Abstract
Background:
Obesity is associated with poor outcomes in many diseases, although recent data suggest that acute lung injury (ALI) is an exception. This is particularly interesting because obesity is marked by increased levels of proinflammatory mediators associated with increased morbidity and mortality in ALI. We hypothesized that cytokine response might be attenuated in patients who are obese and critically ill or that obesity might modify the relationship between plasma cytokines and clinical outcomes in ALI.
Methods:
We analyzed plasma biomarker levels (interleukin [IL]-6, IL-8, tumor necrosis factor-α receptor 1, surfactant protein D [SP-D], soluble intracellular adhesion molecule, von Willebrand factor (vWF), protein C, and plasminogen activator inhibitor-1) collected at baseline and day 3 in 1,409 participants in prior National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome Network (ARDSNet) trials. BMI was calculated for each patient, and associations with cytokine levels and ventilator-free days (VFDs), organ failure-free days (OFDs), and mortality were investigated in regression models adjusting for confounders.
Results:
In adjusted analyses, plasma IL-6 (P = .052), IL-8 (P = .001), and SP-D (P < .001) were inversely related to BMI, whereas vWF (P = .001) and WBC count (P = .042) increased proportionally with BMI. BMI was not associated with increased morbidity or mortality and did not modify the association between baseline biomarker levels and mortality, VFDs, or OFDs.
Conclusions:
Patients who are obese and have ALI have lower levels of several proinflammatory cytokines, suggesting that the inflammatory response may be altered in patients with ALI and a high BMI. Lower SP-D but higher vWF suggests decreased epithelial and increased endothelial injury in the lung of patients who are obese. Mechanisms by which obesity may modulate innate immunity in critical illness are unclear, and future studies should elucidate such mechanisms.
Obesity is a major epidemic in the United States and is associated with increased morbidity related to cardiovascular disease, diabetes, and asthma.1,2 These morbidities are thought to result, in part, from the production of pathogenic mediators by adipose tissue. Adipose tissue in the individual who is obese produces multiple proinflammatory cytokines, including tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), IL-6, and IL-8. Elevated levels of these cytokines in patients who are obese have been linked to the development of atherosclerotic cardiovascular disease, hypertension, and insulin resistance, and it is believed they may have systemic effects mediating many of the other medical complications of obesity.1,3-7 Thus, obesity represents a chronic inflammatory state.
Acute lung injury (ALI) and its more severe form, acute respiratory distress syndrome, are characterized by an overexuberant inflammatory response in the lung associated with and perhaps driven by elevated plasma levels of inflammatory cytokines.8 Previous clinical investigations have demonstrated an association between increased mortality and high circulating levels of TNF-α, IL-1β, IL-6, and IL-8 in ALI.9-13 Such an association is also seen with biomarkers of epithelial injury (surfactant protein D [SP-D]) and vascular injury (soluble intracellular adhesion molecule [sICAM] and von Willebrand factor [vWF]), as well as disordered coagulation (plasminogen activator inhibitor 1 [PAI 1] and protein C).14-18 Because many of these same inflammatory cytokines are elevated at baseline in individuals who are obese,19 one might anticipate that individuals who are obese would have more severe lung injury and worse clinical outcomes from ALI. However, prior studies of patients with ALI have reported that mortality does not increase, and may even be lower with increasing BMI.20-22 Thus, an apparent paradox exists that patients who are obese, who manifest a proinflammatory state with increased circulating cytokines in their normal state, do not have increased mortality in the setting of critical illness with ALI, which is a condition driven, at least in part, by a proinflammatory cytokine milieu.
Given this paradox in patients with ALI, we hypothesized that one of the following scenarios may be occurring: (1) increasing BMI is associated with decreased levels of circulating inflammatory cytokines, (2) increasing BMI is associated with more rapid attenuation of cytokine release during the course of ALI, or (3) BMI alters the relationship between baseline cytokine levels and mortality and morbidity from ALI. In order to address these hypotheses and to examine the effects of obesity on other biomarkers associated with outcomes in ALI, we analyzed data from prior National Heart, Lung, and Blood Institute (NHLBI) Acute Respiratory Distress Syndrome Network (ARDSNet) clinical trials in ALI because they present a large, well-characterized patient population with plasma biomarker measurements (at enrollment and Day 3) and information on outcomes from ALI.
Materials and Methods
Patient Selection
We examined data from patients who participated in four randomized controlled trials (RCTs) conducted by the NHLBI ARDSNet.23-26 Of the 1,451 patients in these four studies, 861 participated in the RCT of mechanical ventilation comparing lower tidal volume with higher tidal volume (6 mL/kg of predicted body weight vs 12 mL/kg).23 This RCT was conducted simultaneously with two other clinical trials in which ketoconazole or lisofylline were compared with placebo in a factorial design.25,26 An additional group of 41 patients continued in the lisofylline study after the tidal volume study was completed and were assigned to receive 6 cc/kg tidal volume. The remaining 549 patients participated in the RCT of lower vs higher positive end-expiratory pressure (PEEP), and these patients also received 6 mL/kg tidal volume.24 Details of these RCTs are described elsewhere.23-26 Briefly, patients were eligible if they required mechanical ventilation and met American-European Consensus Conference criteria for ALI.27 Patients were excluded if their body weight was > 1 kg/cm height. For each participant, an Acute Physiologic and Chronic Health Evaluation (APACHE) III score from the time of ICU admission was calculated, and the physician investigator identified the primary risk factor for the development of ALI (sepsis, trauma, pneumonia, aspiration, multiple transfusions, or other), as previously described.28 In the classification of sepsis, investigators were instructed to use the Society of Critical Care Medicine definition of clinical sepsis, which includes a known or suspected source of systemic infection and at least two of the following: (1) temperature < 36°C or > 38°C; (2) heart rate > 90 bpm; (3) respiratory rate > 20 breaths/ min or Paco2 < 32 mm Hg; and (4) WBC count > 12,000/mm3, < 4,000/mm3, or > 10% bands. Known infection is a documented source of infection (eg, positive blood cultures). Suspected infection is evidenced by one or more of the following: white cells in a normally sterile body fluid, perforated viscus, radiographic evidence of pneumonia plus purulent sputum, or a syndrome associated with a high risk of infection (eg, ascending cholangitis).29
Of the 1,451 patients, 1,409 had both height and weight recorded and formed the cohort for this study. The study was approved by the University of Vermont Committee on Human Research in the Medical Sciences (#08-051) as well as the NHLBI ARDSNet.
