Abstract
The amount of information one can maintain in working memory (WM) increases between childhood and adulthood (Gathercole, 1994; Gathercole, 1999; Klingberg, 1998; Luciana, 1998; Luciana & Nelson, 1998). In addition to cognitive changes that occur early in life, childhood and adolescence are periods marked by significant neuroanatomical changes that are thought to underlie cognitive maturation. This study used a mixed state-item design and a parametric “n-back” task to explore the relationship between WM load and neural activity changes with age. Thirty-five participants from two age groups (9 to 13 years and 18 to 23 years) were recruited. Our behavioral results indicated that children performed significantly worse than adults at loads of 2-back, but not 0- and 1-back. Our imaging results indicated that during performance of the 2-back task, children showed evidence for increased transient, but decreased sustained activity, in comparison to adults. These results suggest that for the 2-back condition, children had more difficulty maintaining task relevant information across trials and seemed to engage in a more reactive strategy wherein they reactivated context information on a trial-by-trial basis rather than maintaining over a delay. These results have important implications for understanding the development of specific processes within the WM system.
Keywords: fMRI, development, working memory, n-back, state-item
1. Introduction
A number of studies have shown that working memory performance (WM) improves during childhood (Gathercole, 1994; Gathercole, 1999; Klingberg, 1998; Luciana, 1998; Luciana & Nelson, 1998). In addition, studies that have assessed the relationship between brain-activity and age-related changes in WM from childhood to adulthood have shown that children activate many of the same regions as adults during WM tasks. However, these studies have also shown differences in the level of WM related activity between adults and children, with somewhat variable results across studies. It is possible that some of the variable results across studies reflect differences in the types of tasks used, and the degree to which such tasks tap different components of working memory (e.g., sustained activity that may reflect the maintenance of information versus item related activity that may reflect updating, manipulation, etc.) or use different memory loads. Thus, to better understand how WM related brain activity changes with increasing age, the present study used: 1) a state-item design that allowed us to differentiate between neural correlates of sustained and transient processes associated with WM; and 2) multiple memory loads to examine how age-related brain activity varied as a function of load.
Behavioral studies examining age related change in WM have fairly consistently shown that working memory capacity increases with age. For example, studies using variants of an Sternberg item recognition task, a task that clearly taps the ability to maintain items in WM, have shown that children perform less accurately than adolescents and/or adults at high load levels (de Belleroche & Neal, 1982; De Luca et al., 2003; Luciana, 1998; O’Hare, Lu, Houston, Bookheimer & Sowell, 2008; Thomason et al., 2008; Tsujimoto, Yamamoto, Kawaguchi, Koizumi & Sawaguchi, 2004). Similar results have also been found using WM tasks that tap updating and temporal coding in WM, as well as the maintenance of information. For example, several studies have examined performance on the n-back task (Kwon, Reiss & Menon, 2002; Vuontela, 2003), in which participants must look for items that match a stimulus present n-trials back (e.g., 1, 2 or 3 trials back). This task necessitates the updating of the contents of the memory set on each trial, and also requires individuals to code the order of items within memory. These studies have also consistently found reduced performance in children as compared to adults.
A growing number of studies have also examined the neurobiological mechanisms that may support age related changes in WM function. Although children attain adult-like levels of total cerebral volume by approximately 5 years (Giedd et al., 1996; Kretschmann, Kammradt, Krauthausen, Sauer & Wingert, 1986; Reiss, Abrams, Singer, Ross & Denckla, 1996), grey and white matter in the brain continue to develop through adolescence, with maturational trajectories that differ across regions of the brain. White matter density increases linearly with age from childhood to adulthood (Giedd et al., 1999; Nagy, Westerberg & Klingberg, 2004; Schmithorst, Wilke, Dardzinski & Holland, 2002; Snook, Paulson, Roy, Phillips & Beaulieu, 2005). In contrast, grey matter density is thought to increase and then decrease during pre- and post-adolescence, respectively (Giedd et al., 1999; Giedd et al., 1996; Gogtay et al., 2004; Sowell, Thompson, Holmes, Jernigan & Toga, 1999). Interestingly, maturation of regions thought to subserve WM (i.e., prefrontal, superior parietal, and temporal cortices) occurs last (Gogtay et al., 2004; Sowell et al., 2004; Sowell, Trauner, Gamst & Jernigan, 2002). Further, a number of studies have shown that maturation of these regions is positively correlated with performance on cognitive tasks (Nagy et al., 2004; Sowell et al., 2004; Sowell, Thompson, Tessner & Toga, 2001).
A number of studies have also used functional neuroimaging to compare developmental changes in the neural correlates of WM (Casey et al., 1995; Ciesielski, Lesnik, Savoy, Grant & Ahlfors, 2006; Crone, Wendelken, Donohue, van Leijenhorst & Bunge, 2006; Klingberg, Forssberg & Westerberg, 2002; Kwon et al., 2002; Nelson et al., 2000; Olesen, Nagy, Westerberg & Klingberg, 2003; Schweinsburg, Nagel & Tapert, 2005; Thomas et al., 1999; Tsujimoto et al., 2004). Many of these studies have found that children activate a number of the same regions as adults during WM tasks. However, there are mixed results with regard to the direction of age related changes in functional brain activation during WM tasks. For example, some studies report positive correlations between prefrontal brain activity and age (Casey et al., 1995; Ciesielski et al., 2006; Klingberg et al., 2002; Kwon et al., 2002; Schweinsburg et al., 2005), such that activity is greater in adults than children. In contrast, other studies report negative correlations (Klingberg et al., 2002; Schweinsburg et al., 2005; Tsujimoto et al., 2004), such that activity is greater in children than adults.
One possible explanation for this discrepancy across studies is that the majority have used blocked fMRI designs. Blocked fMRI designs confound activity related to processes associated with each individual trial within a task block with processes that are sustained across trials (Visscher, 2003). In the context of a WM task, the use of such a blocked design can confound activity related to sustaining information in WM memory across trials with activity related to processing each individual trial (response selection, updating, temporal coding). It is possible that developmental changes in WM are more associated with one component than another (e.g., maintenance of information versus updating/temporal coding), or that the pattern of age-related change in brain activity associated with one component versus the other might differ (e.g., increased or decreased activity associated with sustained or item related processes). Thus, using a block design may produce variable results across studies if tasks differ in the degree to which they emphasize WM processes such as maintenance of information versus updating/temporal coding. In order to separately examine these different components of brain activity with fMRI, one needs to use a state-item design that allows for separate estimates of sustained versus item-related functional brain activity. Using such a design, if we find age differences in sustained activity, it may suggest that children have relative difficulty actively maintaining items and order information. In contrast, if we find age-related differences in transient or item-related activity, it would suggest that children have difficultly with more item-specific processes, such as updating, temporal coding, or response selection.
