Abstract
Background:
Unrecognized obstructive sleep apnea (OSA) may lead to poor asthma control despite optimal therapy. Our objective was to evaluate the relationship between OSA risk and asthma control in adults.
Methods:
Patients with asthma seen routinely at tertiary-care clinic visits completed the validated Sleep Apnea Scale of the Sleep Disorders Questionnaire (SA-SDQ) and Asthma Control Questionnaire (ACQ). An ACQ score of ≥ 1.5 defined not-well-controlled asthma, and an SA-SDQ score of ≥ 36 for men and ≥ 32 for women defined high OSA risk. Logistic regression was used to model associations of high OSA risk with not-well-controlled asthma (ACQ full version and short versions).
Results:
Among 472 subjects with asthma, the mean ± SD ACQ (full version) score was 0.87 ± 0.90, and 80 (17%) subjects were not well controlled. Mean SA-SDQ score was 27 ± 7, and 109 (23%) subjects met the definition of high OSA risk. High OSA risk was associated, on average, with 2.87-times higher odds for not-well-controlled asthma (ACQ full version) (95% CI, 1.54-5.32; P = .0009) after adjusting for obesity and other factors known to worsen asthma control. Similar independent associations were seen when using the short ACQ versions.
Conclusions:
High OSA risk is significantly associated with not-well-controlled asthma independent of known asthma aggravators and regardless of the ACQ version used. Patients who have difficulty achieving adequate asthma control should be screened for OSA.
Current guidelines for the management of asthma recommend that therapy be targeted to achieve asthma control,1‐3 which has been shown to improve health-related quality of life.4 However, large community-based surveys show that this goal is rarely achieved.5,6
Data suggest that obstructive sleep apnea (OSA) is an important contributor to asthma control. Continuous positive airway pressure (CPAP) treatment of OSA in patients with asthma improves outcomes, including asthma symptoms,7‐9 rescue bronchodilator use,7 peak expiratory flow rates (PEFRs),7 and asthma-specific quality of life assessed with validated instruments.10,11 Although important, these studies included small numbers of participants selected primarily for nocturnal symptoms and used nonrandomized designs. Additionally, OSA was recently identified as an important risk factor for frequent exacerbations in patients with difficult-to-treat asthma.12 The National Asthma Education and Prevention Program guidelines recommend evaluation for OSA in patients with asthma with suboptimal control.1 However, evidence contributed only to a grade D (expert panel) recommendation, highlighting the need for more research.
Asthma control involves multiple components of subjective and objective measures.1,2,13‐15 The Asthma Control Questionnaire (ACQ) is a composite instrument that demonstrates strong discriminating ability between well-controlled and not-well-controlled asthma.16 Current guidelines recommend using the ACQ in routine clinical practice.1,3 A full version and three shortened versions of the ACQ (designed with exclusion of spirometry, β2-agonist data, and both) were recently validated.17,18 The Sleep Apnea Scale of the Sleep Disorders Questionnaire (SA-SDQ) is a validated screening tool to identify patients at high risk for OSA.19
Using these questionnaires, we sought to determine whether a high OSA risk is associated with not-well-controlled asthma apart from other characteristics known to be associated with poor asthma outcomes.1,12 Further, because information on spirometry and β2-agonist use may not always be available, we wished to determine whether similar relationships exist between OSA risk and the three ACQ short versions as for the ACQ full version. We hypothesized that high OSA risk will independently predict a higher likelihood for not-well-controlled asthma and that comparable associations will be observed with the ACQ short versions. Preliminary data from this study were presented in abstract form.20,21
Materials and Methods
Study Population
The population consisted of patients with asthma aged 18 to 75 years managed at routine follow-up visits at the allergy and pulmonary subspecialty clinics of the University of Wisconsin–Madison between July 2007 and January 2009. Subjects were enrolled as part of an ongoing study on the relationship between OSA and asthma, which received University of Wisconsin Health Sciences Institutional Review Board approval. Written informed consent was obtained from each subject. The diagnosis of asthma was made by allergy and pulmonary physicians staffing these tertiary-care clinics and was based on established criteria.22 Standard of care at follow-up visits includes history, physical examination, asthma control assessment, and spirometry. Patients having urgent asthma visits and pregnant women were excluded.
Survey Instruments and Medical Records Review
The full version of ACQ contains items on symptoms (including activities) and rescue β2-agonist use in the prior week, and percent-predicted FEV1 (recorded from prebronchodilator spirometry performed at clinic visit), all rated on a 7-point (0-6) Likert scale.17 Scores are obtained by averaging the responses from each question, with higher scores indicating worse asthma control.17 The three shortened versions of the ACQ are: (1) symptoms and percent-predicted FEV1, (2) symptoms and short-acting β2-agonist use, and (3) symptoms alone. The measurement properties of all four versions of the ACQ are very similar.18 The validated cut-point of ≥ 1.5 differentiates between well-controlled and not-well-controlled asthma.16 The minimal clinically important difference for all ACQ versions has been established at 0.5.18
The SA-SDQ contains eight OSA symptom items rated on a 5-point Likert scale, including loud snoring disruptive to the bed partner, breathing pauses during sleep, sudden gasping arousals, worsening of snoring while supine or after alcohol consumption, nocturnal nasal congestion and sweating, and history of hypertension.19 Four anthropometric variables (age, smoking, weight, and BMI) are rated on a scale of 1 to 5. To define high OSA risk, we used the SA-SDQ cutoff scores of ≥ 36 for men and ≥ 32 for women, which have been validated with polysomnography (PSG) in a large sample of sleep patients.19
Medical records were reviewed for established diagnoses of comorbid lung diseases (eg, allergic bronchopulmonary aspergillosis, COPD, interstitial lung diseases); sleep-disordered breathing, including treatment; and comorbid illnesses known to worsen asthma control (gastroesophageal reflux disease [GERD]; rhinitis; chronic sinusitis; nasal polyposis; and psychiatric disease, such as depression, anxiety, and panic or bipolar disorders),1,12 spirometry data from clinic visits, and current asthma and allergy medications.
