Abstract
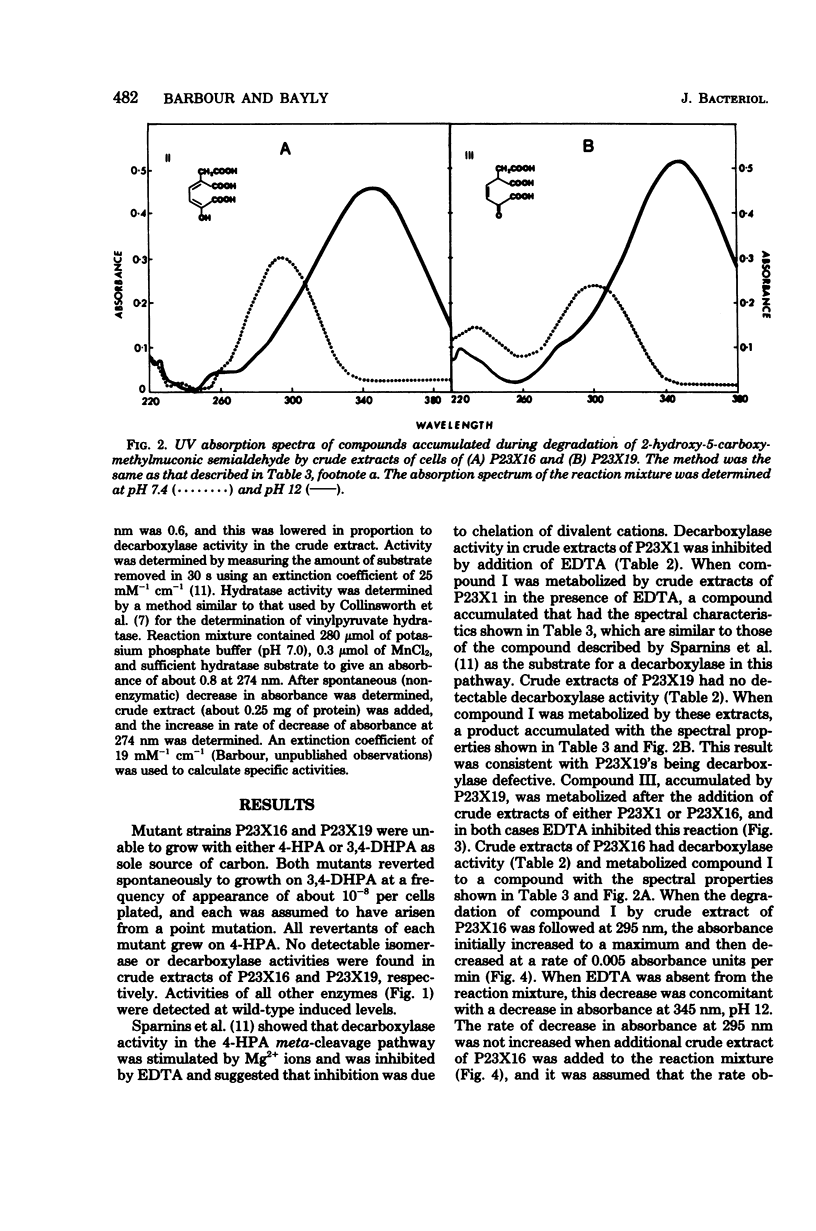
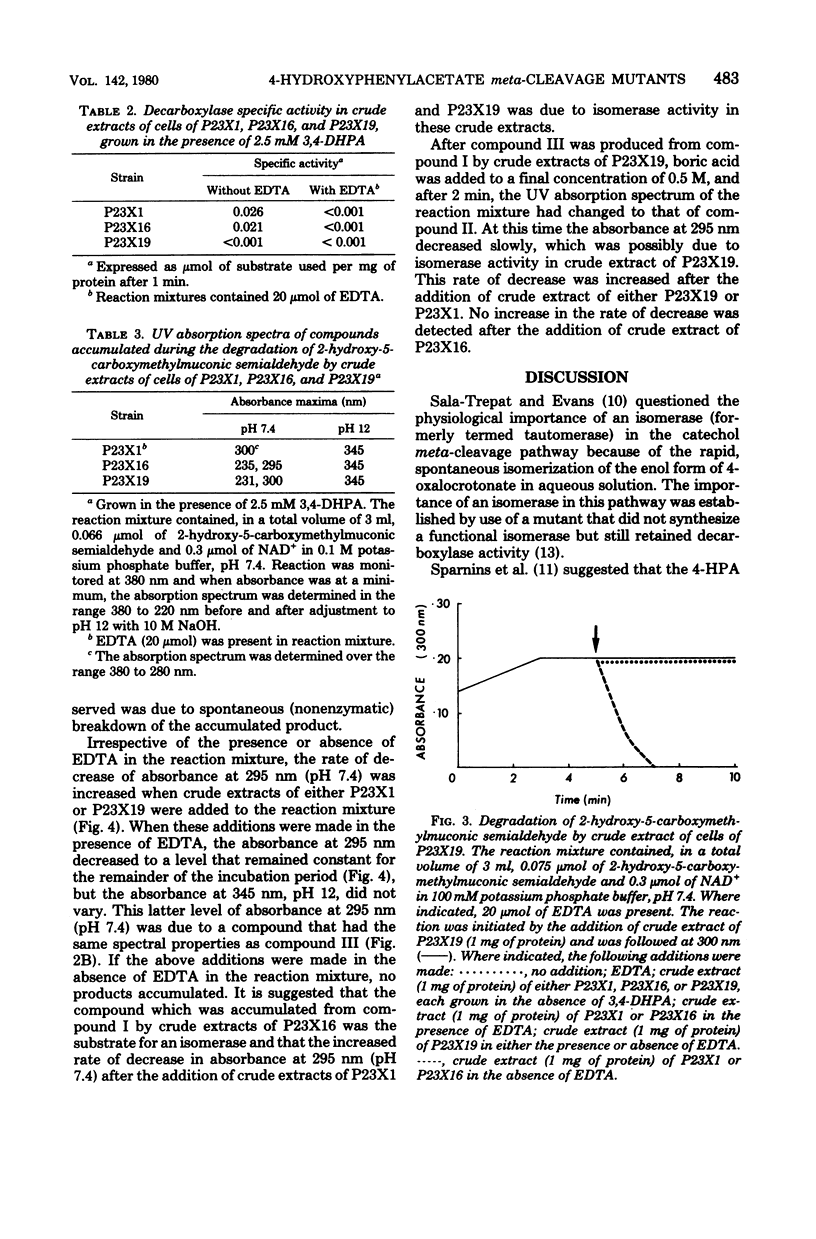
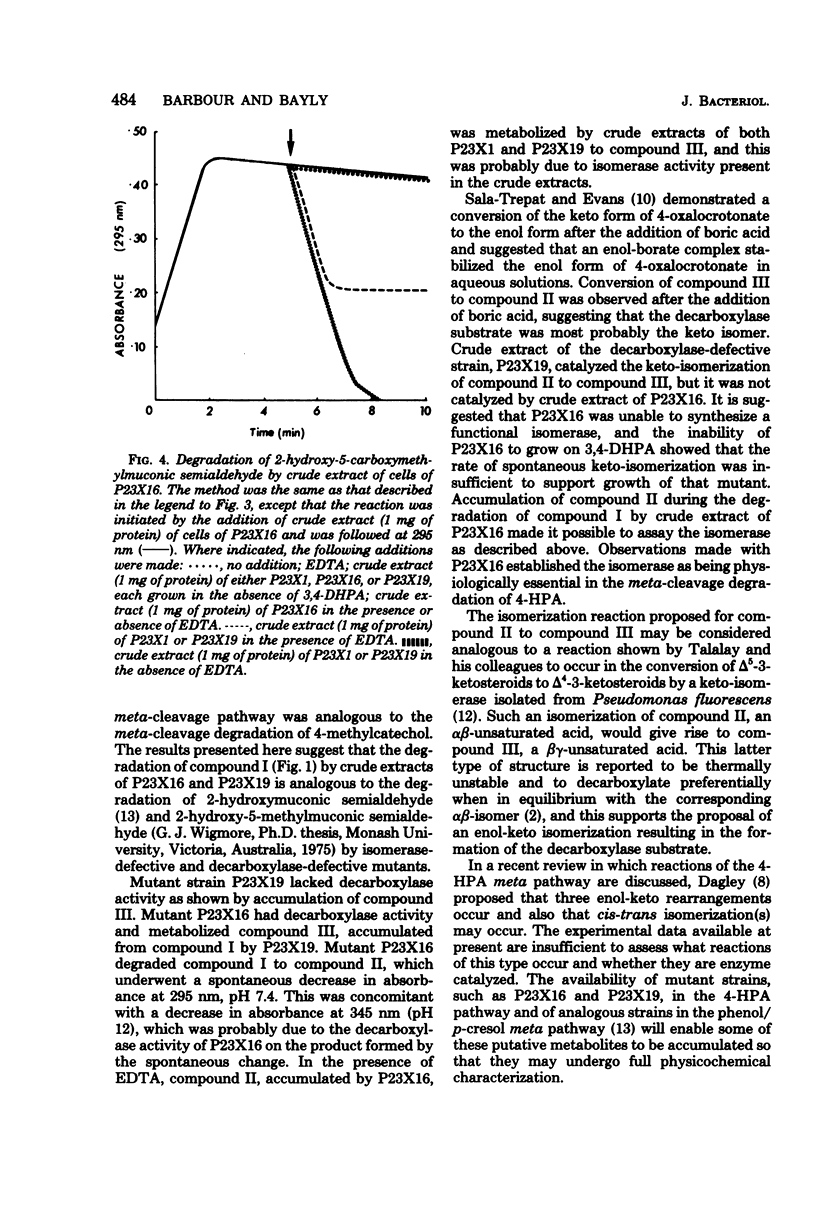
Degradation of 2-hydroxy-5-carboxymethylmuconic semialdehyde, the ring fission product of the 4-hydroxyphenylacetate meta-cleavage pathway, by mutant strains P23X19 and P23X16 of Pseudomonas putida NCI B 9865 was studied. Both mutants were unable to grow on either 4-hydroxyphenylacetate of 3,4-dihydroxyphenylacetate. Cell extracts of P23X19, grown in the presence of 3,4-dihydroxyphenylacetate, degraded the ring fission product to a compound that accumulated and had maximum UV absorption at 300 nm, pH 7.4, and 345 nm, pH 12. These are the spectral characteristics of 2-keto-5-carboxymethylhex-3-ene-1,6-dioate, the substrate for the decarboxylase in this pathway. This observation is consistent with P23X19's being decarboxylase defective. Cell extracts of P23X16, grown in the presence of 3,4-dihydroxyphenylacetate, degraded the ring fission product to a compound that accumulated and has maximum UV absorption at 295 nm, pH 7.4, and 345 nm, pH 12. This compound spontaneously degraded to a compound with the spectral properties of the decarboxylase substrate. The compound accumulated by P23X16 was also obtained when the decarboxylase substrate was treated with borate. It is suggested that the compound accumulated by P23X16 is