Another possible explanation for the discrepancy in the direction of age-related differences in functional brain activation across WM studies is that age differences in the magnitude of activation could reflect age differences in neural efficiency. The hyperactivation in children compared with adults found in some studies may be the consequence of children having to recruit additional cognitive resources to accomplish the same task demands as adults. This phenomenon may be particularly apparent at lower loads that are still within the WM abilities of children. In contrast, hypoactivation in children may represent decreased neural activity when their WM abilities are exceeded at higher load levels. Such hypotheses related to changes in neural efficiency associated with WM have been suggested to account for functional brain activation changes in older adults, but may be equally applicable to development at the beginning of the life span (Reuter-Lorenz, Stanczak & Miller, 1999). Of those studies reporting increased activity in regions for children versus adults, few have compared activation levels in children and adults at multiple load levels. To our knowledge, only a handful of studies have looked at the relationship of WM capacity and load level in children with functional imaging (Klingberg et al., 2002; O’Hare et al., 2008; Thomason et al., 2008). Of these studies, two (Thomason et al., 2008) looked specifically at age related differences in activation across multiple load levels. Thomason et al. (2008) examined activation differences between children (ages 7 to 12) and adults across multiple loads (spans of 2, 4, or 6 in a parametric Sternberg paradigm) and found greater increases in activity within a number of regions in adults versus children. O’Hare et al. (2008) examined activation differences between children (ages 7 to 10), adolescents (ages 11 to 15) and young adults (ages 20 to 28) across multiple loads (spans of 1, 3, and 6 in a parametric Sternberg paradigm) and found that linear load-dependent activity was significant increased in adolescents and young adults relative to children in left superior parietal lobe (BA7) as well as in right superior and inferior cerebellum. Findings from both studies suggest an “immature” WM system in children. However, both studies used a blocked design that did not allow them to examine sustained versus transient components of activation.
Given the issues raised above, the goal of the present study was to explore the relationship between WM demands and neural activity changes with age using: 1) a mixed state-item fMRI design that allowed us to dissociate between sustained and transient WM-related brain activity; and 2) multiple memory loads to examine whether the nature of age-related changes in brain activity (hypo- versus hyperactivity) varied as a function of memory load. We administered a letter n-back task with loads of 0-, 1-, and 2-back to children (ages 9 to 13 years) and young adults (ages 18 to 23 years). We predicted that if developmental changes in WM reflected increases in the ability to maintain information in WM or to sustain task control, we should see age related differences in sustained brain activity. In contrast, if developmental changes in WM reflect improvements in item-specific processes (e.g., updating, temporal coding, and/or response selection), we should see age related differences in transient components of brain activity. In addition, if age-related changes in WM brain activity also reflect changes in neural efficiency, then the pattern may vary across memory loads. Specifically, we would predict increased WM related brain activity at lower memory loads in children, but decreased WM related brain activity at higher loads. Importantly, the present study examined whether brain activity associated with sustained and transient processes differ across development, but could not differentiate between different types of sustained (WM maintenance or sustained task control) or transient processes (e.g., updating, response selection, etc.). Should our results suggest that there are developmental differences in one or both types of processing, future studies using task designs that allow for the differentiation between these specific processes would be useful to further clarify the underlying components of any developmental differences between sustained and transient activity.
2. Results
Behavioral Data
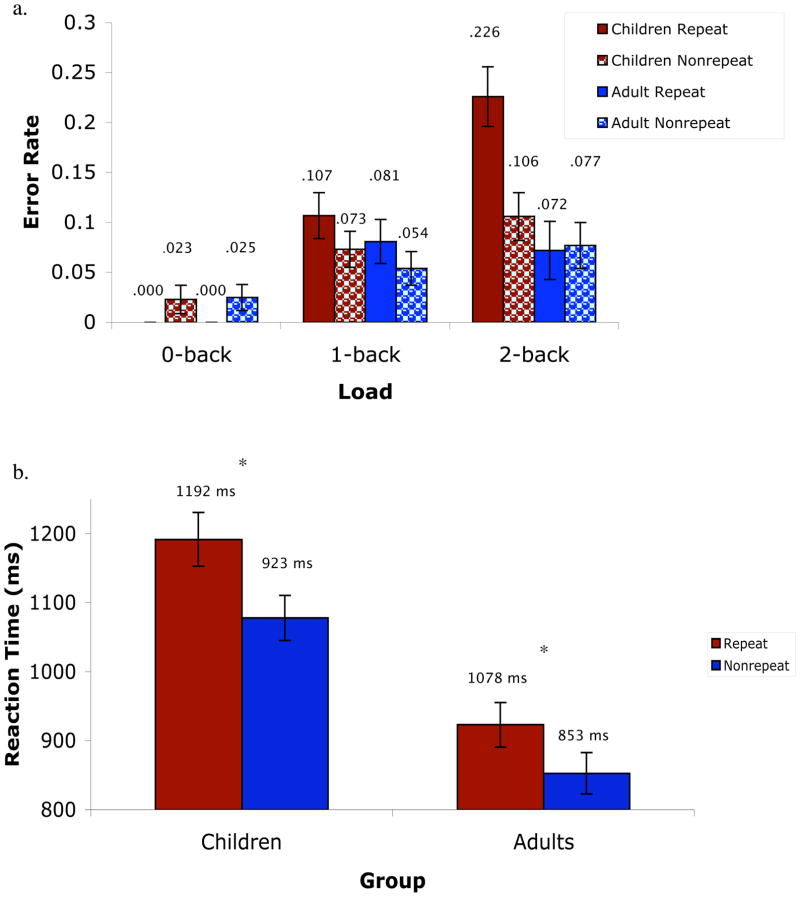
The repeated measures ANOVA for accuracy indicated significant main effects of load (0-, 1-, and 2-back errors; F(2, 66) = 24.45, p = .000, Eta2 = .43), repeat trials (F(2, 33) = 8.6, p = .006, Eta2 = .21), and age (children vs. adults; F(1, 33) = 4.7, p = .04, Eta2 = .13). Participants made increasingly more errors for 0-back; (errors = .01/1% of trials), 1-back (errors = .08/8% of trials) and 2-back (errors = .12/12% of trials) loads. They also made more errors for repeat (errors = .08/8% of trials) than nonrepeat (errors = .06/6% of trials) trials.
The following interactions were also significant: load by age (F(2, 66) = 4.8, p = .02, Eta2 = .13), repeat trials by age (F(1, 33) = 9.6, p = .004, Eta2 = .23), and repeat trials by load by age (F(2, 66) = 7.2, p = .001, Eta2 = .18). Planned contrasts to determine the source of these interactions indicated that performance accuracy was significantly lower in children than adults for the 2-back load but not for the 0- and 1-back loads (see Table 1). Planned contrasts also indicated that children performed significantly worse on repeat trials than nonrepeat trials, whereas performance accuracy for repeat versus nonrepeat trials did not significantly differ in adults. Only the child group showed a significant repeat trial by load interaction, with the greatest number of errors occurring on repeat trials for the 2-back load (see Figure 1a).
Table 1.
Behavioral Data for Groups
| Young (Ages 9 to 13) | Old (Ages 18 to 23) | Significance | ||||
|---|---|---|---|---|---|---|
| Characteristic | M | SD | M | SD | F | p |
| 0-back Accuracy (errors) | .010 | .003 | .009 | .003 | .074 | .787 |
| 1-back Accuracy (errors) | .063 | .014 | .047 | .014 | .603 | .443 |
| 2-back Accuracy (errors) | .113 | .022 | .058 | .021 | 3.19 | .083 |
| Total Accuracy (errors) | .062 | .009 | .038 | .009 | 3.23 | .082 |
| 0-back Reaction Time (ms) | 988 | 116 | 786 | 111 | 21.7 | .000 |
| 1-back Reaction Time (ms) | 1111 | 158 | 884 | 116 | 23.7 | .000 |
| 2-back Reaction Time (ms) | 1242 | 175 | 958 | 171 | 23.7 | .000 |
| Total Reaction Time (ms) | 1114 | 32 | 876 | 31 | 28.6 | .000 |
Figure 1.