Data Analysis
Obesity was defined as a BMI ≥ 30 kg/m2 (Centers for Disease Control and Prevention criteria). Baseline variables were summarized as mean ± SD for continuous variables and percentages for categorical variables. Two-sample t tests were used to analyze differences in ACQ scores between subjects with and without high OSA risk. Logistic regression was used to test for univariate relationships of not-well-controlled asthma with high OSA risk and other contributors to asthma control (demographics [age, sex, and black race], obesity, nasal diseases [rhinitis, chronic sinusitis, and polyps], GERD, and psychiatric disease).1,12 Multivariate logistic regression models were then fitted with not-well-controlled asthma as the outcome and high OSA risk as the predictor, with stepwise adjustment for the aforementioned covariates regardless of their univariate associations with not-well-controlled asthma. Two-sided P values < .05 indicated statistical significance. Analyses were performed using SAS statistical software, version 9.1 (SAS Institute; Cary, NC).
Results
Subject Characteristics
Of the 567 subjects recruited to participate, 539 (95%) completed questionnaires, and 28 were excluded because of comorbid lung disease. Of the remaining 511, 63 had previously diagnosed OSA, and of those, 39 being treated at the time of the survey were excluded from further analyses.
Demographic, physiologic, and clinical characteristics of the remaining 472 subjects are shown in Table 1. The majority of our subjects were white. In subsequent analyses, black subjects were compared with white and subjects and those from all other races combined. Among the 450 subjects with rhinitis, 360 (80%) were using a nasal corticosteroid spray, 280 (62%) an oral antihistamine, 14 (3%) nasal ipratropium, 12 (3%) a topical antihistamine, and 3 (1%) topical cromolyns; 409 (91%) were taking either a nasal corticosteroid or an antihistamine (oral or topical), with 243 (54%) taking both. Overall, 411 (91%) subjects with rhinitis were using at least one of the types of medications enumerated above.
Table 1.
—Demographic, Physiologic, and Clinical Characteristics Patients With Asthma (N = 472)
| Characteristic | Value |
| Age, y | 47 ± 14 |
| Female sex | 300 (64) |
| BMI, kg/m2 (range) | 28.4 ± 6.4 (16.7-60.5) |
| Obese (BMI ≥ 30 kg/m2) | 149 (32) |
| Race | |
| Black | 15 (3.2) |
| White | 446 (94.5) |
| Othera | 11 (2.3) |
| Current-smoker | 20 (4) |
| FEV1, % predicted | 93.9 ± 18.5 |
| FVC, % predicted | 91.1 ± 16.5 |
| FEV1/FVC | 73.5 ± 8.5 |
| FEF25-75%, % predicted | 69.2 ± 30.6 |
| History | |
| GERD | 201 (43) |
| Rhinitis | 450 (95) |
| Chronic sinusitis | 173 (37) |
| Nasal polyps | 77 (16) |
| Psychiatric disease | 107 (23) |
| Medications | |
| Inhaled corticosteroid | 366 (78) |
| Inhaled long-acting bronchodilator | 275 (58) |
| Antileukotriene agents | 88 (19) |
| Theophylline | 8 (2) |
| Oral corticosteroid | 31 (7) |
Data are presented as mean ± SD or No. (%). FEF25-75% = forced expiratory flow, midexpiratory rate; GERD = gastroesophageal reflux disease.
Includes Asians, Hawaiian/Pacific Islanders, American Indians, and Alaskan Natives.
The ACQ scores and frequency of patients meeting criteria for not-well-controlled asthma are shown in Table 2. The mean SA-SDQ score was 27 ± 7, with 109 (23%) achieving scores that placed them at high risk for OSA.
Table 2.
—Asthma Control Questionnaire Scores and Frequency of Not-Well-Controlled Asthma for Each Asthma Control Questionnaire Version
| ACQ Version | ACQ Scores | Not-Well-Controlled Asthma (ACQ ≥ 1.5) |
| ACQ full | 0.87 ± 0.90 | 80 (17) |
| ACQ symptoms and FEV1 % predicted | 0.92 ± 0.94 | 100 (21) |
| ACQ symptoms and β2-agonist use | 0.80 ± 0.94 | 88 (19) |
| ACQ symptoms alone | 0.86 ± 1.00 | 91 (19) |
Data are presented as mean ± SD or No. (%). ACQ = Asthma Control Questionnaire.
Associations of High OSA Risk With Not-Well-Controlled Asthma (From ACQ Full Version)
In univariate analyses, a high OSA risk was associated with a 3.60 times higher odds for having not-well-controlled asthma (95% C, 2.16-5.98; P < .0001) (Table 3). Obesity, black race, nasal polyps, GERD, and psychopathology also were associated with higher odds for not-well-controlled asthma, whereas rhinitis was associated with lower odds. Progressive adjustment for these characteristics (as single variables or as a group for nasal diseases) only slightly altered the relationship of not-well-controlled asthma with high OSA risk (Table 4). Independent of all these characteristics, a high OSA risk was associated with 2.87 times higher odds for not-well-controlled asthma (95% CI, 1.54-5.32; P = .0009). In all models, rhinitis, a frequently diagnosed condition in our population, was inversely related to asthma control.
