Abstract
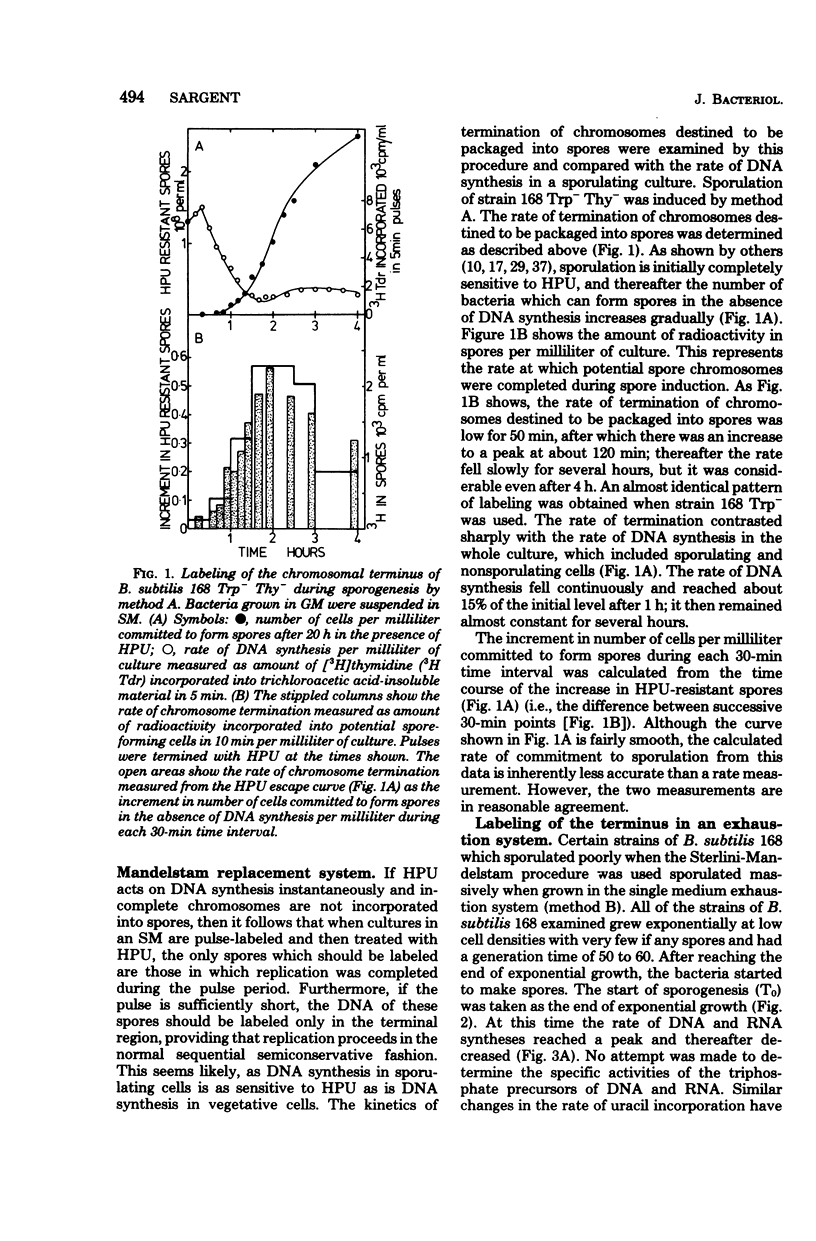
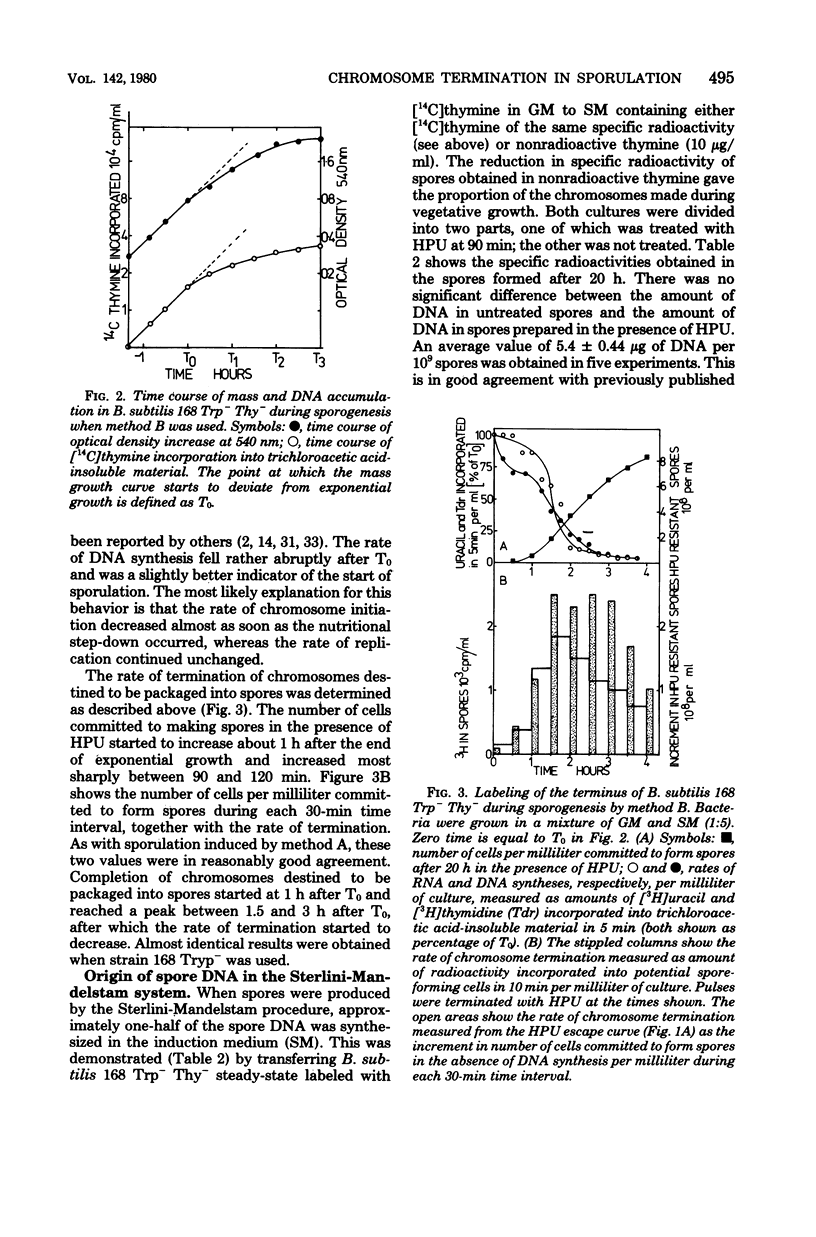
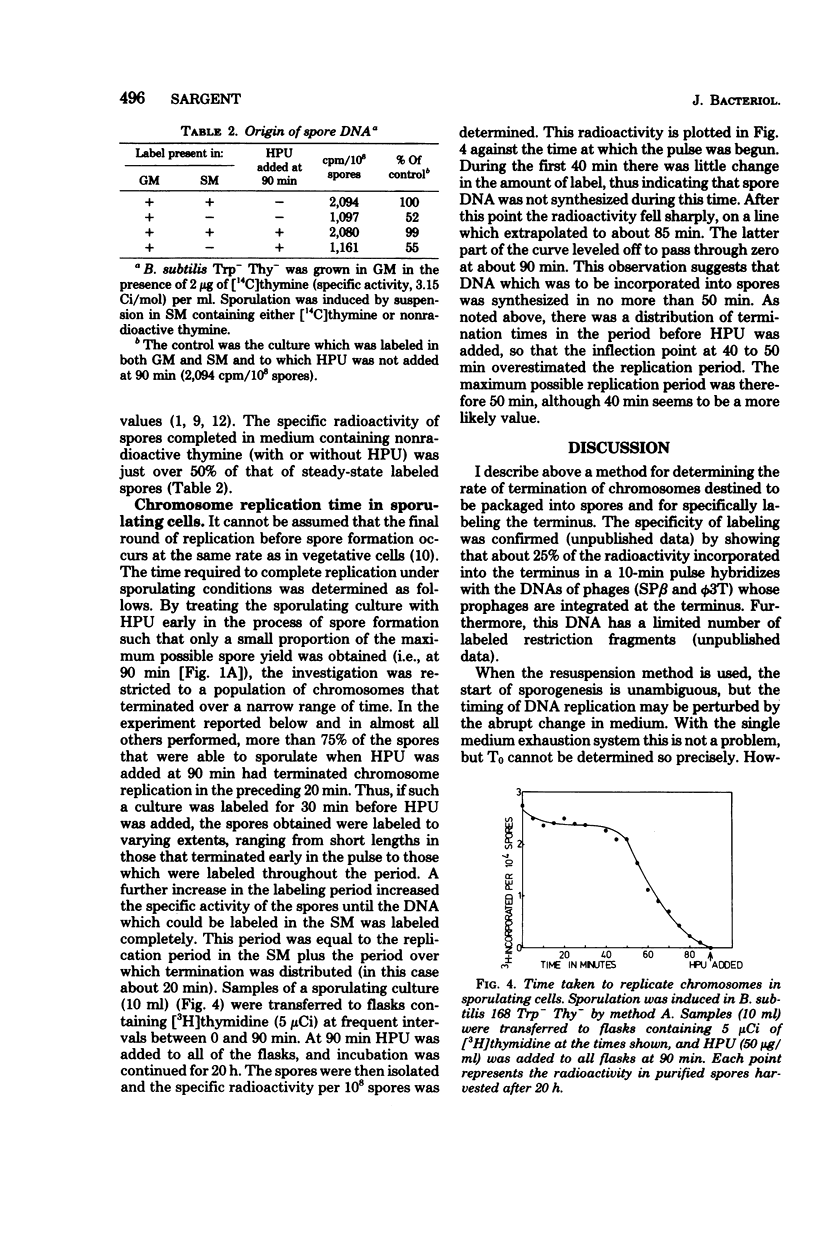
A method of specifically labeling the chromosomal terminus of Bacillus subtilis is described. When sporulating cultures were pulse-labeled with [3H]thymidine and then treated with 6-(p-hydroxyphenylazo)uracil, a drug which inhibits deoxyribonucleic acid (DNA) synthesis rapidly and completely, the only labeled spores formed were those that had completed replication during the pulse period. DNA-mediated transformation was used to show that the DNA of spores formed in the presence of 6-(p-hydroxyphenylazo)uracil had the same ratio of origin to terminus markers as DNA from untreated spores. Furthermore, spores formed in the presence of 6-(p-hydroxyphenylazo)uracil had the same DNA content as untreated spores. These two observations indicated that spores formed in the presence of 6-(hydroxyphenylazo)uracil contained completed chromosomes. The rate of termination of chromosomes destined to be packaged into spores was determined by this method, using the Sterlini-Mandelstam replacement system and a single medium exhaustion system for inducing sporulation. With both systems the rate of termination reached a broad peak 2 h after the start of sporogenesis. This was measured from the time of resuspension by using the replacement system and from the point where exponential growth ceased in the exhaustion system. The amount of spore DNA synthesized in the Sterlini-Mandelstam sporulation-inducing medium was very close to one-half the amount of the DNA present in mature spores. This suggests that chromosomes destined to be packaged into spores were replicated from close to the origin and possibly initiated in the sporulation-inducing medium. A method was devised for estimating the time taken to complete replication of the chromosomes destined to be packaged into spores. This was probably no more than 50 min. Whereas starvation must have occurred almost simultaneously in most cells in the population, the chromosome replication that was essential for sporogenesis was distributed over a wide time span. Thus, in some cells, replication started within 10 min of the nutritional step-down, but the peak rate was not reached for 1 h; thereafter replication continued at a substantial rate.