(a) Behavioral accuracy for the different working memory trial types. (b) Behavioral reaction times for the different working memory trial types (collapsed across 0-, 1-, and 2-back trials).
The repeated measures ANOVA for reaction time (for correct trials only) indicated significant main effects of load (F(2, 66) = 86.5, p = .000, Eta2 = .72), target trials (F(1, 33) = 26.8, p = .000, Eta2 = .45), repeat trials (F(1, 33) = 84.0, p = .000, Eta2 = .72), and age (F(1, 33) = 28.5, p = .000, Eta2 = .46). Participants responded increasingly more slowly to 0-back (875ms), 1-back (1016ms), and 2-back (1143ms) loads. They also performed more slowly for repeat (1057 ms) than nonrepeat (965 ms) trials and for nontarget (1054 ms) than target (968 ms) trials. The significant effect of age indicated that children (1135ms) responded more slowly than adults (888ms).
The age by repeat trials interaction was significant (F(1, 33) = 4.7, p = .04, Eta2 = .13). Planned contrasts to determine the source of this interaction indicated that RTs were significantly longer in children than adults for both repeat and nonrepeat trial types. Additionally, RTs for repeat trials were significantly longer than RTs for nonrepeat trials for both child and adult groups; however, the size of the effect was greater for the child than the adult group (partial eta square of .753 and .667, respectively; see Figure 1b).
Functional Magnetic Resonance Imaging Data (fMRI)
Sustained State-Related Effects
As is shown in Table 2, four regions showed a main effect of load on sustained activity (left inferior frontal gyrus; BA47, right supramarginal gyrus; BA40, and bilateral superior parietal lobe; BA7) that did not interact with age. The magnitude of sustained activity in bilateral superior parietal lobe increased across 0-, 1-, and 2-back loads. The magnitude of activity in left inferior frontal gyrus and right supramarginal gyrus did not follow the expected pattern of progressively increasing activity with increasing load. Instead, the magnitude of activity in right supramarginal gyrus for the 2-back load fell between that of 0- and 1-back loads, with activity being lowest for the 0-back load and highest for the 1-back load. The magnitude of activity in left inferior frontal gyrus for the 0-back task fell between that of 1- and 2-back loads, with activity being lowest for the 1-back load and highest for the 2-back load.
Table 2.
Regions showing a main effect of load
| Analysis Approach | Regions of Interest | Brodmann’s Area | X | Y | Z | F Value |
|---|---|---|---|---|---|---|
| Sustained | Left Inferior Frontal Gyrus | 47 | −33 | +18 | −14 | 9.2 |
| Sustained | Right Supramarginal Gyrus | 40 | +47 | −21 | +20 | 8.2 |
| Sustained | Right Superior Parietal Lobe | 7 | +19 | −66 | +43 | 11.0 |
| Sustained | Left Superior Parietal Lobe | 7 | −11 | −70 | +39 | 8.2 |
| Transient | Right Cerebellum | +28 | −78 | −43 | 9.2 | |
| Transient | Right Inferior Temporal Gyrus | 20 | +63 | −36 | −20 | 8.5 |
| Transient | Left Precentral Gyrus | 44 | −54 | +11 | +12 | 9.2 |
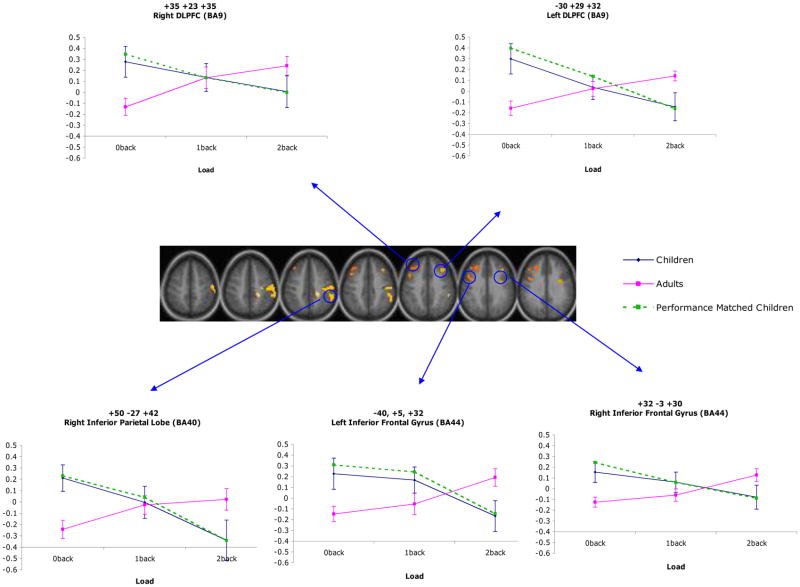
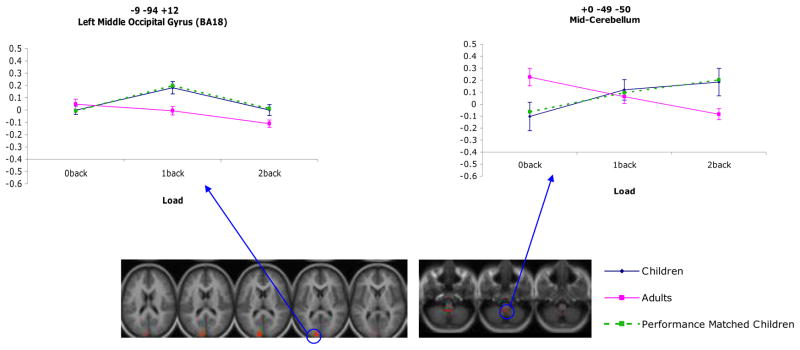
There were four regions that showed a main effect of age for sustained activity (right superior temporal gyrus; BA38, left inferior frontal gyrus; BA47, and right anterior cingulate cortex; BA32 and BA24; see Table 3). In all of these regions, the magnitude of activity was greater for children than adults. There were also number of regions that showed age differences in sustained activity that varied as a function of load (see Table 4). These regions included left cerebellum, right inferior temporal cortex (BA 20), left superior temporal gyrus (BA 22), bilateral supramarginal gyrus (BA 40), bilateral inferior frontal gyrus (BA 44), right middle frontal gyrus (BA 9), right and middle superior parietal lobe (BA 7), right precentral gyrus (BA 4), as well as right pre- and post-central gyrus (BA 6 and 3, respectively). Interestingly, in all of these regions, the younger participants showed hyperactivation in comparison with the adults for the 0-back load and hypoactivation in comparison with adults for the 2-back load. In contrast, the magnitude of activation in most regions did not differ between age groups for the 1-back load (see Figure 2). These regions continued to show an age by load interaction once performance differences between groups were accounted for.
Table 3.