Table 3.
—Univariate Associations of Not-Well-Controlled Asthma (on ACQ Full Version) With High Obstructive Sleep Apnea Risk and Other Characteristics Known To Worsen Asthma
| Characteristic | OR (95% CI) | P Value |
| High OSA risk | 3.60 (2.16-5.98) | < .0001 |
| Age | 1.01 (0.99-1.03) | .14 |
| Sex, female vs male | 0.69 (0.42-1.13) | .14 |
| Obesity (BMI ≥ 30 kg/m2) | 2.46 (1.50-4.01) | .0003 |
| Black (vs all other) | 3.45 (1.19-9.99) | .02 |
| Nasal condition | ||
| Rhinitis | 0.27 (0.11-0.66) | .004 |
| Chronic sinusitis | 1.11 (0.68-1.83) | .67 |
| Polyps | 1.95 (1.10-3.48) | .02 |
| GERD | 3.03 (1.83-5.01) | < .0001 |
| Psychiatric disease | 1.99 (1.18-3.36) | .01 |
Not-well-controlled asthma is defined by an ACQ score of ≥ 1.5. High OSA risk is defined as a Sleep Apnea Scale of the Sleep Disorders Questionnaire (SA-SDQ) score of ≥ 36 for men and ≥ 32 for women. OR = odds ratio; OSA = obstructive sleep apnea. See Table 1 and 2 legends for expansion of other abbreviations.
Table 4.
—Multivariate Logistic Regression Models of Not-Well-Controlled Asthma (Defined Based on ACQ Full Version) on High OSA Risk, With Adjustment for Factors Known To Worsen Asthma Control
| Characteristic | Adjusted for Demographicsa | Adjusted for Demographics and Obesity | Adjusted for Demographics, Obesity, and GERD | Adjusted for Demographics, Obesity, GERD, and Nasal Diseases | Adjusted for Demographics, Obesity, GERD, Nasal Diseases, and Psychiatric Disease | |||||||||
| OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | |||||
| High OSA risk | 3.92 (2.27-6.76) | < .0001 | 3.29 (1.82-5.95) | < .0001 | 3.11 (1.71-5.68) | .0002 | 3.01 (1.62-5.60) | .0005 | 2.87 (1.54-5.32) | .0009 | ||||
| Obesity | … | … | 1.52 (0.87-2.65) | .15 | 1.38 (0.78-2.43) | .27 | 1.38 (0.77-2.46) | .27 | 1.43 (0.80-2.54) | .23 | ||||
| GERD | … | … | … | … | 2.87 (1.67-4.94) | .0001 | 3.20 (1.82-5.65) | < .0001 | 3.00 (1.70-5.31) | .0002 | ||||
| Nasal diseases | ||||||||||||||
| Rhinitis | … | … | … | … | … | … | 0.33 (0.12-0.86) | .02 | 0.38 (0.14-1.02) | .05 | ||||
| Sinusitis | … | … | … | … | … | … | 0.56 (0.29-1.07) | .08 | 0.55 (0.29-1.06) | .08 | ||||
| Nasal polyps | … | … | … | … | … | … | 2.28 (1.04-5.01) | .04 | 2.37 (1.08-5.24) | .03 | ||||
| Psychiatric disease | … | … | … | … | … | … | … | … | 1.79 (0.97-3.27) | .06 | ||||
Associations of High OSA Risk With Short ACQ Versions
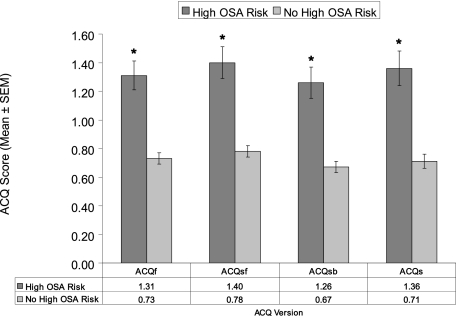
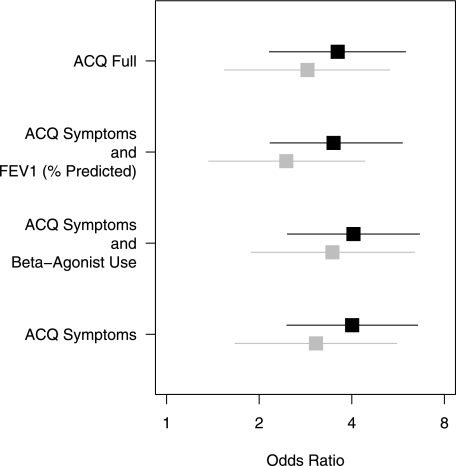
Subjects with high OSA risk compared with those without high OSA risk had higher scores on all ACQ versions, with differences uniformly greater than the validated minimal clinically important difference of 0.5 (P < .0001 in all cases) (Fig 1). We found analogous associations of high OSA risk with not-well-controlled asthma on the ACQ short versions as for the full version (Fig 2) (each P < .0001). Reiteration of the final multivariate regression model (from Table 4) using not-well-controlled asthma defined on the basis of each ACQ short version showed similar independent associations with high OSA risk as the ACQ full version (Table 5).