Study Outcome Variables
Our primary predictor was BMI, and patients were categorized according to NHLBI guidelines: < 18.5 (underweight), 18.5 to 24.9 (normal), 25 to 29.9 (overweight), 30.0 to 39.9 (obese), and ≥ 40.0 (severely obese).30 Demographic information and variables of interest that were selected a priori included age, gender, comorbid diabetes,31 ALI risk factor, APACHE III score32 at admission, and tidal volume per kilogram predicted body weight that patients were receiving before randomization into the parent RCTs. Outcomes of interest included plasma inflammatory biomarker levels at study enrollment (day 0) and at study day 3, mortality at 90 days, ventilator-free days (VFDs), and organ-failure-free days (OFDs). VFDs and OFDs were calculated as the number of days during the first 28 after study enrollment that a participant was alive and breathing unassisted for ≥ 48 h (VFDs) and free of nonpulmonary organ failure (OFDs), respectively.33 Organ failure was determined as defined in the Brussels Organ Failure Table.34 Patients who died prior to achieving unassisted breathing or to being classified as organ failure free before day 28 were assigned zero VFDs and OFDs, respectively, as in previous studies.23,25,26 All participants had blood samples collected at study enrollment and again at day 3, and plasma levels of the following inflammatory biomarkers were measured in duplicate using commercially available enzyme-linked immunoassay kits in previous ancillary studies: IL-6, TNF-α receptor 1, SP-D, sICAM, vWF, protein C, and PAI-1.9,14-17,35 Sensitivities, specificities, limits of detection, and coefficients of variation of the various assays have been published previously.9,14-17,35
Statistical Analysis
Univariate analyses were performed with Wilcoxon rank sum tests for continuous variables and χ2 tests for categorical variables. Trends in variables by BMI categories were analyzed with the Wilcoxon rank sum test for trend for continuous variables and the Cochrane-Armitage test for trend for categorical variables. Multivariable linear (for continuous variables) and logistic (for categorical variables) regressions with robust standard errors were used to evaluate the associations between BMI and our outcomes of interest (baseline biomarker levels, mortality, VFDs, and OFDs) with adjustment for confounders. Multivariable regression was also used to determine if BMI modified the relationship between inflammatory cytokine level and mortality, VFDs, and OFDs. Analysis of covariance was used to analyze day 3 inflammatory biomarker levels while adjusting for baseline levels.
In our multivariable models, BMI was fit as a linear continuous variable. Inflammatory biomarker levels were natural log-transformed because of nonnormality. Because age is a large component of the total APACHE III score, we included only the APACHE III score in our models to avoid colinearity, and it was fit as a linear continuous variable. Gender, ALI risk factor, and comorbid diabetes were also included. Risk factor for ALI was fit as an indicator variable. We chose not to include prerandomization tidal volume per kilogram predicted body weight in our multivariable models because it may be in the causal pathway between obesity and cytokine levels or outcomes.20 In the models of day 3 cytokine levels, mortality, OFDs, and VFDs, we also adjusted for tidal volume group assignment (6 mL/kg or 12 mL/kg) because this was shown to affect outcome.23 All patients in the RCT of higher vs lower PEEP received the tidal volume of 6 cc/kg predicted body weight per protocol. Because the RCTs of lisofylline, ketoconazole, and higher vs lower PEEP found no difference between groups, we did not include treatment group assignment in these RCTs in our models.24-26
Additionally, interaction terms (with BMI as a continuous linear variable) were included in our models to assess for effect modification by BMI on the relationships between baseline biomarker levels and death, VFDs, and OFDs. Furthermore, because we were concerned that patients who were underweight (BMI < 18.5) may represent a very different population from patients who were heavier (often with chronic illnesses such as malignancy), thus potentially skewing our results, we performed sensitivity analyses by excluding the 61 patients in the underweight category from our univariate analyses and regression analyses of baseline cytokine levels.
All analyses were performed with Stata 9.0 or newer (StataCorp LP; College Station, TX). Statistical significance was defined as a two-sided P value ≤ .05.
Results
Baseline Characteristics
The 42 patients that we excluded because of lack of either height or weight measurement, when compared with the 1,409 included patients, had lower severity of illness at admission (APACHE III score 75.8 ± 27.1 vs 87.6 ± 30.4, P = .01), had less diabetes (2.4% vs 17.5%, P = .02), and had more trauma (28.6% vs 9.2%, P < .001) and less sepsis (9.5% vs 25.0%, P = .03) as their ALI risk factor. Among the study population, we did not find a significant difference in age between BMI groups, but we did find significant differences in gender, severity of illness, prevalence of comorbid diabetes, and ALI risk factor (Table 1). With increasing BMI, patients were more often women, had lower severity of illness, more commonly had diabetes, and more often had sepsis as their ALI risk factor. When the 61 patients with BMI < 18.5 were excluded from these analyses (also shown in Table 1), the results were similar except that patients who were obese had significantly higher Pao2/Fio2 ratios and significantly more OFDs, while mortality was not different.
Table 1.
—Demographic Characteristics and Unadjusted Outcomes of Patients in 5 BMI Groups
| Characteristics | Underweight (BMI < 18.5) (n = 61) | Normal Weight (18.5 ≤ BMI < 25) (n = 542) | Overweight (25 ≤ BMI < 30) (n = 417) | Obese (30 ≤ BMI < 40) (n = 315) | Morbidly Obese (BMI > 40) (n = 74) | P Value Including All BMI Groups | P Value Excluding Underweight Group |
| Age,a y | 50.2 ± 16.8 | 51.6 ± 17.9 | 52.1 ± 18.3 | 51.0 ± 15.5 | 49.3 ± 13.1 | .76b | .51b |
| Gender, % women | 41.0 | 41.7 | 36.0 | 45.7 | 68.9 | < .001c | < .001c |
| Pao2/Fio2d | 131.0 ± 60.3 | 125.3 ± 55.3 | 129.1 ± 58.5 | 129.5 ± 59.1 | 148.1 ± 65.3 | .065 | .031 |
| APACHE III scorea | 97.2 ± 35.2 | 89.9 ± 29.8 | 87.2 ± 30.1 | 83.7 ± 30.5 | 81.5 ± 28.6 | < .001b | .001b |
| Prerandomization tidal volume per kilogram predicted body weight | 8.5 ± 2.1 | 9.1 ± 2.0 | 9.5 ± 2.2 | 9.5 ± 2.4 | 10.2 ± 2.6 | .001 | .008 |
| Diabetes, % | 13.1 | 11.8 | 13.2 | 17.5 | 35.1 | < .001c | < .001c |
| ALI risk factor | |||||||
| Trauma, % | 3.2 | 6.7 | 11.3 | 11.7 | 9.5 | .004c | .006c |
| Sepsis, % | 25.0 | 21.7 | 25.4 | 28.9 | 31.1 | .03c | .03c |
| Pneumonia, % | 44.2 | 37.8 | 33.8 | 32.4 | 35.1 | .004c | .004c |
| 90-d mortality, % | 45.9 | 33.4 | 30.2 | 27.6 | 32.4 | .03e | .17e |
| VFDsf | 2 (0-20) | 15 (0-23) | 14 (0-23) | 17 (0-24) | 15 (0-22) | .23b | .85b |
| OFDsf | 9 (0-19) | 16 (0-25) | 20 (1-26) | 17 (0-25) | 23 (8-27) | .005b | .07b |
ALI = acute lung injury; APACHE = Acute Physiologic and Chronic Health Evaluation; ODFs = organ-failure-free days; VFDs = ventilator-free days.
mean ± SD.