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonamy C., Hirschbein L., Szulmajster J. Synthesis of ribosomal ribonucleic acid during sporulation of Bacillus subtilis. J Bacteriol. 1973 Mar;113(3):1296–1306. doi: 10.1128/jb.113.3.1296-1306.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown N. C. Inhibition of bacterial DNA replication by 6-(p-hydroxyphenylazo)-uracil: differential effect on repair and semi-conservative synthesis in Bacillus subtilis. J Mol Biol. 1971 Jul 14;59(1):1–16. doi: 10.1016/0022-2836(71)90409-8. [DOI] [PubMed] [Google Scholar]
- CANFIELD R. E., SZULMAJSTER J. TIME OF SYNTHESIS OF DEOXYRIBONUCLEIC ACID AND PROTEIN IN SPORES OF B. SUBTILIS. Nature. 1964 Aug 8;203:596–598. doi: 10.1038/203596a0. [DOI] [PubMed] [Google Scholar]
- Callister H., Wake R. G. Completed chromosomes in thymine-requiring Bacillus subtilis spores. J Bacteriol. 1974 Nov;120(2):579–582. doi: 10.1128/jb.120.2.579-582.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callister H., Wake R. G. Homogeneity in Bacillus subtilis spore DNA content. J Mol Biol. 1976 Apr 5;102(2):367–371. doi: 10.1016/s0022-2836(76)80060-5. [DOI] [PubMed] [Google Scholar]
- Dawes I. W., Mandelstam J. Sporulation of Bacillus subtilis in continuous culture. J Bacteriol. 1970 Sep;103(3):529–535. doi: 10.1128/jb.103.3.529-535.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis E. S., Wake R. G. Autoradiography of the Bacillus subtilis chromosome. J Mol Biol. 1966 Feb;15(2):435–439. doi: 10.1016/s0022-2836(66)80119-5. [DOI] [PubMed] [Google Scholar]
- Ephrati-Elizur E., Borenstein S. Velocity of chromosome replication in thymine-requiring and independent strains of Bacillus subtilis. J Bacteriol. 1971 Apr;106(1):58–64. doi: 10.1128/jb.106.1.58-64.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FITZ-JAMES P. C., YOUNG I. E. Comparison of species and yarieties of the genus Bacillus. Structure and nucleic acid content of spores. J Bacteriol. 1959 Dec;78:743–754. doi: 10.1128/jb.78.6.743-754.