Figure 1.
Scores on each version of the ACQ in subjects with and without high obstructive sleep apnea risk, which is defined by Sleep Apnea Scale of the Sleep Disorders Questionnaire (SA-SDQ) scores ≥ 36 for men and ≥ 32 for women. * P < .0001. ACQ = Asthma Control Questionnaire; ACQf = ACQ full version; ACQs = ACQ symptoms alone; ACQsb = ACQ symptoms and short-acting β2-agonist use; ACQsf = ACQ symptoms and percent predicted FEV1; OSA = obstructive sleep apnea.
Figure 2.
Unadjusted (black) and adjusted* (gray) odds ratios (95% CIs) for not-well-controlled asthma defined on the basis of each ACQ version, with high OSA risk (SA-SDQ scores ≥ 36 for men and ≥ 32 for women) as the predictor. * Adjustment performed for the variables included in the final model shown in Table 4. See Figure 1 legend for expansion of abbreviation.
Table 5.
—Multivariate Logistic Regression Models of Not-Well-Controlled Asthma (Defined Based on Each ACQ Version) on High OSA Risk, Controlling for Other Factors Known To Worsen Asthma Control
| Characteristic | ACQ Full | ACQ Symptoms and FEV1% | ACQ Symptoms and β2-Agonist Use | ACQ Symptoms | |||||||
| OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | ||||
| High OSA risk | 2.87 (1.54-5.32) | .0009 | 2.45 (1.37-4.41) | .003 | 3.46 (1.88-6.39) | < .0001 | 3.06 (1.67-5.60) | .0003 | |||
| Obesity | 1.43 (0.80-2.54) | .23 | 1.80 (1.06-3.08) | .03 | 1.17 (0.66-2.07) | .60 | 1.43 (0.81-2.51) | .21 | |||
| GERD | 3.00 (1.70-5.31) | .0002 | 2.84 (1.68-4.82) | .0001 | 2.68 (1.55-4.64) | .0004 | 3.10 (1.79-5.37) | < .0001 | |||
| Nasal diseases | |||||||||||
| Rhinitis | 0.38 (0.14-1.02) | .055 | 0.26 (0.10-0.70) | .008 | 0.20 (0.07-0.54) | .001 | 0.21 (0.08-0.57) | .002 | |||
| Sinusitis | 0.55 (0.29-1.06) | .08 | 0.74 (0.41-1.34) | .32 | 0.91 (0.50-1.67) | .76 | 0.97 (0.53-1.76) | .92 | |||
| Nasal polyps | 2.37 (1.08-5.24) | .03 | 1.78 (0.84-3.76) | .13 | 1.41 (0.65-3.05) | .38 | 1.35 (0.62-2.93) | .45 | |||
| Psychiatric disease | 1.79 (0.97-3.27) | .06 | 2.12 (1.23-3.77) | .007 | 2.06 (1.16-3.67) | .01 | 2.06 (1.16-3.65) | .01 | |||
Discussion
In this large, well-characterized, specialty clinic-based asthma population, an association of high OSA risk with not-well-controlled asthma emerged. This finding was independent of obesity and other factors known to worsen asthma control, such as GERD, nasal diseases, and psychopathology, with similar associations observed for the ACQ short versions.
Our asthma population is likely representative of patients who have unidentified or untreated OSA managed in tertiary-care clinic settings. There was a high rate (95%) of participation in the survey. The characteristics of these patients are comparable to previous studies,23,24 except for a lower rate of obesity and black race. Additionally, the high prevalence (95%) of patients with rhinitis reflects that this sample was primarily accrued from allergy clinics. Rhinitis decreased the odds ratios for not-well-controlled asthma likely because of its rigorous management by expert providers, counteracting its detrimental influence on asthma control.
Our results add to the evidence of OSA as a potential contributor to overall asthma control but on a much larger scale and independent of other known contributors to asthma control. Studies of CPAP treatment of PSG-diagnosed OSA in patients with asthma reported improved asthma outcomes, but in small numbers of participants selected primarily for nocturnal asthma symptoms. Chan et al7 reported reduced asthma symptoms and bronchodilator use, and improved PEFR after 2 weeks of CPAP in nine patients. Cessation of CPAP returned PEFR to baseline levels. In a study by Guilleminault et al8 of 10 subjects with OSA, 6 months of CPAP reduced the overall number of asthma attacks and eliminated nocturnal asthma. In 16 patients with nocturnal asthma, Ciftci et al9 found that 2 months of CPAP for OSA resulted in a significant reduction in asthma symptoms. Lafond et al10 reported improved asthma-specific quality of life after 6 weeks of CPAP in 20 patients with asthma and OSA. Several other factors (demographics, comorbidities such as obesity, nasal diseases, GERD, and psychopathology) can influence asthma control.1 The potential contribution of OSA has been concomitantly assessed in only one study of patients with difficult-to-control asthma and found to be an important risk factor for frequent exacerbations in the prior year.12 Our findings extend this observation in specialty-managed patients with asthma overall because they demonstrate, for the first time to our knowledge, associations of OSA risk with not-well-controlled asthma independent of these well-recognized asthma aggravators. Results suggest that when other contributors to asthma control are addressed, a new focus on untreated OSA may make a difference in asthma control.