Wilcoxon rank sum test for trend.
χ2 test.
ratio of partial pressure of arterial to fraction of inspired oxygen.
Cochrane-Armitage test for trend.
median (intraquartile range).
Association Between BMI and Biomarker Levels in ALI
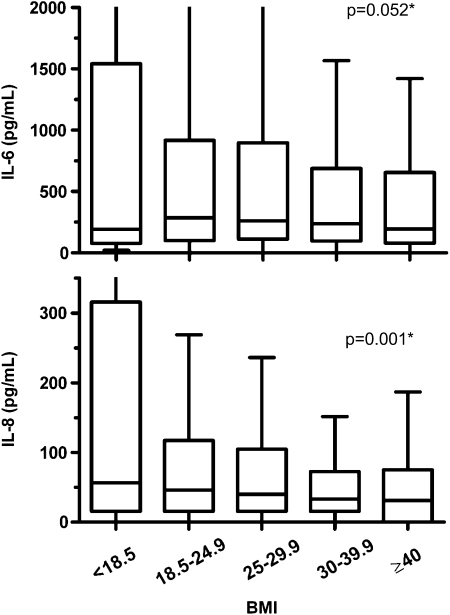
Unadjusted analyses revealed that the levels of several inflammatory biomarkers at baseline (Day 0) significantly varied by BMI group (Fig 1, Table 2). Of particular note, IL-8 and TNF-α receptor 1 significantly decreased with increasing BMI, and IL-6 trended toward decrease with increasing BMI (P = .08). Additionally, SP-D significantly decreased, while WBC count, protein C, and vWF significantly increased with increasing BMI.
Figure 1.
Box plots of IL-6 and IL-8 plasma levels at baseline in each BMI category. Plotted data are unadjusted. P values (*) result from multivariable linear regression including log-transformed cytokine levels and BMI as a continuous variable with adjustment for gender, Acute Physiologic and Chronic Health Evaluation (APACHE) III score, comorbid diabetes, and acute lung injury (ALI) risk factor. IL = interleukin.
Table 2.
—Association of BMI With Plasma Biomarker Measurements and WBC Count at Baseline
| Underweight (BMI < 18.5) |
Normal (18.5 ≤ BMI < 25) |
Overweight (25 ≤ BMI < 30) |
Obese (30 ≤ BMI < 40) |
Morbidly Obese (BMI ≥ 40) |
||||||||
| Variable | No. | pg/mLc | No. | pg/mLc | No. | pg/mLc | No. | pg/mLc | No. | pg/mLc | P Valuea (Unadjusted) | P Valueb (Adjusted) |
| IL-6 | 55 | 193 (78-1,542) | 502 | 285 (101-916) | 362 | 261.5 (112-894) | 277 | 238 (102-687) | 70 | 194.5 (81-636) | .08 | .052 |
| IL-8 | 55 | 56.5 (15.6-315.8) | 505 | 46 (15.6-117) | 363 | 40.2 (15.6-104.7) | 279 | 33 (15.6-72.5) | 70 | 31.1 (0-74.9) | < .001 | .001 |
| TNF-α receptor 1 | 41 | 3,615 (2,439-7,023) | 396 | 3,897 (2,368-7,727) | 304 | 3,668 (2,392-7,371) | 250 | 3,127 (2,157-6,639) | 64 | 3,318 (2,169-8,315) | .04 | .98 |
| SP-D | 41 | 112 (57-201) | 388 | 99.5 (48.5-227.5) | 301 | 83 (42-190) | 246 | 81 (42-165) | 64 | 92.5 (33-178) | .007 | < .001 |
| sICAM | 55 | 716 (345-1,236) | 501 | 732 (455-1,172) | 364 | 749 (425-1,174) | 279 | 698 (396-1,198) | 70 | 794 (437-1,221) | .89 | .25 |
| vWF | 41 | 344 (194-517) | 393 | 307 (187-527) | 304 | 340 (201-531) | 250 | 364 (225-505) | 63 | 353 (215-686) | .008 | .001 |
| Protein C | 54 | 44.8 (27.2-63.8) | 502 | 51.4 (33.2-77.9) | 365 | 52.8 (35.3-82.2) | 279 | 61.6 (42.2-94.3) | 70 | 53.2 (41.8-91.9) | < .001 | .009 |
| PAI-1 | 54 | 65.1 (32.8-118.9) | 499 | 65.9 (34.5-146.1) | 365 | 75.4 (39.7-161.7) | 278 | 61.4 (36.9-125.7) | 70 | 81.5 (36.9-184.1) | .75 | .66 |
| WBC | 61 | 10,000 (6,100-14,200) | 540 | 11,345 (7,100-16,150) | 415 | 11,800 (7,700-15,900) | 311 | 12,300 (8,800-16,700) | 74 | 11,630 (7,900-16,800) | .002 | .042 |
IL = interleukin; PAI-1 = plasminogen activator inhibitor-1; sICAM = soluble intracellular adhesion molecule; SP-D = surfactant protein D; TNF-α = tumor necrosis factor-α; vWF = von Willebrand factor. See Table 1 for expansion of other abbreviations.
Wilcoxon rank sum test for trend, unadjusted.
Baseline multivariable linear regression model includes log-transformed cytokine levels and BMI as a continuous variable with adjustment for gender, APACHE III score, comorbid diabetes, and ALI risk factor.
Median (intraquartile range).
These associations generally held true after adjusting for gender, APACHE III score, comorbid diabetes, and ALI risk factor. After adjustment, lower baseline biomarker plasma levels of IL-8 and SP-D were significantly associated with increasing BMI, and there was a strong trend toward lower levels of IL-6 (P = .052) being associated with increasing BMI (Table 2). Higher WBC count and baseline levels of vWF and protein C were significantly associated with increasing BMI. When sensitivity analyses were performed by repeating these same regression analyses excluding the patients who were underweight (BMI < 18.5), results were unchanged except that rather than a trend, IL-6 significantly decreased with increasing BMI (P = .03).
