Abstract
Previous findings in rats suggest that the rostral basolateral amygdala (rBLA) and prelimbic prefrontal cortex (plPFC) are likely components of cue reinstatement circuitry based on bilateral inactivation of each site alone. In the present investigation, we examined whether the rBLA and plPFC interact to regulate reinstatement of cocaine-seeking behavior elicited by re-exposure to a combination of discrete and contextual cocaine-paired cues. After establishing stable baseline responding under a second-order schedule of cocaine reinforcement and cue presentation, rats underwent response-extinction training in which cocaine and cocaine-paired cues were withheld. To test the interaction, rats with asymmetric cannulae placements in the rBLA and plPFC received vehicle or lidocaine infusions prior to reinstatement testing during which cocaine-paired cues were presented, in the absence of cocaine availability, under a second-order schedule. Asymmetric inactivation of the rBLA and plPFC significantly attenuated reinstatement of cocaine-seeking behavior relative to vehicle treatment. As expected, inactivation of the rBLA or plPFC in rats with unilateral cannulae placements did not disrupt reinstatement relative to vehicle treatment. Findings propose critical intrahemispheric interaction between the rBLA and plPFC in regulating reinstatement of cocaine-seeking behavior elicited by re-exposure to drug-paired cues.
Keywords: Asymmetric, Basolateral amygdala, Cocaine, Cocaine-seeking behavior, Lidocaine, Prelimbic prefrontal cortex, Reinstatement circuitry, Second-order schedule, Unilateral
1. Introduction
Past research is suggestive of an interaction between the rostral basolateral amygdala (rBLA) and dorsomedial prefrontal cortex (dmPFC) in regulating reinstatement of cocaine-seeking behavior elicited by re-exposure to contextual cues previously paired with cocaine (Fuchs et al., 2007). This idea, however, has never been tested directly in relation to reinstatement of cocaine-seeking behavior elicited by re-exposure to response-contingent cocaine-paired cues presented discretely within a specific cocaine-associated context. By combining discrete (response-contingent) and contextual (response-independent) cocaine-paired cues in a laboratory setting to assess reinstatement of cocaine-seeking behavior, it is possible to utilize stimuli that are provocatively similar to drug-associated stimuli that can precede relapse in humans (Shiffman et al., 1996; Childress et al., 1999). Indirect evidence for an interaction is found in studies where bilateral inactivation of either the rBLA or the prelimbic PFC (plPFC) attenuated reinstatement of cocaine-seeking behavior elicited by re-exposure to discrete or a combination of discrete and contextual cocaine-paired cues (Meil and See, 1997; Kantak et al., 2002; McLaughlin and See, 2003; Di Pietro et al., 2006). The involvement of the BLA in regulating cue reinstatement may be subregion-selective, as a recent investigation using dopamine D1 receptor ligands and a second-order schedule of drug delivery and cue presentation elegantly demonstrated regulation of cocaine-seeking behavior by the caudal BLA (cBLA) only during the cocaine self-administration maintenance phase of testing and by the rBLA only during the cue reinstatement phase of testing (Mashhoon et al., 2009).
Importantly, a neuroanatomical framework for an interaction between the rBLA and plPFC during cue reinstatement testing exists as shown by retrograde tract tracing studies revealing efferent projections from the rBLA to the plPFC (Hoover and Vertes, 2007) and from the plPFC to the rBLA (Gabbott et al., 2005). Typically, the most efficient and effective way to show that connections between two sites in the same hemisphere functionally interact to regulate behavior is to use an asymmetric or interhemispheric “crossed” disconnection procedure rather than an ipsilateral or intrahemispheric disconnection procedure (e.g., Olton et al., 1982; Seamans et al., 1998; Warburton et al., 2000; Dunnett et al., 2005; Floresco and Ghods-Sharifi, 2007). Ipsilateral manipulation results in intact functioning of the two sites on the contralateral side, which generally is sufficient to maintain the behavior under study. The asymmetric manipulation results in disruption of neural activity between the origin of a pathway in one hemisphere and the termination of an efferent pathway in the contralateral hemisphere (Seamans et al., 1998). Therefore, an asymmetric lidocaine inactivation procedure was used in rats trained to self-administer cocaine under a second-order schedule for which response-contingent drug-paired light cues were presented against a background of continuous white noise serving as a drug-paired contextual sound cue. Lidocaine is a sodium channel blocker that reversibly inhibits neural conductance and signaling (Catterall and Mackie, 1996). If lidocaine inactivation of the rBLA in one hemisphere together with lidocaine inactivation of the plPFC in the contralateral hemisphere disrupts reinstatement of cocaine-seeking behavior in the same way as bilateral lidocaine inactivation of each site alone (Kantak et al., 2002; Di Pietro et al., 2006), then the two sites within each hemisphere may interact directly to regulate cocaine-seeking rather than function independently (see Gaffan et al., 1993 and Di Ciano and Everitt, 2004). To examine whether the effects of asymmetric inactivation were due simply to unilateral inactivation of one or the other site, unilateral rBLA and plPFC control groups were also tested to observe whether cocaine-seeking behavior would, as hypothesized, remain undisturbed following unilateral inactivation of either the rBLA or plPFC. We predicted that disruption of the connectivity between the rBLA and plPFC on both sides of the brain via asymmetric manipulation would significantly attenuate reinstatement of cocaine-seeking behavior elicited by re-exposure to discrete and contextual drug-paired cues.
2. Materials and Methods
2.1. Subjects
Male Wistar rats [Crl(WI)BR rats; Charles River Laboratories, Portage, MI, USA], approximately 276-300 g upon arrival, were maintained at 90% of a growth adjusted ad libitum body weight throughout the duration of the study by providing 16 g of food per day. Body weights were recorded daily. Between experimental sessions, rats were allowed unlimited access to water in their home cages. Rats were housed individually in clear plastic cages (43 × 22 × 20 cm) in a temperature- (21-23 °C) and light- (08:00 h on, 20.00 h off) controlled vivarium. Each experimental group consisted of 5-6 animals to permit a meaningful statistical analysis and interpretation of the behavioral and Fos protein expression data. The policies and procedures set forth in the “Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research” were followed, as well as specific national laws. All protocols were approved by the Boston University Institutional Animal Care and Use Committee.
