Abstract
Objectives:
The majority of women with lymphangioleiomyomatosis (LAM) present with cystic lung disease, and most require lung biopsy for definitive diagnosis. The purpose of this study was to determine the prospective diagnostic usefulness of a serologic test for vascular endothelial growth factor-D (VEGF-D), a lymphangiogenic growth factor.
Methods:
We prospectively measured serum VEGF-D levels by enzyme-linked immunoassay in 48 women presenting with cystic lung disease. Diagnostic test performance was determined from a cohort of 195 women, with tuberous sclerosis complex (TSC), TSC-LAM, sporadic LAM (S-LAM), and other cystic lung diseases in the differential diagnosis, including biopsy-proven or genetically proven pulmonary Langerhans cell histiocytosis, emphysema, Sjögren syndrome, or Birt-Hogg-Dubé syndrome.
Results:
Serum VEGF-D levels were significantly greater in S-LAM (median 1,175 [interquartile range (IQR): 780-2,013] pg/mL; n = 56) than in other cystic lung diseases (median 281 [IQR 203-351] pg/mL; n = 44, P < .001). In the cohort evaluated prospectively, 12 of the 15 individuals ultimately diagnosed with LAM by biopsy had VEGF-D levels of > 800 pg/mL, whereas levels were < 600 pg/mL in all 18 subjects later diagnosed with other causes of cystic lung disease. Receiver operating characteristic curves demonstrated that VEGF-D effectively identified LAM, with an area under the curve of 0.961(95% CI, 0.923-0.992). A VEGF-D level of > 600 pg/mL was highly associated with a diagnosis of LAM (specificity 97.6%, likelihood ratio 35.2) and values > 800 pg/mL were diagnostically specific. Serum VEGF-D levels were significantly elevated in women with TSC-LAM (median 3,465 [IQR 1,970-7,195] pg/mL) compared with women with TSC only (median 370 [IQR 291-520] pg/mL), P < .001).
Conclusions:
A serum VEGF-D level of > 800 pg/mL in a woman with typical cystic changes on high-resolution CT (HRCT) scan is diagnostically specific for S-LAM and identifies LAM in women with TSC. A negative VEGF-D result does not exclude the diagnosis of LAM. The usefulness of serum VEGF-D testing in men or in women who do not have cystic lung disease on HRCT scan is unknown.
Lymphangioleiomyomatosis (LAM) is a rare, progressive, frequently fatal cystic lung disease that affects women almost exclusively.1,2 LAM occurs in up to 40% of women with tuberous sclerosis complex (TSC-LAM),3-5 a tumor suppressor syndrome associated with seizures, cognitive impairment, and hamartomas in multiple organs, and in a nonheritable sporadic form (S-LAM) that involves only the lung, lymphatics, and kidney. High-resolution CT (HRCT) scanning in LAM demonstrates round, relatively uniform, thin-walled cysts randomly distributed throughout the lungs. Fat- and smooth muscle-rich tumors of the kidney, called angiomyolipomas (AMLs), occur in approximately 80% and 30% of patients with TSC-LAM and S-LAM, respectively. Lymphatic obstruction leads to chylous fluid collections in the pleural, peritoneal, and pericardial spaces in approximately 30% of patients with S-LAM and < 10% of patients with TSC-LAM.6
In the presence of a compatible clinical presentation and typical changes on HRCT scan, a clinical diagnosis of LAM is often made without obtaining a biopsy specimen if a renal AML, chylous fluid collection, or TSC are present.7 However, the majority of patients who present for evaluation of LAM do not have these clinical features. In these patients with “lone” S-LAM, a definitive diagnosis cannot be based on HRCT scan alone, because HRCT scan accuracy is estimated at ≤ 80% in the hands of expert radiologists.8 Tissue confirmation is often required to distinguish lone S-LAM from other lung diseases commonly considered in the differential diagnosis, including pulmonary Langerhans cell histiocytosis (PLCH), emphysema, Sjögren syndrome, systemic lupus, or other connective tissue disease with associated follicular bronchiolitis or lymphocytic interstitial pneumonitis, or Birt-Hogg-Dubé (BHD) syndrome.
Vascular endothelial growth factors C (VEGF-C) and D (VEGF-D) are ligands for the lymphatic growth factor receptor VEGFR-2 and VEGFR-3/Flt-4 that induce formation of lymphatics and promote the spread of tumor cells to lymph nodes in mouse models and in humans.9,10 Seyama et al11 reported that serum levels of VEGF-D, but not VEGF-C, are elevated in patients with S-LAM in comparison with normal controls.