Plasma levels of inflammatory biomarkers on day 3 were also associated with BMI in unadjusted analyses, and these relationships were similar to those of baseline biomarker levels (Table 3). Unlike the previously demonstrated effects of low vs high tidal volume ventilation to accelerate decreases in plasma IL-6 and IL-8 over time,9 BMI did not influence the rate of change between baseline and day 3 levels of IL-6, IL-8, TNF-α receptor 1, sICAM, vWF, protein C, or PAI-1 after adjusting for gender, APACHE III score, comorbid diabetes, ALI risk factor, tidal volume group assignment, and baseline cytokine level in an analysis of covariance model (adjusted P value in Table 3). BMI did, however, significantly influence the rate of change of SP-D such that increasing obesity was associated with smaller increases between baseline and day 3 (P < .001).
Table 3.
—Association of BMI With Plasma Biomarker Measurements at Study Day 3
| Underweight (BMI < 18.5) |
Normal (18.5 ≤ BMI < 25) |
Overweight (25 ≤ BMI < 30) |
Obese (30 ≤ BMI < 40) |
Morbidly Obese (BMI ≥ 40) |
||||||||
| Variable | No. | Pg/mLc | No. | pg/mLc | No. | pg/mLc | No. | pg/mLc | No. | pg/mLc | P Valuea (Unadjusted) | P Valueb (Adjusted) |
| IL-6 | 51 | 129 (44-468) | 453 | 99 (51-264) | 338 | 105 (49-272) | 245 | 80 (41-211) | 64 | 90.5 (33-153) | .005 | .07 |
| IL-8 | 50 | 47.8 (15.6-98) | 455 | 35.6 (15.6-76) | 336 | 31 (15.6-67.5) | 246 | 19.6 (2-58.1) | 64 | 15.6 (0-54.6) | < .001 | .92 |
| TNF-α receptor 1 | 37 | 3,878 (2,650-7,660) | 351 | 3,613.1 (2,391-6,727.7) | 280 | 3,807.5 (2,308-6,714.1) | 216 | 3,139.5 (2,167.4-6,768.4) | 57 | 3,001.2 (2,024-5,823.1) | .04 | .92 |
| SP-D | 38 | 214 (103-338) | 342 | 211 (110-388) | 272 | 164.5 (85.5-300) | 214 | 137.5 (72-244) | 57 | 111 (60-243) | < .001 | < .001 |
| sICAM | 47 | 838.2 (447.5-1,399) | 442 | 766.5 (508.6-1,255.8) | 329 | 879 (523-1,360) | 242 | 812 (405.6-1,398) | 61 | 895 (474.4-1,395) | .76 | .73 |
| vWF | 37 | 287.3 (161-480.2) | 345 | 342.8 (203-521.8) | 278 | 392.2 (238.2-607.3) | 220 | 384.0 (250.1-551.8) | 55 | 407 (255.8-587.0) | .001 | .09 |
| Protein C | 47 | 54.2 (34.3-89.1) | 444 | 62.2 (40.5-97.6) | 331 | 62.8 (42.1-95.2) | 241 | 76.6 (48.7-121.6) | 61 | 75.2 (47.2-121.8) | < .001 | .20 |
| PAI-1 | 47 | 41.4 (29.3-83.8) | 442 | 46.9 (29.9-102.2) | 331 | 48.5 (30.7-103.1) | 241 | 52.7 (32.4-114.6) | 61 | 67.5 (36.8-110.7) | .04 | .10 |
Wilcoxon rank sum test for trend, unadjusted.
When adjusted analyses of day 3 biomarker measurements were performed with an analysis of covariance model, BMI did not affect the rate of change between baseline and day 3 biomarker levels. That model includes log-transformed cytokine levels and BMI as a continuous variable with adjustment for gender, APACHE III score, comorbid diabetes, ALI risk factor, tidal volume assignment, and baseline cytokine level.
Median (intraquartile range).
Association Between BMI and Clinical Outcomes in ALI
Unadjusted 90-day mortality was highest in patients who were underweight (45.9%) and lowest in patients who were obese (27.6%), and this trend was statistically significant (Table 1). There were no significant trends in unadjusted VFDs, but participants with higher BMIs had significantly more OFDs (P = .005).
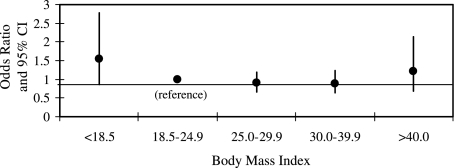
Importantly, after adjustment for gender, APACHE III score, comorbid diabetes, ALI risk factor, and tidal volume group assignment, BMI was not significantly associated with mortality, either in a logistic regression model with BMI expressed as a continuous linear variable (odds ratio of death with each unit increase in BMI equal to 0.994 [95% CI = 0.98-1.01]) or as a categorical variable (Fig 2). Similarly, after adjusting for the same confounders, there was no association between BMI and VFDs (β = −0.015; 95% CI = −0.095 to 0.063; P = .70) or OFDs (β = 0.001; 95% CI = −0.077 to 0.080; P = .98). These results are similar to those obtained in a prior study examining associations between BMI and clinical outcomes in a subset of the current cohort (those patients who participated in the RCT of mechanical ventilation comparing lower tidal volume with higher tidal volume).22
Figure 2.
Odds ratio of death in each BMI category relative to patients who were of normal weight, after adjusting for gender, APACHE III score, ALI risk factor, comorbid diabetes, and tidal volume group assignment. There was no statistically significant difference in mortality between any BMI category and patients of normal weight. Error bars show 95% CI. See Figure 1 legend for expansion of abbreviations.
Association Between Baseline Cytokine Biomarkers and Outcomes in ALI
After confirming that our clinical outcomes findings were similar to prior reports, we aimed to also confirm that baseline levels of several inflammatory biomarkers were associated with the odds of death as previously reported in patients in the RCT of low vs high tidal volume ventilation.9 Our analyses verified that after adjustment for gender, APACHE III score, comorbid diabetes, ALI risk factor, and tidal volume assignment, increasing levels of IL-6, IL-8, TNF-α receptor 1, SP-D, vWF, and PAI-1 were significantly associated with increasing odds of death, while an increased level of protein C was associated with decreased odds of death (Table 4). Similarly, baseline cytokine levels were also associated with VFDs and OFDs (Table 4).
Table 4.