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillin F. D., Ganesan A. T. Control of chromosome replication in thymine-requiring strains of Bacillus subtilis 168. J Bacteriol. 1975 Sep;123(3):1055–1067. doi: 10.1128/jb.123.3.1055-1067.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussey C., Losick R., Sonenshein A. L. Ribosomal RNA synthesis is turned off during sporulation of Bacillus subtilis. J Mol Biol. 1971 Apr 14;57(1):59–70. doi: 10.1016/0022-2836(71)90119-7. [DOI] [PubMed] [Google Scholar]
- Keynan A., Berns A. A., Dunn G., Young M., Mandelstam J. Resporulation of outgrowing Bacillus subtilis spores. J Bacteriol. 1976 Oct;128(1):8–14. doi: 10.1128/jb.128.1.8-14.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighton T. J. An RNA polymerase mutation causing temperature-sensitive sporulation in Bacillus subtilis. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1179–1183. doi: 10.1073/pnas.70.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love E., D'Ambrosio D., Brown N. C. Mapping of the gene specifying DNA polymerase III of Bacillus subtilis. Mol Gen Genet. 1976 Mar 30;144(3):313–321. doi: 10.1007/BF00341730. [DOI] [PubMed] [Google Scholar]
- Mandelstam J., Higgs S. A. Induction of sporulation during synchronized chromosome replication in Bacillus subtilis. J Bacteriol. 1974 Oct;120(1):38–42. doi: 10.1128/jb.120.1.38-42.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelstam J., Sterlini J. M., Kay D. Sporulation in Bacillus subtilis. Effect of medium on the form of chromosome replication and on initiation to sporulation in Bacillus subtilis. Biochem J. 1971 Nov;125(2):635–641. doi: 10.1042/bj1250635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mychajlonka M., Slepecky R. A. Requirement of deoxyribonucleic acid synthesis for microcycle sporulation in Bacillus megaterium. J Bacteriol. 1974 Dec;120(3):1331–1338. doi: 10.1128/jb.120.3.1331-1338.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OISHI M., YOSHIKAWA H., SUEOKA N. SYNCHRONOUS AND DICHOTOMOUS REPLICATIONS OF THE BACILLUS SUBTILIS CHROMOSOME DURING SPORE GERMINATION. Nature. 1964 Dec 12;204:1069–1073. doi: 10.1038/2041069a0. [DOI] [PubMed] [Google Scholar]