We previously reported the concept that serum VEGF-D levels might have diagnostic potential, based on preliminary data derived from a mixed population of male and female subjects with LAM and other cystic lung diseases.12 That study included only seven women with other causes of cystic lung disease (four with emphysema and three with PLCH). Our objective in this study was to prospectively validate VEGF-D as a diagnostic test in a much larger, clinically relevant referral population of female patients with cystic lung disease. We also added the other two diseases that are typically considered in the diagnosis of LAM and specifically analyzed the diagnostic usefulness of serum VEGF-D in patients with lone LAM. The additional patient recruitments and prospective/subset analyses in this study led us to conclude that the use of VEGF-D can obviate the need for surgical lung biopsy in approximately 70% of patients with lone LAM who present for diagnostic evaluation.
Materials and Methods
Study Population
Prospective VEGF-D testing was performed in 48 women presenting for clinical evaluation who did not meet criteria for definite LAM diagnosis at the time of initial evaluation. In comparison, results of retrospectively performed VEGF-D testing were analyzed from 122 women with known diagnoses. Subjects were recruited from pulmonary clinics and LAM and TSC clinics at the University of Cincinnati and Cincinnati Children’s Hospital Medical Center, and via referral from other physicians and several patient foundations. Stored samples from adult healthy volunteers were obtained from a normal donor repository. Archived samples were provided by study investigators for a subset of patients with PLCH, BHD, and Sjögren syndrome with cystic lung disease. Diagnoses were verified through review of clinical, radiographic, and pathologic material. This study was approved by the institutional review boards at The University of Cincinnati College of Medicine (05-12-19-01) and Cincinnati Children’s Hospital Medical Center (2008-0932).
Inclusion criteria for S-LAM required either biopsy-proven LAM, or cystic lung disease with an AML or chylous fluid collection. The diagnosis of TSC-LAM was based on the presence of cystic lung disease in a woman with TSC diagnosed using clinical criteria or by genetic analysis.13 Women with TSC and no evidence of cystic lung disease (“TSC only”) had a normal chest HRCT scan within the prior 18 months. Only female subjects were included in the Other Disease comparison groups in this study. The diagnosis of BHD was based on clinical and/or genetic criteria.14 Of the seven subjects with serologic diagnosis of Sjögren syndrome, all had radiographic findings of cystic lung disease, and four had undergone lung biopsy. PLCH and emphysema cases were selected for inclusion in this study specifically based on establishment of a definitive diagnosis by prior lung biopsy.
A portion of these data were previously reported.12 The numbers of overlapping subjects with cystic lung disease were 18/56 with S-LAM, 3/10 with emphysema, 4/15 with PLCH, 0/12 with BHD, and 0/7 with Sjögren syndrome. Seven of the 10 subjects with lymphangiomatosis, 17/28 women with TSC-LAM and 10/17 women with TSC only were previously reported.
VEGF-D Measurements
Serum samples were obtained in serum separator tubes and allowed to clot for 30 min prior to centrifugation at 3,500 rpm for 10 min. VEGF-D levels were evaluated using the Quantikine Human VEGF-D Immunoassay (R&D Systems; Minneapolis, MN) according to the manufacturer’s instructions. Results were quantified using the chromogenic substrate tetramethylbenzidine and analysis with a microtiter plate spectrophotometer (Perkin Elmer; Waltham, MA) at wavelength of 450 nm, with correction at 570 nm. Samples were assayed on two different days, and quality control samples with known values were included in each assay.
Statistical Analysis
All statistical analyses were performed using SAS 9.1 (SAS Institute; Cary, NC). VEGF-D levels in patient groups are expressed as medians and interquartile range (IQR). Groups were compared for age and VEGF-D levels by analysis of variance and Mann-Whitney tests, respectively. Association between age and VEGF-D was measured using Spearman correlation. A two-sided probability value of < .05 was regarded as significant.
Results
Study Subjects
Serum samples were evaluated from 195 female subjects with definite S-LAM or other proven causes of cystic lung disease that may mimic LAM (emphysema, PLCH, BHD, and Sjögren), TSC-LAM, TSC only, lymphangiomatosis, and healthy volunteers. The clinical characteristics of the study population are shown in Table 1. The presentations and disease severity of subjects with LAM and those with other cystic lung diseases were similar. There was no significant difference in the age of subjects with LAM compared with the aggregate of all other cystic lung disease groups (43.2 ± 11.7 vs 40.8 ± 11.9 years, respectively, P = .255). Subjects with TSC-LAM were older than women with TSC only (P < .05). Current cigarette smoking was common in subjects with PLCH. A greater proportion of women with TSC-LAM were current or former smokers than those with TSC only. For both TSC-LAM and S-LAM, we found no significant association between VEGF-D levels and age, most contemporaneous FEV1 value, or use of supplemental oxygen.