—Association of Plasma Biomarker Measurements at Baseline With Death
| Variable (pg/mL) | Odds Ratio of Death (95% CI)a | VFDs (Mean Difference, Days) (95% CI)b | OFDs (Mean Difference, Days) (95% CI)b |
| IL-6 | 1.10 (1.02-1.19) | −0.99 (−1.33 to −0.65) | −1.23 (−1.56 to −0.90) |
| IL-8 | 1.30 (1.17-1.44) | −1.34 (−1.77 to −0.90) | −1.62 (−2.05 to −1.20) |
| TNF-α receptor 1 | 1.48 (1.24-1.76) | −1.07 (−1.86 to −0.27) | −4.02 (−4.76 to −3.28) |
| SP-D | 1.35 (1.18-1.55) | −1.08 (−1.65 to −0.50) | −0.64 (−1.21 to −0.07) |
| sICAM | 1.11 (0.94-1.33) | −0.62 (−1.37 to 0.14) | −0.93 (−1.67 to −0.19) |
| vWF | 1.50 (1.21-1.85) | −0.53 (−1.42 to 0.35) | −1.08 (−1.95 to −0.21) |
| Protein C | 0.74 (0.61-0.91) | 2.24 (1.36-3.13) | 2.74 (1.88-3.61) |
| PAI-1 | 1.20 (1.06-1.36) | −0.91 (−1.48 to −0.34) | −0.98 (−1.54 to −0.41) |
Logistic regression models include natural log-transformed cytokine levels with adjustment for gender, APACHE III score, comorbid diabetes, ALI risk factor, and tidal volume assignment. Odds ratio is expressed per natural log increment of cytokine level.
Linear regression models include natural log-transformed cytokine levels with adjustment for gender, APACHE III score, comorbid diabetes, ALI risk factor, and tidal volume assignment. Days are expressed per natural log increment of cytokine level.
Effect Modification by BMI on the Association of Baseline Plasma Biomarkers and Outcomes in ALI
To evaluate whether BMI modified the association between circulating inflammatory cytokine levels and mortality, VFDs, and OFDs, we created eight separate regression models for each outcome (24 models total). The independent variable in these models was the individual cytokine level, and BMI was fit as a linear continuous variable. These models also included an interaction term between BMI and cytokine level, as well as gender, APACHE III score, ALI risk factor, presence of diabetes, and tidal volume group assignment. As shown in Table 5, BMI did not modify the association between the baseline cytokine level and mortality (ie, none of the coefficients for the interaction terms in the models were statistically significant). In the regression models, BMI did modify the association between vWF and both VFDs (P = .04) and OFDs (P = .01) as well as the association between sICAM and OFDs (P = .04). However, when the Bonferroni method36 was conservatively used to determine the significance of these P values (0.05/24 = 0.0021), they were not statistically significant.
Table 5.
—Effect Modification by BMI on the Association of Plasma Biomarker Measurements at Baseline With Clinical Outcomes
| Odds Ratio and 95% CI of Interaction Term |
Coefficient and 95% CI of Interaction Term |
||
| Interaction Term in Regression Model | Logistic Regression Models of Mortalitya | Linear Regression Models of VFDsb | Linear Regression Models of OFDsb |
| IL-6 × BMI | 1.00 (0.99-1.01) | −0.01 (−0.06 to 0.03) | −0.03 (−0.08 to 0.01) |
| IL-8 × BMI | 1.01 (0.99-1.03) | −0.01 (−0.08 to 0.06) | −0.05 (−0.12 to 0.02) |
| TNF-α receptor 1 × BMI | 0.99 (0.97-1.01) | −0.003 (−0.10 to 0.09) | −0.03 (−0.12 to 0.06) |
| SP-D × BMI | 0.99 (0.97-1.01) | 0.07 (−0.01 to 0.14) | 0.06 (−0.02 to 0.13) |
| sICAM × BMI | 1.00 (0.98-1.03) | 0.04 (−0.07 to 0.14) | −0.11 (−0.21 to −0.005) |
| vWF × BMI | 1.03 (0.99-1.06) | −0.12 (−0.24 to −0.003) | −0.15 (−0.26 to −0.04) |
| Protein C × BMI | 0.98 (0.96-1.01) | 0.08 (−0.05 to 0.20) | 0.03 (−0.09 to 0.15) |
| PAI-1 × BMI | 1.00 (0.98-1.02) | 0.02 (−0.06 to 0.10) | 0.02 (−0.06 to 0.10) |
Logistic regression models of mortality include natural log-transformed cytokine levels with adjustment for BMI (as a continuous linear variable) and an interaction term between cytokine level and BMI in addition to gender, APACHE III score, comorbid diabetes, ALI risk factor, and tidal volume assignment. Odds ratio is expressed per natural log increment of cytokine level.
Linear regression models include natural log-transformed cytokine levels with adjustment for BMI (as a continuous linear variable) and an interaction term between cytokine level and BMI in addition to gender, APACHE III score, comorbid diabetes, ALI risk factor, and tidal volume assignment. Days are expressed per natural log increment of cytokine level.
Discussion
Individuals who are obese but otherwise healthy have elevated levels of proinflammatory cytokines relative to individuals who are healthy and of normal weight. In the obese state, adipose tissue is a metabolically active tissue consisting of adipocytes with infiltrating macrophages that produce a variety of mediators thought to be related to many of the metabolic consequences of obesity.19,37 These cytokines include many of the proinflammatory mediators associated with worse outcomes in patients with ALI, such as IL-6, IL-8, and TNF-α, and thereby are believed to partially drive ALI pathogenesis. Because patients who are obese have a heightened inflammatory state when healthy, it might be anticipated that they would have worse outcomes after ALI, yet recent evidence has suggested otherwise.
In this study, we found that increasing BMI is associated with lower levels of key biomarkers of inflammation and injury, particularly IL-6, IL-8, and SP-D, in patients with ALI. These results are opposite those found in people who are healthy and obese, where IL-6 and IL-8 are increased in proportion to BMI,38,39 thus suggesting that patients who are obese may have an attenuated inflammatory response in the setting of ALI that leads to a reduction in alveolar epithelial injury. Further, we have demonstrated that higher BMI is associated with a blunted rise in SP-D, thus suggesting that reduced alveolar epithelial injury persists over time in patients who are obese. Additionally, our finding that higher BMI is associated with a rising WBC count, simultaneous with reduced levels of inflammatory cytokines classically associated with leukocytosis, further suggests that obesity in critical illness may be associated with fundamental alterations in the innate immune response.
