Inhaler/CPD status so as to enable the principal focus of our analyses to be the subset of individuals reporting both inhaler use and CPD. Second, although we had detailed data on medication nonadherence, we did not have data on which specific medications were being skipped. Since adjustment for inhaler-specific costs eliminated the increased skipping risk conferred by Inhaler+/CPD+ status, it is likely that inhalers are being skipped. However, it is also possible that high inhaler costs may cause individuals to skip other medications. Third, our assessment of CRN was based on self-report with a 1-year recall period; thus our data are susceptible to recall bias and social desirability bias. In addition, the use of a 30-day period as the recall window for out-of-pocket costs may result in misclassification of costs for individuals who fill prescriptions at 90-day intervals. However, given the strength of our findings we feel that it is unlikely that these biases would be large enough to invalidate the associations present in our sample. Finally, we cannot know from our data what the consequences of this skipping were, such as whether these patients had more hospitalizations or worse health status.
In conclusion, our study provides evidence that out-of-pocket inhaler costs remain a significant barrier to compliance with inhaled medications in Medicare seniors, even after the implementation of Medicare Part D. Policy makers should be aware that high inhaler co-pays for persons with asthma and COPD may be a strong driver of medication nonadherence, and they may want to consider reducing co-pays, particularly given strong evidence of adverse and costly outcomes of nonadherence, such as frequent hospitalization for respiratory reasons. Clinicians may want to question their patients who use inhalers about trouble paying for them, particularly when there is evidence of possible nonadherence, such as poor disease control.
Acknowledgments
Author contributions: Dr Castaldi: contributed to the design of this project and took the lead in data analysis and manuscript preparation.
Dr Rogers: contributed to initial survey development and overall survey design as well as manuscript preparation.
Dr Safran: contributed to initial survey development and overall survey design as well as manuscript preparation.
Dr Wilson: contributed to the design of this study, initial survey development, and review of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Safran is employed by Blue Cross Blue Shield of Massachusetts (BCBSMA). BCBSMA is a not-for-profit health-care company whose products include a variety of plans available to Medicare beneficiaries. Drs Castaldi, Rogers, and Wilson have reported that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Other contributions: We thank the National Institute of Aging for their participation in the funding of the earlier phase of this work (1998-2002); the Robert Wood Johnson Foundation (grant 047164) for funding the 2004 survey; and Hong Chang for his analytic support.
Abbreviations
- CPD
chronic pulmonary disease
- CRN
cost-related nonadherence
- OR
odds ratio
- PCS
physical component score
Funding/Support: This work was funded by a grant from the Agency for Healthcare Research and Quality [Grant 5R01HS009622], the National Institutes of Health [Grants T32HS00060, F32 HL094035], and the National Center for Research Resources [Tufts CTSA, Grant 5UL1RR025752].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (http://www.chestpubs.org/site/misc/reprints.xhtml).
References
- 1.DiMatteo MR. Variations in patients’ adherence to medical recommendations: a quantitative review of 50 years of research. Med Care. 2004;42(3):200–209. doi: 10.1097/01.mlr.0000114908.90348.f9. [DOI] [PubMed] [Google Scholar]
- 2.Elliott RA, Ross-Degnan D, Adams AS, Safran DG, Soumerai SB. Strategies for coping in a complex world: adherence behavior among older adults with chronic illness. J Gen Intern Med. 2007;22(6):805–810. doi: 10.1007/s11606-007-0193-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soumerai SB, Pierre-Jacques M, Zhang F, et al. Cost-related medication nonadherence among elderly and disabled medicare beneficiaries: a national survey 1 year before the medicare drug benefit. Arch Intern Med. 2006;166(17):1829–1835. doi: 10.1001/archinte.166.17.1829. [DOI] [PubMed] [Google Scholar]
- 4.Wilson IB, Rogers WH, Chang H, Safran DG. Cost-related skipping of medications and other treatments among Medicare beneficiaries between 1998 and 2000. Results of a national study. J Gen Intern Med. 2005;20(8):715–720. doi: 10.1111/j.1525-1497.2005.0128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heisler M, Langa KM, Eby EL, Fendrick AM, Kabeto MU, Piette JD. The health effects of restricting prescription medication use because of cost. Med Care. 2004;42(7):626–634. doi: 10.1097/01.mlr.0000129352.36733.cc. [DOI] [PubMed] [Google Scholar]
- 6.Neuman P, Strollo MK, Guterman S, et al. Medicare prescription drug benefit progress report: findings from a 2006 national survey of seniors. Health Aff. 2007;26(5):w630–w643. doi: 10.1377/hlthaff.26.5.w630. [DOI] [PubMed] [Google Scholar]
- 7.Madden JM, Graves AJ, Zhang F, et al. Cost-related medication nonadherence and spending on basic needs following implementation of Medicare Part D. JAMA. 2008;299(16):1922–1928. doi: 10.1001/jama.299.16.1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piette JD, Heisler M, Wagner TH. Cost-related medication underuse among chronically ill adults: the treatments people forgo, how often, and who is at risk. Am J Public Health. 2004;94(10):1782–1787. doi: 10.2105/ajph.94.10.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tarkan L. Rough transition to a new asthma inhaler New York Times Online. http://www.nytimes.com/2008/05/13/health/13asth.html. Posted May 13, 2008. Accessed December 18, 2009.