Regions showing a main effect of age
| Analysis Approach | Regions of Interest | Brodmann’s Area | X | Y | Z | F Value | Group Pattern |
|---|---|---|---|---|---|---|---|
| Sustained | Right Superior Temporal Gyrus | 38 | +35 | +10 | −40 | 18.9 | young > old |
| Sustained | Left Inferior Frontal Gyrus | 47 | −28 | +30 | −4 | 24.4 | young > old |
| Sustained | Right Anterior Cingulate | 32 | +22 | +20 | −7 | 13.5 | young > old |
| Sustained | Right Anterior Cingulate | 24 | +8 | +32 | +3 | 15.3 | young > old |
| Transient | Left Postcentral Gyrus | 1/3 | −58 | −22 | +41 | 17.3 | old > young |
| Transient | Left Supramarginal Gyrus | 40 | −48 | −34 | +40 | 13.6 | old > young |
| Transient | Left Superior Parietal Lobe | 7 | −15 | −68 | +50 | 14.9 | old > young |
Table 4.
Regions showing an age by load interaction
| Analysis Approach | Regions of Interest | Brodmann’s Area | X | Y | Z | F Value |
|---|---|---|---|---|---|---|
| Sustained | Left Cerebellum | −29 | −48 | −53 | 10.5 | |
| Sustained | Right Inferior Temporal Gyrus | 20 | +63 | −34 | −19 | 9.8 |
| Sustained | Left Superior Temporal Gyrus | 22 | −54 | +10 | +0 | 9.0 |
| Sustained | Left Middle Frontal Gyrus | 9 | −30 | +29 | +32 | 10.3 |
| Sustained | Left Inferior Frontal Gyrus | 44 | −40 | +5 | +32 | 10.1 |
| Sustained | Right Inferior Frontal Gyrus | 44 | +32 | −3 | +30 | 8.0 |
| Sustained | Right Middle Frontal Gyrus | 9 | +35 | +23 | +35 | 8.6 |
| Sustained | Right Inferior Parietal Lobe | 40 | +50 | −27 | +42 | 11.3 |
| Sustained | Right Superior Parietal Lobe | 7 | +19 | −35 | +43 | 8.6 |
| Sustained | Right Superior Parietal Lobe | 7 | +38 | −52 | +58 | 10.7 |
| Sustained | Right Precentral Gyrus | 4 | +36 | −17 | +60 | 10.0 |
| Sustained | Right Superior Parietal Lobe | 7 | +0 | −69 | +58 | 9.4 |
| Sustained | Right Precentral Gyrus | 6 | +4 | −10 | +61 | 8.0 |
| Sustained | Right Postcentral Gyrus | 3 | +21 | −28 | +65 | 8.8 |
| Transient | Mid-Cerebellum | +0 | −49 | −50 | 10.7 | |
| Transient | Left Middle Occipital Gyrus | 18 | −9 | −94 | +12 | 12.3 |
Figure 2.
Regions demonstrating a significant load by age interaction for sustained activity.
Transient Item-Related Effects
As is shown in Table 2, three regions showed a main effect of load for item-related activity that did not further interact with age (right cerebellum, right inferior temporal gyrus; BA20, and left precentral gyrus; BA44). The magnitude of item-related activity for the 2-back load fell between that of the 0-back and 1-back loads in both right cerebellum and left inferior frontal gyrus, with activity being lowest for the 0-back load and highest for the 1-back load in both cases. The magnitude of item-related activity for the 0-back load fell between that of the 2-back and 1-back loads in right inferior temporal cortex, with activity being lowest for the 2-back load and highest for the 1-back load. There were three regions that showed a main effect of age for item-related activity (right postcentral gyrus; BA1/3, left supramarginal gyrus; BA40, and right superior parietal lobe; BA7; see Table 3). In all of these regions, the magnitude of activity was less in children than adults. There were also two regions that showed an age by load interaction, such that age differences in transient item-related activity varied across load (see Table 4). These regions continued to show an age by load interaction when performance differences between groups were accounted for. Within the mid-cerebellar region, the children showed hypoactivation compared with adults for the 0-back load and hyperactivation compared with the adults for the 2-back load. The magnitude of activity did not significantly differ between age groups for the 1-back load. Within left lingual gyrus, the magnitude of activity did not significantly differ between groups for the 0-back load; however, children showed hyperactivation compared with adults for both the 1-back and 2-back loads (see Figure 3). Examples of timecourses for item effects (age, load, and age by load) are attached as supplemental data.
Figure 3.
Regions demonstrating a significant load by age interaction for transient activity.
Correlations between brain activity and behavior
We examined correlations between activity in regions showing a significant group by load interaction for either sustained state-related or transient item-related activity and behavioral performance. Only two regions, right inferior parietal lobe (coordinates +50, −27, +42, p = .04, r = −.35) and right superior parietal lobe (coordinates +38, −52, +58, p = .04, r = .36) showed significant negative correlations between reaction time and brain activity. The correlation between left inferior frontal gyrus (coordinates –40, +5, +32) and reaction time was marginally significant (p = .06, r = −.33). No region showed a significant correlation between performance accuracy and brain activity.
3. Discussion
The goals of the present project were two-fold. First, we were interested in examining age related differences in WM related brain activity associated with processes that are sustained across a task block (e.g., maintenance of information in WM) versus processes that are more transient and item-specific (e.g, updating, temporal coding). Second, we were interested in examining how these differences in functional brain activity varied as a function of memory load, as a means of testing hypotheses related to changes in neural efficiency that may occur with development. As a secondary goal, we were also interested in examining behavioral differences during WM, including age related differences in performance accuracy for specific trial types (e.g., repeat and non-repeat trial types) in order to better understand the nature of WM difficulties in children versus adults at higher load levels.
In terms of behavior, we found that performance accuracy significantly differentiated child and adult groups only at the 2-back level, the load with the greatest demands on maintenance, updating and temporal coding. In addition, planned contrasts indicated that the children performed significantly worse on repeat trials than nonrepeat trials, whereas performance accuracy for repeat versus nonrepeat trials did not significantly differ in adults. Only the child group showed a significant repeat by load interaction, with the greatest number of errors occurring on repeat trials for the 2-back load. In other words, children were more likely to make false alarms when an item had been presented earlier but not in the correct n-back position. As described by Gray, Chabris, & Braver (2003), these recently but incorrectly positioned items require greater demand for control, and are more susceptible to errors if the participant is not accurately encoding an item’s “precise position in the temporal sequence of recent stimuli.” Thus, poorer performance by the children on the 2-back load suggests that they have greater difficulty in maintaining and updating the temporal order of items within WM, and may instead rely on familiarity of items when responding.
In terms of the imaging results, there were a number of brain regions that did not show age differences. For example, we found that sustained activity in left inferior frontal gyrus (BA47), right supramarginal gyrus (BA40), and bilateral superior parietal lobe and transient activity in right cerebellum, right inferior temporal gyrus (BA20), and left precentral gyrus (BA44) showed a main effect of load that did not further interact with age. Thus, activity in these regions significantly differed across load levels, but did not significantly differ between groups. Such findings support the theory that the neural network important for WM is in place at an early age, although it may continue to show refinement and development as a function of age.