Multiple putative pathways for OSA aggravation of asthma exist. First, OSA could promote GERD,25 a well-recognized asthma trigger.26 Second, direct links may exist through OSA-related increase in the resistive load on lower airways27 overimposed on an already more challenged airway system especially during sleep,28 upper-airway-triggered vagally mediated bronchoconstriction,8 and increased bronchial responsiveness29; additionally, through altered chemical28 arousal thresholds or to resistive loading,28 thus allowing more bronchoconstriction to occur. Although these pathways remain to be tested in patients with asthma and coexistent OSA, experimental studies of sustained hypoxia have shown vagally mediated bronchoconstriction,30 increased bronchial responsiveness,31 cough suppression,32 altered arousal thresholds to resistive loading,28 and impaired symptom perception in asthma.33 Finally, OSA may lead to oxidative stress and inflammation in the lower airway as it does in the cardiovascular system.34 Increased exhaled 8-isoprostane and interleukin-635,36 and a neutrophilic inflammatory cellular type in induced sputum have been recently demonstrated,37,38 the latter correlated with OSA severity.38 Preliminary work from our laboratory has found that symptomatic patients with asthma and comorbid OSA have lower levels of exhaled nitric oxide,24 an indicator of eosinophilic inflammation. This observation suggests that OSA may contribute to the noneosinophilic phenotype increasingly recognized among patients with uncontrolled asthma.39
Our study substantiates the utility of all ACQ formats for this relationship. ACQ versions had similar associations with OSA risk because there was nearly complete overlap of CIs (Fig 2, Table 5). These findings are consistent with the reported lack of benefit in FEV1 observed with up to 2 months of CPAP9,10 and collectively suggest that such an effect may take longer or is less likely to develop.
OSA has been postulated to be a mediator of the relationship between obesity and asthma control.11,40 The evidence from our study supports such a hypothesis. In univariate analysis, obesity was significantly associated with not-well-controlled asthma; however, when accounting for OSA risk (Table 4 [data on obesity not shown]), as well as in the final models (Table 5), this association was almost always lost. Recent studies in patients with asthma and obesity found less-severe airway obstruction41 and inflammation41,42 or no association with airway inflammation43,44 but increased airway markers of oxidative stress.42 The one study that systematically ascertained OSA syndrome by either PSG or symptoms reported an increased risk for OSA (OR, 3.1; 95% CI, 1.1-9.0) and other asthma-aggravating comorbidities in patients with obesity and concluded that factors other than airway inflammation alone explain the relationship between obesity and asthma.41
There are limitations to our study, the main relating to use of a questionnaire-based assessment of OSA, since PSG is the gold standard to diagnose OSA. Prospective use of a costly technology such as PSG would have been prohibitive for this large study, but our results substantiate a need for such PSG-based studies. The SA-SDQ demonstrated high internal validity, good sensitivity, and specificity in a large sample of sleep study patients.19 Furthermore, this questionnaire has a high diagnostic value compared with other sleep apnea screening instruments.45 Second, the SA-SDQ has not been specifically validated in patients with asthma. In other primary non-lung disease patient populations, the SA-SDQ was found to be a good predictor for OSA on PSG46,47 such that lower cutoff scores than used herein have been proposed for use in these patients.47 However, when these lower cutoff scores were applied to a population of patients with another chronic lung condition (ie, pulmonary fibrosis), although a good correlation was noted between SA-SDQ score and OSA severity, this instrument did not perform as well as48 in the original validation study.19 These findings raise the possibility that the original cutoff scores,19 which we used in our study, may be more appropriate for patients with lung diseases. Further studies are necessary to validate this scale specifically in patients with asthma. A third limitation stems from the cross-sectional design, preventing any conclusion about causality. Although asthma also may be a risk factor for the development of OSA,49 our data, corroborated by other interventional studies7‐10 along with the magnitude of independent effects observed and the plausible mechanistic basis presented, suggest an effect of OSA on asthma control. It is also possible that our study population, which was based in a highly specialized clinic, may contribute to an underestimation of the true relationship of OSA with asthma control. The nose is the main breathing route during sleep, and nasal congestion is a well-established risk factor for OSA.50 A large proportion of our study subjects had a history of rhinitis, with the majority (91%) receiving at least one treatment at the time of survey. Such a high proportion of treatment use, through its effect, is a likely explanation for the “protective” association of rhinitis with asthma control. These observations suggest that this treatment effect also may have contributed to a reduction of the true OSA risk in this cohort and perhaps attenuated the impact of OSA on asthma control. Group analyses such as ours may not entirely describe control of asthma in individual patients, such as those with a poor perception of airway narrowing. Therefore, in such patients, using the full-version ACQ for determining its relationship with OSA risk may provide the best estimate of asthma control. Finally, none of the ACQ versions can separate between nocturnal from daytime asthma control. Because interventional studies for OSA in asthma have preferentially selected patients with nocturnal symptoms,7‐10 the OSA effect specifically on daytime asthma remains largely unknown. Understanding this relationship may have important implications on the mechanisms underlying this interaction and is worthy of future study.
In summary, this first large study of a population of well-characterized patients with asthma finds an association of OSA risk with not-well-controlled asthma, as incorporated in the current guidelines, independent of obesity and other recognized asthma aggravators. These data strengthen the evidence of the role of OSA in asthma control and suggest that OSA may prove to be a treatable target in patients affected by these highly prevalent and interacting conditions. Prospective studies with objective sleep assessments are needed if this relationship and its mechanistic basis are to be better understood.
Acknowledgments
Author contributions: Dr M. Teodorescu: contributed to the study concept and design; data collection, analysis, and interpretation; and drafting of the article.