It is notable that increasing obesity was associated with higher prerandomization tidal volume per kilogram predicted body weight in our study, a finding that strengthens our results. Because higher tidal volumes have been shown to lead to increased circulating cytokines,9 one might expect that a higher prerandomization tidal volume might cause increased plasma proinflammatory cytokine levels, worse ventilator-associated lung injury,40 and poorer outcomes. However, although patients who were obese received higher tidal volumes before being enrolled in the parent RCTs, we found lower levels of proinflammatory cytokines and outcomes no different than for patients who were lean.
In contrast to our findings of decreased circulating markers of inflammation (IL-8 and IL-6) and alveolar epithelial injury (SP-D) in patients who were obese, we did find that vWF, a marker of endothelial injury, was increased in patients who were obese and critically ill. This may suggest that although alveolar epithelial injury is attenuated in patients who are obese through a blunted inflammatory response, this might be counterbalanced by heightened endothelial injury in the setting of underlying microvascular disease. Such a mixed effect of obesity on markers of lung injury may explain why our study and several prior studies have not found that patients who are obese have better outcomes after critical illness than their counterparts who are lean.12-14
We also found that circulating levels of the endogenous anticoagulant protein C are significantly higher in patients who are obese with ALI and, as expected based on prior data,14 are associated with decreased mortality. Circulating protein C levels have been shown to be elevated in volunteers who are healthy and obese,41 and our finding increased levels of protein C in patients who are obese with ALI may simply reflect this usual state and may partially explain why patients who are obese seem to be “protected” from poor outcomes in the ICU (ie, why their outcomes are not worse than those of patients with normal weight, as we might expect).
While our results suggest that alveolar epithelial injury in patients who are obese and critically ill may be blunted, these findings did not correspond with detectable improvements in clinical outcomes in patients who are obese. The results from our study that obesity is not associated with mortality are similar to several previous publications.20,22,42 However, other prior studies have found that obesity is associated with decreased ICU mortality.21,43 Possible explanations for these differences among studies include varying patient populations (ALI vs general critical illness), different categorizations of BMI, and limited sample size of groups of patients who were severely obese. We also did not find an association between BMI and either VFDs or OFDs, a result that is inconsistent with some prior studies.20,42 This difference might be explained by the fact that the ventilator and weaning strategies for all participants in the parent RCTs occurred according to a standardized protocol. It may be that physicians are slower to wean patients who are obese from the ventilator in the absence of a standardized protocol because of perceptions that these patients are at higher risk of adverse events compared with patients of normal weight.
In keeping with prior studies in this patient population,9 we also found that plasma levels of several key inflammatory biomarkers were associated with risk of death, VFDs, and OFDs. Importantly, however, the relationships between higher inflammatory baseline biomarker levels and poorer clinical outcomes were not modified by BMI. In other words, patients with high cytokine levels had similarly poor outcomes regardless of BMI, thus suggesting that this is not the mechanism by which BMI might influence mortality in patients who are critically ill.
Mechanisms by which obesity may affect the immune response are the subject of intense investigation, and much further research is needed in this area. It has been suggested that extra nutritional stores available to patients who are obese during critical illness may be partially protective, but this hypothesis does not explain immune alteration.44 It has also been recently suggested that obesity may impair host defense in the lung and attenuate the inflammatory response to exogenous insults.45 If this is true, the patient who is obese may be at increased risk of such insults as infection (eg, pneumonia),46 but may also exhibit reduced capabilities to mount the overwhelming pulmonary inflammatory response that characterizes ALI.
While our hypothesis that obesity may fundamentally alter the innate immune system is interesting and novel, there are also additional explanations for our finding decreased circulating levels of proinflammatory biomarkers. One possibility is that patients who are obese have less severe lung injury at presentation and therefore have reduced levels of circulating cytokines. The Pao2/Fio2 ratio was not significantly higher with increasing obesity, thus suggesting that ALI was not less severe with increasing BMI (Table 1). However, when Pao2/Fio2 was evaluated across BMI groups after exclusion of patients were underweight, increasing BMI was associated with increasing Pao2/Fio2. Another possibility is that patients who are obese are physiologically more fragile and therefore may have an exaggerated response to lower levels of inflammatory cytokines, thus becoming just as ill as patients who are lean with a smaller physiologic insult. If this were the case, though, we would expect to find that BMI modifies the association between cytokine levels and outcomes. A final possibility is that lower plasma concentrations of mediators may simply be a result of hemodilution, as has been shown to be true with prostatic-specific antigen in men who are obese.47 If this latter possibility were true, however, we might expect to find all mediators uniformly decreased among patients who are obese, which we did not detect. Additionally, it is important to note that while patients who are obese and otherwise healthy have elevated circulating levels of inflammatory cytokines, these levels are typically in the 1.0 to 5.0 pg/mL range.48 In patients who are critically ill, whether lean or obese, these levels are markedly elevated, often by 50 to 100 times those found in patients who are healthy and obese.
There are several limitations of our study. BMI may be inaccurate in patients who are critically ill because of fluid resuscitation or erroneous measurements of height in patients who were supine. Additionally, discerning the presence of bilateral pulmonary infiltrates on a chest radiograph may be especially difficult in patients who are obese, thus rendering the diagnosis of ALI in patients who are obese potentially inaccurate. Such misclassification of ALI in patients who are obese could have led to incorrect case ascertainment. It should be noted, however, that we have found consistently decreasing levels of IL-6, IL-8, and SP-D across all BMI groups, even among participants with mild-to-moderate obesity (BMI 25-40) where these differences in appearance of the chest radiograph are less likely to occur. Furthermore, the sample size of patients who were severely obese in this study is relatively small because of the parent RCTs excluding participants with a body weight > 1 kg/cm height. Thus, our power to detect differences in the group of patients who were severely obese was limited.
Conclusions
In summary, we have found that increasing BMI is associated with both decreasing plasma inflammatory biomarkers and increasing WBC count, but not with a change in mortality, in patients who are critically ill with ALI. These findings suggest that innate immunity could be altered in patients who are obese. However, the mechanisms by which obesity may modulate innate immunity in critical illness are not clear. Future studies are needed to understand how obesity may alter the inflammatory response in patients with ALI. These investigations should include both animal and human studies comparing neutrophil trafficking, cell signaling, and differences in innate and adaptive immune responses in subjects who are obese and of normal weight.
Acknowledgments
Author contributions: Drs Stapleton and Suratt had full access to all study data and take responsibility for the accuracy and integrity of the data and data analysis.
Dr Stapleton: drafted the manuscript and contributed to the statistical analysis, study supervision, study design, data acquisition and analysis, results interpretation, and review of the manuscript for intellectual content.