Further, our imaging results provided evidence that both the distinction between sustained and transient activation and the manipulation of load are critical for understanding developmental changes in the neural systems associated with working memory. In terms of main effects of age, we found approximately equal evidence for alterations in both sustained and transient activation. However, we found much stronger evidence for age related changes in sustained activity that varied as a function of load. Specifically, we found regions across a number of different areas of cortex that showed altered sustained activity in children. These included regions in the cerebellum and the temporal, frontal, and parietal cortices. In contrast, we only found age related changes in transient activity as a function of load in two regions, which were mid-cerebellum and left middle occipital gyrus (BA18). These results are consistent with the hypothesis that processes that are sustained across items within task blocks are a key aspect of developmental changes in WM associated with modulations of brain activity as a function of increasing age. As discussed above, such sustained processes include the maintenance of information within working memory, but may also involve sustained task control processes that specify the key task parameters that need to be maintained (Dosenbach, Fair, Cohen, Schlaggar & Petersen, 2008; Dosenbach et al., 2007). Importantly, developmental changes in sustained activity have been demonstrated in a study of inhibitory control (Velanova, Wheeler & Luna, 2009) which further supports the idea of continued developmental change in sustained task control processes that are not specific to working memory, per se. Our data alone cannot isolate which of these processes are driving the age related changes in sustained activity, but do point to the need for additional studies that can untangle these different components.
Our results also support the hypothesis that developmental changes in WM function also involved alterations in neural efficiency. Specifically, at higher load levels (e.g., 2-back), children showed reduced sustained activity compared to the adults, but enhanced activity compared to adults at the 0-back level in a wide range of regions. In contrast, children showed greater transient activity compared to adults in mid-cerebellum and left middle occipital gyrus at the 2-back level, and reduced activity compared to adults in mid-cerebellum at the 0-back level. Our findings suggest that at least for sustained activity children may need to recruit additional cognitive resources to accomplish task goals (e.g., maintaining task relevant information) at low load levels; however, when WM abilities are exceeded at higher load levels, they show decreased neural activity. These results are different from those reported by Thomason et al. (2008), who primarily found regions whose activity did not differ between children and adults at low loads but that showed greater activity in adults at high loads. It is not clear why Thomason et al did not see hyperactivity in children at low loads, as we did. It is possible that our ability to isolate sustained activity allowed greater power to detect this pattern, as the block design used by Thomason et al. averaged together sustained and transient activity, thus potentially masking subtle differences in one component versus another.
Collectively, our results point to changes in sustained activity and neural efficiency as key aspects of WM function that change across the course of development. As such, an important question is what underlying neurobiological mechanisms drive these developmental processes. As was discussed earlier, both white matter (Giedd et al., 1999; Nagy et al., 2004; Schmithorst et al., 2002; Snook et al., 2005) and grey matter density is thought to change during this period (Giedd et al., 1999; Giedd et al., 1996; Gogtay et al., 2004; Sowell et al., 1999), with maturation of prefrontal, superior parietal, and temporal cortices occurring last (Gogtay et al., 2004; Sowell et al., 2004; Sowell et al., 2002). In addition to maturation of individual regions within the brain, there are also changes in connectivity between regions during childhood and adolescence.
A growing literature has focused on the development of connections between cognitive control regions during childhood and adolescence. Work by Goldman-Rakic suggests that prefrontal efferents (i.e., cortico-cortical and cortico-subcortical connections) begin to form by the second trimester of pregnancy (see Goldman-Rakic, 1987 for review). However, during childhood and adolescence, several maturational processes (synaptic strengthening and pruning as well as axonal myelination) are occurring in the brain as is it becomes more refined to perform complex cognitive functions (Huttenlocher, 1979; Huttenlocher & Dabholkar, 1997; Yakovlev & Lecours, 1967).
Edin, Macoveanu, Olesen, Tegner & Klingberg (2007) found that synaptic strengthening, which resulted in increased synaptic connections between frontal and parietal cortex, accounted for an observed increase in WM related activity between children (mean age of 13 years) and adults (mean age of 23 years). Some research has suggested that the active maintenance of information in WM also depends on recurrent connections between prefrontal and parietal regions, though the contributions to active maintenance may differ between prefrontal and parietal cortex (Curtis, 2006). Thus, the ability to engage in active maintenance or sustained task control may be dependent on the strength of prefrontal to parietal connections. Interestingly, Fair et al. (2007) looked at connectivity among prefrontal-parietal regions involved in cognitive control, and found that the strength of PFC to parietal connections increased as a function of age (see Figure 3 in Fair et al. 2007). Thus, while this theory is only speculative, it is possible that decreased sustained activity in children during performance of the 2-back task in our study may reflect immaturity in connections (both strength of connections, and number of connections) that may lead to more inefficient processing in children. Future studies examining developmental changes in connectivity and its relationship to brain activation during performance of a WM task would help to further explore this hypothesis.
In summary, we found that while children in our study activated a number of regions that adults do during performance of a WM task, there were clearly differences in the pattern of sustained and transient activity across load. Specifically, during performance of the most complex condition (2-back), which requires that the participant maintain context relevant information in the face of intervening distracting information, children showed evidence for increased transient, but decreased sustained activity, in comparison to adults. Additionally, their behavioral performance showed decreased performance accuracy for repeat trials, but not nonrepeat trials, in comparison to adults. Collectively, our results suggest that for the 2-back condition, children had a more difficult time maintaining task relevant information across trials. Decreased reliance on a more ”sustained” approach may be the consequence of an “underdeveloped” system in children, with increased connectivity between prefrontal and parietal regions potentially supporting the enhanced use of sustained processing with increased age. Future studies looking at the relationship between structural and functional changes across a broader age range (childhood, adolescence, and adulthood) is necessary to better understand these important questions.
4. Experimental Procedure
Participants
Thirty-five participants were recruited from a volunteer database of participants from previous studies at Washington University in St. Louis and from Volunteers for Health. Participants were excluded if they had a history of neurologic disorder, major medical disorder, learning disorder, and/or mental retardation. Participants comprised two age groups: (1) 17 children between the ages of 9 and 13 years (M = 10.5, SD = 1.5) and (2) 18 adults between the ages of 18 and 23 years (M = 20.6, SD = 0.9). The child and adult groups were 59% and 44% female, respectively. Estimated IQs for the child (M = 120.1, SD = 12.4) and adult (M = 121.6, SD = 9.9) groups were based on combined scores from the Vocabulary and Matrix Reasoning subtests of the Wechsler Abbreviated Scale of Intelligence. There were no significant between-group differences in either gender or IQ.