2.2. Apparatus
Experimental chambers (model ENV-008CT; Med Associates, St. Albans, VT, USA) were each equipped with two response levers positioned 8 cm to the left and right of a center mounted food receptacle and 7 cm from the grid floor, and outfitted with a single channel fluid swivel (Instech Solomon, Plymouth Meeting, PA, USA) and spring leash assembly connected to a counterbalanced arm assembly (Med Associates), allowing the animal to move freely in the chamber. A white stimulus light was mounted 7 cm above each lever. A sound-attenuating cubicle (model ENV-108 M; Med Associates) equipped with a houselight to provide general illumination, a fan to provide ventilation, and an 8 ohm speaker to provide white noise, enclosed each chamber. Motor-driven syringe pumps (model PHM-100, Med Associates) located within each sound-attenuating cubicle were used for intravenous drug delivery. A standard personal computer programmed in Medstate Notation™ and connected to an interface (Med Associates) controlled experimental events.
2.3. Drugs and Intracranial Infusion Procedures
Drugs used were cocaine hydrochloride (gift from the National Institute on Drug Abuse, Bethesda, MD, USA) and lidocaine hydrochloride (Sigma, St. Louis, MO, USA). Cocaine was dissolved in sterile 0.9% saline solution containing 3 IU heparin/ml to a final concentration of 2.68 mg/ml. For all self-administration sessions, a 1.0 mg/kg unit infusion dose of cocaine was used and delivered intravenously at a rate of 1.8 ml/min. To attain a dose of 1.0 mg/kg, infusion volume was adjusted for body weight, resulting in drug delivery times of 1.2 sec/100 g body weight in individual rats. Lidocaine was dissolved in sterile 0.9% saline to make 6% (60 mg/ml) and 20% (200 mg/ml) solutions. A total volume of 0.5 μL, resulting in lidocaine doses of 30 and 100 μg, respectively, was infused at a rate of 0.59 μL/min. Different doses of lidocaine were used because previous research demonstrated that 100 μg was maximally effective in rBLA (Kantak et al., 2002) and 30 μg was maximally effective in plPFC (Di Pietro et al., 2006) for reducing cocaine-seeking behavior.
2.4. Surgery and Histology
Rats were anesthetized with an intraperitoneal (i.p.) injection of 90 mg/kg ketamine plus 10 mg/kg xylazine. To enable intravenous delivery of cocaine, a catheter made of silicon tubing was implanted into the right jugular vein. The catheter ran subcutaneously under the neck, exited through an incision at the top of the head, and was attached to an L-shaped pedestal mount (Plastics One, Roanoke, VA). Subsequent to catheter implantation, 0.1 ml of a solution containing 1.0 mg methohexital sodium (Brevital; King Pharmaceuticals, Briston, TN, USA) was infused intravenously as needed to maintain anesthesia. After suturing the neck incision, the rat was placed in a stereotaxic frame and asymmetrically or unilaterally placed 22 gauge stainless steel guide cannulae (Plastics One) were implanted. Guide cannulae were positioned 1 mm above the intended sites and placements were based on the bregma coordinate system provided by the atlas of Swanson (1992). For rats with unilateral rBLA placements (n=12), cannulae were implanted into either the right or left rBLA (anteroposterior (AP) -2.0 mm, lateral (L) ±4.5 mm, dorsoventral (DV) -7.6 mm). For rats with unilateral plPFC placements (n=12), cannulae were implanted into either the right or left plPFC at 15° angles (AP +2.8 mm, L ±1.4 mm, DV - 2.9 mm). Angled placements for the plPFC were used to avoid breach of the medial wall of the cortex (Di Pietro et al., 2006). For rats with asymmetric rBLA/plPFC placements (n=12), the same stereotaxic coordinates as above for the unilateral rBLA and plPFC placements were used to implant a single cannula into each site on opposite sides of the brain. Placements for each site were counterbalanced to the right and left sides. We did not include bilateral placement groups in this study, as previous behavioral findings from our laboratory demonstrated that bilateral lidocaine inactivation of the rBLA or the plPFC significantly attenuated cue-induced reinstatement of cocaine-seeking behavior in rats trained under a second-order schedule of drug delivery and contextual and discrete cue presentation (Kantak et al., 2002; Di Pietro et al., 2006). The guide cannulae, pedestal, and three stainless steel anchoring screws were attached to the skull and permanently embedded in dental cement. Two 28 gauge obturators (Plastics One) were used to occlude guide cannulae between infusions. Wounds were treated daily with topical furazolidone powder (Veterinary Products Laboratories, Phoenix, AZ, USA) until healed and rats were allowed one week of recovery from surgery before initiation of the study. Catheters were maintained by daily flushing (Monday-Friday) with 0.1 ml of 0.9% saline solution containing 3 IU heparin (Baxter Healthcare Corporation, Deerfield, IL, USA) and 6.7 mg Timentin (GlaxoSmithKline, Research Triangle Park, NC, USA). On Fridays, a locking solution consisting of glycerol and undiluted (1000 IU/ml) heparin (3:1) was used to fill the catheter dead space and minimize blockages. This solution remained in catheters until Monday, when it was removed and replaced with the heparin/saline solution prior to the start of behavioral sessions. Additionally, catheters were checked weekly for patency by infusing a 1.0 mg/0.1 ml solution of Brevital intravenously, which produces a rapid temporary loss of muscle tone. A new catheter was implanted into either the left jugular vein or right femoral vein to replace a leaking or non-functional catheter, and rats were allowed four days of recovery from surgery before resuming the study.