Dr Polomis: contributed to the data collection and drafting of the article.
Ms Hall: contributed to the data collection and critical revision of the article for important intellectual content.
Dr M. C. Teodorescu: contributed to the data collection and interpretation, and critical revision of the article for important intellectual content.
Dr Gangnon: contributed to the data analysis and interpretation, and critical revision of the article for important intellectual content.
Ms Peterson: contributed to the data collection and interpretation, and critical revision of the article for important intellectual content.
Dr Xie: contributed to the data interpretation and critical revision of the article for important intellectual content.
Dr Sorkness: contributed to the data interpretation and critical revision of the article for important intellectual content.
Dr Jarjour: contributed to the data interpretation and critical revision of the article for important intellectual content.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Sorkness is on the advisory board for GlaxoSmithKline (< $10,000). Dr Jarjour is on the advisory board for GlaxoSmithKline and received speaker honoraria (each < $10,000) from Novartis and Merck and consulting fees (> $10,000) from Asthmatx. He received funding support from the National Institutes of Health, GlaxoSmithKline, Medimmune, and Genentech. Drs M. Teodorescu, Polomis, M. C. Teodorescu, Gangnon, and Xie and Mss Hall and Peterson have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The contents of this article do not represent the views of the Department of Veterans Affairs or the US government.
Other contributions: We thank Whitney Stalsberg, Roman Aydiko, and Padau Yang for their assistance with subject enrollment. We also thank Dr William Busse and the other providers and staff in the allergy and pulmonary clinics at the University of Wisconsin–Madison for their assistance with patient recruitment.
Abbreviations
- ACQ
Asthma Control Questionnaire
- CPAP
continuous positive airway pressure
- GERD
gastroesophageal reflux disease
- OSA
obstructive sleep apnea
- PEFR
peak expiratory flow rate
- PSG
polysomnography
- SA-SDQ
Sleep Apnea Scale of the Sleep Disorders Questionnaire
Funding/Support: This work was performed at the University of Wisconsin–Madison and was funded by the University of Wisconsin School of Medicine and Public Health, Department of Medicine, and Medical Education and Research Committee–New Investigator Award; 1UL1RR025011 from the Clinical and Translational Science Award program of the National Center for Research Resources, National Institutes of Health; and additional resources from the William S. Middleton Memorial Veterans Hospital, Madison, WI (to Dr M. Teodorescu).
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (http://www.chestpubs.org/site/misc/reprints.xhtml).
References
- 1.US Department of Health and Human Services National Institutes of Health National Heart, Lung and Blood Institute National Asthma Education and Prevention Program Expert panel report 3 (EPR-3): Guidelines for the diagnosis and management of asthma. Full report 2007. http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.pdf. Accessed August 15, 2009.
- 2.Global Initiative for Asthma Global strategy for asthma management and prevention. http://www.ginasthma.org/Guidelineitem.asp?l1=2&l2=1&intId=1561. Accessed August 15, 2009.
- 3.British Thoracic Society Scottish Intercollegiate Guidelines Network British guideline on the management of asthma: a national clinical guideline. Rev ed. http://www.brit-thoracic.org.uk/Portals/0/Clinical%20Information/Asthma/Guidelines/asthma_final2008.pdf. Accessed August 15, 2009.
- 4.Bateman ED, Bousquet J, Keech ML, Busse WW, Clark TJ, Pedersen SE. The correlation between asthma control and health status: the GOAL study. Eur Respir J. 2007;29(1):56–62. doi: 10.1183/09031936.00128505. [DOI] [PubMed] [Google Scholar]
- 5.Fuhlbrigge AL, Adams RJ, Guilbert TW, et al. The burden of asthma in the United States: level and distribution are dependent on interpretation of the national asthma education and prevention program guidelines. Am J Respir Crit Care Med. 2002;166(8):1044–1049. doi: 10.1164/rccm.2107057. [DOI] [PubMed] [Google Scholar]
- 6.Lai CK, De Guia TS, Kim YY, et al. Asthma Insights and Reality in Asia-Pacific Steering Committee Asthma control in the Asia-Pacific region: the Asthma Insights and Reality in Asia-Pacific Study. J Allergy Clin Immunol. 2003;111(2):263–268. doi: 10.1067/mai.2003.30. [DOI] [PubMed] [Google Scholar]
- 7.Chan CS, Woolcock AJ, Sullivan CE. Nocturnal asthma: role of snoring and obstructive sleep apnea. Am Rev Respir Dis. 1988;137(6):1502–1504. doi: 10.1164/ajrccm/137.6.1502. [DOI] [PubMed] [Google Scholar]
- 8.Guilleminault C, Quera-Salva MA, Powell N, et al. Nocturnal asthma: snoring, small pharynx and nasal CPAP. Eur Respir J. 1988;1(10):902–907. [PubMed] [Google Scholar]