Task and Materials
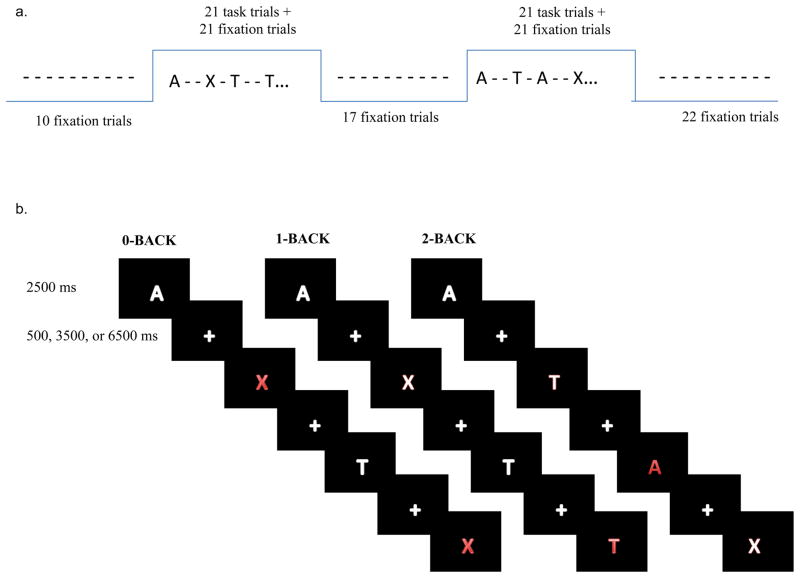
Participants were scanned while performing 0-, 1-, and 2-back loads of the n-back task. For the 0-back condition, participants were asked to press one button (target) when they saw the letter “X” and another button (nontarget) when they saw any other letter. For the 1-back condition, participants were asked to press one button (target) when they saw a letter that was the same as the letter presented one trial earlier and another button (nontarget) when the letter was not the same as that presented one trial earlier. Finally, for the 2-back condition, participants were asked to press one button (target) when they saw a letter that was the same as the letter presented two trials earlier and another button (nontarget) when the letter was not the same as that presented two trials earlier (see Figure 4a).
Figure 4.
(a) FMRI design comprised of 2 task blocks with 42 trials (21 task + 21 fixation) with interleaved fixation blocks of 10, 17, and 22 fixation frames (b) Examples of 0-back, 1-back, and 2-back tasks. Red letters represent targets in each of the specified conditions.
Subjects performed each task condition in a run lasting ~6 minutes (3 runs total). Each run was comprised of 4 blocks, with 2 “task” blocks and 2 “fixation” blocks in alternating order. Each task block lasted 110 seconds (44 acquisitions at a TR of 2.5 seconds), and consisted of 21 task trials pseudo randomly interleaved with 21 fixation trials, as well as a start cue at the beginning of each task block and an end cue at the end of each task block. Task blocks were interleaved with fixation blocks of 10, 17, and 22 frames. The stimuli were presented for 2.5 seconds (see Figure 4b). Participants also performed episodic encoding tasks in the same session, the data from which are the focus of a different report (McAuley et al., in preparation).
Visual stimuli were generated by an Apple PowerMac and PsyScope (Cohen, MacWhinney, Flatt & Provost, 1993) and projected using a Sharp LCD projector onto a screen positioned at the head end of the MR bore. Participants viewed the screen through a mirror attached to the top of the MR head coil. A fiber-optic key press interfaced with the PsyScope Button box was used to record participant’s behavioral performance.
Each participant completed a practice block outside of the scanner. The practice, which consisted of 24 trials (evenly split between 0-, 1-, and 2-back conditions), was repeated until an adequate level of performance was achieved. The practice block was followed by 252 task trials. The condition presented first was counterbalanced across participants. Participants also performed episodic encoding tasks in the same session, the data from which are the focus of a different report (McAuley et al., in preparation).
Neuroimaging Procedure
Scanning was performed on the 1.5T Siemens VISION system at the Research Imaging Center of the Mallinckrodt Institute of Radiology at Washington University Medical School. Functional images were collected using an asymmetric spin-echo echo-planar sequence sensitive to blood oxygenation level-dependent (BOLD) contrast (T2*) (TR = 3000ms, TE = 50ms, FOV = 24cm, flip=90°). During each functional run, 137 sets of oblique axial images were acquired parallel to the anterior-posterior commissure plane (3.75×3.75mm in plane resolution). Nineteen 7 mm thick slices were acquired in each image. Structural images were acquired using a coronal MP-RAGE 3D T1-weighted sequence (TR=9.7ms, TE=4ms, flip=10°; voxel size=1×1×1.2mm). These structural images were used for between subject registration and anatomic localization.
Preprocessing of the fMRI data included: (1) compensation for slice-dependent time shifts; (2) elimination of odd/even slice intensity differences due to interpolated acquisition; (3) realignment of data acquired in each subject within and across runs to compensate for rigid body motion (Ojemann et al., 1997); (4) intensity normalization to a whole brain mode value of 1000; and (5) spatial smoothing with an 8-mm FWHM Gaussian kernel. Functional data were transformed into stereotaxic atlas space (Talairach & Tournoux, 1988) by computing a sequence of affine transforms and resampled to 3 mm cubic voxels. Methods for movement correction and cross subject registration are analogous to the linear methods used in AIR (Woods, Grafton, Holmes, Cherry & Mazziotta, 1998).
Statistical Analysis
Behavioral Data
In addition to assessing overall performance differences between the two age groups, we assessed group differences in performance for various trial types in planned contrasts. An additional manipulation (repeated versus nonrepeated trials) contained within the n-back task allowed us to further investigate group differences in performance for different trial types post-hoc. More specifically, repeated non-target trials represented items whose prior presentation was not in the correct n-back position (i.e., 1-back distracters during the 0-back task, 2-back and 3-back distracters during the 1-back task, and 1-back and 3-back distracters during the 2-back task). Repeated targets were items whose more recent prior presentation was in the correct n-back position, but which also had at least one additional prior presentation within the same block. Participants with inefficiencies in temporal coding would be expected to make false alarms to repeat nontarget trials more frequently (Perlstein, Carter, Noll & Cohen, 2001) because they may be responding on the basis of familiarity rather than accurate encoding of the item within WM (Brahmbhatt, Haut, Csernansky & Barch, 2006). These individuals are also more likely to benefit from repeated presentations of target trials. Thus, age differences in performance for specific trial types (i.e., repeated non-targets) suggest that children have difficulties encoding the temporal order of items in WM and instead may be relying more on familiarity of the items when responding. To assess this hypothesis, accuracy and RTs to correct trials were analyzed using repeated measures ANOVAs with group as a between subjects factor and repeat trials (repeat vs. nonrepeat), target trials (target vs. nontarget), and load (0, 1, and 2 back) as within subject factors.
Functional Magnetic Resonance Imaging Data (fMRI)
FMRI was analyzed using in-house software (FIDL, http://www.nil.wustl.edu/~fidl). To estimate blocked and trial-related neuroimaging effects separately, we used a mixed design (Donaldson, Petersen, Ollinger & Buckner, 2001; Visscher, 2003), with blocks of task trials, interleaved with blocks of rest. Within each task block, trials were jittered so that crosshair fixation trials were randomly inserted in between each letter trial for either 500, 3500, or 6500 milliseconds. Stimuli remained on the screen for 2500 ms. During fixation blocks, a cross hair appeared continuously and participants were told to fixate. The preprocessed fMRI data were analyzed using a general linear model (GLM), coding regressors for transient effects at each load level, regressors for sustained effects at each load level, and two regressors for the baseline signal and the slope of linear drift, all modeled simultaneously. Each of the sustained effects was modeled by a single regressor defined to have the shape of a gamma function convolved with a boxcar function lasting the length of the task block. This effect was estimated as any systematic variance in the signal that was not accounted for by transient effects. For the sustained effect, a single magnitude was calculated for each voxel for the entire length of a task block (for each of two task blocks per run, and excluding the fixation blocks.) Transient effects were estimated using seven time-point regressors (starting at trial onset), for each of the load levels. These seven regressors were delta function regressors that modeled the time course of an event-related timecourse without assuming a hemodynamic response function. This approach reduces co-linearity between the transient and sustained regressors, while minimizing the potential for misestimation of sustained versus transient effects (Visscher et al., 2003). The transient effect was then summarized as a magnitude parameter based on a cross-correlation of these seven timepoints with a contrast derived by convolving a boxcar function lasting 2.5 seconds with a standard hemodynamic response. This approach allows us to obtain a single estimate for the transient effects (to enhance power), but provides protection against misestimation by first separating sustained and transient effects using the delta function approach described above.