2.5. Self-Administration Training under a Second-Order Schedule
Prior to surgery, rats underwent an overnight session for acquisition of lever pressing under an FR1 schedule of food pellet delivery. After rats learned to rapidly press the lever for 50 pellets, right jugular vein catheters and guide cannulae were implanted as described above. After a week of recovery from surgery, hour-long cocaine self-administration sessions began. Rats were incrementally trained to self-administer 1.0 mg/kg cocaine under a fixed-interval (FI) 5-min [FR5:S] second-order schedule of drug delivery, as previously described (Kantak et al., 2002). Under the FI 5-min [FR5: S] schedule of drug delivery, every fifth response (FR5) on the active lever during the FI resulted in a 2-sec presentation of the stimulus light located above the active lever. The 2-sec light presentation served as a brief cocaine-associated conditioned stimulus (brief CS+). Responses on the inactive lever were counted separately, but produced no scheduled consequences. Acquisition of the FI 5-min [FR5: S] schedule took approximately six weeks (30 sessions) of training. For half the rats, the right lever was designated as the active lever and the left lever was designated as the inactive lever. This order was reversed for the remaining rats. Intravenous cocaine delivery was contingent upon completion of five responses on the active lever after the 5-min FI elapsed. The light above the active lever remained illuminated for the duration of the infusion as well as for the 20-s timeout period that followed each infusion (prolonged CS+), while the house light was extinguished during the timeout. White noise (70 db), initiated at the start of the session, was present for the duration of each session and served as a response-independent cocaine-paired contextual sound cue. Baseline training sessions were conducted five days a week during the light phase, and continued until cocaine intake was stable (number of infusions did not deviate by more than 20%) and the number of responses on the inactive lever was no greater than 25 for each session over a 5-day period. Criterion was typically reached after approximately three weeks (15 sessions) of baseline training.
2.6. Response-Extinction Training
Following establishment of stable baseline responding, rats underwent 14 response-extinction training sessions to extinguish lever responding. Cocaine delivery, the contextual white noise and the response-contingent light cue were withheld during response-extinction training sessions.
2.7. Reinstatement Testing under a Second-Order Schedule
Following response-extinction training, rats underwent a single 1-hour cue-induced reinstatement test during which intravenous cocaine was not available for self-administration. The contextual sound cue was restored and the response-contingent brief CS+ (2-sec light) was presented after every fifth response on the active lever during the 5-min FI and the response-contingent prolonged CS+ (20-sec light) was presented upon completion of five responses after the 5-min FI elapsed. There was a 20-s timeout period during which the house light was extinguished. Vehicle or lidocaine was infused either unilaterally into the plPFC or rBLA or asymmetrically into the plPFC and rBLA 5-min prior to testing (n=6 for each placement/treatment group).
2.8. Histology
Rats were given an overdose of sodium pentobarbital 30 minutes following the 1-hr reinstatement test and then perfused intracardially with saline and 4% paraformaldehyde solution. Brains were extracted, post-fixed in 4% paraformaldehyde for 4 hours, and stored in 30% sucrose at 4 °C for 2-3 days. Subsequently, brains were flash frozen using isopentane and stored at -80°C. Coronal sections (40 μm) targeting the plPFC and the rBLA were collected using a cryostat, mounted on gelatin-coated slides, and stained with thionin. Slides were examined under a microscope to verify infusion cannulae placements.
2.9. Data Analysis
Three behavioral measures were calculated: (1) the number of active lever responses (2) the number of inactive lever responses and (3) the number of reinforcers earned (cocaine infusions or prolonged CS+ presentations). Measures of baseline performance were based on data averaged over the last five cocaine self-administration sessions and last five response extinction sessions. Drug-seeking behavior on test day was operationally defined as active lever responding maintained by drug-associated cues during the second-order schedule of cue presentation (Spealman et al., 1999).
To ensure that there were no preexisting behavioral differences among the six groups prior to reinstatement testing, one-factor (group) ANOVAs were used to separately analyze self-administration and response extinction baseline data. In addition, responding across the 14 sessions of extinction training was examined to compare rates of extinction learning in the six groups using two-factor (group X extinction session) ANOVA, with repeated measures on the extinction session factor. Behavioral differences during reinstatement testing were determined by two-factor ANOVAs. Factors included placement site (unilateral rBLA, unilateral plPFC, and asymmetric rBLA/plPFC) and treatment (vehicle and lidocaine). Significant ANOVAs were followed by simple main effects tests or the post-hoc Tukey test where appropriate.
3. Results
3.1. Histology
Histological verification of placements was confirmed for all rats in the six experimental groups; two groups with asymmetric cannulae aimed at the rBLA and plPFC (n=12), two groups with unilateral cannulae aimed at the plPFC (n=12), and two groups with unilateral cannulae aimed at the rBLA (n=12). Depiction of cannulae placements are shown in Figure 1 (left panels). Placements were within 0.6 – 0.8 mm of the intended position in the AP plane and within the plPFC and rBLA anatomical ranges (Swanson, 1992). A 0.5 μl volume of lidocaine is estimated to spread spherically with a radius of 0.5 mm from the infusion site (Tehovnik and Sommer, 1997). Representative photomicrographs of asymmetric and unilateral cannulae tracks also are depicted in Figure 1 (right panels).
Figure 1.