- 9.Ciftci TU, Ciftci B, Guven SF, Kokturk O, Turktas H. Effect of nasal continuous positive airway pressure in uncontrolled nocturnal asthmatic patients with obstructive sleep apnea syndrome. Respir Med. 2005;99(5):529–534. doi: 10.1016/j.rmed.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 10.Lafond C, Sériès F, Lemière C. Impact of CPAP on asthmatic patients with obstructive sleep apnoea. Eur Respir J. 2007;29(2):307–311. doi: 10.1183/09031936.00059706. [DOI] [PubMed] [Google Scholar]
- 11.Boulet LP, Hamid Q, Bacon SL, et al. Symposium on obesity and asthma–November 2, 2006. Can Respir J. 2007;14(4):201–208. doi: 10.1155/2007/342618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.ten Brinke A, Sterk PJ, Masclee AA, et al. Risk factors of frequent exacerbations in difficult-to-treat asthma. Eur Respir J. 2005;26(5):812–818. doi: 10.1183/09031936.05.00037905. [DOI] [PubMed] [Google Scholar]
- 13.Woolcock A, Rubinfeld AR, Seale JP, et al. Thoracic Society of Australia and New Zealand. Asthma management plan, 1989. Med J Aust. 1989;151(11-12):650–653. [PubMed] [Google Scholar]
- 14.Guidelines for management of asthma in adults: I—Chronic persistent asthma. Statement by the British Thoracic Society, Research Unit of the Royal College of Physicians of London, King’s Fund Centre, National Asthma Campaign. BMJ. 1990;301(6753):651–653. doi: 10.1136/bmj.301.6753.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lemière C, Bai T, Balter M, et al. Canadian Adult Consensus Group of the Canadian Thoracic Society Adult Asthma Consensus Guidelines update 2003. Can Respir J. 2004;11(suppl A):9A–18A. doi: 10.1155/2004/271362. [DOI] [PubMed] [Google Scholar]
- 16.Juniper EF, Bousquet J, Abetz L, Bateman ED. GOAL Committee Identifying ‘well-controlled’ and ‘not well-controlled’ asthma using the Asthma Control Questionnaire. Respir Med. 2006;100(4):616–621. doi: 10.1016/j.rmed.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 17.Juniper EF, O’Byrne PM, Guyatt GH, Ferrie PJ, King DR. Development and validation of a questionnaire to measure asthma control. Eur Respir J. 1999;14(4):902–907. doi: 10.1034/j.1399-3003.1999.14d29.x. [DOI] [PubMed] [Google Scholar]
- 18.Juniper EF, Svensson K, Mörk AC, Ståhl E. Measurement properties and interpretation of three shortened versions of the asthma control questionnaire. Respir Med. 2005;99(5):553–558. doi: 10.1016/j.rmed.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 19.Douglass AB, Bornstein R, Nino-Murcia G, et al. The Sleep Disorders Questionnaire. I: Creation and multivariate structure of SDQ. Sleep. 1994;17(2):160–167. doi: 10.1093/sleep/17.2.160. [DOI] [PubMed] [Google Scholar]
- 20.Hall SV, Sorkness CA, Peterson AG, et al. Association of obstructive sleep apnea (OSA) symptoms and asthma control. Am J Respir Crit Care Med. 2008;177(abstract suppl):A671. [Google Scholar]
- 21.Teodorescu M, Hall SV, Peterson AG, et al. Do shorter and full versions of the Asthma Control Questionnaire have similar correlations with obstructive sleep apnea risk? Sleep. 2008;31(abstract suppl):A307. [Google Scholar]
- 22.Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease (COPD) and asthma. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, November 1986. Am Rev Respir Dis. 1987;136(1):225–244. doi: 10.1164/ajrccm/136.1.225. [DOI] [PubMed] [Google Scholar]
- 23.Auckley D, Moallem M, Shaman Z, Mustafa M. Findings of a Berlin Questionnaire survey: comparison between patients seen in an asthma clinic versus internal medicine clinic. Sleep Med. 2008;9(5):494–499. doi: 10.1016/j.sleep.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 24.Teodorescu M, Peterson AG, Teodorescu MC, et al. Airway inflammation in asthma patients with obstructive sleep-disordered breathing (oSDB) Am J Respir Crit Care Med. 2009;179(abstract suppl):A2874. [Google Scholar]
- 25.Zanation AM, Senior BA. The relationship between extraesophageal reflux (EER) and obstructive sleep apnea (OSA) Sleep Med Rev. 2005;9(6):453–458. doi: 10.1016/j.smrv.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 26.Harding SM. Gastroesophageal reflux: a potential asthma trigger. Immunol Allergy Clin North Am. 2005;25(1):131–148. doi: 10.1016/j.iac.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 27.Bijaoui EL, Champagne V, Baconnier PF, Kimoff RJ, Bates JH. Mechanical properties of the lung and upper airways in patients with sleep-disordered breathing. Am J Respir Crit Care Med. 2002;165(8):1055–1061. doi: 10.1164/ajrccm.165.8.2107144. [DOI] [PubMed] [Google Scholar]
- 28.Ballard RD. Sleep, respiratory physiology, and nocturnal asthma. Chronobiol Int. 1999;16(5):565–580. doi: 10.3109/07420529908998729. [DOI] [PubMed] [Google Scholar]
- 29.Lin CC, Lin CY. Obstructive sleep apnea syndrome and bronchial hyperreactivity. Lung. 1995;173(2):117–126. doi: 10.1007/BF02981471. [DOI] [PubMed] [Google Scholar]