These estimates (the single magnitude for sustained, and the single magnitude for item effects) were then entered into appropriately designed ANOVAs and t-tests (described below) that treated subjects as a random factor. Images were subjected to a stringent threshold to control for false-positive rates, using a cluster-size threshold of 13 contiguous voxels and a per-voxel alpha of .0001, corresponding to a corrected whole brain false positive rate of approximately .05 based on Monte-Carlo simulations. We conducted voxel-wise ANOVAs with group as a between-subject factor and load (0-, 1-, and 2-back) as a within subject factor, with one set of ANOVAs for state effects, and a different set of ANOVAs for item effects. To examine similarities between children and adults in WM task related activation, we masked out those regions that showed a load by group interaction from the main effect of load. To examine differences between children and adults in WM task related activation, we identified regions that showed a main effect of age or an age by load interaction. Imaging data was reanalyzed using a subset of children (n = 14) and adults (n = 18) whose performance accuracy did not significantly differ at load levels of 0 (kids mean error rate = .010, adults mean error rate = .009; p = .875), 1 (kids mean error rate = .060, adults mean error rate = .047; p = .562), and 2 (kids mean error rate = .094, adults mean error rate = .058; p = .271).
Supplementary Material
Acknowledgments
This work was supported by grants by a Conte Center for Translational Neuroscience Research (MH071616-01) and a Narsad Established Investigator’s Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Brahmbhatt SB, Haut K, Csernansky JG, Barch DM. Neural correlates of verbal and nonverbal working memory deficits in individuals with schizophrenia and their high-risk siblings. Schizophrenia Research. 2006;87(1–3):191–204. doi: 10.1016/j.schres.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Cohen JD, Jezzard P, Turner R, Noll DC, Trainor RJ, Giedd J, Kaysen D, Hertz-Pannierk L, Rapoport JL. Activation of prefrontal cortex in children during a nonspatial working memory task with functional MRI. Neuroimage. 1995;2:221–229. doi: 10.1006/nimg.1995.1029. [DOI] [PubMed] [Google Scholar]
- Ciesielski KT, Lesnik PG, Savoy RL, Grant EP, Ahlfors SP. Developmental neural networks in children performing a Categorical N-Back Task. Neuroimage. 2006;33(3):980–90. doi: 10.1016/j.neuroimage.2006.07.028. [DOI] [PubMed] [Google Scholar]
- Cohen J, MacWhinney B, Flatt M, Provost J. PsyScope: an interactive graphic system for designing and controlling experiments in the psychology laboratory using Macintosh computers. Behavior Research Methods, Instruments, and computers. 1993;25(2):257–271. [Google Scholar]
- Crone EA, Wendelken C, Donohue S, van Leijenhorst L, Bunge SA. Neurocognitive development of the ability to manipulate information in working memory. Proceedings of the National Academy of Sciences. 2006;103(24):9315–20. doi: 10.1073/pnas.0510088103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis CE. Prefrontal and parietal contributions to spatial working memory. Neuroscience. 2006;28(1):173–80. doi: 10.1016/j.neuroscience.2005.04.070. [DOI] [PubMed] [Google Scholar]
- de Belleroche JS, Neal MJ. The contrasting effects of neuroleptics on transmitter release from the nucleus accumbens and corpus striatum. Neuropharmacology. 1982;21:529–537. doi: 10.1016/0028-3908(82)90043-0. [DOI] [PubMed] [Google Scholar]
- De Luca CR, Wood SJ, Anderson V, Buchanan JA, Proffitt TM, Mahony K, Pantelis C. Normative data from the CANTAB. I: development of executive function over the lifespan. Journal of Clinical and Experimental Neuropsychology. 2003;25(2):242–54. doi: 10.1076/jcen.25.2.242.13639. [DOI] [PubMed] [Google Scholar]
- Donaldson DI, Petersen SE, Ollinger JM, Buckner RL. Dissociating item and state components of recognition memory using fMRI. NeuroImage. 2001;13:129–142. doi: 10.1006/nimg.2000.0664. [DOI] [PubMed] [Google Scholar]
- Dosenbach NUF, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-network architecture of top-down control. Trends in Cognitive Sciences. 2008;12(3):99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NUF, Visscher KM, Palmer ED, Miezin FM, Burgund ED, Grimes AL, Schlaggar BL, Petersen SE. A Core System for the Implementation of Task Sets. Neuron. 2007;(50):799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edin F, Macoveanu J, Olesen P, Tegner J, Klingberg T. Stronger synaptic connectivity as a mechanism behind development of working memory-related brain activity during childhood. Journal of Cognitive Neuroscience. 2007;19(5):750–760. doi: 10.1162/jocn.2007.19.5.750. [DOI] [PubMed] [Google Scholar]
- Fair DA, Dosenbach NUF, Church JA, Cohen AL, Brahmbhatt SB, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL. Development of distinct control networks through segregation and integration. Proceedings of the National Academy of Sciences. 2007;104(33):13507–13512. doi: 10.1073/pnas.0705843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gathercole SE. Neuropsychology and working memory: A review. Neuropsychology. 1994;8(4):494–505. [Google Scholar]
- Gathercole SE. Cognitive approaches to the development of short-term memory. Trends in Cognitive Sciences. 1999;3:410–419. doi: 10.1016/s1364-6613(99)01388-1. [DOI] [PubMed] [Google Scholar]
- Giedd J, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: A longitudinal MRI study. Nature Neuroscience. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giedd J, Snell JW, Lange N, Rajapaske J, Casey BJ, Kaysen D, Vaituzis CK, Vaus YC, Hamburger SD, Kozuch PL, Rapoport JL. Quantitative MRI of human brain development: Ages 4–18. Cerebral Cortex. 1996;6(4):551–60. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences. 2004;101(21):8174–9. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Development of corical circuitry and cognitive function. Child Development. 1987;58(58):601–622. [PubMed] [Google Scholar]
- Gray JR, Chabris CF, Braver TS. Neural mechanisms of general fluid intelligence. Nature Neuroscience. 2003;6(3):316–22. doi: 10.1038/nn1014. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR. Synaptic density in human frontal cortex: Developmental changes and effects of aging. Brain Research. 1979;163:195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. Journal of Computational Neurology. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Klingberg T. Concurrent performance of two working memory tasks: Potential mechanisms of interference. Cerebral Cortex. 1998;8:593–601. doi: 10.1093/cercor/8.7.593. [DOI] [PubMed] [Google Scholar]
- Klingberg T, Forssberg H, Westerberg H. Increased brain activity in frontal and parietal cortex underlies the development of visuospatial working memory capacity during childhood. Journal of Cognitive Neuroscience. 2002;14(1):1–10. doi: 10.1162/089892902317205276. [DOI] [PubMed] [Google Scholar]