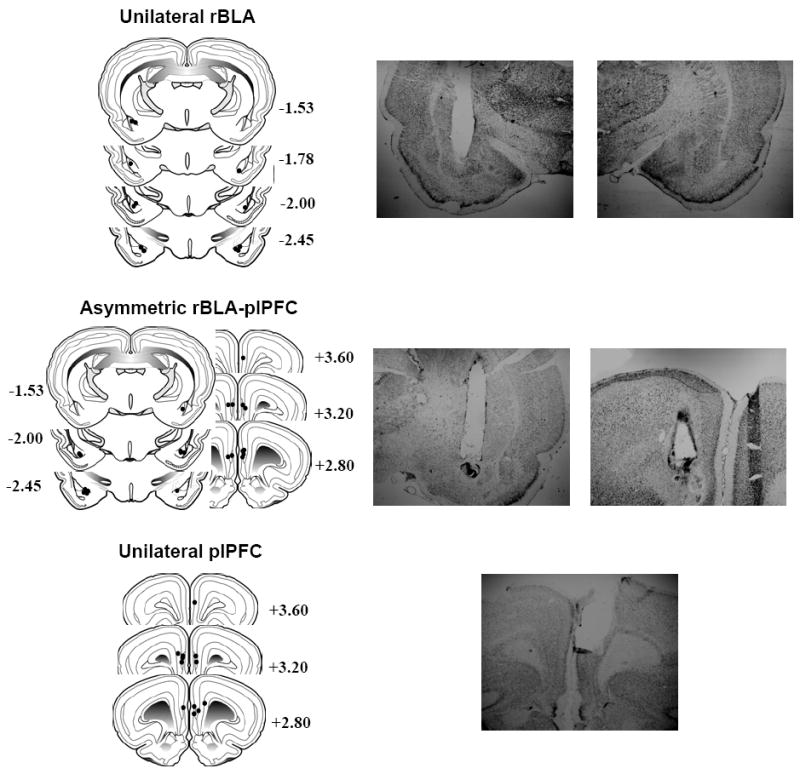
Schematic drawings representing coronal sections of the rBLA and plPFC subregions (left panels). Circles indicate the diameter (1.0 mm) of the theoretical diffusion of lidocaine from the cannulae tips of rats with unilateral rBLA (top), asymmetric rBLA/plPFC (middle), and unilateral plPFC (bottom) placements. All drawings are based on the atlas of Swanson (1992), with the anterior-posterior references measured from bregma. Each placement is shown at the midpoint of its anterior-posterior extent. Representative photomicrographs of asymmetric and unilateral cannulae tracks also are depicted (right panels).
3.2. Baseline Cocaine Self-Administration Sessions
By the end of training, the six groups of rats had similar baseline levels of cocaine self-administration, and successfully discriminated the active from the inactive lever. One-factor ANOVA analyses comparing the six groups that would later undergo reinstatement testing following either vehicle or lidocaine treatment revealed that active lever responses during baseline cocaine self-administration sessions did not differ significantly between groups (Figure 2, top panel, bars above the letter A). Like active lever responses, the number of inactive lever responses (Figure 2, top panel, bars above the letter I) and cocaine infusions earned (Figure 2, bottom panel) did not differ significantly between the six groups of rats.
Figure 2.
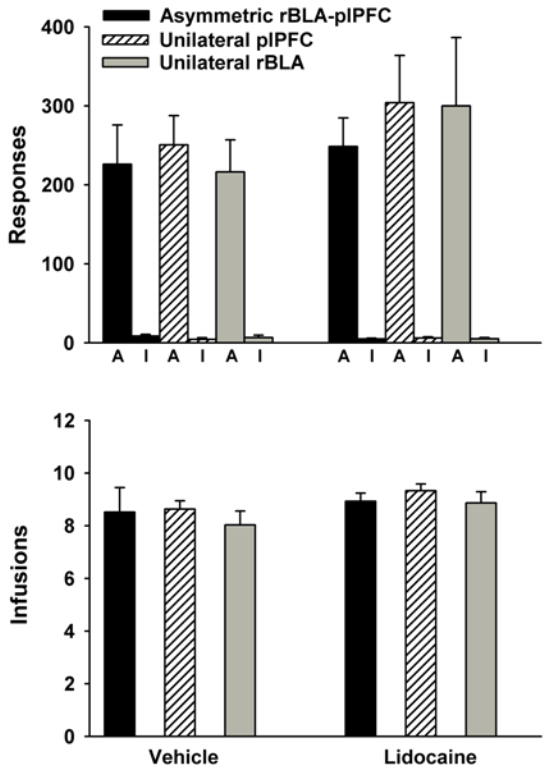
Behavior during cocaine self-administration baseline sessions in rats with asymmetric or unilateral rBLA and/or plPFC placements and designated for later pretreatment with vehicle (left) or lidocaine (right) prior to reinstatement test sessions. Values are the mean ± SEM number of active (A) and inactive (I) lever responses (top panel) and number of infusions earned (bottom panel).
3.3. Response-Extinction Sessions
Across the course of 14 sessions of extinction training, the six groups of rats demonstrated significantly diminished responding on the active lever and inactive lever. Two-factor ANOVA analyses of active lever responses revealed a significant main effect of extinction session (F [13, 390] = 39.7, p ≤ 0.001), but not of group or the extinction session X group interaction. As expected, the significant main effect of session indicated that responding decreased as extinction training progressed in all six groups (Figure 3, top and bottom panels). Overall, group comparisons revealed similar extinction learning curves across all six groups. Additional analysis of active lever responses averaged over the last five extinction sessions in each of the six groups indicated that there were no significant between-group differences (data not shown). Thus, active lever responding was attenuated to a similar level in the six groups during the last five sessions of extinction training that immediately preceded the reinstatement test.
Figure 3.
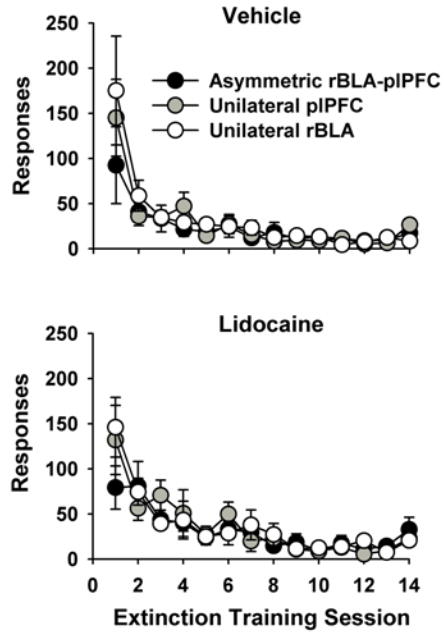
Behavior during the 14 response-extinction training sessions in rats with asymmetric or unilateral rBLA and/or plPFC placements and designated for later pretreatment with vehicle (top panel) or lidocaine (bottom panel) prior to reinstatement test sessions. Values are the mean ± SEM number of active lever responses.