Table 1.
—Clinical Characteristics of the Study Population
| Category | No. | Age, y | FEV1, % predicted | Smoking History, % Never/Former/Current | SupplementalOxygen Use, % | History of Spontaneous Pneumothorax, % |
| S-LAM | 56 | 45.7 ± 10.7 | 63.6 ± 23.5 | 80/16/4 | 36 | 45 |
| TSC with LAM | 28 | 38.1 ± 11.9 | 66.6 ± 23.2 | 56/32/12 | 32 | 50 |
| Emphysema | 10 | 42.3 ± 4.6 | 64.1 ± 29.9 | 40/50/10 | 20 | 50 |
| PLCH | 15 | 33.2 ± 9.3 | 75.6 ± 20.1 | 7/13/80 | 13 | 0 |
| BHD | 12 | 50.5 ± 14.5 | 96.5 (n = 2 only) | 67/8/25 | 0 | 83 |
| Sjögren with cystic lung disease | 7 | 38.7 ± 7.5 | 73.0 ± 15.7 | 100/0/0 | 0 | 0 |
| TSC only | 17 | 28.8 ± 11.1 | Unknown | 93/7/0 | 0 | 0 |
| Lymphangiomatosis | 10 | 37.9 ± 12.7 | Unknown | 80/20/0 | 0 | 0 |
| Healthy volunteers | 40 | 31.3 ± 4.8 | Not applicable | Unknown | NA | NA |
BHD = Birt-Hogg-Dubé; LAM = lymphangioleiomyomatosis; NA = not applicable; S-LAM = sporadic lymphangioleiomyomatosis; PLCH = pulmonary Langerhans cell histiocytosis; TSC = tuberous sclerosis complex.
Serum VEGF-D Levels in a Prospective Clinical Context
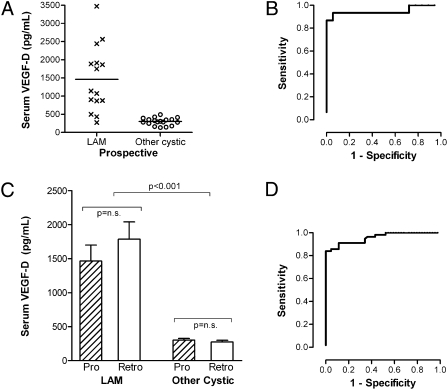
To test how VEGF-D would perform in a clinical setting, we conducted VEGF-D testing prospectively in 48 women presenting as diagnostic unknowns without clinically definite criteria for LAM at the time of testing. All patients had HRCT scan evidence of cystic lung disease and were referred for evaluation of suspected LAM. For this prospective cohort, 15 individuals were later diagnosed with LAM (by biopsy) and 18 were diagnosed with other cystic lung diseases (based on biopsy, genetic, or serologic testing), whereas 15 others had not pursued definitive diagnostic evaluations at the time of this writing. Serum VEGF-D levels were significantly greater in prospectively identified S-LAM cases than in women later proven to have other causes of cystic lung disease (Fig 1A), with an area under the receiver operating characteristic (ROC) curve of 0.948 ± 0.048 (95% CI, 0.855-1.0). Twelve of the 15 patients who proved to have LAM had VEGF-D levels of 800 pg/mL or greater. None of the 18 individuals confirmed to have other lung diseases had VEGF-D levels > 600 (Fig 1A). When results of prospective VEGF-D testing were compared with those from the larger cross-sectional (retrospective) cohort, there was no significant difference in VEGF-D levels in the two cohorts (Fig 1C), and the test performance was similar (0.961 ± 0.018 [95% CI, 0.923-0.992]) (Fig 1D).
Figure 1.
Serum VEGF-D levels prospectively distinguish sporadic LAM (S-LAM) from other cystic lung diseases in patients presenting as diagnostic unknowns. (A) Results of prospective serum VEGF-D testing in patients presenting prior to definitive diagnosis. A total of 15 individuals were subsequently diagnosed with LAM by biopsy, and VEGF-D levels were > 800 pg/mL in 12 of these 15 cases. VEGF-D levels were normal in the 18 individuals later diagnosed with other causes of cystic lung disease (Other Cystic). Lines denote median values for each group. (B) ROC curve based on this prospective cohort only. The area under the ROC curve is 0.948 ± 0.048 (95% CI, 0.855-1.0). (C) Comparison of serum VEGF-D levels in the prospective cohort vs the retrospective cohort demonstrates similar findings in both cohorts. (D) ROC curve for LAM vs other cystic lung diseases. Data included are from combined retrospective and prospective cohort of subjects with definite S-LAM (n= 56) in comparison with females with the other cystic lung diseases (emphysema, pulmonary Langerhans cell histiocytosis [PLCH], Birt-Hogg-Dubé [BHD] syndrome, and Sjögren syndrome with follicular bronchiolitis, n = 44 total). Healthy volunteers are not included in this analysis. Area under the ROC curve is 0.961 ± 0.018 (95% CI, 0.923-0.992). LAM = lymphangioleiomyomatosis; Pro = prospective cohort; Retro = retrospective cohort; ROC = receiver operating characteristic; VEGF-D = vascular endothelial growth factor D.