- 10.The national campaign to save CFC inhalers http://www.savecfcinhalers.org. Accessed September 4, 2009.
- 11.Adams NP, Bestall JB, Malouf R, Lasserson TJ, Jones PW. Inhaled beclomethasone versus placebo for chronic asthma. Cochrane Database Syst Rev. 2005;(1):CD002738. doi: 10.1002/14651858.CD002738.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adams NP, Bestall JC, Lasserson TJ, Jones P, Cates CJ. Fluticasone versus placebo for chronic asthma in adults and children. Cochrane Database Syst Rev. 2008;(4):CD003135. doi: 10.1002/14651858.CD003135.pub4. [DOI] [PubMed] [Google Scholar]
- 13.Nannini L, Cates CJ, Lasserson TJ, Poole P. Combined corticosteroid and long-acting beta-agonist in one inhaler versus placebo for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2007;(4):CD003794. doi: 10.1002/14651858.CD003794.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Safran DG, Strollo M, Guterman S, Li A, Rogers WH, Neuman P. Prescription coverage, use and spending before and after Part D implementation: a national longitudinal panel study. J Gen Intern Med. 2010;25(1):10–17. doi: 10.1007/s11606-009-1134-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Safran DG, Neuman P, Schoen C, et al. Prescription drug coverage and seniors: findings from a 2003 national survey. Health Aff. 2005;(suppl W5):152–166. doi: 10.1377/hlthaff.w5.152. [DOI] [PubMed] [Google Scholar]
- 16.Dillman DA. Mail and Telephone Surveys: The Total Design Method. New York, NY: John Wiley and Sons; 1978. [Google Scholar]
- 17.Fowles JB, Fowler EJ, Craft C. Validation of claims diagnoses and self-reported conditions compared with medical records for selected chronic diseases. J Ambul Care Manage. 1998;21(1):24–34. doi: 10.1097/00004479-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Tisnado DM, Adams JL, Liu H, et al. What is the concordance between the medical record and patient self-report as data sources for ambulatory care? Med Care. 2006;44(2):132–140. doi: 10.1097/01.mlr.0000196952.15921.bf. [DOI] [PubMed] [Google Scholar]
- 19.Ware JE, Kosinski M, Bayliss MS, McHorney CA, Rogers WH, Raczek A. Comparison of methods for scoring and statistical analysis of SF-36 health profiles and summary measures: summary of results from the Medical Outcomes Study. Med Care. 1995;33(suppl 4):AS264–AS279. [PubMed] [Google Scholar]
- 20.Wilson IB, Schoen C, Neuman P, et al. Physician-patient communication about prescription medication nonadherence: a 50-state study of America’s seniors. J Gen Intern Med. 2007;22(1):6–12. doi: 10.1007/s11606-006-0093-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soumerai SB, Avorn J, Ross-Degnan D, Gortmaker S. Payment restrictions for prescription drugs under Medicaid. Effects on therapy, cost, and equity. N Engl J Med. 1987;317(9):550–556. doi: 10.1056/NEJM198708273170906. [DOI] [PubMed] [Google Scholar]
- 22.Mojtabai R, Olfson M. Medication costs, adherence, and health outcomes among Medicare beneficiaries. Health Aff. 2003;22(4):220–229. doi: 10.1377/hlthaff.22.4.220. [DOI] [PubMed] [Google Scholar]
- 23. http://www.drugstore.com/. Accessed August 15, 2008.
- 24.Roblin DW, Platt R, Goodman MJ, et al. Effect of increased cost-sharing on oral hypoglycemic use in five managed care organizations: how much is too much? Med Care. 2005;43(10):951–959. doi: 10.1097/01.mlr.0000178216.23514.b7. [DOI] [PubMed] [Google Scholar]