Regarding inactive lever responses during response-extinction training, two-factor ANOVA analyses revealed a significant main effect of extinction session (F [13, 390] = 2.6, p ≤ 0.01), but not of group or the extinction session X group interaction. As expected, the significant main effect of session indicated that inactive lever responding also decreased as extinction training continued (from a maximum average 14.8 ± 3.7 and a minimum average of 7.0 ± 4.6 on Day 1 to a maximum average of 9.2 ± 3.8 and a minimum average of 0.5 ± 0.3 on day 14; data not shown).
3.4. Reinstatement Testing
Asymmetric infusion of lidocaine into the rBLA and plPFC profoundly attenuated cocaine-seeking behavior, while unilateral infusion of lidocaine into either the rBLA or plPFC did not alter cocaine-seeking behavior. Analyses of active lever responses emitted during test sessions (Figure 4) revealed significant main effects of placement (F [2, 30] = 7.6, p ≤ 0.01) and treatment (F [1, 30] = 8.6, p ≤ 0.01), as well as a significant placement X treatment interaction (F [2, 30] = 10.1, p ≤ 0.001). Post-hoc analyses of the placement X treatment interaction indicated that for the asymmetric rBLA/plPFC placements, active lever responding was significantly less in lidocaine-pretreated rats that underwent reinstatement testing compared to vehicle-pretreated rats that underwent reinstatement testing (p ≤ 0.001). Further post-hoc analysis revealed that for the unilateral rBLA and unilateral plPFC placements, active lever responding was not significantly different between lidocaine-and vehicle-pretreated rats that underwent reinstatement testing. A post-hoc between-placement comparison of lidocaine-pretreated rats that underwent reinstatement testing also indicated that active lever responses were significantly lower in rats with asymmetric rBLA/plPFC placements compared to rats with unilateral rBLA or unilateral plPFC placements (p ≤ 0.001). Similar post-hoc comparisons of vehicle-pretreated rats that underwent reinstatement testing indicated no significant between-placement differences in active lever responding. Inactive lever responses averaged fewer than 10 (a maximum average of 8.5 ± 5.3 and a minimum average of 1.2 ± 0.7) in each of the six groups, with no significant differences among placements, between treatments, or for the interaction of placement X treatment.
Figure 4.
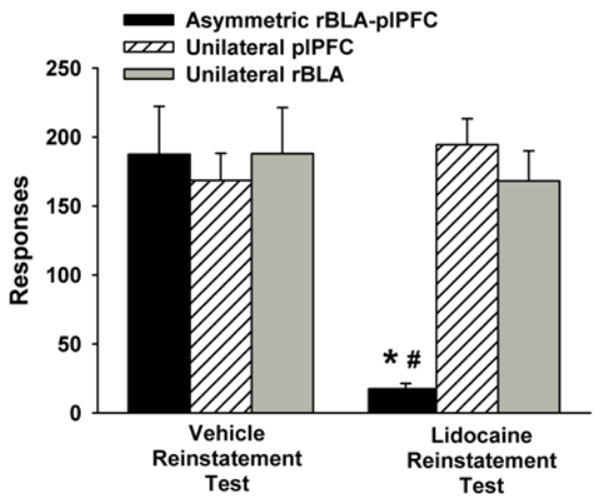
Behavior during reinstatement test sessions in rats with asymmetric or unilateral rBLA and/or plPFC placements and pretreated with vehicle (left) or lidocaine (right). Values are the mean ± SEM number of active lever responses. * p ≤ 0.001 compared to the corresponding vehicle-treated group that underwent reinstatement testing, and # p≤ 0.01 compared to the unilateral lidocaine-treated groups that underwent reinstatement testing.
For the number of prolonged CS+ presentations earned (i.e., the 20-sec stimulus light presented upon completion of five responses after each 5-min FI elapsed), there was a significant main effect of placement (F [2, 30] = 32.3, p ≤ 0.001) and treatment (F [1, 30] = 23.5, p ≤ 0.001), as well as a significant interaction of placement X treatment (F [2, 30] = 23.6, p ≤ 0.001). Post-hoc analysis of the placement X treatment interaction revealed that for the asymmetric rBLA/plPFC placements, significantly (p ≤ 0.001) fewer prolonged CS+ presentations were earned in lidocaine-pretreated rats that underwent reinstatement testing (1.7 ± 0.3) compared to vehicle-pretreated rats that underwent reinstatement testing (6.7 ± 0.6) (data not shown). Further post-hoc analysis revealed that for the unilateral rBLA and unilateral plPFC placements, the number of prolonged CS+ presentations earned was not significantly different between lidocaine-pretreated (7.2 ± 0.4 and 7.0 ± 0.4, respectively) and vehicle-pretreated (7.0 ± 0.4 and 7.2 ± 0.3, respectively) rats that underwent reinstatement testing. A post-hoc between-placement comparison of lidocaine-pretreated rats that underwent reinstatement testing also revealed that the number of prolonged CS+ presentations earned was significantly lower in rats with asymmetric rBLA/plPFC placements compared to rats with unilateral rBLA or unilateral plPFC placements (p ≤ 0.001). A similar post-hoc comparison of vehicle-pretreated rats that underwent reinstatement testing indicated no significant between-placement differences in the number of prolonged CS+ presentations earned.
4. Discussion
4.1. Interaction of the rBLA and plPFC in Regulating Reinstatement of Cocaine-Seeking Behavior
The present findings revealed that following a period of response-extinction training, asymmetric inactivation of the rBLA and plPFC, known to be densely interconnected brain regions (Gabbott et al., 2005; Hoover and Vertes, 2007), profoundly disrupted reinstatement of cocaine-seeking behavior studied under a second-order schedule. Results also demonstrated a concomitant decrease in prolonged CS+ presentations earned following asymmetric inactivation of the rBLA and plPFC. Unilateral inactivation of each site alone did not alter either behavior. Thus, intact communication between the rBLA and plPFC on at least one side of the brain appears to be sufficient for the generation of cocaine-seeking behavior elicited by re-exposure to a combination of discrete and contextual drug-paired cues. Furthermore, the robust reinstatement following unilateral inactivation of the rBLA or plPFC supports the idea that the rBLA and plPFC do not independently regulate cocaine-seeking behavior following their asymmetric inactivation. Collectively, it appears that under a second-order schedule, reinstatement of cocaine-seeking behavior is disrupted if this circuit is interrupted at the same loci (bilateral rBLA or bilateral plPFC inactivation) in both hemispheres (Kantak et al., 2002; Di Pietro et al., 2006) or at different loci (asymmetric rBLA and plPFC inactivation) in opposite hemispheres (present findings).