- Kretschmann HJ, Kammradt G, Krauthausen I, Sauer B, Wingert F. Brain growth in man. Bibl Anat. 1986;28:1–26. [PubMed] [Google Scholar]
- Kwon H, Reiss AL, Menon V. Neural basis of protracted developmental changes in visuo-spatial working memory. PNAS. 2002;99(20):13336–133341. doi: 10.1073/pnas.162486399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciana M, Nelson The functional emergence of prefrontally-guided working memory systems in four-to-eight-year-old children. Neuropsychologia. 1998;36:273–293. doi: 10.1016/s0028-3932(97)00109-7. [DOI] [PubMed] [Google Scholar]
- Luciana M, Nelson CA. The functional emergence of prefrontally-guided working memory systems in four- to eight-year-old children. Neuropsychologia. 1998;36(3):273–293. doi: 10.1016/s0028-3932(97)00109-7. [DOI] [PubMed] [Google Scholar]
- Nagy Z, Westerberg H, Klingberg T. Maturation of white matter is associated with the development of cognitive functions during childhood. Journal of Cognitive Neuroscience. 2004;16(7):1227–33. doi: 10.1162/0898929041920441. [DOI] [PubMed] [Google Scholar]
- Nelson CA, Monk CS, Lin J, Carver LJ, Thomas KM, Truwit CL. Functional neuroanatomy of spatial working memory in children. Developmental Psychology. 2000;36(1):109–16. doi: 10.1037//0012-1649.36.1.109. [DOI] [PubMed] [Google Scholar]
- O’Hare ED, Lu LH, Houston SM, Bookheimer SY, Sowell ER. Neurodevelopmental changes in verbal working memory load-dependency: an fMRI investigation. Neuroimage. 2008 doi: 10.1016/j.neuroimage.2008.05.057. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojemann J, Akbudak E, Snyder A, McKinstry R, Raichle M, Conturo T. Anatomic localization and quantitative analysis of gradient refocused echo-planar fMRI susceptibility artifacts. Neuroimage. 1997;6:156–167. doi: 10.1006/nimg.1997.0289. [DOI] [PubMed] [Google Scholar]
- Olesen PJ, Nagy Z, Westerberg H, Klingberg T. Combined analysis of DTI and fMRE data reveals a joing maturation of white and grey matter in a fronto-parietal network. Brain Res Cogn Brain Res. 2003;18(1):48–57. doi: 10.1016/j.cogbrainres.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Perlstein WH, Carter CS, Noll DC, Cohen JD. Relation of prefrontal cortex dysfunction to working memory and symptoms in schizophrenia. American Journal of Psychiatry. 2001;158(7):1105–1113. doi: 10.1176/appi.ajp.158.7.1105. [DOI] [PubMed] [Google Scholar]
- Reiss AL, Abrams MT, Singer HS, Ross JL, Denckla MB. Brain development, gender, and IQ in children. A volumetric imaging study. Brain. 1996;119(Pt 5):1763–74. doi: 10.1093/brain/119.5.1763. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Stanczak L, Miller AC. Neural recruitment and cognitive aging: Two hemispheres are better than one, especially as you age. Psychological Science. 1999;10(6):494–499. [Google Scholar]
- Schmithorst VJ, Wilke M, Dardzinski BJ, Holland SK. Correlation of white matter diffusivity and anisotropy with age during childhood and adolescence: a cross-sectional diffusion-tensor MR imaging study. Radiology. 2002;222(1):212–8. doi: 10.1148/radiol.2221010626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg AD, Nagel BJ, Tapert SE. fMRI reveals alteration of spatial working memory networks across adolescence. Journal of the International Neuropsychological Society. 2005;11(5):631–44. doi: 10.1017/S1355617705050757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snook L, Paulson LA, Roy D, Phillips L, Beaulieu C. Diffusion tensor imaging of neurodevelopment in children and young adults. Neuroimage. 2005;26(4):1164–73. doi: 10.1016/j.neuroimage.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Batth R, Jernigan TL, Toga AW. Localizing age-related changes in brain structure between childhood and adolescence using statistical parametric mapping. Neuroimage. 1999;9(6):587–97. doi: 10.1006/nimg.1999.0436. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nature Neuroscience. 1999;2(10):859–61. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in children. Journal of Neuroscience. 2004;24(38):8223–31. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Tessner KD, Toga AW. Improved memory functioning and frontal lobe maturation between childhood and adolescence: a structural MRI study. Journal of International Neuropsychology. 2001;7(3):312–22. doi: 10.1017/s135561770173305x. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Trauner DA, Gamst A, Jernigan TL. Development of cortical and subcortical brain structures in childhood and adolescence: a structural MRI study. Developmental Medicine and Child Neurology. 2002;44(1):4–16. doi: 10.1017/s0012162201001591. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme; 1988. [Google Scholar]
- Thomas KA, King SW, Franzen PL, Welsh TF, Berkowitz AL, Noll DC, Birmaher V, Casey BJ. A developmental functional MRI study of spatial working memory. Neuroimage. 1999;10:327–338. doi: 10.1006/nimg.1999.0466. [DOI] [PubMed] [Google Scholar]
- Thomason ME, Race E, Burrows B, Whitfield-Gabrieli S, Glover GH, Gabrieli JD. Development of spatial and verbal working memory capacity in the human brain. Journal of Cognitive Neuroscience. 2008:1–17. doi: 10.1162/jocn.2008.21028. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto S, Yamamoto T, Kawaguchi H, Koizumi H, Sawaguchi T. Prefrontal cortical activation associated with working memory in adults and preschool children: an even-related optical topography study. Cerebral Cortex. 2004;14(7):703–12. doi: 10.1093/cercor/bhh030. [DOI] [PubMed] [Google Scholar]
- Velanova K, Wheeler ME, Luna B. The maturation of task set-related activation supports late developmental improvements in inhibitory control. Journal of Neuroscience. 2009;29(40):12558–67. doi: 10.1523/JNEUROSCI.1579-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visscher KM, Miezin FM, Kelly JE, Buckner RL, Donaldson DI, McAvoy MP, Bhalodia VM, Peterson SE. Mixed blocked/event related designs separate transient and sustained activity in fMRI. Neuroimage. 2003;19:1694–1708. doi: 10.1016/s1053-8119(03)00178-2. [DOI] [PubMed] [Google Scholar]
- Vuontela V, Steenari MR, Carlson S, Koivisto J, Fjallberg M, Aronen ET. Audiospatial and visuospatial working memory in 6–13 year old school children. Learning and Memory. 2003;10:74–81. doi: 10.1101/lm.53503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods RP, Grafton ST, Holmes CJ, Cherry SR, Mazziotta JC. Automated image registration: I. general methods and intrasubject, intramodality validation. Journal of Computer Assisted Tomography. 1998;22(1):139–152. doi: 10.1097/00004728-199801000-00027. [DOI] [PubMed] [Google Scholar]
- Yakovlev PI, Lecours AR. Regional development of the brain in early life. M. A. Oxford (UK): Blackwell Scientific; 1967. The myelogenetic cycles of regional maturation of the brain; pp. 3–70. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.