Serum VEGF-D Levels in Relationship to Disease Features
We investigated potential relationships between VEGF-D and LAM-associated clinical disease features. The median serum VEGF-D level was elevated in S-LAM subjects without AMLs (1,142 [IQR 659-1,912] pg/mL) and not significantly different from S-LAM subjects with AMLs (1,191 [IQR 878-2,243] pg/mL). There was a trend toward greater VEGF-D elevation in subjects with S-LAM with a history of chylous effusions compared with those without this disease manifestation, but the difference did not reach statistical significance (1,855 [IQR 917-3,014] pg/mL vs 1,142 [IQR 691-1,885] pg/mL, P= .086).
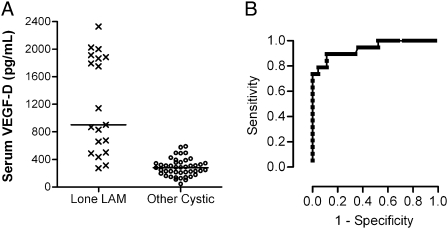
The availability of a diagnostic test for LAM would be most beneficial for the subset of patients with cystic lung disease who present without other corroborating disease features (eg, an AML or chylothorax). For these subjects with lone S-LAM, the median serum VEGF-D level was significantly greater than other cystic lung disease groups (P < .0001) (Fig 2A). The ROC curve (Fig 2B) shows that VEGF-D remains an effective diagnostic test in the lone LAM subgroup. At a VEGF-D value of 600 pg/mL, the test sensitivity is 73.7% and specificity 100%. The sensitivity is 60% at a more conservative cut-off level of 800 pg/mL.
Figure 2.
Serum VEGF-D levels are elevated in S-LAM in the absence of angiomyolipomas (AMLs) or chylous effusions and distinguish lone LAM from other cystic lung diseases that mimic LAM. (A) Serum VEGF-D levels in lone LAM vs other cystic lung diseases in the differential diagnosis. Subjects with lone LAM (n = 19) lacked other corroborating disease features, such as tuberous sclerosis complex (TSC), AMLs, or chylous effusions, and were all diagnosed by biopsy. Lines denote median values for each group. (B) ROC curve for lone LAM vs other cystic lung diseases. The area under the ROC curve is 0.940 ± 0.034 (95% CI, 0.873-1.0). See Figure 1 legend for expansion of abbreviations.
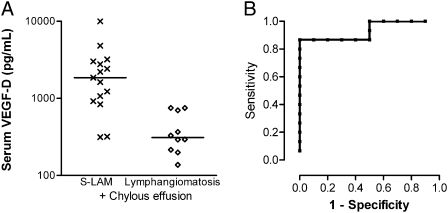
To determine whether other causes of lymphatic dysfunction alone could lead to elevated VEGF-D levels, we obtained samples from women with biopsy-proven lymphangiomatosis, a lymphatic dysplasia syndrome. Although bilateral cystic lung disease has not been observed in lymphangiomatosis, it is often considered in the differential diagnosis of LAM when chylous effusions or ascites are present. VEGF-D levels were significantly greater in patients with LAM with a history of chylous effusions than in the lymphangiomatosis group (Fig 3). Three of the 10 women with lymphangiomatosis had mildly elevated VEGF-D levels of 700 to 750 pg/mL, suggesting that careful radiographic evaluation for bilateral cystic lung disease and a VEGF-D cutoff of ≥ 800 pg/mL is also optimal for this subpopulation.
Figure 3.
In subjects with chylous effusions, serum VEGF-D levels distinguish subjects with LAM from those with lymphangiomatosis. (A) Comparison of serum VEGF-D levels in subjects with S-LAM and history of chylous effusions vs women with lymphangiomatosis, a lymphatic disorder also associated with chylous effusions. Lines denote median values for each group. (B) ROC curve for LAM with chylous effusions vs lymphangiomatosis. The area under the ROC curve is 0.933 ± 0.051 (95% CI, 0.834-1.0). See Figure 1 legend for expansion of abbreviations.