The relationship between the rBLA and plPFC extends previous single-site studies hypothesizing that these brain regions are important components of cue reinstatement circuitry (Shalev et al., 2002; Kalivas and McFarland, 2003; McLaughlin and See, 2003; See, 2005). A study assessing the role of the rBLA and the dmPFC (targeting the anterior cingulate and prelimbic cortex subregions) in regulating reinstatement of cocaine-seeking elicited by re-exposure to response-independent drug-paired contextual cues found significant impairment following asymmetric inactivation (Fuchs et al., 2007). The present study adds to these findings to suggest that intrahemispheric connectivity between the rBLA and plPFC is critical for reinstatement of cocaine-seeking behavior elicited by re-exposure to a combination of discrete and contextual drug-paired cues.
It is unlikely that regions nearby the rBLA (i.e. the cBLA or central amygdala) or the plPFC (i.e. the infralimbic cortex) were inactivated while infusing lidocaine into these discrete sites. Rats pretreated asymmetrically with lidocaine prior to the reinstatement test would not have shown such a profound attenuation of cocaine-seeking responses if the effects of lidocaine were due to diffusion outside the regions of interest. Notably, previous studies have shown no involvement of the cBLA, central amygdala, and infralimbic cortex in regulating reinstatement of cocaine-seeking behavior (Kruzich and See, 2001; Kantak et al., 2002; McLaughlin and See, 2003; Mashhoon et al., 2009). Although the central nucleus and cBLA are close in proximity to the rBLA, and the infralimbic cortex is close in proximity to the plPFC, studies measuring the spread of 0.5 μl lidocaine, which was the infusion volume used in the present study, found that the boundaries for neural inactivation were limited to a spherical area with a radius of approximately 0.5 mm from the infusion site (Tehovnik and Sommer, 1997). Previous studies using microinjection procedures, infusion volumes, and rates of infusion similar to those used in the present study have shown the spread of lidocaine, which is volume-dependent and not concentration-dependent, to be consistent with values calculated from the spherical volume equation (Sandkuhler and Gebhart, 1984; Martin, 1991; Tehovnik and Sommer, 1997; Martin and Ghez, 1999). Additionally, Kantak and colleagues (2002) showed distinct differences in the regulation of cocaine-seeking behavior during maintenance and reinstatement phases by anatomically proximal structures, the cBLA and rBLA, following lidocaine inactivation of each nucleus, respectively. Thus, in the current experiment, it is improbable that lidocaine spread outside the rBLA or plPFC, but was instead confined to specific regions of interest. It is also important to note that it is unlikely that the effects of asymmetric inactivation of the rBLA and plPFC were related to motor impairment (Kantak et al., 2002; Di Pietro et al., 2006).
4.2. Connectivity between the rBLA, plPFC, and NAcc Core
It is highly likely that the rBLA and plPFC interact with other elements of cue reinstatement circuitry. Past research has shown the nucleus accumbens (NAcc) core, but not shell, to consistently play a critical role in regulating reinstatement of cocaine-seeking behavior (Kalivas and McFarland, 2003; Fuchs et al., 2004; Bäckström and Hyytiä, 2007). Neuroanatomically, the NAcc core shares connections with both the rBLA (Wright et al., 1996) and the plPFC (Gabbott et al., 2005). As the NAcc receives excitatory synaptic input from both the BLA and PFC (O’Donnell and Grace, 1995; Gruber and O’Donnell, 2009), reinstatement of cocaine-seeking behavior elicited by re-exposure to discrete and contextual drug-paired cues may be associated with neuronal activation of the NAcc core. Fos protein expression is thought to be a marker for neuronal activation (Chaudhuri, 1997; Harlan and Garcia, 1998). Previously, Fos protein expression was found to be greatly enhanced in the NAcc core and enhanced to a lesser extent in the NAcc shell of rats undergoing reinstatement of cocaine-seeking behavior elicited by re-exposure to response-contingent drug-paired cues (Kufahl et al., 2009). Preliminary findings from our laboratory have demonstrated enhanced Fos protein expression in the NAcc core (basal cell counts: 39.5 ± 8.3; reinstatement cell counts: 62.4 ± 6.4; t[9] = 2.2, p≤0.05), but not shell (basal cell counts: 34.5 ± 6.2; reinstatement cell counts: 43.3 ± 4.9; t[9] = 1.1, n.s.), of rats re-exposed to discrete and contextual cocaine-paired cues under a second-order schedule (unpublished data). Findings of enhanced Fos expression in the nucleus accumbens core, but not as greatly in the shell, coupled with previous neuroanatomical and neurophysiological findings (O’Donnell and Grace, 1995; Wright et al., 1996; Gabbott et al., 2005; Hoover and Vertes, 2007) suggest that a circuit involving the NAcc core, rBLA and plPFC may be engaged to regulate reinstatement of cocaine-seeking behavior elicited by re-exposure to discrete and contextual drug-paired cues. The identity of potential excitatory or inhibitory activated cells in these regions, as well as the characterization of projection pathways between the rBLA, plPFC, and NAcc core remains uncertain. However, some insights into the interaction of the BLA, the plPFC, and the NAcc core have already been advanced. Kalivas and McFarland (2003) hypothesized that response-contingent cues associated with cocaine self-administration activate a projection from the rBLA to the anterior cingulate/plPFC (i.e. dmPFC), the excitatory output of which converges onto the NAcc core to initiate and maintain cocaine-seeking behavior. In contrast, Miller and Marshall (2005) proposed that contextual cues associated with conditioned place preference activate BLA projections to the plPFC and NAcc core. The BLA projection is thought to activate inhibitory GABA interneurons in the plPFC (Miller and Marshall, 2004), which attenuates excitatory output from the plPFC to the NAcc core. They suggest that the BLA provides significant excitatory drive to the NAcc core to initiate and maintain cocaine-seeking behavior. One explanation for these divergent hypotheses is that the critical final pathway underlying the initiation and maintenance of cocaine-seeking behavior (plPFC to NAcc core or BLA to NAcc core) may depend on whether response-contingent or contextual cues are available to elicit cocaine-seeking behavior. Another explanation involves the conceptual and methodological differences inherent in the self-administration and conditioned place preference procedures. However, Fuchs et al., (2007) concluded that bilateral input from the dmPFC to the rBLA may be required to maintain contextual cue-induced reinstatement of cocaine-seeking behavior in rats trained to self-administer cocaine. This view is distinct from the hypothesis that the BLA accesses relapse circuitry exclusively via the dmPFC (Kalivas and McFarland, 2003), but is also distinct from the hypothesis that inputs from the BLA modulate plPFC and NAcc core activity to regulate contextual cue-induced cocaine-seeking behavior (Miller and Marshall, 2005).