- 30.Nadel JA, Widdicombe JG. Effect of changes in blood gas tensions and carotid sinus pressure on tracheal volume and total lung resistance to airflow. J Physiol. 1962;163:13–33. doi: 10.1113/jphysiol.1962.sp006956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.D’Brot J, Ahmed T. Hypoxia-induced enhancement of nonspecific bronchial reactivity: role of leukotrienes. J Appl Physiol. 1988;65(1):194–199. doi: 10.1152/jappl.1988.65.1.194. [DOI] [PubMed] [Google Scholar]
- 32.Eckert DJ, Catcheside PG, Stadler DL, McDonald R, Hlavac MC, McEvoy RD. Acute sustained hypoxia suppresses the cough reflex in healthy subjects. Am J Respir Crit Care Med. 2006;173(5):506–511. doi: 10.1164/rccm.200509-1455OC. [DOI] [PubMed] [Google Scholar]
- 33.Eckert DJ, Catcheside PG, Smith JH, Frith PA, McEvoy RD. Hypoxia suppresses symptom perception in asthma. Am J Respir Crit Care Med. 2004;169(11):1224–1230. doi: 10.1164/rccm.200305-630OC. [DOI] [PubMed] [Google Scholar]
- 34.Mehra R, Redline S. Sleep apnea: a proinflammatory disorder that coaggregates with obesity. J Allergy Clin Immunol. 2008;121(5):1096–1102. doi: 10.1016/j.jaci.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carpagnano GE, Kharitonov SA, Resta O, Foschino-Barbaro MP, Gramiccioni E, Barnes PJ. Increased 8-isoprostane and interleukin-6 in breath condensate of obstructive sleep apnea patients. Chest. 2002;122(4):1162–1167. doi: 10.1378/chest.122.4.1162. [DOI] [PubMed] [Google Scholar]
- 36.Carpagnano GE, Kharitonov SA, Resta O, Foschino-Barbaro MP, Gramiccioni E, Barnes PJ. 8-Isoprostane, a marker of oxidative stress, is increased in exhaled breath condensate of patients with obstructive sleep apnea after night and is reduced by continuous positive airway pressure therapy. Chest. 2003;124(4):1386–1392. doi: 10.1378/chest.124.4.1386. [DOI] [PubMed] [Google Scholar]
- 37.Salerno FG, Carpagnano E, Guido P, et al. Airway inflammation in patients affected by obstructive sleep apnea syndrome. Respir Med. 2004;98(1):25–28. doi: 10.1016/j.rmed.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 38.Li AM, Hung E, Tsang T, et al. Induced sputum inflammatory measures correlate with disease severity in children with obstructive sleep apnoea. Thorax. 2007;62(1):75–79. doi: 10.1136/thx.2006.060657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Foley SC, Hamid Q. Images in allergy and immunology: neutrophils in asthma. J Allergy Clin Immunol. 2007;119(5):1282–1286. doi: 10.1016/j.jaci.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 40.Shore SA, Johnston RA. Obesity and asthma. Pharmacol Ther. 2006;110(1):83–102. doi: 10.1016/j.pharmthera.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 41.van Veen IH, Ten Brinke A, Sterk PJ, Rabe KF, Bel EH. Airway inflammation in obese and nonobese patients with difficult-to-treat asthma. Allergy. 2008;63(5):570–574. doi: 10.1111/j.1398-9995.2007.01597.x. [DOI] [PubMed] [Google Scholar]
- 42.Komakula S, Khatri S, Mermis J, et al. Body mass index is associated with reduced exhaled nitric oxide and higher exhaled 8-isoprostanes in asthmatics. Respir Res. 2007;8:32. doi: 10.1186/1465-9921-8-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Todd DC, Armstrong S, D’Silva L, Allen CJ, Hargreave FE, Parameswaran K. Effect of obesity on airway inflammation: a cross-sectional analysis of body mass index and sputum cell counts. Clin Exp Allergy. 2007;37(7):1049–1054. doi: 10.1111/j.1365-2222.2007.02748.x. [DOI] [PubMed] [Google Scholar]
- 44.Sutherland TJ, Cowan JO, Young S, et al. The association between obesity and asthma: interactions between systemic and airway inflammation. Am J Respir Crit Care Med. 2008;178(5):469–475. doi: 10.1164/rccm.200802-301OC. [DOI] [PubMed] [Google Scholar]
- 45.Kryger MH, Roth T, Dement WC. Principles and Practice of Sleep Medicine. Philadelphia, PA: Elsevier; 2005. pp. 603–608. [Google Scholar]
- 46.Bassetti C, Aldrich MS, Quint D. Sleep-disordered breathing in patients with acute supra- and infratentorial strokes. A prospective study of 39 patients. Stroke. 1997;28(9):1765–1772. doi: 10.1161/01.str.28.9.1765. [DOI] [PubMed] [Google Scholar]
- 47.Weatherwax KJ, Lin X, Marzec ML, Malow BA. Obstructive sleep apnea in epilepsy patients: the Sleep Apnea scale of the Sleep Disorders Questionnaire (SA-SDQ) is a useful screening instrument for obstructive sleep apnea in a disease-specific population. Sleep Med. 2003;4(6):517–521. doi: 10.1016/j.sleep.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 48.Lancaster LH, Mason WR, Parnell JA, et al. Obstructive sleep apnea is common in idiopathic pulmonary fibrosis. Chest. 2009;136(3):772–778. doi: 10.1378/chest.08-2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Knuiman M, James A, Divitini M, Bartholomew H. Longitudinal study of risk factors for habitual snoring in a general adult population: the Busselton Health Study. Chest. 2006;130(6):1779–1783. doi: 10.1378/chest.130.6.1779. [DOI] [PubMed] [Google Scholar]
- 50.Young T, Finn L, Palta M. Chronic nasal congestion at night is a risk factor for snoring in a population-based cohort study. Arch Intern Med. 2001;161(12):1514–1519. doi: 10.1001/archinte.161.12.1514. [DOI] [PubMed] [Google Scholar]