Determination of Overall Test Performance
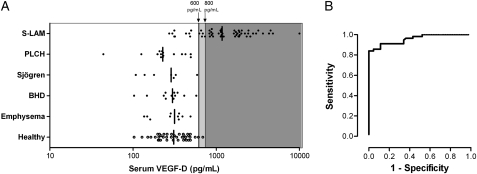
We used the entire retrospective and prospective study cohorts to determine VEGF-D test performance. Median serum VEGF-D levels were elevated in S-LAM compared with healthy volunteers (1,175 [IQR 780-2,013] pg/mL vs 309 [IQR 211-433] pg/mL, P < .001) (Fig 4). Furthermore, serum VEGF-D levels were normal in emphysema, PLCH, Sjögren with cystic lung disease, and BHD. The ROC curve demonstrates an area under the curve of 0.961 (95% CI, 0.923-0.992) for S-LAM in comparison with other cystic lung disease controls (Fig 4B). In this context, a VEGF-D cut-off value of > 607 pg/mL was associated with 83.9% sensitivity (95% CI, 71.7%-92.4%) and 100% specificity for LAM. When the comparison group was expanded to include the 40 healthy female volunteers, the area under the ROC curve was 0.950 (95% CI, 0.914-0.987). At 600 pg/mL, the sensitivity was unchanged, but specificity decreased slightly to 97.6% (95% CI, 91.7%-99.7%) with a positive likelihood ratio of 35.3 for diagnosis of LAM.
Figure 4.
Test performance of serum VEGF-D levels in distinguishing women with S-LAM from those with other cystic lung diseases that mimic LAM. (A) Serum VEGF-D levels in the combined prospective and retrospective cohorts of subjects with definite S-LAM vs female subjects with other cystic lung diseases, as proven by biopsy and/or genetic testing, and healthy volunteers. Lines denote median values for each group, and proposed cut-off values are shown. (B) ROC curve for LAM vs other cystic lung diseases. Data included are from subjects with definite S-LAM (n = 56) in comparison with women with the other cystic lung diseases (emphysema, PLCH, BHD, and Sjögren syndrome with follicular bronchiolitis, n = 44 total). Healthy volunteers are not included in this analysis. Area under the ROC curve is 0.961 ± 0.018 (95% CI, 0.923-0.992). See Figure 1 legend for expansion of abbreviations.
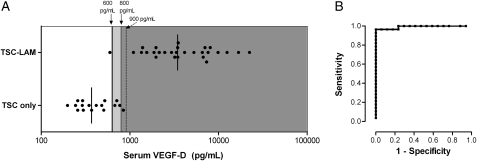
We also evaluated VEGF-D test performance as a potential screening tool for LAM in women with TSC. VEGF-D levels were significantly greater in women with TSC-LAM (median 3,465 [IQR 1,970-7,195] pg/mL) than in women with S-LAM (1,175 [IQR 780-2,013] pg/mL, P < .0001). Serum VEGF-D levels were significantly elevated in women with TSC-LAM in comparison with women with TSC only (Fig 5). However, specificity for TSC-LAM, as based on chest CT scan evidence of cysts, was incomplete at a cut-off value of 800 pg/mL.
Figure 5.
Test performance of serum VEGF-D levels in discriminating the presence or absence of LAM in women with TSC, an at-risk population. (A) Results of VEGF-D testing in women with TSC-LAM (n = 28) vs women with TSC only (n = 17). Women with TSC only were defined as those with the absence of cysts on chest scan within 18 months of VEGF-D testing. Lines denote median values for each group, and performance at proposed cut-off values is shown. Note that the dotted line indicates that a higher cut-off value of 900 pg/mL would be needed to achieve 100% specificity in this setting. (B) ROC curve for VEGF-D in women with TSC-LAM vs TSC only. Area under the ROC curve is 0.992 ± 0.010 (95% CI, 0.973-1.0). See Figure 1 and 2 legends for expansion of abbreviations.
Discussion
The objective of this study was to prospectively evaluate the test characteristics and diagnostic usefulness of serum VEGF-D for LAM. We found that VEGF-D effectively discriminated LAM and lone elevated serum LAM from other cystic lung diseases that are commonly considered in the differential diagnosis, and was associated with the presence of cystic lung disease in women with TSC. We conclude that serum VEGF-D is a useful diagnostic test for S-LAM and a promising screening tool for LAM in women with TSC.