- Piggot P. J., De Lencastre H. A rapid method for constructing multiply marked strains of Bacillus subtilis. J Gen Microbiol. 1978 May;106(1):191–194. doi: 10.1099/00221287-106-1-191. [DOI] [PubMed] [Google Scholar]
- Ryter A., Aubert J. P. Etude autoradiographique de la synthèse de l'ADN aucours de la sporulation de Bacillus subtilis. Ann Inst Pasteur (Paris) 1969 Nov;117(5):601–611. [PubMed] [Google Scholar]
- Sargent M. G. A procedure for isolating high quality DNA from spores of Bacillus subtilis 168. J Gen Microbiol. 1980 Feb;116(2):511–514. doi: 10.1099/00221287-116-2-511. [DOI] [PubMed] [Google Scholar]
- Sargent M. G. Macromolecular synthesis in chromosome initiation mutants of Bacillus subtilis. Mol Gen Genet. 1977 Oct 24;155(3):329–338. doi: 10.1007/BF00272813. [DOI] [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterlini J. M., Mandelstam J. Commitment to sporulation in Bacillus subtilis and its relationship to development of actinomycin resistance. Biochem J. 1969 Jun;113(1):29–37. doi: 10.1042/bj1130029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumida-Yasumoto C., Doi R. H. Bacillus subtilis ribonucleic acid polymerase mutants conditionally temperature sensitive at various stages of sporulation. J Bacteriol. 1977 Jan;129(1):433–444. doi: 10.1128/jb.129.1.433-444.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson G. A., Bott K. F. Nutritional factors influencing the development of competence in the Bacillus subtilis transformation system. J Bacteriol. 1968 Apr;95(4):1439–1449. doi: 10.1128/jb.95.4.1439-1449.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson G. A., Bott K. F. Nutritional factors influencing the development of competence in the Bacillus subtilis transformation system. J Bacteriol. 1968 Apr;95(4):1439–1449. doi: 10.1128/jb.95.4.1439-1449.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lencastre H., Piggot P. J. Identification of different sites of expression for spo loci by transformation of Bacillus subtilis. J Gen Microbiol. 1979 Oct;114(2):377–389. doi: 10.1099/00221287-114-2-377. [DOI] [PubMed] [Google Scholar]