the substrate of an isomerase. The results are consistent with P23X16's being unable to synthesize a functional isomerase while retaining decarboxylase activity and establish the physiological importance of an enzyme-catalyzed isomerization in the meta-cleavage degradation of 4-hydroxyphenylacetate.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADACHI K., TAKEDA Y., SENOH S., KITA H. METABOLISM OF P-HYDROXYPHENYLACETIC ACID IN PSEUDOMONAS OVALIS. Biochim Biophys Acta. 1964 Dec 9;93:483–493. doi: 10.1016/0304-4165(64)90332-0. [DOI] [PubMed] [Google Scholar]
- Barbour M. G., Bayly R. C. Regulation of the meta-cleavage of 4-hydroxyphenylacetic acid by Pseudomonas putida. Biochem Biophys Res Commun. 1976 May 23;76(2):565–571. doi: 10.1016/0006-291x(77)90761-6. [DOI] [PubMed] [Google Scholar]
- Bayly R. C., McKenzie D. I. Catechol oxygenases of Pseudomonas putida mutant strains. J Bacteriol. 1976 Sep;127(3):1098–1107. doi: 10.1128/jb.127.3.1098-1107.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayly R. C., Wigmore G. J. Metabolism of phenol and cresols by mutants of Pseudomonas putida. J Bacteriol. 1973 Mar;113(3):1112–1120. doi: 10.1128/jb.113.3.1112-1120.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayly R. C., di Berardino D. Purification and properties of 2-hydroxy-6-oxo-2,4-heptadienoate hydrolase from two strains of Pseudomonas putida. J Bacteriol. 