4.3. Conclusions
Clearly, future investigations are needed to investigate whether the flow of information between the plPFC, rBLA and NAcc core during reinstatement of cocaine-seeking behavior elicited by re-exposure to discrete and contextual drug-paired cues is top-down or bottom-up. Nonetheless, the present investigation provides important behavioral evidence supporting critical connectivity between the rBLA and plPFC, as well as preliminary immunohistochemical findings that support a potential important role of downstream signaling mechanisms in the NAcc core, for regulating reinstatement of cocaine-seeking behavior following re-exposure to discrete and contextual drug-paired cues. Elucidating both the behavioral and molecular mechanisms involved in this circuitry has important implications for developing and evaluating treatments for cocaine relapse prevention (Zavala et al., 2008).
Acknowledgments
This project was supported by grant number R01 DA011716 from the National Institute on Drug Abuse. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Abuse or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Yasmin Mashhoon, Email: ymashhoon@mclean.harvard.edu.
Audrey M. Wells, Email: wellsam@email.unc.edu.
Kathleen M. Kantak, Email: kkantak@bu.edu.
References
- Bäckström P, Hyytiä P. Involvement of AMPA/kainate, NMDA, and mGlu5 receptors in the nucleus accumbens core in cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2007;192:571–80. doi: 10.1007/s00213-007-0753-8. [DOI] [PubMed] [Google Scholar]
- Catterall W, Mackie K. Local Anesthetics. In: Hardman J, Limbird L, Molinoff P, Ruddon R, Goodman A, editors. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. 9. New York: 1996. pp. 331–347. [Google Scholar]
- Chaudhuri A. Neural activity mapping with inducible transcription factors. Neuroreport. 1997;8:v–ix. [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP. Limbic activation during cue-induced cocaine-craving. Am J Psychiatry. 1999;156:11–8. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Direct interactions between the basolateral amygdala and nucleus accumbens core underlie cocaine-seeking behavior by rats. J Neurosci. 2004;24:7167–73. doi: 10.1523/JNEUROSCI.1581-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pietro NC, Black YD, Kantak KM. Context-dependent prefrontal cortex regulation of cocaine self-administration and reinstatement behaviors in rats. Eur J Neurosci. 2006;24:3285–98. doi: 10.1111/j.1460-9568.2006.05193.x. [DOI] [PubMed] [Google Scholar]
- Dunnett SB, Meldrum A, Muir JL. Frontal-striatal disconnection disrupts cognitive performance of the frontal-type in the rat. Neuroscience. 2005;135:1055–65. doi: 10.1016/j.neuroscience.2005.07.033. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Ghods-Sharifi S. Amygdala-prefrontal cortical circuitry regulates effort-based decision making. Cereb Cortex. 2007;17:251–60. doi: 10.1093/cercor/bhj143. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Parker MC, See RE. Differential involvement of the core and shell subregions of the nucleus accumbens in conditioned cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2004;176:459–65. doi: 10.1007/s00213-004-1895-6. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Eaddy JL, Su ZI, Bell GH. Interactions of the basolateral amygdala with the dorsal hippocampus and dorsomedial prefrontal cortex regulate drug context-induced reinstatement of cocaine-seeking in rats. Eur J Neurosci. 2007;26:487–98. doi: 10.1111/j.1460-9568.2007.05674.x. [DOI] [PubMed] [Google Scholar]
- Gabbott PL, Warner TA, Jays PR, Salway P, Busby SJ. Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. J Comp Neurol. 2005;492:145–77. doi: 10.1002/cne.20738. [DOI] [PubMed] [Google Scholar]
- Gaffan D, Murray EA, Fabre-Thorpe M. Interaction of the amygdala with the frontal lobe in reward memory. Eur J Neurosci. 1993;5:968–75. doi: 10.1111/j.1460-9568.1993.tb00948.x. [DOI] [PubMed] [Google Scholar]
- Gruber AJ, O’Donnell P. Bursting activation of prefrontal cortex drives sustained up states in nucleus accumbens spiny neurons in vivo. Synapse. 2009;63:173–80. doi: 10.1002/syn.20593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlan RE, Garcia MM. Drugs of abuse and immediate-early genes in the forebrain. Mol Neurobiol. 1998;16:221–67. doi: 10.1007/BF02741385. [DOI] [PubMed] [Google Scholar]
- Hoover WB, Vertes RP. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct. 2007;212:149–79. doi: 10.1007/s00429-007-0150-4. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology (Berl) 2003;168:44–56. doi: 10.1007/s00213-003-1393-2. [DOI] [PubMed] [Google Scholar]
- Kantak KM, Black Y, Valencia E, Green-Jordan K, Eichenbaum HB. Dissociable effects of lidocaine inactivation of the rostral and caudal basolateral amygdala on the maintenance and reinstatement of cocaine-seeking behavior in rats. J Neurosci. 2002;22:1126–36. doi: 10.1523/JNEUROSCI.22-03-01126.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruzich PJ, See RE. Differential contributions of the basolateral and central amygdala in the acquisition and expression of conditioned relapse to cocaine-seeking behavior. J Neurosci. 2001;21(RC155):1–5. doi: 10.1523/JNEUROSCI.21-14-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kufahl PR, Zavala AR, Singh A, Thiel KJ, Dickey ED, Joyce JN, Neisewander JL. c-Fos expression associated with reinstatement of cocaine-seeking behavior by response-contingent conditioned cues. Synapse. 2009;63:823–35. doi: 10.1002/syn.20666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JH. Autoradiographic estimation of the extent of reversible inactivation produced by microinjection of lidocaine and muscimol in the rat. Neurosci Lett. 1991;127:160–4. doi: 10.1016/0304-3940(91)90784-q. [DOI] [PubMed] [Google Scholar]