The finding of thin-walled cysts on HRCT scanning of the chest can represent a significant diagnostic and management dilemma for the pulmonary clinician. The most likely diseases in the differential are PLCH, emphysema, and LAM. Less common disorders that should also be considered include Sjögren syndrome or systemic lupus associated with follicular bronchiolitis, lymphocytic interstitial pneumonitis, or BHD syndrome, a genodermatosis associated with kidney tumors, lung cysts, and spontaneous pneumothorax. Unfortunately, the clinical presentation is not sufficient to make a definitive diagnosis in the majority of patients with S-LAM, and pathologic confirmation is often considered. Although diagnosis by transbronchial biopsy has been reported, the yield is considered to be low, and lung biopsy via video-assisted thoracoscopic surgery is the procedure of choice. The risks of surgical biopsy and general anesthesia, including prolonged air leak, prolonged chest wall pain or numbness, and death, must be weighed against the potential benefits.
Serum VEGF-D was elevated above the upper limit of values in healthy volunteers or cystic lung disease controls in about 78% of patients with S-LAM and in 96% of women with TSC-LAM. LAM is “highly likely” in a woman with a VEGF-D level of 600 pg/mL and typical cystic change on HRCT scan, and clinically definite in a patient with a level of 800 pg/mL. These criteria proved to be equally robust in both a retrospective cohort and a prospective set of patients who had VEGF-D levels drawn before the diagnosis was established. In the prospective cohort, the test performed as predicted and could have obviated the need for biopsy in > 70% of patients. There were no false positives from individuals who were later shown to have other causes of cystic lung disease.
We also found that serum VEGF-D levels are elevated in the most relevant subset of patients with LAM, those with lone LAM, although values tended to be higher (though not significantly) in those patients with S-LAM with a history of chylous effusion or an AML. In a recent report, Glasgow et al15 concluded in a subanalysis that serum VEGF-D was only a “fair predictor” of LAM in patients without lymphadenopathy as defined by Einstein et al.16 However, in the Glasgow et al study,15 the patients with LAM were compared only to healthy volunteers and not to patients with HRCT scan evidence of cystic lung disease, which is the clinically relevant comparison. In addition, the mean VEGF-D in their normal control population was more than twice that reported in our study, the Seyama et al11 series, or the manufacturer’s literature, resulting in overlap with disease subjects that significantly compromised the diagnostic value of the test. From our data and test interassay variability, we find that a serum VEGF-D level of 800 pg/mL is a conservative threshold for a definitive diagnosis of LAM in patients with typical thin-walled cystic change on HRCT scan. This cut-point is based on the assay conditions and reagents used in this study. To our knowledge, this test is not available in clinically certified laboratories at this time.
Although we did not perform pulmonary function testing coincident with VEGF-D testing in the present study, we did not find any association between serum VEGF-D level and subject age, supplemental oxygen use, or most recent FEV1% predicted. Similarly, Glasgow et al15 did not find any association between VEGF-D levels and a number of pulmonary function parameters, including FEV1, FVC, FEV1/FVC ratio, total lung capacity, residual volume, or decline in FEV1 or diffusing capacity of the lung for carbon monoxide (Dlco) per year. That group did find that higher VEGF-D levels were associated with a higher severity score on CT scan analysis and lower Dlco or Dlco/alveolar volume in the subset of patients with lymphatic involvement. Seyama et al11 found that Dlco/alveolar volume, and FEV1/FVC but not FEV1, total lung capacity, or residual volume correlated with VEGF-D levels. Should future studies aim to incorporate VEGF-D testing as a potential biomarker of therapeutic response, it will be important to validate changes in VEGF-D levels with clinically relevant outcome measures.
For women with TSC, elevation in serum VEGF-D levels was strongly associated with the presence of cystic lesions on chest HRCT scan. These data suggest that serum VEGF-D may be useful for screening young women with TSC for the development of LAM, and could reduce the burden of radiation exposure imposed using the current empirical practices of periodic HRCT scanning in this population.17 Longitudinal studies to evaluate the predictive value of elevations in serum VEGF-D for TSC-LAM are underway. Although we have observed very elevated VEGF-D levels (up to 3,500 pg/mL) in five men with TSC and normal chest CT scans and in two men with TSC-LAM,12 the potential implications of VEGF-D levels in men with TSC are unknown.
VEGF-D is expressed by LAM cells, and dissemination of LAM cells has been demonstrated to occur via local lymphangiogenesis.18-20 VEGF-D has also been implicated in LAM cell proliferation via the Akt/mTOR pathway.21 We speculate that VEGF-D promotes metastasis in LAM and that therapeutic strategies that target lymphangiogenic pathways have promise.