Dr Dixon: contributed to the statistical analysis, study supervision, study design, data acquisition and analysis, results interpretation, and review of the manuscript for intellectual content.
Dr Parsons: contributed to the study design, data acquisition and analysis, results interpretation, and review of the manuscript for intellectual content.
Dr Ware: contributed to the study design, data acquisition and analysis, results interpretation, and review of the manuscript for intellectual content.
Dr Suratt: contributed to the statistical analysis, study supervision, study design, data acquisition and analysis, results interpretation, and review of the manuscript for intellectual content.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Abbreviations
- ALI
acute lung injury
- APACHE
Acute Physiologic and Chronic Health Evaluation
- ARDSNet
Acute Respiratory Distress Syndrome Network
- IL
interleukin
- NHLBI
National Heart, Lung, and Blood Institute
- ODFs
organ-failure-free days
- PAI-1
plasminogen activator inhibitor-1
- PEEP
positive end-expiratory pressure
- RCTs
randomized controlled trials
- sICAM
soluble intracellular adhesion molecule
- SP-D
surfactant protein D
- TNF-α
tumor necrosis factor-α
- VFDs
ventilator-free days
- vWF
von Willebrand factor
Footnotes
Funding/Support: This research was partially supported by the National Institutes of Health [Grants HL084200, P20 RR015557, HL081332, and NO1HR46064].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (http://www.chestpubs.org/site/misc/reprints.xhtml).
References
- 1.Yudkin JS. Adipose tissue, insulin action and vascular disease: inflammatory signals. Int J Obes Relat Metab Disord. 2003;27(Suppl 3):S25–S28. doi: 10.1038/sj.ijo.0802496. [DOI] [PubMed] [Google Scholar]
- 2.Shore SA, Johnston RA. Obesity and asthma. Pharmacol Ther. 2006;110(1):83–102. doi: 10.1016/j.pharmthera.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Yudkin JS, Kumari M, Humphries SE, Mohamed-Ali V. Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? Atherosclerosis. 2000;148(2):209–214. doi: 10.1016/s0021-9150(99)00463-3. [DOI] [PubMed] [Google Scholar]
- 4.Yudkin JS, Stehouwer CD, Emeis JJ, Coppack SW. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol. 1999;19(4):972–978. doi: 10.1161/01.atv.19.4.972. [DOI] [PubMed] [Google Scholar]
- 5.Tracy RP. Is visceral adiposity the “enemy within”? Arterioscler Thromb Vasc Biol. 2001;21(6):881–883. doi: 10.1161/01.atv.21.6.881. [DOI] [PubMed] [Google Scholar]
- 6.Ramos EJ, Xu Y, Romanova I, et al. Is obesity an inflammatory disease? Surgery. 2003;134(2):329–335. doi: 10.1067/msy.2003.267. [DOI] [PubMed] [Google Scholar]
- 7.Lee YH, Nair S, Rousseau E, et al. Microarray profiling of isolated abdominal subcutaneous adipocytes from obese vs non-obese Pima Indians: increased expression of inflammation-related genes. Diabetologia. 2005;48(9):1776–1783. doi: 10.1007/s00125-005-1867-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 9.Parsons PE, Eisner MD, Thompson BT, et al. NHLBI Acute Respiratory Distress Syndrome Clinical Trials Network Lower tidal volume ventilation and plasma cytokine markers of inflammation in patients with acute lung injury. Crit Care Med. 2005;33(1):1–6. doi: 10.1097/01.ccm.0000149854.61192.dc. [DOI] [PubMed] [Google Scholar]
- 10.Meduri GU, Headley S, Kohler G, et al. Persistent elevation of inflammatory cytokines predicts a poor outcome in ARDS. Plasma IL-1 beta and IL-6 levels are consistent and efficient predictors of outcome over time. Chest. 1995;107(4):1062–1073. doi: 10.1378/chest.107.4.1062. [DOI] [PubMed] [Google Scholar]
- 11.Hack CE, Hart M, van Schijndel RJ, et al. Interleukin-8 in sepsis: relation to shock and inflammatory mediators. Infect Immun. 1992;60(7):2835–2842. doi: 10.1128/iai.60.7.2835-2842.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Casey LC, Balk RA, Bone RC. Plasma cytokine and endotoxin levels correlate with survival in patients with the sepsis syndrome. Ann Intern Med. 1993;119(8):771–778. doi: 10.7326/0003-4819-119-8-199310150-00001. [DOI] [PubMed] [Google Scholar]
- 13.Pinsky MR, Vincent JL, Deviere J, Alegre M, Kahn RJ, Dupont E. Serum cytokine levels in human septic shock. Relation to multiple-system organ failure and mortality. Chest. 1993;103(2):565–575. doi: 10.1378/chest.103.2.565. [DOI] [PubMed] [Google Scholar]
- 14.Ware LB, Matthay MA, Parsons PE, Thompson BT, Januzzi JL, Eisner MD. National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome Clinical Trials Network Pathogenetic and prognostic significance of altered coagulation and fibrinolysis in acute lung injury/acute respiratory distress syndrome. Crit Care Med. 2007;35(8):1821–1828. doi: 10.1097/01.CCM.0000221922.08878.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ware LB, Eisner MD, Thompson BT, Parsons PE, Matthay MA. Significance of von Willebrand factor in septic and nonseptic patients with acute lung injury. Am J Respir Crit Care Med. 2004;170(7):766–772. doi: 10.1164/rccm.200310-1434OC. [DOI] [PubMed] [Google Scholar]
- 16.Eisner MD, Parsons P, Matthay MA, Ware L, Greene K. Acute Respiratory Distress Syndrome Network Plasma surfactant protein levels and clinical outcomes in patients with acute lung injury. Thorax. 2003;58(11):983–988. doi: 10.1136/thorax.58.11.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calfee CS, Eisner MD, Ware LB, et al. Acute Respiratory Distress Syndrome Network, National Heart, Lung, and Blood Institute Trauma-associated lung injury differs clinically and biologically from acute lung injury due to other clinical disorders. Crit Care Med. 2007;35(10):2243–2250. doi: 10.1097/01.ccm.0000280434.33451.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ware LB, Conner ER, Matthay MA. von Willebrand factor antigen is an independent marker of poor outcome in patients with early acute lung injury. Crit Care Med. 2001;29(12):2325–2331. doi: 10.1097/00003246-200112000-00016. [DOI] [PubMed] [Google Scholar]
- 19.Ferrante AW., Jr Obesity-induced inflammation: a metabolic dialogue in the language of inflammation. J Intern Med. 2007;262(4):408–414. doi: 10.1111/j.1365-2796.2007.01852.x. [DOI] [PubMed] [Google Scholar]