- Martin JH, Ghez C. Pharmacological inactivation in the analysis of the central control of movement. J Neurosci Methods. 1999;86:145–59. doi: 10.1016/s0165-0270(98)00163-0. [DOI] [PubMed] [Google Scholar]
- Mashhoon Y, Tsikitas LA, Kantak KM. Dissociable effects of cocaine-seeking behavior following D1 receptor activation and blockade within the caudal and rostral basolateral amygdala in rats. Eur J Neurosci. 2009;29:1641–53. doi: 10.1111/j.1460-9568.2009.06705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin J, See RE. Selective inactivation of the dorsomedial prefrontal cortex and the basolateral amygdala attenuates conditioned-cued reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2003;168:57–65. doi: 10.1007/s00213-002-1196-x. [DOI] [PubMed] [Google Scholar]
- Meil WM, See RE. Lesions of the basolateral amygdala abolish the ability of drug associated cues to reinstate responding during withdrawal from self-administered cocaine. Behav Brain Res. 1997;87:139–48. doi: 10.1016/s0166-4328(96)02270-x. [DOI] [PubMed] [Google Scholar]
- Miller CA, Marshall JF. Altered prelimbic cortex output during cue-elicited drug seeking. J Neurosci. 2004;24:6889–97. doi: 10.1523/JNEUROSCI.1685-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CA, Marshall JF. Altered Fos expression in neural pathways underlying cue-elicited drug seeking in the rat. Eur J Neurosci. 2005;21:1385–93. doi: 10.1111/j.1460-9568.2005.03974.x. [DOI] [PubMed] [Google Scholar]
- O’Donnell P, Grace AA. Synaptic interactions among excitatory afferents to nucleus accumbens neurons: hippocampal gating of prefrontal cortical input. J Neurosci. 1995;15:3622–39. doi: 10.1523/JNEUROSCI.15-05-03622.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olton DS, Walker JA, Wolf WA. A disconnection analysis of hippocampal function. Brain Res. 1982;233:241–53. doi: 10.1016/0006-8993(82)91200-8. [DOI] [PubMed] [Google Scholar]
- Sandkuhler J, Gebhart GF. Relative contributions of the nucleus raphe magnus and adjacent medullary reticular formation to the inhibition by stimulation in the periaqueductal gray of a spinal nociceptive reflex in the pentobarbital-anesthetized rat. Brain Res. 1984;305:77–87. doi: 10.1016/0006-8993(84)91121-1. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Floresco SB, Phillips AG. D1 receptor modulation of hippocampal-prefrontal cortical circuits integrating spatial memory with executive functions in the rat. J Neurosci. 1998;18:1613–21. doi: 10.1523/JNEUROSCI.18-04-01613.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See RE. Neural substrates of cocaine-cue associations that trigger relapse. Eur J Pharmacol. 2005;526:140–6. doi: 10.1016/j.ejphar.2005.09.034. [DOI] [PubMed] [Google Scholar]
- Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev. 2002;54:1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty JA, Gnys M, Kassel JA, Hickcox M. First lapses to smoking: within subjects analysis of real-time reports. J Consult Clin Psychol. 1996;64:366–379. doi: 10.1037//0022-006x.64.2.366. [DOI] [PubMed] [Google Scholar]
- Spealman RD, Barrett-Larimore RL, Rowlett JK, Platt DM, Khroyan TV. Pharmacological and environmental determinants of relapse to cocaine-seeking behavior. Pharmacol Biochem Behav. 1999;64:327–36. doi: 10.1016/s0091-3057(99)00049-0. [DOI] [PubMed] [Google Scholar]
- Swanson L. Brain Maps: Structure of the Rat Brain. Amsterdam: Elsevier; 1992. [Google Scholar]
- Tehovnik EJ, Sommer MA. Effective spread and timecourse of neural inactivation caused by lidocaine injection in monkey cerebral cortex. J Neurosci Methods. 1997;74:17–26. doi: 10.1016/s0165-0270(97)02229-2. [DOI] [PubMed] [Google Scholar]
- Warburton EC, Baird AL, Morgan A, Muir JL, Aggleton JP. Disconnecting hippocampal projections to the anterior thalamus produces deficits on tests of spatial memory in rats. Eur J Neurosci. 2000;12:1714–26. doi: 10.1046/j.1460-9568.2000.00039.x. [DOI] [PubMed] [Google Scholar]
- Wright CI, Beijer AV, Gröenewegen HJ. Basal amygdaloid complex afferents to the rat nucleus accumbens are compartmentally organized. J Neurosci. 1996;16:1877–93. doi: 10.1523/JNEUROSCI.16-05-01877.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young ST, Porrino LJ, Iadarola MJ. Cocaine induces striatal c-fos-immunoreactive proteins via dopaminergic D1 receptors. Proc Natl Acad Sci USA. 1991;88:1291–5. doi: 10.1073/pnas.88.4.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala AR, Browning JR, Dickey ED, Biswas S, Neisewander JL. Region-specific involvement of AMPA/Kainate receptors in Fos protein expression induced by cocaine-conditioned cues. Eur Neuropsychopharmacol. 2008;18:600–11. doi: 10.1016/j.euroneuro.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]