In summary, we find that serum VEGF-D is a robust diagnostic test for S-LAM and promising screening test for TSC-LAM in women with TSC. We suggest performing serum VEGF-D testing in women who present with typical cystic lung disease on HRCT scan in the absence of other diagnostic elements, such as TSC, angiomyolipomata or chylous effusion. Based on our assay conditions, for women with levels > 800 pg/mL, a diagnosis of S-LAM may be established without biopsy. For patients with levels between 600 and 800 pg/mL, the diagnosis is considered highly likely, and the decision to perform a biopsy should be based on the clinical context. Normal VEGF-D levels occur in approximately one-quarter of women with LAM, and therefore a normal VEGF-D level does not exclude the diagnosis of LAM. The usefulness of VEGF-D testing in women without cystic lung disease and in men is unknown. Prospective studies to determine if elevation of serum VEGF-D presages the development of LAM in women with TSC are underway.
Acknowledgments
Author contributions: Dr Young: contributed to designing and conducting the study and writing the manuscript.
Dr VanDyke: contributed to performing data analysis and assisting with writing the manuscript.
Mr Gulleman: contributed to manuscript preparation and performing research assays.
Dr Inoue: contributed to manuscript preparation and providing critical patient samples and data.
Dr Brown: contributed to manuscript preparation and providing critical patient samples and data.
Dr Schmidt: contributed to manuscript preparation and providing critical patient samples and data.
Dr Linehan: contributed to manuscript preparation and providing critical patient samples and data.
Dr Hajjar: contributed to manuscript preparation and collecting and analyzing research data.
Dr Kinder: contributed to performing data analysis and assisting with writing the manuscript.
Dr Trapnell: contributed to manuscript preparation and providing critical patient samples and data.
Dr Bissler: contributed to manuscript preparation and providing critical patient samples and data.
Dr Franz: contributed to manuscript preparation and providing critical patient samples and data.
Dr McCormack: contributed to designing and conducting the study and writing the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Kinder received grant monies of > $100,000 from the National Institutes of Health. Drs Young and McCormack have received federal and university grant monies and funding from Wyeth for clinical trials in LAM. They have filed a patent application for the development of the VEGF-D assay as a diagnostic tool. They have waived their rights to royalties generated by the performance of the assay. Mr Gulleman and Drs VanDyke, Inoue, Brown, Schmidt, Linehan, Hajjar, Trapnell, Bissler, and Franz have reported that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The content of this publication does not necessarily reflect the views or policies of the Department of Heath and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Other contributions: We thank the many physicians and nurses who referred patients for this study, and the research coordinators at all sites who assisted with subject enrollment and sample collection. We also thank all patients who contributed to the study and The LAM Foundation, The LYMF Foundation, The Lymphangiomatosis Foundation, the Birt Hogg Dubé Family Alliance, and the Myrovlytis Trust for assistance with recruitment.
Abbreviations
- AML
angiomyolipoma
- BHD
Birt-Hogg-Dubé
- Dlco
diffusing capacity of the lung for carbon monoxide
- HRCT
high-resolution CT
- IQR
interquartile range
- LAM
lymphangioleiomyomatosis
- PLCH
pulmonary Langerhans cell histiocytosis
- ROC
receiver operating characteristic
- S-LAM
sporadic lymphangioleiomyomatosis
- TSC
tuberous sclerosis complex
- VEGF-D
vascular endothelial growth factor D
Funding/Support: This study was funded in part by a pilot project grant from The LAM Foundation, The Tante Mela Foundation, and NIH/NHLBI RR19498. This research was also supported in part by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research. This project has been funded in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract HHSN261200800001E (to L. S. S.).
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (http://www.chestpubs.org/site/misc/reprints.xhtml).
References
- 1.Juvet SC, McCormack FX, Kwiatkowski DJ, Downey GP. Molecular pathogenesis of lymphangioleiomyomatosis: lessons learned from orphans. Am J Respir Cell Mol Biol. 2007;36(4):398–408. doi: 10.1165/rcmb.2006-0372TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson SR. Lymphangioleiomyomatosis. Eur Respir J. 2006;27(5):1056–1065. doi: 10.1183/09031936.06.00113303. [DOI] [PubMed] [Google Scholar]