- 20.Morris AE, Stapleton RD, Rubenfeld GD, Hudson LD, Caldwell E, Steinberg KP. The association between body mass index and clinical outcomes in acute lung injury. Chest. 2007;131(2):342–348. doi: 10.1378/chest.06-1709. [DOI] [PubMed] [Google Scholar]
- 21.O’Brien JM, Jr, Phillips GS, Ali NA, Lucarelli M, Marsh CB, Lemeshow S. Body mass index is independently associated with hospital mortality in mechanically ventilated adults with acute lung injury. Crit Care Med. 2006;34(3):738–744. doi: 10.1097/01.CCM.0000202207.87891.FC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Brien JM, Jr, Welsh CH, Fish RH, Ancukiewicz M, Kramer AM. National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome Network Excess body weight is not independently associated with outcome in mechanically ventilated patients with acute lung injury. Ann Intern Med. 2004;140(5):338–345. doi: 10.7326/0003-4819-140-5-200403020-00009. [DOI] [PubMed] [Google Scholar]
- 23.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342(18):1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 24.Brower RG, Lanken PN, MacIntyre N, et al. National Heart, Lung, and Blood Institute ARDS Clinical Trials Network Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351(4):327–336. doi: 10.1056/NEJMoa032193. [DOI] [PubMed] [Google Scholar]
- 25.Ketoconazole for early treatment of acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. The ARDS Network. JAMA. 2000;283(15):1995–2002. doi: 10.1001/jama.283.15.1995. [DOI] [PubMed] [Google Scholar]
- 26.Randomized, placebo-controlled trial of lisofylline for early treatment of acute lung injury and acute respiratory distress syndrome. Crit Care Med. 2002;30(1):1–6. doi: 10.1097/00003246-200201000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149(3 Pt 1):818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 28.Eisner MD, Thompson T, Hudson LD, et al. Acute Respiratory Distress Syndrome Network Efficacy of low tidal volume ventilation in patients with different clinical risk factors for acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;164(2):231–236. doi: 10.1164/ajrccm.164.2.2011093. [DOI] [PubMed] [Google Scholar]
- 29.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20(6):864–874. [PubMed] [Google Scholar]
- 30.National Institutes of Health Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults—The Evidence Report. Obes Res. 1998;6(Suppl 2):51S–209S. [PubMed] [Google Scholar]
- 31.Moss M, Guidot DM, Steinberg KP, et al. Diabetic patients have a decreased incidence of acute respiratory distress syndrome. Crit Care Med. 2000;28(7):2187–2192. doi: 10.1097/00003246-200007000-00001. [DOI] [PubMed] [Google Scholar]
- 32.Knaus WA, Wagner DP, Draper EA, et al. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest. 1991;100(6):1619–1636. doi: 10.1378/chest.100.6.1619. [DOI] [PubMed] [Google Scholar]
- 33.Schoenfeld DA, Bernard GR. ARDS Network Statistical evaluation of ventilator-free days as an efficacy measure in clinical trials of treatments for acute respiratory distress syndrome. Crit Care Med. 2002;30(8):1772–1777. doi: 10.1097/00003246-200208000-00016. [DOI] [PubMed] [Google Scholar]
- 34.Bernard G. The Brussels Score. Sepsis. 1997;1(1):43–44. [Google Scholar]
- 35.Parsons PE, Matthay MA, Ware LB, Eisner MD. National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome Clinical Trials Network Elevated plasma levels of soluble TNF receptors are associated with morbidity and mortality in patients with acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2005;288(3):L426–L431. doi: 10.1152/ajplung.00302.2004. [DOI] [PubMed] [Google Scholar]
- 36.Bland JM, Altman DG. Multiple significance tests: the Bonferroni method. BMJ. 1995;310(6973):170. doi: 10.1136/bmj.310.6973.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park HS, Park JY, Yu R. Relationship of obesity and visceral adiposity with serum concentrations of CRP, TNF-alpha and IL-6. Diabetes Res Clin Pract. 2005;69(1):29–35. doi: 10.1016/j.diabres.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 39.Kim CS, Park HS, Kawada T, et al. Circulating levels of MCP-1 and IL-8 are elevated in human obese subjects and associated with obesity-related parameters. Int J Obes (Lond) 2006;30(9):1347–1355. doi: 10.1038/sj.ijo.0803259. [DOI] [PubMed] [Google Scholar]
- 40.Frank JA, Parsons PE, Matthay MA. Pathogenetic significance of biological markers of ventilator-associated lung injury in experimental and clinical studies. Chest. 2006;130(6):1906–1914. doi: 10.1378/chest.130.6.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Solá E, Navarro S, Medina P, et al. Activated protein C levels in obesity and weight loss influence. Thromb Res. 2009;123(5):697–700. doi: 10.1016/j.thromres.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 42.Akinnusi ME, Pineda LA, El Solh AA. Effect of obesity on intensive care morbidity and mortality: a meta-analysis. Crit Care Med. 2008;36(1):151–158. doi: 10.1097/01.CCM.0000297885.60037.6E. [DOI] [PubMed] [Google Scholar]
- 43.Oliveros H, Villamor E. Obesity and mortality in critically ill adults: a systematic review and meta-analysis. Obesity (Silver Spring) 2008;16(3):515–521. doi: 10.1038/oby.2007.102. [DOI] [PubMed] [Google Scholar]
- 44.Druml W. ICU patients: fatter is better? Intensive Care Med. 2008;34(11):1961–1963. doi: 10.1007/s00134-008-1246-x. [DOI] [PubMed] [Google Scholar]
- 45.Mancuso P. Obesity and lung inflammation. J Appl Physiol. 2010;108(3):722–728. doi: 10.1152/japplphysiol.00781.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Falagas ME, Kompoti M. Obesity and infection. Lancet Infect Dis. 2006;6(7):438–446. doi: 10.1016/S1473-3099(06)70523-0. [DOI] [PubMed] [Google Scholar]
- 47.Bañez LL, Hamilton RJ, Partin AW, et al. Obesity-related plasma hemodilution and PSA concentration among men with prostate cancer. JAMA. 2007;298(19):2275–2280. doi: 10.1001/jama.298.19.2275. [DOI] [PubMed] [Google Scholar]
- 48.Bastard JP, Jardel C, Bruckert E, et al. Elevated levels of interleukin 6 are reduced in serum and subcutaneous adipose tissue of obese women after weight loss. J Clin Endocrinol Metab. 2000;85(9):3338–3342. doi: 10.1210/jcem.85.9.6839. [DOI] [PubMed] [Google Scholar]