1978 Apr;134(1):30–37. doi: 10.1128/jb.134.1.30-37.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collinsworth W. L., Chapman P. J., Dagley S. Stereospecific enzymes in the degradation of aromatic compounds by pseudomonas putida. J Bacteriol. 1973 Feb;113(2):922–931. doi: 10.1128/jb.113.2.922-931.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Sala-Trepat J. M., Evans W. C. The meta cleavage of catechol by Azotobacter species. 4-Oxalocrotonate pathway. Eur J Biochem. 1971 Jun 11;20(3):400–413. doi: 10.1111/j.1432-1033.1971.tb01406.x. [DOI] [PubMed] [Google Scholar]
- Sparnins V. L., Chapman P. J., Dagley S. Bacterial degradation of 4-hydroxyphenylacetic acid and homoprotocatechuic acid. J Bacteriol. 1974 Oct;120(1):159–167. doi: 10.1128/jb.120.1.159-167.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG S. F., KAWAHARA F. S., TALALAY P. The mechanism of the delta5-3-ketosteroid isomerase reaction: absorption and fluorescence spectra of enzyme-steroid complexes. J Biol Chem. 1963 Feb;238:576–585. [PubMed] [Google Scholar]
- Wigmore G. J., Bayly R. C., Di Berardino D. Pseudomonas putida mutants defective in the metabolism of the products of meta fission of catechol and its methyl analogues. J Bacteriol. 1974 Oct;120(1):31–37. doi: 10.1128/jb.120.1.31-37.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]