- 3.Franz DN, Brody A, Meyer C, et al. Mutational and radiographic analysis of pulmonary disease consistent with lymphangioleiomyomatosis and micronodular pneumocyte hyperplasia in women with tuberous sclerosis. Am J Respir Crit Care Med. 2001;164(4):661–668. doi: 10.1164/ajrccm.164.4.2011025. [DOI] [PubMed] [Google Scholar]
- 4.Moss J, Avila NA, Barnes PM, et al. Prevalence and clinical characteristics of lymphangioleiomyomatosis (LAM) in patients with tuberous sclerosis complex. Am J Respir Crit Care Med. 2001;164(4):669–671. doi: 10.1164/ajrccm.164.4.2101154. [DOI] [PubMed] [Google Scholar]
- 5.Crino PB, Nathanson KL, Henske EP. The tuberous sclerosis complex. N Engl J Med. 2006;355(13):1345–1356. doi: 10.1056/NEJMra055323. [DOI] [PubMed] [Google Scholar]
- 6.Ryu JH, Moss J, Beck GJ, et al. NHLBI LAM Registry Group The NHLBI lymphangioleiomyomatosis registry: characteristics of 230 patients at enrollment. Am J Respir Crit Care Med. 2006;173(1):105–111. doi: 10.1164/rccm.200409-1298OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCormack FX. Lymphangioleiomyomatosis: a clinical update. Chest. 2008;133(2):507–516. doi: 10.1378/chest.07-0898. [DOI] [PubMed] [Google Scholar]
- 8.Koyama M, Johkoh T, Honda O, et al. Chronic cystic lung disease: diagnostic accuracy of high-resolution CT in 92 patients. AJR Am J Roentgenol. 2003;180(3):827–835. doi: 10.2214/ajr.180.3.1800827. [DOI] [PubMed] [Google Scholar]
- 9.Achen MG, Jeltsch M, Kukk E, et al. Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (Flt4) Proc Natl Acad Sci U S A. 1998;95(2):548–553. doi: 10.1073/pnas.95.2.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stacker SA, Caesar C, Baldwin ME, et al. VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nat Med. 2001;7(2):186–191. doi: 10.1038/84635. [DOI] [PubMed] [Google Scholar]
- 11.Seyama K, Kumasaka T, Souma S, et al. Vascular endothelial growth factor-D is increased in serum of patients with lymphangioleiomyomatosis. Lymphat Res Biol. 2006;4(3):143–152. doi: 10.1089/lrb.2006.4.143. [DOI] [PubMed] [Google Scholar]
- 12.Young LR, Inoue Y, McCormack FX. Diagnostic potential of serum VEGF-D for lymphangioleiomyomatosis. N Engl J Med. 2008;358(2):199–200. doi: 10.1056/NEJMc0707517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roach ES, Gomez MR, Northrup H. Tuberous sclerosis complex consensus conference: revised clinical diagnostic criteria. J Child Neurol. 1998;13(12):624–628. doi: 10.1177/088307389801301206. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt LS, Warren MB, Nickerson ML, et al. Birt-Hogg-Dubé syndrome, a genodermatosis associated with spontaneous pneumothorax and kidney neoplasia, maps to chromosome 17p11.2. Am J Hum Genet. 2001;69(4):876–882. doi: 10.1086/323744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glasgow CG, Avila NA, Lin JP, Stylianou MP, Moss J. Serum vascular endothelial growth factor-D levels in patients with lymphangioleiomyomatosis reflect lymphatic involvement. Chest. 2009;135(5):1293–1300. doi: 10.1378/chest.08-1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Einstein DM, Singer AA, Chilcote WA, Desai RK. Abdominal lymphadenopathy: spectrum of CT findings. Radiographics. 1991;11(3):457–472. doi: 10.1148/radiographics.11.3.1852937. [DOI] [PubMed] [Google Scholar]
- 17.Hyman MH, Whittemore VH. National Institutes of Health consensus conference: tuberous sclerosis complex. Arch Neurol. 2000;57(5):662–665. doi: 10.1001/archneur.57.5.662. [DOI] [PubMed] [Google Scholar]
- 18.Kumasaka T, Seyama K, Mitani K, et al. Lymphangiogenesis in lymphangioleiomyomatosis: its implication in the progression of lymphangioleiomyomatosis. Am J Surg Pathol. 2004;28(8):1007–1016. doi: 10.1097/01.pas.0000126859.70814.6d. [DOI] [PubMed] [Google Scholar]
- 19.Kumasaka T, Seyama K, Mitani K, et al. Lymphangiogenesis-mediated shedding of LAM cell clusters as a mechanism for dissemination in lymphangioleiomyomatosis. Am J Surg Pathol. 2005;29(10):1356–1366. doi: 10.1097/01.pas.0000172192.25295.45. [DOI] [PubMed] [Google Scholar]
- 20.Mitani K, Kumasaka T, Takemura H, et al. Cytologic, immunocytochemical and ultrastructural characterization of lymphangioleiomyomatosis cell clusters in chylous effusions of patients with lymphangioleiomyomatosis. Acta Cytol. 2009;53(4):402–409. doi: 10.1159/000325340. [DOI] [PubMed] [Google Scholar]
- 21.Issaka RB, Oommen S, Gupta SK, et al. Vascular endothelial growth factors C and D induces proliferation of lymphangioleiomyomatosis cells through autocrine crosstalk with endothelium. Am J Pathol. 2009;175(4):1410–1420. doi: 10.2353/ajpath.2009.080830. [DOI] [PMC free article] [PubMed] [Google Scholar]